Abstract
RIP1 is an adaptor serine/threonine kinase associated with the signaling complex of death receptors (DRs) including Fas, TNFR1, and TRAIL-Rs which can initiate apoptosis. While DRs are dispensable throughout development, RIP1 deletion results in perinatal lethality. The developmental defect caused by absence of RIP1 remains unexplained. In previous studies, RIP1-deficient hematopoietic progenitors failed to reconstitute the T cell compartment and our recent data indicate a new role for RIP1 in TCR-induced activation of the prosurvival NF-κB pathway. Here we show that RIP1 is also critical for B cell development. In addition, RIP1−/− B cells stimulated through LPS/TLR4 are impaired in NF-κB activation but have no major defect in the Akt pathway. Recently, RIP1 has also emerged as a critical player in necrosis-like death, necroptosis, in various cell lines. We have demonstrated that RIP1 deficiency can reverse the embryonic and T cell proliferation defects in mice lacking FADD, a caspase adaptor protein, which indicates a potential role for RIP1 in mediating in vivo necroptosis. We provide an overview and discussion of the accumulating data revealing insights into the diverse functions of RIP1 in survival and death signaling in lymphocytes.
Key wards: RIP1, lymphocytes, apoptosis, necroptosis, NF-κB
Introduction
Members of the neural growth factor receptor (NGFR)/tumor necrosis factor receptor (TNFR) superfamily are single transmembrane-domain proteins characterized by the presence of cysteine-rich repeats in the extracellular domain (1, 2). These receptors have diverse functions, which include signaling cell growth, survival and proliferation, inflammatory responses, and cell death. Several of these receptors are also known as “death receptors” (DRs) including Fas (Apo-1 or CD95), TNFR1, TNF-related apoptosis-inducing ligand receptors (TRAIL-Rs or DR4/5), DR3 and DR6, as they are capable of inducing apoptosis when overexpressed or triggered by their cognate ligands (3, 4). Unlike other members of the NGFR/TNFR family, DRs contain an intracellular motif designated the death domain (DD) which consists of six anti-parallel α-helices and is important for apoptotic signal transduction (5, 6).
In a study of signaling induced by Fas, Stanger et al. discovered the receptor interacting protein (RIP) which can interact with the Fas intracellular domain in yeast cells (7). RIP was also cloned independently as a protein which binds to TNFR1-associated death domain protein (TRADD) (8). Subsequently, additional RIP-like proteins were isolated, all of which contain a serine/threonine kinase domain (9). Accordingly, RIP has since also been frequently referred to as RIP1 or RIPK1. We and others isolated the Fas-associated death domain (FADD or Mort1) protein using Fas as bait (10–12). We have repeatedly isolated RIP1 in searches for proteins that interact with FADD using yeast two-hybrid systems (unpublished data). Fas plays a critical role in maintaining homeostasis in the immune system. Mutations in the Fas gene cause lymphoproliferation (lpr) diseases in mice characterized by progressive lymphadenopathy, splenomegaly and accumulation of autoantibodies, as well as a CD3lo T cell population which also expresses the B cell marker B220 (1, 13). A similar autoimmune-lymphoproliferative syndrome (ALPS) was also reported in human patients carrying mutations in Fas (14, 15). TNFR1 deletion does not lead to lpr-like symptoms in mice but results in compromised pathogen clearance capability (16, 17).
Through FADD, Fas recruits and activates the initiator caspase 8 (in mice and humans) and caspase 10 (only in humans), which in turn process the pro-forms of the downstream executioner caspases 3, 6 and 7 (10, 18–20). The action of caspases leads to the apoptotic demise of a cell. However, we found that deletion of FADD in mice not only blocks Fas-induced apoptosis but also leads to impaired survival and proliferative responses in lymphocytes (21–24). Our recent data show that some of the defects present in FADD-deficient lymphocytes is due to a RIP1-mediated process, likely necrosis-like death (25). Conversely, some of the RIP1-deficient lymphocyte defects could be corrected by FADD deletion (25). We provide an overview of the diverse functions of RIP1 and present our recent data, particularly from the analysis of RIP1−/− B cells.
The RIP1 protein and its expression
The mouse and human RIP1 proteins are 656 and 671 amino acids (aa) in length with predicted molecular weights of 74 and 76 kDa, respectively (7, 8). The first 300 aa encode the serine/threonine kinase domain (KD) at the amino terminus (Fig. 1 A). Towards the carboxy terminus, there is the death domain (DD; aa 559–671), which is homologous to the DD in the Fas and TNFR1 intracellular domains (Fig. 1 A) (5, 6). Within the intermediate sequence between the KD and DD, a potential caspase 8 cleavage site (D324) has been described (26–29). A sequence designated RIP homotypic interaction motif (RHIM) is present in the intermediate domain of RIP1, which mediates RIP1 interaction with RIP3 (Fig. 1 A) (30). A ubiquitinylation site was identified at lysine (K)377, which may be involved in regulating NF-κB activation (31–33).
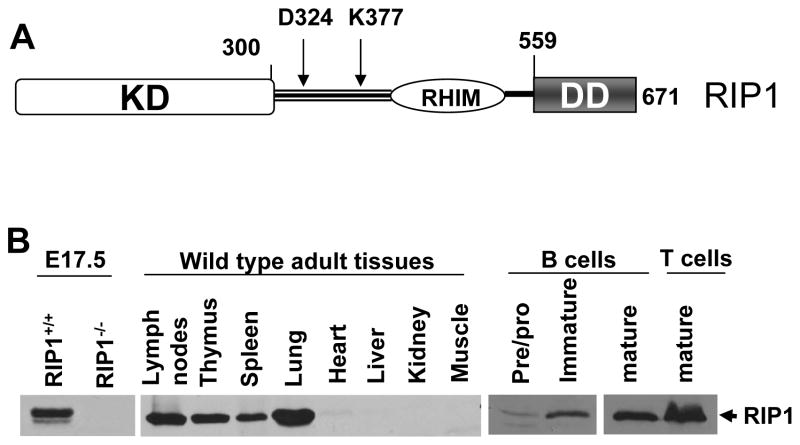
Fig 1. RIP1 protein structure and expression.
A, A diagram of the human RIP1 protein is shown, which consists of an amino terminal serine/threonine kinase domain (KD) and carboxy terminal death domain (DD). The intermediate domain between the KD and DD contains a RIP homotypic interaction motif (RHIM), a caspase 8 cleavage site (D324), and a ubiquitinylation site (K377). B, Western blotting analysis shows the expression of the RIP1 protein in wild type E17.5 mouse embryos, adult mouse tissues, and purified lymphocytes. The RIP1−/− mutant embryo was used as a control.
The RIP1 protein is expressed at low levels at embryonic day (E)14.5 but appears to be upregulated when FADD is deleted (25). RIP1 expression is also readily detectable by western blotting in embryonic tissues at later stages (E17.5; Fig. 1 B). In adult mice, RIP1 is expressed in lymphoid tissues including the lymph nodes, thymus, spleen, as well as the lung (Fig. 1 B). Other adult organs/tissues such as the heart, liver, kidney, and muscle contain little RIP1 protein (Fig. 1 B). We also analyzed RIP1 expression in lymphocyte populations purified by sorting. Pro/pre B-cells in the bone marrow express low levels of the RIP1 protein (Fig. 1 B). Increased expression of RIP1 was observed in bone marrow immature B cells, and in peripheral mature B cells. Peripheral T cells appear to contain higher levels of RIP1 than peripheral mature B cells (Fig. 1 B).
Two distinct roles for RIP1 in T cell apoptosis
In initial studies, overexpression of the full-length RIP1 leads to apoptosis without triggering DRs (7, 8). In addition, overexpression of truncated RIP1 mutants containing the DD, but not the kinase domain, can also lead to apoptosis (8). The function of RIP1 was further investigated using cells lacking RIP1. A RIP1-deficient variant of the human Jurkat T cell line can undergo normal apoptotic responses when stimulated with an agonistic anti-Fas antibody (34). However, a later study found that the same RIP1-deficient Jurkat mutant cells were less sensitive to membrane-bound Fas Ligand (29). In addition, reduction of RIP1 using geldanamycin desensitizes primary human T cells to membrane-bound Fas ligand-induced apoptosis. More recently, RIP1 was suggested to facilitate caspase 8 activation when Fas is stimulated by membrane-bound Fas ligand, but not by a crosslinking anti-Fas antibody (35). It is unclear what role RIP1 plays in primary T cell apoptosis in vivo. Unlike the T cell accumulation phenotype in Fas−/− mice, a T cell maturation defect is present in RIP1-deficient mice (25, 36, 37). RIP1−/− mouse thymocytes isolated from neonates have no defects in apoptosis induced by anti-Fas antibodies, as shown by us and others (25, 36). Although undetected in initial attempts (7, 34), a Fas-RIP1 complex was shown in later studies (35, 38). The results obtained with Jurkat T cells may indicate a requirement for RIP1 in Fas-induced apoptosis in activated mature T cells, which is lacking in the current RIP1 knockout mouse models (25, 36, 37). The requirement for RIP1 in Fas-induced apoptosis in B cells remains to be determined. While TNF is unable to induce apoptosis in wild type thymocytes, we recently showed that absence of RIP1 renders thymocytes hypersensitive to TNF-induced death and this death is blocked by FADD deletion (25). This hypersensitivity to TNF-induced apoptosis may be due to the impairment of the pro-survival signal mediated by NF-κB in RIP1−/− thymocytes, as discussed later. In tumor cells, RIP1 was shown to play an active role in Smac mimetic-induced apoptosis (39). Therefore, RIP1 seems to serve either as an inhibitor or facilitator for apoptosis, in a cell type- and pathway-dependent manner.
RIP1 plays a role in TNFR1-induced activation of NF-κB
Whereas Fas is a professional death receptor, TNFR1 primarily triggers the pro-survival NF-κB pathway. Only when NF-κB is inactivated, is TNFR1 able to induce apoptosis (40, 41). Overexpression of full-length RIP1 not only activates apoptosis but also leads to transcription from an NF-κB reporter promoter (8). The DD of RIP1 alone can induce death and suppress NF-κB activation when overexpressed in HEK293 cells, whereas transient transfection of the serine/threonine kinase domain of RIP1 fails to activate either NF-κB or apoptosis (8). Instead, overexpression of the intermediate region of RIP1 activates NF-κB (8). The intermediate domain of RIP1 can bind to TNFR-associated factor (TRAF) 2 (8) and help recruit IκB kinase (IKK) (42, 43).
When searching for factors controlling TNF-induced activation of NF-κB, Ting et al. mutagenized human Jurkat T lymphoma cells and isolated clones in which TNF was unable to induce NF-κB activation (34). One of the mutant clones lacked RIP1 and has since become a useful cell system which helped reveal several potential functions of RIP1. Functional reconstitution analysis was performed by expressing various mutant forms of RIP1. A kinase-dead mutant of RIP1 fully restored TNF-induced NF-κB activation in RIP1−/− Jurkat T cells, while RIP1 mutants lacking the intermediate domain failed to do so (34). Interestingly, removal of the DD of RIP1 led to constitutive NF-κB activity (34). More recent studies using the RIP−/− Jurkat cells show that K377 is ubiquitinylated, which helps recruit the NEMO subunit of the inhibitor of kappaB kinase (IKK) complex (31–33). A defect in TNF-induced activation of NF-κB was also detected in a transformed RIP1-deficient pre-B cell line (36). A requirement for RIP1 in TNF-induced activation in a mouse embryonic fibroblast (MEF) cell line as shown previously (36, 37, 44) was disputed by a recent study (45). We prepared additional primary RIP1−/− MEFs and observed a defect in TNF-induced phosphorylation of p65 NF-κB in these mutant MEFs (25). These results indicate that in T cells, B cells, and MEFs, RIP1 is required for TNFR1-induced activation of NF-κB.
The function of RIP1 in T cell development and TCR-induced NF-κB activation
Flow cytometric analysis indicates normal thymocyte populations present in RIP1−/− neonates (36) or in E18.5 fetuses (unpublished data). Early postnatal lethality precludes further analysis of the effect of RIP1 deficiency on adult T cells. When adoptively transferred, RIP1−/− fetal liver progenitor cells fail to reconstitute the T cell compartment of lethally irradiated wild type or lymphocyte-deficient Rag1−/− mutant recipient mice (25, 37). Apoptosis has been observed in neonatal RIP1−/− thymus (36). It was suggested that the developmental defect in RIP1−/− T cells was due to impaired pro-survival NF-κB signaling, which leads to TNF-induced apoptosis. Indeed, RIP1−/− thymocytes are hypersensistive to in vitro TNF-induced death (25, 37). However, we found that blocking apoptosis by FADD deletion only partially rescued the developmental defect in RIP1−/− T cells (25). Interestingly, our recent studies have shown that the few mature T cells generated from RIP1−/− fetal liver progenitors have a defect in T cell antigen receptor (TCR)-induced phosphorylation of p65 NF-κB and this defect could not be rescued by FADD deletion (25). Therefore, in addition to TNFR1, the TCR also requires RIP1 for NF-κB activation. Likely, the RIP1−/− T cell defects are due to not only enhanced apoptosis induced by DRs but also diminished NF-κB survival signaling by the TCR.
RIP1 is required in B cell development
In initial analyses, the RIP1−/− bone marrow of E18.5 fetuses appeared to contain B220+ B cells of similar percentages to control RIP1+/+ littermates (36). Ablation of TNFR1 helped prolong the survival of RIP1−/− mice (37). It was reported that normal percentages of B lineage subsets were observed in the spleen in 2–5 day old neonatal RIP1−/− TNF-R1−/− double knockout mice (46). In an earlier study, RIP1−/− fetal liver hematopoietic stem cells were transferred into lethally irradiated wild type or lymphocyte-deficient Rag-1−/− mutant mice (37). The resulting chimeras appeared to contain B220+ B cells in the spleen, which was comparable to chimeras receiving wild type control fetal liver cells (37). Based on these data, it was concluded that RIP1 is dispensable for B cell development, while playing a critical role in T cell development.
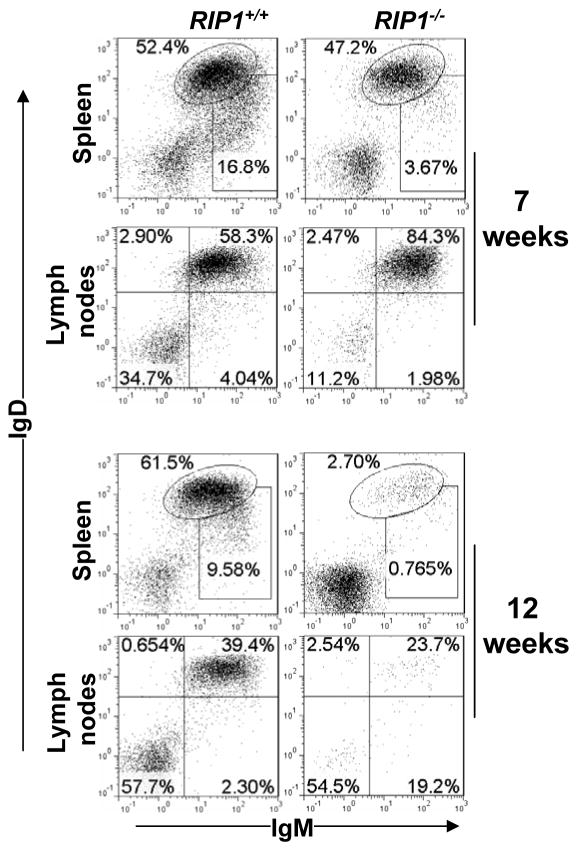
Recently, we performed further analysis of RIP1 function in the B lineage. We adoptively transferred RIP1−/− fetal liver cells into immunodeficient recipient Prkdcscid Il2rgtm1Wjl/SzJ (NSG ) mice (47), as we previously described (25). Consistent with earlier data (37), IgM+IgD+ mature B cells were readily detectable in the spleen and lymph nodes from the resulting hematopoietic chimeras that received RIP1−/− fetal liver cells at 7 weeks post adoptive transfer (Fig. 2). However, at 12 weeks post transfer, peripheral B cell numbers were considerably decreased in RIP1−/− mutant chimeric mice when compared to control RIP1+/+ chimeras (Fig. 2).
Fig. 2. RIP1 deficiency results in a time-dependent decline in the size of the peripheral B cell pool.
RIP1−/− fetal liver cells were adoptively transferred to immunodeficient NSG recipient mice irradiated (200 RAD), as described previously (25, 77). At 7 or 12 weeks post transfer, the resulting hematopoietic chimeras were used to prepare single cell suspensions from the specified lymphoid organs. Reconstitution of the peripheral B cell compartment was determined by flow cytometric analysis of the IgM+ and/or IgD+ populations in the indicated organs. Gates were set to indicate immature (IgDloIgM+) and mature (IgD+IgM+) B cell populations. The numbers are percentages of the gated populations. Data are representative of 5 independent experiments. RIP1+/+ fetal liver cell-transferred recipients were used as a control.
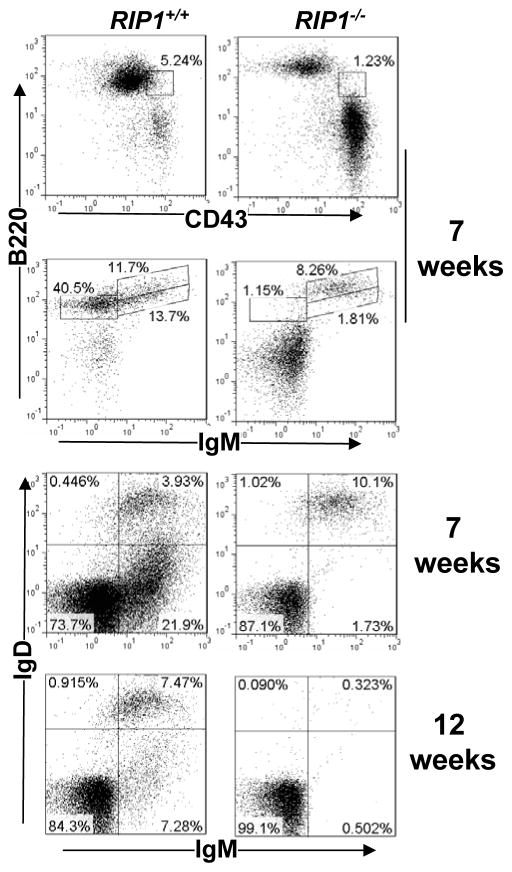
The phenotype of a time-dependent decrease of RIP1−/− B-cells may indicate that RIP1 is required for the maintenance of the peripheral B cell pool. However, it is also possible that there is a defect in the peripheral replenishment of B cells by the adoptively transferred bone marrow precursors. To determine the effect of RIP1 deficiency on the early developmental stages of the B lineage, bone marrow cells from RIP1−/− mutant and RIP1+/+ control chimeras were stained for B220, CD43, IgM and IgD, and flow cytometric analysis performed. A dramatic decrease in the pro-B (CD43+B220lo), pro/pre-B (B220loIgM−), and immature (IgM+IgDlo) B cell populations were detected in RIP1−/− mutant chimeras at 7 weeks post transfer when compared to control RIP1+/+ chimeras (Fig. 3). At 12 weeks post transfer, this defect in early bone marrow B cell development became more severe in RIP1−/− chimeras (data not shown). The recirculating mature (IgM+IgD+) B cell population, while present at 7 weeks post transfer, was depleted in RIP1−/− mutant chimeras at 12 weeks post transfer (Fig. 3). In RIP+/+ control chimeric mice, recirculating mature B cells in the bone marrow were present at both 7 weeks and 12 weeks post transfer of fetal liver cells. Therefore, RIP1 is essential for early B cell development in the bone marrow.
Fig. 3. RIP1 deficiency leads to impaired B lineage cell development in the bone marrow.
At 7 or 12 weeks post transfer of RIP1−/− or RIP1+/+ fetal liver cells, bone marrow cells were isolated from NSG recipients and were stained for B220, CD43, IgM, and IgD, and analyzed by flow cytometry. Gates were set to indicate pro-B (CD43+B220lo), pro/pre-B (B220loIgM−), immature B (IgM+IgD−), and recirculating mature B cell (IgM+IgD+) populations in NSG recipients transferred with RIP1−/− mutant and RIP1+/+ wild type control fetal liver cells. The percentages of each gated populations are shown. The data are representative of 5 independent experiments.
The time-dependent depletion of B cells in RIP1−/− fetal liver cell chimeric mice shown in the current study is reminiscent of the developmental defect observed in RIP1−/− T cells, as shown in a previous study (37). Thymic T cell subsets were normal at 4 weeks following adoptive transfer of fetal liver cells. However, T cell numbers decline dramatically thereafter (37). We confirmed the time-dependent T cell depletion phenotype in our RIP1−/− NSG chimeric mutant mice (25). While present in the thymus at 4 weeks after adoptive transfer of RIP1−/− fetal liver cells, RIP1−/− T cells became undetectable at 12 weeks post adoptive transfer (25) and (data not shown).
A selective defect in TLR-induced responses in RIP1−/− B cells
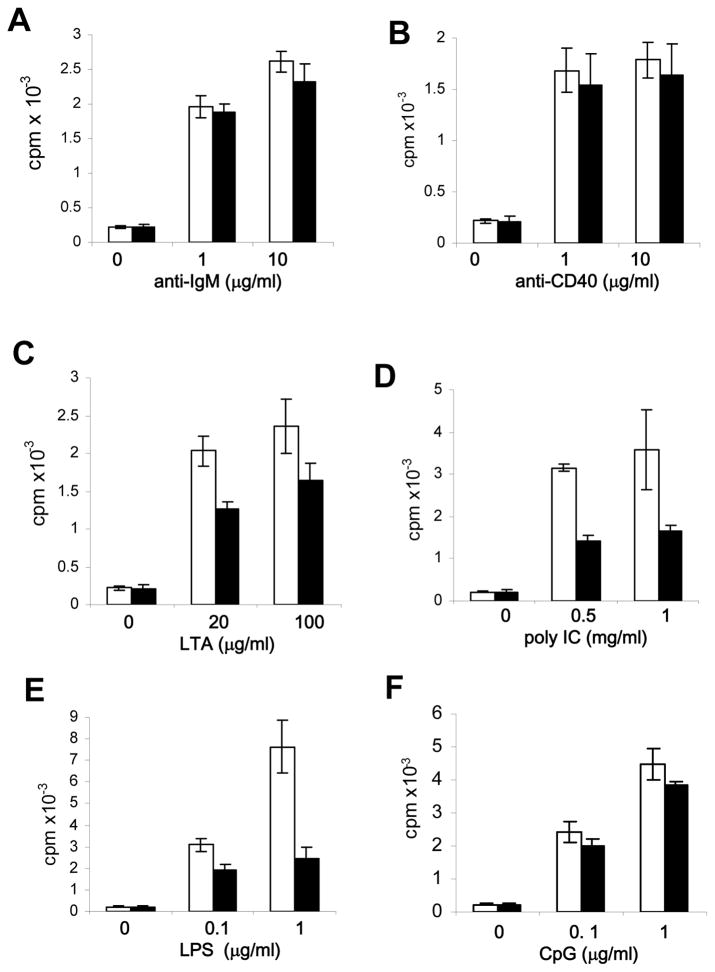
Toll-like receptors (TLRs) are pattern recognition receptors capable of sensing microorganisms by binding to various conserved structures present in macromolecules of pathogens (48–50). B cells express most of the known TLRs. LPS specifically binds to TLR4, and can induce proliferation of B cells. Other pathogen-expressed molecules, including lipoteichoic acid (LTA), viral double stranded (ds)RNA and unmethylated CpG motifs in bacterial DNA, can be recognized by TLR2, TLR3 and TLR9, respectively. RIP1 has been previously shown to play a role in TLR3- and TLR4-induced responses in MEFs (51, 52). Splenic B cells from RIP1−/−TNF-R1−/− double knockout neonates were shown to be defective in TLR4- and TLR9-induced proliferation responses (46). We isolated single knockout RIP1−/− B cells from fetal liver cell chimeras and performed analyses of their proliferation in response to a panel of stimuli. As shown in Figure 4 A and B, no significant proliferation defect was detected in RIP1−/− B cells treated with anti-IgM and anti-CD40 antibodies. However, when compared to control RIP1+/+ B cells, proliferation responses of RIP1−/− B cells were dramatically reduced when stimulated with LTA, poly I:C, or LPS, which are ligands for TLR2, TLR3, and TLR4, respectively, (Fig. 4 C, D, and E). In contrast, TLR9 stimulation with CpG resulted in similar proliferation responses in RIP1−/− and RIP1+/+ B cells (Fig. 4 F). Therefore, while dispensable for proliferation induced by BCR, CD40 and TLR-9, RIP1 is required for B cell proliferation induced by TLR2, TLR3, and TLR4.
Fig. 4. Selective defects in TLR-induced proliferation in RIP1−/− B cells.
B cells were isolated from the spleen and lymph nodes of NSG recipients transferred with RIP1+/+ (open bars) or RIP1−/− (filled bars) fetal liver cells, and stimulated with the indicated agonists. Proliferation was determined by the incorporation of radioactivity of [3H] thymidine into activated B cells (cpm, counts per minute). Error bars are ± SEM of triplicates. The data are representative of 4 independent experiments.
RIP1 is essential for LPS-induced activation of NF-κB and induction of activation markers
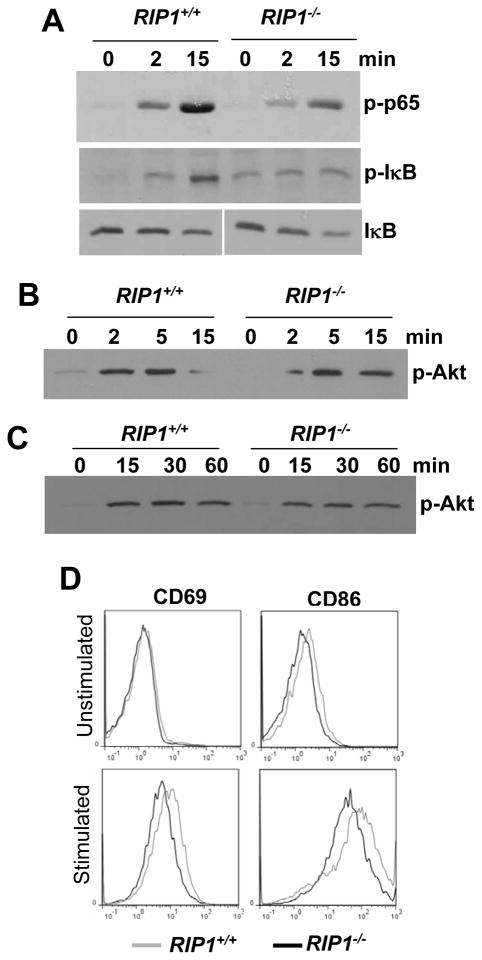
A major signaling event initiated downstream of many TLRs is the activation of NF-κB (48, 50). In MEFs, RIP1 is required for TLR3- and TLR4-induced activation of NF-κB (51). However, in another study, the NF-κB pathway appeared to be unaffected in RIP1−/−TNF-R1−/− double knockout B cells (46). Instead, it was suggested that the proliferation defect in neonatal RIP1−/−TNF-R1−/− B cells stimulated with LPS was due to impaired activation of the Akt pathway, which is crucial for cell survival (46). We performed analysis of LPS-induced signaling in RIP1+/+ and RIP1−/− B cells from NSG chimeric mice receiving the respective fetal liver cells. As shown in Figure 5 A, LPS-induced phosphorylation of p65 NF-κB as well as IκB was consistently reduced in RIP1−/− mutant B cells in comparison with control RIP1+/+ B cells. No major difference in LPS-induced degradation of IκB was detected in RIP1+/+ and RIP1−/− B cells (Fig. 5 A). We also found that RIP1 deficiency has no major impact on the induction of Akt phosphorylation in LPS-treated B cells and MEFs (Fig. 5 B and C).
Fig. 5. LPS-induced signaling in RIP1−/− B cells.
Peripheral B cells purified from NSG recipients transferred with RIP1−/− or RIP1+/+ fetal liver cells (A and B) or MEFs of the indicated genotypes (C) were stimulated with LPS (10 μg/ml), and western blotting analysis was performed using Abs specific for p-p65 NF-κB, p-IκB, IκB, and p-Akt. D, The CD69 and CD86 activation markers on B cells were analyzed by flow cytometry 12 hours post stimulation with LPS (1 μg/ml). Unstimulated B cells were used as a control. Data shown are representative of at least 4 independent experiments.
Cell surface proteins, including CD69 and the costimulatory molecule CD86 (B7.2), can be induced in B cells upon stimulation with LPS (53). Splenic and lymph node B cells were isolated and stimulated with LPS, and upregulation of activation markers were determined. As shown in Figure 5 D representing 4 independent experiments, the induction of both CD69 and CD86 were compromised in RIP1−/− B cells stimulated with LPS.
Our data obtained using adult RIP1−/− single knockout B cells contrast with an earlier study using neonatal double knockout RIP1−/−TNF-R1−/− B cells which presumably have normal induction of CD86 when stimulated with LPS (46). Both CD69 and CD86 are targets of NF-κB (54, 55) (http://bioinfo.lifl.fr/NF-kB/). Induction of CD69 and CD86 is dependent on TIR-domain-containing adapter-inducing interferon-β(TRIF) (53) and therefore, the compromised expression of CD69 and CD86 in RIP1−/− B cells (Fig. 5 D) is indicative of defective TRIF-mediated signaling as well as impaired NF-κB activity (Fig. 5 A). An earlier study using RIP1−/− MEFs also showed a role for RIP1 in TRIF-dependent activation of NF-κB through TLR4 (52). In total, the current study reveals a novel role for RIP1 in B cell development and LPS-induced NF-κB activation in B cells, which was not been appreciated in previous studies (36, 37, 46).
A role of RIP1 in necroptosis revealed using in vitro cell systems
Accumulating evidence indicates that RIP1 is a key player in necroptosis (or programmed necrosis or aponecrosis), which is so designated as it shares certain similarities with, yet is distinct from, apoptosis and classical necrosis (38) (56–58). Apoptosis and necroptosis can be triggered by the same inducers, such as DRs and cellular stress. Whereas apoptosis requires caspases, necroptosis occurs when caspases are inhibited. Necroptotic cells undergo swelling and membrane rupture, characteristics similar to classical necrosis caused by trauma and physical damage. Historically, necrosis is considered an uncontrolled cell death process, whereas necroptosis is an ordered cell explosion regulated by specific proteins including RIP1, RIP3, FADD and caspase 8, as discussed below.
Both apoptosis and necrosis have long been observed in TNF-treated cells (59). In L929 fibrosarcoma cells, TNF induces primarily necrosis in the presence of caspase inhibitors (60). Similarly, Fas can also induce necrosis in L929 cells in the presence of caspase inhibitors. Interestingly, dimerization of the caspase adaptor, FADD, can lead to necrosis in caspase 8-deficient Jurkat cells (61). Furthermore, dimerization of the FADD DED alone is sufficient to induce necrosis in caspase 8−/− Jurkat T cells (62). The landmark work by Tschopp and colleagues provided the first evidence that these types of necrosis involve a specific protein, i.e. RIP1 (38). In particular, RIP1−/− Jurkat mutant T cells are unable to undergo TNF-induced necroptosis. The kinase domain of RIP1 plays an obligatory role in TNF-induced necroptosis (57, 63). RIP1 is also required for oxidative stress-induced necroptic cell death (64). Further analysis provided evidence that RIP1-mediated necroptosis is involved in viral control (56). Chemical screen studies identified a small molecule, necrostatin 1 (Nec-1), which specifically targets the kinase active site of RIP1 and blocks necroptosis in various in vitro cell systems (57, 65). Subsequent work showed that RIP3 is also critical for necroptosis induced by TNF (66–68).
Evidence that RIP1-dependent necrosis occurs in vivo
Unlike RIP1−/− cells, FADD-deficient Jurkat cells are hypersensitive to TNF-induced necroptosis (38). In addition, FADD−/− MEFs readily undergo ROS-induced necrotic death (25, 64). Although embryonic lethality has long been observed in FADD−/− mice, it is not clear what causes death of the mutant embryo (21). There appear to be heart defects including thinner ventricular myocardium in E10.5 FADD−/− embryos (69). However, overall cellular proliferation seems normal in E10.5 FADD−/− embryos. At later stages (E14.5), FADD−/− embryo disintegration and massive tissue necrosis become apparent (25). Importantly, RIP1 deficiency appears to completely correct the embryonic defects caused by FADD deletion.
FADD−/− T cells not only are defective in DR-induced apoptosis but also fail to proliferate effectively in response to stimulation of the TCR (21). This paradoxical phenotype is likely not due to abnormal development of T cells, as indicated by studies using conditional FADD knockout mice (22). Strikingly, the absence of RIP1 fully restores normal TCR-induced proliferative responses in FADD−/− T cells (25). In addition, treatment with the RIP1 inhibitor, Nec-1, corrects FADD−/− T cell proliferation defects (25, 70). Unlike the TCR-induced proliferation defect, FADD−/− B cells proliferate normally in response to stimulation of the BCR. However, TLR3/4-induced responses are defective in FADD−/− B cells (23). Interestingly, this TLR signaling defect remains in FADD−/−RIP1−/− double knockout B cells (25). Taken together, these findings suggest that RIP1 plays differential roles in T cells and B cells.
Conclusions
Although RIP1 was originally isolated as a Fas-interacting protein, it is also involved in signaling by the TCR, TNFR1, and TLRs. RIP1 is necessary for efficient apoptosis triggered by membrane-anchored Fas ligand, but not by soluble Fas ligand. In addition, RIP1 is required for activation of NF-κB induced by the TCR, TNFR1, and TLRs, which is critical for lymphocyte survival. Without RIP1, T and B cells die at immature stages, likely due to impaired NF-κB activation in the TCR, TNFR1 and other yet unidentified pathways, which results in enhanced apoptosis and diminished survival. However, these need to be confirmed in the future using approaches including reverse genetics by introducing hypomorphic RIP1 mutants into RIP1−/− lymphocytes.
Whereas RIP1 mediates apoptosis, NF-κB activation, and necroptosis, its relative, RIP3 has no role in NF-κB or apoptosis, but is critical for necroptosis (71). However, RIP3 knockout mice have no developmental or lymphocyte defects which may be due to necrosis (71). Caspase 8-deficient mice have essentially identical phenotypes to FADD−/− mice (21, 72–74). Interestingly, RIP3 deficiency completely rescues the embryonic defects in caspase 8−/− mice (75, 76). Furthermore, the DKO mice develop lpr-like symptoms as seen in Fas mutant mice. Further studies are necessary to generate FADD−/−RIP3−/− double knockout mice which may have normal development but exhibit lpr diseases as well. A plausible model is that RIP1 and RIP3 mediate a potent necroptotic pathway in both embryonic cells and lymphocytes which is kept at bay by FADD-caspase 8 to ensure proper embryogenesis and lymphocyte function. While dispensable in embryos, RIP1/RIP3-mediated necroptosis serves as a critical backup mechanism to ensure homeostasis is maintained in lymphocytes if apoptosis fails. The molecular details regarding how RIP1 and RIP3 carry out downstream signaling leading to necroptosis remain largely unknown. Further studies are also necessary to determine the mechanism involved in the regulation of RIP1/RIP3-mediated necroptosis by FADD and caspase 8.
Acknowledgments
The authors thank Dr. M. Kelliher for providing the RIP1+/− mice, Erik Ronzone for technical help, Eric Zhang for critical reading of the manuscript, the Kimmel Cancer Center Flow Cytometry Facility and Jefferson Research Animal Facility for technical support. This study was supported in part by NIH grants (CA95454, AI083915, and AI076788), a TJU Enhancement grant to J.Z; and an NCI core grant (CA137494) to the Kimmel Cancer Center.
Contributor Information
Jianke Zhang, Department of Microbiology and Immunology and Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107 USA.
Haibing Zhang, Department of Microbiology and Immunology and Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107 USA.
Jinghe Li, The Second Hospital of Jilin University, China.
Stephen Rosenberg, Department of Microbiology and Immunology and Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107 USA.
Emily C. Zhang, Department of Microbiology and Immunology and Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107 USA
Xiaohui Zhou, Shanghai Public Health Center, Fudan University, China.
Fengsong Qin, Department of Microbiology and Immunology and Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107 USA.
Mathew Farabaugh, Department of Microbiology and Immunology and Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107 USA.
References
- 1.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1455. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 2.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF Receptor Superfamilies. Integrating Mammalian Biology Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 3.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 5.Itoh N, Nagata S. A novel protein domain required for apoptosis. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 6.Tartaglia LA, Ayres TM, Wong GHW, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 7.Stanger BZ, Leder P, Lee T-H, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 8.Hsu H, Huang J, Shu H-B, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 9.Meylan E, Tschopp J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151–159. doi: 10.1016/j.tibs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Winoto A. A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-induced apoptosis. Mol Cell Biol. 1996;16:2756–2763. doi: 10.1128/mcb.16.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 12.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 13.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Ann Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 14.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 15.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 17.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 18.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Kramer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 19.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 20.Slee EA, Adrain C, Martin SJ. Executioner Caspase-3, -6, and -7 Perform Distinct, Non-redundant Roles during the Demolition Phase of Apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Rosenberg S, Wang H, Imtiyaz HZ, Hou YJ, Zhang J. Conditional Fas-Associated Death Domain Protein (FADD):GFP Knockout Mice Reveal FADD Is Dispensable in Thymic Development but Essential in Peripheral T Cell Homeostasis. J Immunol. 2005;175:3033–3044. doi: 10.4049/jimmunol.175.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imtiyaz HZ, Rosenberg S, Zhang Y, Rahman ZS, Hou YJ, Manser T, Zhang J. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176:6852–6861. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg S, Zhang H, Zhang J. FADD deficiency impairs early hematopoiesis in the bone marrow. J Immunol. 2011;186:203–213. doi: 10.4049/jimmunol.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinon F, Holler N, Richard C, Tschopp J. Activation of a pro-apoptotic amplification loop through inhibition of NF-kappaB-dependent survival signals by caspase-mediated inactivation of RIP. FEBS Lett. 2000;468:134–136. doi: 10.1016/s0014-5793(00)01212-6. [DOI] [PubMed] [Google Scholar]
- 28.Kim JW, Choi EJ, Joe CO. Activation of death-inducing signaling complex (DISC) by pro-apoptotic C-terminal fragment of RIP. Oncogene. 2000;19:4491–4499. doi: 10.1038/sj.onc.1203796. [DOI] [PubMed] [Google Scholar]
- 29.Barcia RN, Valle NS, McLeod JD. Caspase involvement in RIP-associated CD95-induced T cell apoptosis. Cell Immunol. 2003;226:78–85. doi: 10.1016/j.cellimm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a Novel Homotypic Interaction Motif Required for the Phosphorylation of Receptor-interacting Protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 31.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J Biol Chem. 2006;281:13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-intitiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan MJ, Kim Y-S, Liu Z-g. Membrane-Bound Fas Ligand Requires RIP1 for Efficient Activation of Caspase-8 within the Death-Inducing Signaling Complex. J Immunol. 2009;183:3278–3284. doi: 10.4049/jimmunol.0803428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 37.Cusson N, Oikemus S, Kilpatrick ED, Cunningham L, Kelliher M. The Death Domain Kinase RIP Protects Thymocytes from Tumor Necrosis Factor Receptor Type 2-induced Cell Death. J Exp Med. 2002;196:15–26. doi: 10.1084/jem.20011470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 40.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappa B antipoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 41.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Kang J, Friedman J, Tarassishin L, Ye J, Kovalenko A, Wallach D, Horwitz MS. Identification of a cell protein (FIP-3) as a modulator of NF-kappaB activity and as a target of an adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis. Proc Natl Acad Sci U S A. 1999;96:1042–1047. doi: 10.1073/pnas.96.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000;12:301–311. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- 44.Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 45.Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ. 2010;17:482–487. doi: 10.1038/cdd.2009.178. [DOI] [PubMed] [Google Scholar]
- 46.Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 48.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 49.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 50.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 51.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 52.Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 53.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 54.Zou GM, Hu WY. LIGHT regulates CD86 expression on dendritic cells through NF-kappaB, but not JNK/AP-1 signal transduction pathway. J Cell Physiol. 2005;205:437–443. doi: 10.1002/jcp.20420. [DOI] [PubMed] [Google Scholar]
- 55.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 56.Chan FK-M, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A Role for Tumor Necrosis Factor Receptor-2 and Receptor-interacting Protein in Programmed Necrosis and Antiviral Responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 57.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 59.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–2634. [PubMed] [Google Scholar]
- 60.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumura H, Shimizu Y, Ohsawa Y, Kawahara A, Uchiyama Y, Nagata S. Necrotic death pathway in fas receptor signaling. J Cell Biol. 2000;151:1247–1256. doi: 10.1083/jcb.151.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 64.Shen HM, Lin Y, Choksi S, Tran J, Jin T, Chang L, Karin M, Zhang J, Liu ZG. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol. 2004;24:5914–5922. doi: 10.1128/MCB.24.13.5914-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 66.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 68.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Yeh W-C, Pompa JL, McCurrach ME, Shu H-B, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry WS, Lowe SW, Goeddel DV, Mak TW. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 70.Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci U S A. 2010;107:13034–13039. doi: 10.1073/pnas.1005997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varfolomeev EE, Schuchmann M, Luria V, Chainnilkulchai N, Beckmann SJ, Mett I, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham kB, Goncharov T, Holtman H, Lonai P, Wallach D. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 73.Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, Wakeham A, Bouchard D, Yeh WC, McGlade JC, Ohashi PS, Hakem R. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beisner DR, Ch’en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 75.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kambe N, Hiramatsu H, Shimonaka M, Fujino H, Nishikomori R, Heike T, Ito M, Kobayashi K, Ueyama Y, Matsuyoshi N, Miyachi Y, Nakahata T. Development of both human connective tissue-type and mucosal-type mast cells in mice from hematopoietic stem cells with identical distribution pattern to human body. Blood. 2004;103:860–867. doi: 10.1182/blood-2003-04-1160. [DOI] [PubMed] [Google Scholar]