Abstract
We report that the bone marrow stroma-released LL-37, a member of the cathelicidin family of antimicrobial peptides, primes/increases responsiveness of murine and human hematopoietic stem/progenitor cells (HSPCs) to an α-chemokine stromal-derived factor-1 (SDF-1) gradient. Accordingly, LL-37 is upregulated in irradiated BM cells and enhances the chemotactic responsiveness of hematopoietic progenitors from all lineages to a low physiological SDF-1 gradient as well as increases their i) adhesiveness, ii) SDF-1-mediated actin polymerization, and iii) MAPKp42/44 phosphorylation. Mice transplanted with bone marrow (BM) cells ex vivo primed by LL-37 showed accelerated recovery of platelet and neutrophil counts by ~3–5 days compared to mice transplanted with unprimed control cells. These priming effects were not mediated by LL-37 binding to its receptor and depended instead on incorporation of the CXCR4 receptor into membrane lipid rafts. We propose that LL-37, which has primarily antimicrobial functions and is harmless to mammalian cells, could be clinically applied to accelerate engraftment as ex vivo priming agent for transplanted human HSPCs. This novel approach would be particularly important in cord blood transplantations, where the number of HSCs available is usually limited.
Keywords: LL-37, SDF-1, CXCR4, C1P, S1P, stem cell homing
Introduction
Current strategies to accelerate hematopoietic reconstitution after transplantation include transplantation of greater numbers of hematopoietic stem progenitor cells (HSPCs) or ex vivo expansion of harvested HSPCs before transplantation. However, in clinical settings, the number of HSPCs available for allogeneic or autologous transplantation can be low (e.g., umbilical cord blood or in individuals who are poor mobilizers) and strategies to expand HSCs and maintain equivalent engraftment capability ex vivo are limited 1.
We have reported that some compounds present in leukapheresis products, such as platelet-derived microparticles 2 and the complement cascade cleavage fragment anaphylatoxin C3a 3, enhance the homing responses of HSPCs to a low SDF-1 gradient. Similar effects have been described by other investigators for hyaluronic acid 4, the sphingosine-1-phosphate receptor agonist FTY20 5, uridine triphosphate (UTP) 6, and prostaglandin E2 (PGE2) 7. Collectively, these results demonstrate that homing responses of HSPCs can be positively modulated by several factors related to inflammation or tissue injury.
In our previous work, we demonstrated that conditioned media harvested from granulocytes activated with the fifth complement cascade protein (C5) cleavage fragment, C5a anaphylatoxin, also enhance responsiveness of HSPCs to an SDF-1 gradient 8. Therefore, we became interested in identifying which factors released from C5a-stimulated granulocytes are responsible for this effect and focused on the cationic peptide LL-37, a member of the cathelicidin family 8. Interestingly, LL-37, like anaphylatoxin C3a, which we previously identified, belongs to a group of antimicrobial cationic peptides (AMPs) 3, 9. AMPs are host-defense peptides and are an evolutionarily conserved component of the innate immune response that, as previously demonstrated, destroy bacteria, enveloped viruses, fungi, and even transformed cancerous cells, but do not affect the viability of normal eukaryotic cells. These selective effects of AMPs in destroying bacteria, but not normal eukaryotic cells, depend on differences in electrostatic and hydrophobic properties of cell membranes between prokaryotic and eukaryotic cells.
We have reported that LL-37, like C3a, enhances chemotactic responsiveness of CFUGM to a low SDF-1 gradient 8. This effect, like that described for C3a, is dependent on incorporation of the SDF-1 receptor CXCR4 into membrane lipid rafts. At the mechanistic level, CXCR4 exerts stronger signaling and more robust responsiveness to low doses of SDF-1 after inclusion into lipid rafts 3.
The aim of this report is to shed more light on this LL-37 priming phenomenon by employing appropriate in vitro and in vivo models. First, we provide evidence that LL-37 is upregulated in BM after irradiation and selectively primes the responsiveness of HSPCs from all hematopoietic lineages to SDF-1, but not to other newly identified homing factors, such as sphingosine-1-phosphate (S1P) or ceramide-1-phosphate (C1P) 10, 11. Second, we observed that LL-37 enhances migration of HSPCs even at a very low level of SDF-1 (1–2ng/ml), which supports the notion that the phenomenon of priming modulates responsiveness of HSPCs to physiological concentrations of SDF-1 in tissues without the necessity of increasing SDF-1 secretion. Third, LL-37 enhances the adhesiveness of hematopoietic progenitors, activates MAPKp42/44 in these cells, and induces actin polymerization. Finally, syngeneic BM cells exposed for 30 minutes ex vivo to LL-37 and subsequently transplanted into lethally irradiated recipients accelerated the recovery of platelets and neutrophils by ~3–5 days in transplanted animals compared to mice transplanted with unprimed control BM cells.
Based on the foregoing, we envision that LL-37, which has primarily antimicrobial functions and is harmless to mammalian cells, could be clinically applied to prime ex vivo human HSPCs before transplantation. This novel approach would be particularly important in umbilical cord blood transplantation, where the number of HSPCs available for transplantation is usually limited.
Material and Methods
Animals
Pathogen-free, 4–6-week-old C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). Mice were allowed to adapt for at least 2 weeks and used for experiments at age 6–8 weeks. Animal studies were approved by the Animal Care and Use Committee of the University of Louisville (Louisville, KY).
Bone marrow nucleated cells (BMNCs)
BMNCs were isolated by flushing the femurs and tibias of pathogen-free C57BL/6 mice. Whole bone marrow cells were suspended in BD Pharm Lyse buffer (BD Biosciences, San Jose, CA) to remove RBCs, washed, and resuspended in RPMI medium (Thermo Fisher Scientific, South Logan, Utah) containing 0.5% BSA (Sigma-Aldrich, St. Louis, MO).
Human umbilical cord blood cells
Cord blood (CB) cells were obtained from healthy volunteer donors who had given informed consent and the protocols used were approved by the institutional review board of the University of Louisville. CB nucleated cells were isolated by lysis of RBCs, as described above.
Chemotaxis and colony-forming assays
Medium (650 μl/well) containing resuspension medium, rhSDF-1, LL-37 (AnaSpec, Fremont, CA), and WKYMVM peptide (Tocris Bioscience, Ellisville, MI) was added to the lower chambers of a Costar Transwell with 5-μm-filter, 24-well plates (Costar Corning, Cambridge, MA). Cell suspension (1 × 106 cells/100 μl) was loaded into the upper chambers, the cultures were incubated (37 °C, 95% humidity, and 5% CO2) for 3 hrs, and subsequently cells in the lower chambers were harvested and counted by fluorescence-activated cell sorting (FACS). Migrated cells were assayed for the number of colony-forming units of granulocytes/macrophages (CFU-GM). Briefly, cells were resuspended in human methylcellulose base medium (R&D Systems, Minneapolis, MN), supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF, 25ng/ml) and interleukin-3 (IL-3, 10ng/ml) for CFU-GM, with granulocyte colony stimulating factor (G-CSF, 20ng/ml) for CFU-granulocyte (G), with macrophage colony stimulating factor (M-CSF, 10ng/ml) for CFU macrophage (M), with erythropoietin (EPO, 5 units/ml, Stem cell Tech.) and stem cell factor (SCF, 5ng/ml) for burst-forming units (BFU-E), and with thrombopoietin (TPO, 100ng/ml) for CFU-megakaryocyte (Meg). Cultures were incubated for 6–10 days and scored under an inverted microscope. CFU-GM, CFU-G, CFU-M, CFU-Meg and BFU-E of CB cells were cultured as previous described 3. For toxicity assay, 1–12.5 μg/ml LL-37 was added to the culture systems. In some experiments, to evaluate the influence of lipid raft formation on cell migration, cells were pre-incubated with 2.5 mM methyl-β-cyclodextran (MβCD, Sigma-Aldrich).
Bone Marrow transplantation
Female 6- to 8-week-old C57BL/6 mice were irradiated with a lethal dose of γ-irradiation (950 cGy). Twenty-four hours after irradiation, mice were transplanted with 5 × 105 mBMNCs by tail vein injection. mBMNCs were either pretreated with 2.5 μg/ml of LL-37 or medium for 30 minutes at 37°C, 95% humidity, and 5% CO2. Transplanted mice were bled at intervals (0, 5, 7, 11, 16, and 21 days after transplantation) from the retro-orbital plexus with sterilized Pasteur pipets (Fisher Scientific, Pittsburgh, PA) and Microvette EDTA-coated tubes (Sarstedt Inc., Newton, NC). The white blood cell (WBC) and platelet count were analyzed by Hemavet 950 (Drew Scientific Inc, Oxford, CT). For the colony forming units-spleen (CFU-S) assay, spleens were isolated 12 days after transplantation, fixed in Telesyniczky's solution, and CFU-S colonies counted on the spleen surface. For the CFU-GM assay, femoral BMNCs were isolated 16 days after transplantation and cultured for CFU-GM, as described above.
Sorting of BMNCs
Lin–Sca-1+CD45+ cells and Sca-1+ cells were purified as described elsewhere. Briefly, BMNCs were resuspended in RPMI medium containing 2% FBS and incubated for 30 min on ice with the following antibodies: biotin-conjugated rat anti-mouse Ly-6A/E (Sca-1, clone E13-161.7), streptavidin–PE–Cy5 conjugate, anti-CD45– APC–Cy7 (clone 30-F11), anti-CD45R/B220–PE (clone RA3-6B2), anti-Gr-1–PE (clone RB6-8C5), anti-TCRα/β–PE (clone H57-597), anti-TCRγ/ζ–PE (clone GL3), anti-CD11b–PE (clone M1/70), and anti-Ter-119–PE (clone TER-119). All antibodies were purchased from BD Biosciences and were added at saturating concentrations. Cells were then washed twice and resuspended in RPMI medium containing 2% FBS and sorted by multiparameter, live sterile cell sorting (MoFlo, Dako A/S, Fort Collins, CO).
Phosphorylation of intracellular pathway proteins
Cells were kept in RPMI containing 0.5% BSA overnight in an incubator to achieve quiescence, stimulated with SDF-1 and/or LL-37 for 2 or 5 min at 37°C, and then lysed for 30 min on ice with M-per lysing buffer (Pierce, Rockford, IL) containing protease and phosphatase inhibitors (Sigma-Aldrich). The concentrations of extracted proteins were measured with the BCA Protein Assay Kit, according to the manufacturer's instructions (Thermo Scientific, Rockford, IL), and equal amounts of protein were separated and analyzed for phosphorylation of MAPKp44/42 and AKT (Ser473). Equal loading in the lanes was evaluated by stripping the blots and reprobing with antibodies of MAPKp44/42 and AKT. All phosphospecific antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA). The membranes were developed with an Amersham ECL Western Blotting Detection Reagents and exposed to Amersham Hyperfilm (GE Healthcare, Buckinghamshire, UK).
Lipid raft isolation
Lipid rafts were isolated as previously described 12. Briefly, human acute monocytic leukemia cell line THP-1 cells (5 × 107) were incubated with or without LL-37 for 30 minutes in an incubator. MβCD was applied in some experiments to interrupt formation of lipid rafts. Cells were spun down and lysed in 600ul MEB buffer (20mM MES, 150mM NaCl, 1mM EDTA, pH 6.5), containing 1% Triton X 100, protease inhibitor cocktail, phenylmethylsulfonyl fluoride (PMSF), and sodium orthovanadate, (Santa Cruz Biotechnology, Santa Cruz, CA), for 1 hour on ice. An equal volume of 80% sucrose (w/v, in MEB buffer) was mixed with cell lysate and placed at the bottom of a 5-ml centrifuge tube (Beckman Coulter, Inc, Brea, CA) and overlaid with 30% sucrose and 5% sucrose. Samples were centrifuged at 200,000 g at 4°C for 17 hours with an SW41 rotor (Beckman Coulter, Inc). Fractions were gently removed from the top of the gradient and n-octylglucoside (Sigma-Aldrich) was added to each fraction (100 mM final) to solubilize rafts. An equal volume from each fraction was used for a standard western blot with anti-CXCR4 antibody (Serotec, Oxford, UK) and anti-Lyn antibody (Santa Cruz Biotechnology)
Confocal microscopy assay
To visualize lipid rafts, confocal microscopy assay was performed as previously described 13. Briefly, pre-plated THP-1 cells were incubated with or without LL-37 for 30 minutes, then washed and fixed in 3.7% paraformaldehyde. Cholera toxin B subunit conjugated with fluorescein isothiocyanate (FITC, Sigma Aldrich) was applied to detect ganglioside GM1 and mouse monoclonal anti-hCXCR4 IgG antibody (R&D Systems) and Alexa Fluor 568 goat anti-mouse IgG antibody (Invitrogen, Carlsbad, CA) were applied to detect CXCR4. Stained cells were examined and images were generated using a FLUOVIEW FV1000 laser-scanning confocal microscope (Olympus America Inc., Center Valley, PA)
Actin polymerization
Actin polymerization was determined as previously described 14. Briefly, cells were resuspended in RPMI containing 0.5% BSA at a concentration of 1 × 106cells/ml and incubated with SDF-1 and/or LL37, and then fixed and permeablized. Alexa 488 phalloidin (Invitrogen) was added to visualize F-actin. Mean fluorescence intensity (MFI) was measured with an LSR II flow cytometer (Becton Dickinson, Mountain View, CA). The relative fluorescence change was normalized by the change for unstimulated cells.
mRNA quantification for cathelicidin
Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Messenger RNA was reverse transcribed with 500 U of Moloney murine leukemia virus reverse transcriptase primed with oligodT, and the resulting cDNA fragments were amplified using the SYBR Green system (Applied Biosystems, Carlsbad, CA). Primer sequences for β-2 microglobulin (β-2m) were 5 ’-CAT ACGCCTGCAGAGTTAAGC-3 ’ (F) and 5 ’-GATCACATGTCTCGATCCCAGTAG-3’ (R). For cathelicidin, the primer sequences were 5’-CACAGCCCTTTCGGTTCAAG-3’ (F) and 5’- CAATCTTCTCCCCACCTTTGC-3’ (R). The relative quantity of the target was normalized to an endogenous gene (β-2m) and, relative to a calibrator, is calculated as 2–ddCt (fold difference).
Detection of the LL-37 receptor (FPRL-1) by reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA from BMNCs and purified HSPCs were isolated and reverse transcribed as described above. The resulting cDNA fragments were amplified using 5 U of Thermus aquaticus (Taq) polymerase. For FPRL-1, the primer sequences were 5 ’-TCATTGCCTTGGACCGCTGTAT (F) and 5 ’-AAAAGGGGCAAAGTGAGAATGAGAG-3’ (R). PCR products were run in 2% agarose gels and photographed under UV light. The sizes of PCR products were verified against 100-bp DNA ladder.
Statistical analysis
Arithmetic means and standard deviations were calculated using Instat 1.14 software (Graphpad, San Diego, CA). Statistical significance was defined as P<0.05. Data were analyzed using Student's t-test for unpaired samples and the t-test for paired samples.
Results
LL-37 is upregulated in irradiated BM and increases chemotactic responsiveness of progenitor cells from all hematopoietic lineages to a physiological low SDF-1 gradient
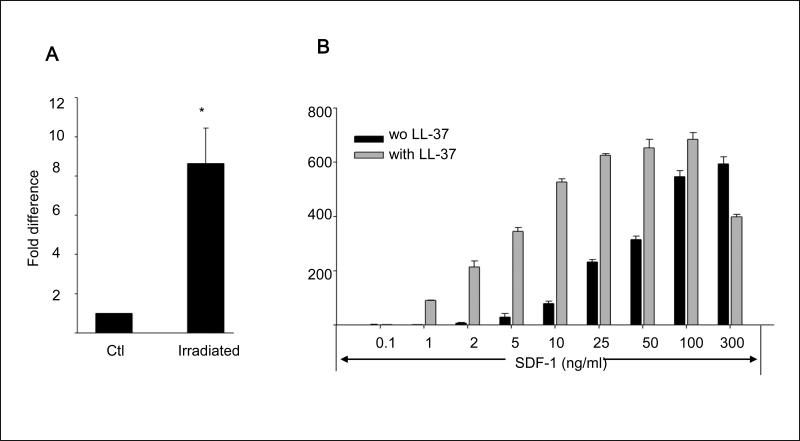
Our recent studies identified activated granulocyte-derived LL-37 as a novel SDF-1–CXCR4 axis-priming factor for HSPCs 8. Since in addition to granulocytes, LL-37 has been reported to be secreted from several other cell types, including BM-stroma cells 9, 15, we evaluated the expression of LL-37 in BM cells isolated from control mice and mice conditioned for transplantation by total body irradiation (1000 cGy). Figure 1 panel A shows that LL-37 mRNA significantly increases in BM cells isolated from the bones of irradiated mice.
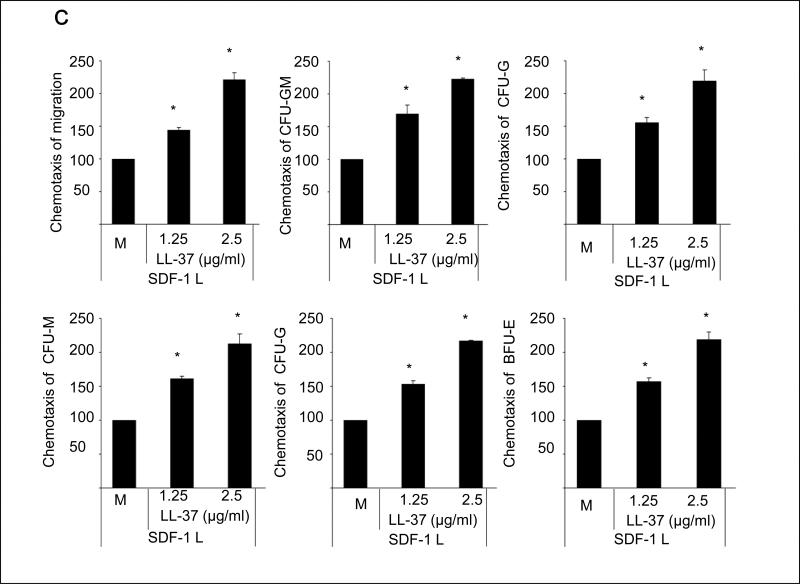
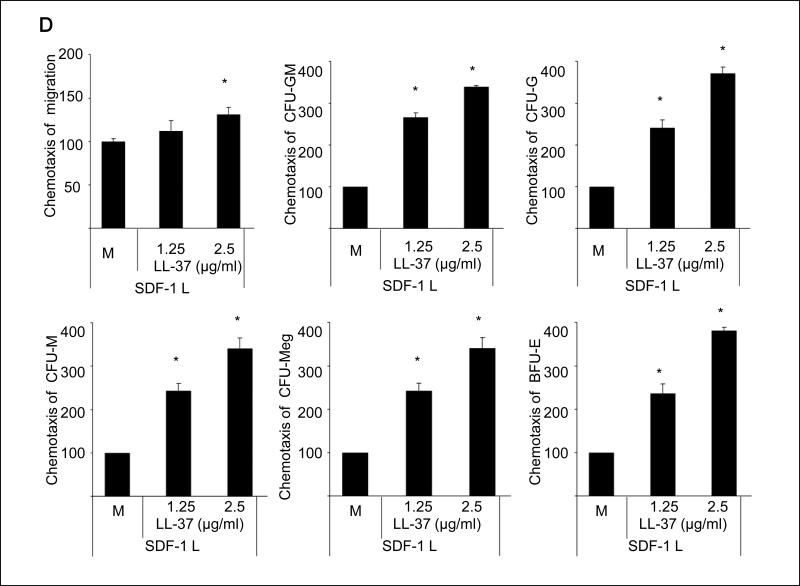
Figure 1. LL-37 enhances responsiveness of mBM- and hCB-derived MNCs and HSPCs to an SDF-1 gradient.
Panel A – Cathelicidin mRNA is upregulated in irradiated BM cells. Whole mBM cells were harvested 24 h after lethal irradiation (myeloablative conditioning for transplantation) and cathelicidin mRNA expression was investigated by RQ-PCR in BM cells from irradiated and control non-irradiated mice. Values are the fold change compared to non-irradiated cells. Data were combined from three independent experiments. *p<0.001. Panel B – Chemotaxis of mBM CFU-GM in response to different concentrations of SDF-1, with and without LL-37. Values are the fold increase of the number of migrated cells compared to the number of migrated cells in medium alone. Gray bars indicate the presence of LL-37 (2.5ug/ml) in the lower Transwell chambers and black bars indicate its absence. The data represent the combined results from three independent experiments performed in duplicate per group (n = 6). Panel C – The priming effect of LL-37 on a low (50ng/ml) SDF-1 gradient in murine BMNCs and clonogenic progenitors. Panel D – The priming effect of LL-37 on a low (50ng/ml) SDF-1 gradient in human BMNCs and clonogenic progenitors. Values are the percentage increase in the number of migrated cells compared with the number of migrated cells in response to SDF-1 alone (M, without LL-37). The data represent the combined results from three independent experiments performed in duplicate per group (n = 6). * p<0.05
In our previous report, we demonstrated that LL-37 enhances the responsiveness of murine CFU-GM to a low SDF-1 gradient that we arbitrary set as 30ng/ml 3, 8. This dose, however, as we recently noticed is ~10x higher than physiological tissue concentration of SDF-1 10. More importantly, as we demonstrate in the current study (Figure 1 panel A), LL-37 increases the chemotactic responsiveness of clonogenic progenitors to much lower doses of SDF-1 (1–3ng/ml) that are within the physiological concentration range of SDF-1 in peripheral blood. Interestingly, we have also learned that the LL-37-mediated priming effect is much stronger than that previously described for C3a anaphylatoxin (data not shown). Furthermore, in the current study we demonstrate that LL-37 exerts its priming effect not only as reported previously for CFU-GM 8 but also for all major hematopoietic lineages and has been effective against both murine BM (Figure 1 panel B) and human umbilical cord blood (UCB) cells (Figure 1 panel C).
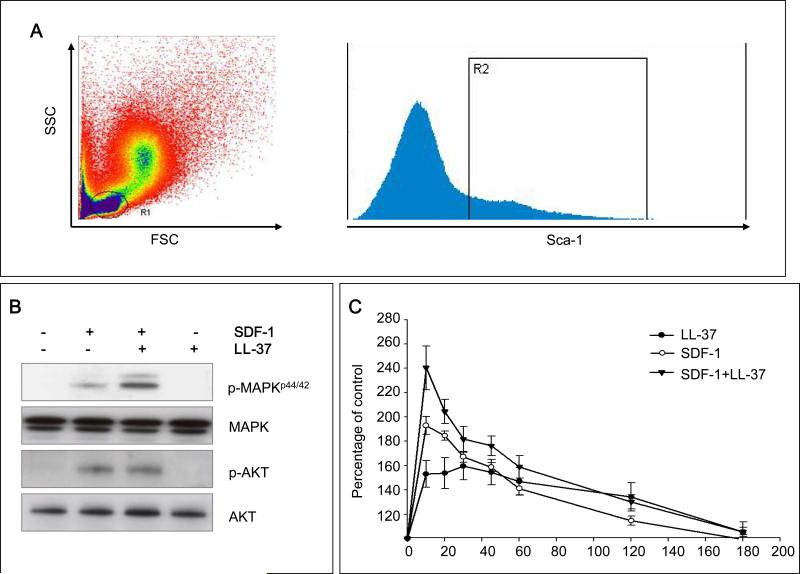
Next, we tested the influence of LL-37 on the activation of pathways related to cell migration in bone marrow nucleated cells (BMNCs). Our signaling studies performed on FACS-sorted Sca-1+ cells (Figure 2 panel A) revealed that, while LL-37 on its own did not affect phosphorylation of MAPKp42/44 and AKT, it increased SDF-1-mediated phosphorylation of MAPKp42/44 (Figure 2 panel B) and actin polymerization (Figure 2 panel C). To our surprise, however, we did not observe any effect of LL-37 on the SDF-1-mediated increase in expression and secretion of MMP-2 and MMP-9 enzymes, which are involved in homing of HSPCs by FACS-purified Sca-1+ cells (data not shown).
Figure 2. LL-37 enhances SDF-1-dependent intracellular signaling and actin polymerization.
Panel A – Sca-1+ BMNCs were sorted from the lymph gate. Panel B – In purified Sca-1+ BMNC, LL-37 increases SDF-1-mediated phosphorylation of MAPKp42/44, but not AKT. Panel C – LL-37 enhances SDF-1-mediated F-actin polymerization in purified Sca-1+ BMNCs. In both experiments, SDF-1 was employed at a dose of 50ng/ml and LL-37 at 2.5μg/ml. Representative data from three independent experiments is shown.
The priming effect of LL-37 is not mediated by known cell surface receptors and depends on lipid raft formation
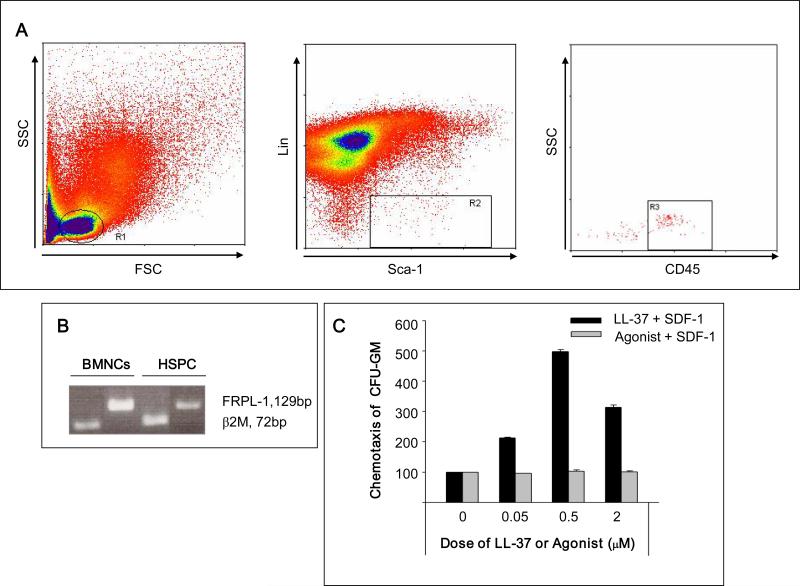
It is known that LL-37 binds to formyl peptide receptor-like-1 receptor (FRPL-1) 16. Figure 3 panel A shows that FACS-sorted Sca-1+Lin–CD45+ cells that are highly enriched in HSPCs express mRNA for this receptor (Figure 3 panel A and B). Therefore, to address whether FRPL-1 is involved in priming of HSPCs by LL-37, we employed the FRPL-1 receptor ligand 16 WKYMVM and evaluated whether this peptide shows a similar priming effect as LL-37. As shown in Figure 3 panel C, WKYMVM, in contrast to LL-37, was not able to prime the response of clonogeneic progenitors to SDF-1. This suggests that the LL-37-mediated priming effect is FRPL-1 independent.
Figure 3. The LL-37-induced priming effect is not mediated by FRPL-1.
Panel A – Sorting of Sca-1+CD45+Lin– BMNCs. Cells from lymph gate (R1) were subjected to lineage-negative, Sca-1-positive (R2) and CD45-positive (R3) selections. Panel B – RTPCR analysis of FPRL-1 mRNA expression in murine BMNCs and BM-purified Sca-1+CD45+Lin– cells. Representative data from two independent experiments is shown. Panel C – Low-dose SDF-1(10ng/ml) chemotaxis priming assay in the presence of different concentrations (0, 0.05, 0.5, 2.0 μM) of LL-37 or the FPRL-1 agonist WKYMVM. LL-37, but not WKYMVM, enhances the responsiveness of clonogenic progenitors to SDF-1 gradients. Values are shown as the percentage increase in the number of migrated cells compared to the number of migrated cells in response to SDF-1 alone. The data represent the combined results from three independent experiments performed in duplicate per group (n = 6).
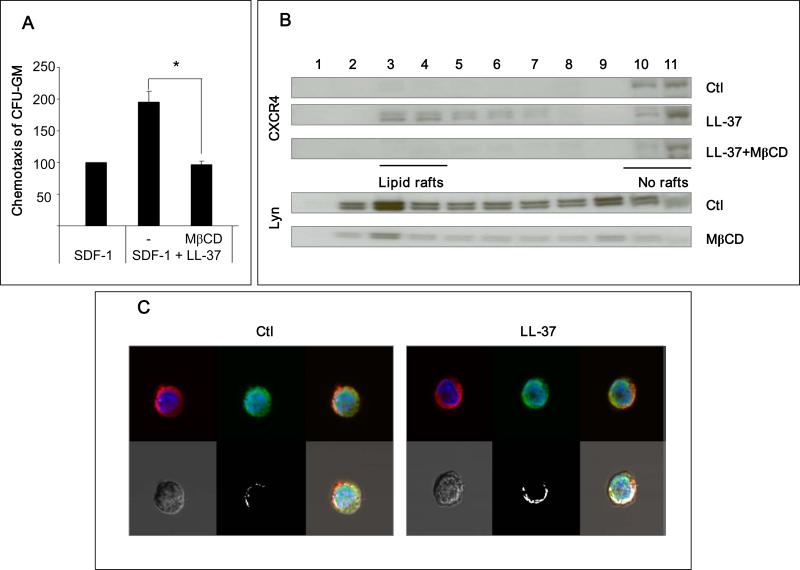
Next, we employed several complementary experimental strategies to confirm that the LL-37-mediated priming effect depends on incorporation of CXCR4 into lipid rafts. First, since membrane cholesterol is crucial for lipid raft formation, cells were depleted of cholesterol before chemotaxis assay by pre-incubating them with methyl-beta-cyclodextrin (MβCD). Figure 4 panel A shows that the SDF-1–CXCR4 axis-priming effect was abrogated after depletion of membrane cholesterol by MβCD. Next, we isolated eleven membrane fractions more or less enriched in lipid rafts from hematopoietic cells i) stimulated with LL-37, ii) stimulated with LL-37 after previous depletion of cholesterol by MβCD, and iii) unprimed as a control. The expression of CXCR4 in these membrane fraction extracts was evaluated by Western blot (Figure 4 panel B – upper panel). To visualize lipid rafts, we also employed Western blot analysis for Lyn expression, which is an established marker for lipid rafts 14 (Figure 4 panel B – lower panel). Our data show that in cells stimulated by LL-37, CXCR4 was detectable in lipid raft-enriched membrane fractions (fractions 3–4, Figure 4 panel B). Finally, we evaluated lipid raft formation in hematopoietic cells exposed or not exposed to LL-37 by employing confocal microscope analysis (Figure 4 panel C). Direct confocal analysis revealed colocalization of CXCR4 receptor with GM-1 protein, which is another marker of lipid rafts 12, in cells exposed to LL-37.
Figure 4. The priming effect of LL-37 is dependent on enhanced incorporation of CXCR4 into lipid rafts.
Panel A – LL-37-mediated enhancement of migration of murine CFU-GM in response to an SDF-1 gradient was significantly inhibited by 1 h pretreatment of cells with MβCD (2.5mM). Values are the percentage increase in the number of migrated cells compared to the number of migrated cells in response to SDF-1 alone. SDF-1 was employed at a dose of 50ng/ml and LL-37 at a dose of 2.5μg/ml. The data represent the combined results from three independent experiments performed in duplicate per group (n = 6). *P<0.01. Panel B – Western blot analysis of the localization of CXCR4 and lipid rafts in different fractions of cell membrane lipids. Membranes were enriched in lipid rafts (fractions 3–4) or depleted of lipid rafts (fractions 10–11). The human monocytic cell line, THP-1, pretreated with or without MβCD (2.5mM), was stimulated with or without LL-37 (2.5μg/ml). CXCR4 was detected in membrane fractions by western blot along with Lyn, a marker of lipid rafts. Representative results are shown from three independent experiments. Panel C – Confocal microscopy analysis of the localization of CXCR4 in lipid rafts. THP-1 cells were cultured for 6 h in serum-free medium and than stimulated without (control) or with LL-37 (2.5 μg/ml). Lipid rafts were stained with cholera toxin B subunit conjugated with fluorescein isothiocyanate (FITC, green) and CXCR4 was stained with mouse monoclonal anti-hCXCR4 IgG and Alexa Fluor 568 goat anti-mouse IgG (red) antibodies. Stained cells were examined and images were generated by using a FLUOVIEW FV1000 laser-scanning confocal microscope. White areas indicate co-localization of lipid rafts with CXCR4. The experiment was repeated three times on different cell preparations and a representative image is shown.
Short-term ex vivo preincubation of BM cells before transplantation with LL-37 enhances and accelerates engraftment of HSPCs in vivo
To evaluate whether the AMP LL-37 could be employed for ex vivo priming before transplantation of HSPCs, we evaluated its potential toxicity in clonogenic assays, and as expected no effect was observed for either murine or human progenitor cells from all the major hematopoietic lineages (data not shown).
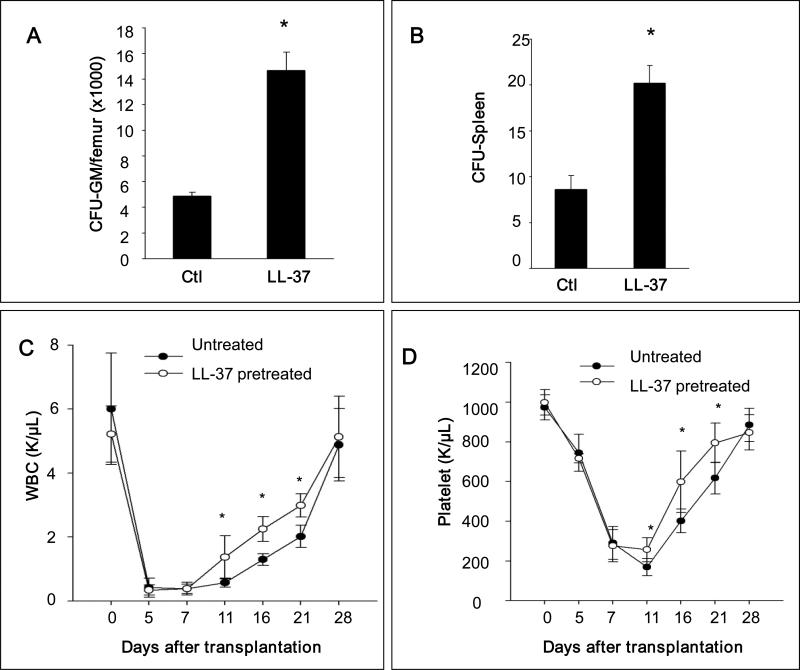
Encouraged by this result, we performed transplantation experiments in vivo. Syngeneic BM cells were exposed or not exposed ex vivo for 30 minutes to LL-37 and subsequently transplanted into lethally irradiated recipients. We observed that mice transplanted with LL-37-primed cells had, by day 11 after transplantation, a higher number of clonogenic progenitors in long bones (Figure 5 panel A) and transplanted LL-37-primed cells formed more spleen colonies in the colony forming units in spleen (CFU-S) assay (Figure 5 panel B).
Figure 5. Pre-incubation of BMNCs with LL-37 before transplantation enhances homing and engraftment of HSPCs.
Panel A – Lethally irradiated mice (6 mice per group) were transplanted with 5 × 105 mBMNCs, pretreated without (control) or with LL-37 (2.5ug/ml for 30 minutes), and 11 days after transplantation femoral BMNCs were harvested and cells isolated for counting CFU-GM. Panel B – In similar experiments mice were lethally irradiated and transplanted with 1 × 105 mBMNCs. Eleven days after transplantation, spleens were harvested and fixed, and CFU-S on the spleen surface was counted. No colonies were formed in lethally irradiated and non-transplanted mice (irradiation control). The data in panel A and B represent the combined results from three independent experiments (n = 9). * p<0.01. Panel C – Lethally irradiated mice were transplanted with 5 × 105 mBMNCs, pretreated without (control) or with LL-37 (2.5μg/ml for 30 minutes). White blood cells (WBC) and platelets were counted at intervals (0, 5, 7, 11, 16, and 21 days after transplantation). Results are combined from two independent experiments (5 mice per group, n = 10). Black circles indicate the un-pretreated mice, and white circles indicate the mice pretreated with LL-37. * p<0.05.
Finally, syngeneic BM cells were exposed ex vivo for 30 minutes to LL-37, transplanted into lethally irradiated recipients, and the kinetics of recovery of peripheral blood parameters was measured. We observed an accelerated recovery of platelets and neutrophils of ~3–5 days in animals transplanted with primed cells compared to animals transplanted with unprimed control cells (Figure 5 panel C).
The priming effect of LL-37 is specific for the SDF-1–CXCR axis
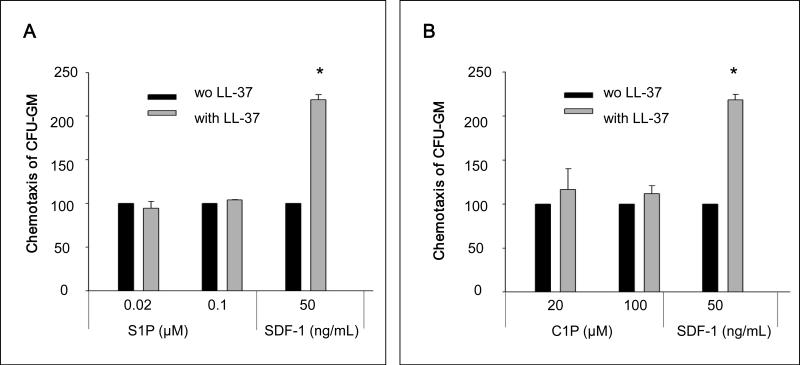
Recently we described an important role for certain bioactive lipids, sphingosine-1-phosphate (S1P) and ceramide-1-phospate (C1P), in homing of HSPCs to BM 10,11. We therefore became interested in whether LL-37 also enhances the responsiveness of HSPCs to these other homing factors and observed that the effect of LL-37 is highly SDF-1-specific (Figure 6 panel A and B).
Figure 6. The LL-37-induced priming effect is specific to the SDF-1–CXCR axis.
Chemotaxis assay of murine CFU-GM in response to different concentrations of S1P (Panel A) and C1P (Panel B) with and without LL-37. Values are the percentage increase in the number of migrated cells in the presence of LL-37 compared to the number migrated cell in the absence of LL-37. Gray bars indicate the presence of LL-37 (2.5μg/ml), while black bars indicate the absence of LL-37 in the lower Transwell chambers. The data represent the combined results from three independent experiments performed in duplicate per group (n = 6). * p<0.001
Next, we wanted to learn whether the priming effect of LL-37 is involved in the chemotactic responsiveness of hematopoietic cells to other chemokines. Since HSPCs do not respond to other chemokines except SDF-1 17-19, we employed the THP-1 cell line as a model system. THP-1 cells, in addition to SDF-1, exhibit chemotaxis in response to MIP-1β, MCP-1, and IL-8. We observed that LL-37 enhances chemotactic responsiveness of THP-1 cells to SDF-1 only (data not shown), which again suggests the high specificity of LL-37 priming for the SDF-1–CXCR4 axis.
Discussion
The SDF-1–CXCR4 receptor axis plays an important role in retention of HSPCs in BM. While HSPCs express CXCR4, SDF-1 is expressed by cells in the BM microenvironment (e.g., osteoblasts and fibroblasts). It is also well known that SDF-1, when employed at supra-physiological concentrations, is a potent in vitro chemoattractant for HSPCs. However, in contrast the SDF-1 concentration in biological fluids is relatively low (e.g., ~1–2ng/ml in PB) 10. Moreover, we have recently demonstrated that myeloablative conditioning for hematopoietic transplantation induces a highly proteolytic microenvironment in BM, and, since SDF-1 is extremely sensitive to degradation by proteolytic enzymes, it is rapidly degraded under these conditions 3, 11, 18, 20. The relatively low physiological concentration of SDF-1 in tissues and its sensitivity to proteolytic enzymes together suggest that some other mechanisms may play an important role in sensitizing the responsiveness of cells to this chemokine.
To support this notion, it has been reported by us and other investigators that platelet-derived microparticles 2, the complement cascade cleavage fragment anaphylatoxin C3a 3, hyaluronic acid 4, the sphingosine-1-phosphate receptor agonist FTY20 5, uridine triphosphate (UTP) 6, and prostaglandin E2 (PGE2) 7 enhance the responsiveness of HSPCs to an SDF-1 gradient. Thus, these observations collectively suggest that the SDF-1-mediated homing responsiveness of HSPCs is positively modulated by several factors related to inflammation or tissue injury.
In this report, we demonstrate that the cationic peptide LL-37 also strongly enhances the responsiveness of HSPCs to an SDF-1 gradient. This priming effect is not receptor mediated since, as shown here, the LL-37 receptor FRPL-1 16, as previously reported for receptors for other AMPs (C3a and desArgC3a) 21-22, are not involved in this process. The molecular mechanism behind this phenomenon is the ability of LL-37, like another cationic peptide anaphylatoxin C3a, to increase incorporation of CXCR4 into lipid rafts 8. We envision that cationic AMPs, which as part of innate immunity may destroy bacterial walls, are harmless to eukaryotic cells which have a different membrane lipid composition, and after exposure perturb only the organization of membrane lipids by non-receptor-mediated mechanisms that promote formation of lipid rafts. Based on this the CXCR4 receptor, when incorporated into lipid rafts, better engages downstream signaling molecules, showing an increase in SDF-1-mediated MAPKp42/44 phosphorylation in the presence of LL-37, as demonstrated in this paper.
Interestingly, since the overall level of SDF-1 in BM after conditioning for transplantation decreases due to induction of a proteolytic environment 10-11, LL-37 secreted by irradiated BM stroma may enhance by a priming effect the responsiveness of HSPCs to decreasing SDF-1 gradient in BM. More importantly, as we demonstrate here for the first time, the strong priming effect of LL-37 is robust in the presence of very low but physiologically relevant doses of SDF-1 (1–3ng/ml). Therefore, this phenomenon significantly potentiates the biological activity of this chemokine in BM. Overall, we hypothesize that several factors induced in irradiated BM during conditioning for transplantation, such as LL-37, C3a anaphylatoxin, PGE2, and UTP, may cooperatively enhance the SDF-1-dependent homing gradient for HSPCs. It is an important mechanism to increase responsiveness to low SDF-1 levels, which additionally decreases after myeloablative conditioning for transplantation 1.
However, while we recently proposed that, in addition to SDF-1, certain bioactive lipids, such as sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) also play an important role in homing of HSPCs after transplantation 11, our results indicate that the effect of LL-37 is specific for the SDF-1–CXCR4 axis only. Furthermore, studies of the THP-1 cell line revealed that, in addition to normal HSPCs, LL-37 may also enhance the responsiveness of malignant cells to an SDF-1 gradient. Again, this effect was specific for SDF-1 and not other chemokine gradients. Based on this finding, LL-37 priming of SDF-1 responsiveness of leukemic cells to an SDF-1 gradient requires further study, as does the role of LL-37 in metastasis of SDF-1-responsive solid tumors 23-25.
In our previous studies, we have proposed that short exposure of BM cells to a priming agent (e.g., C3a anaphylatoxin) before transplantation may enhance homing and, as a consequence, accelerate engraftment of transplanted HSPCs 3. Here we studied whether priming of murine BM by LL-37 before transplantation shows a similar effect and noticed that murine BM cells exposed for 30 minutes to LL-37 show HSPCs engraftment accelerated by 3–5 days in irradiated animals, which has important clinical implications. Since LL-37 is a natural antibacterial peptide that, as we have shown here, does not affect clonogenecity of normal murine and human hematopoietic progenitors, it could be safely employed in the clinic. In fact, the in vitro clinical trials with LL-37 as a novel drug for treatment of bacterial and fungi infection have already been FDA approved 9, 26-27. Thus, priming of HSPCs before transplantation could be a new potential application for this peptide. It would be particularly useful to employ LL-37 priming to accelerate engraftment of UCB HSPCs. It is well known that the number of HSPCs in UCB units is often very low and not optimal for transplantation into an adult recipient 1.
LL-37 in addition to BM stroma cells is also stored in granulocytes and is released from these cells upon activation by C5a 8. It is well known that the leucophoresis products of mobilized peripheral blood HSPCs are enriched for granulocytes and activated complement cascade cleavage fragments, including C5a 8. Therefore, HSPCs in mobilized peripheral blood leucophoresis products, in contrast to UCB or BM cells, are exposed to LL-37 released from C5a activated granulocytes. This may explain why mobilized product engrafts faster after transplantation than, for example, in UCB or BM cells under steady-state conditions.
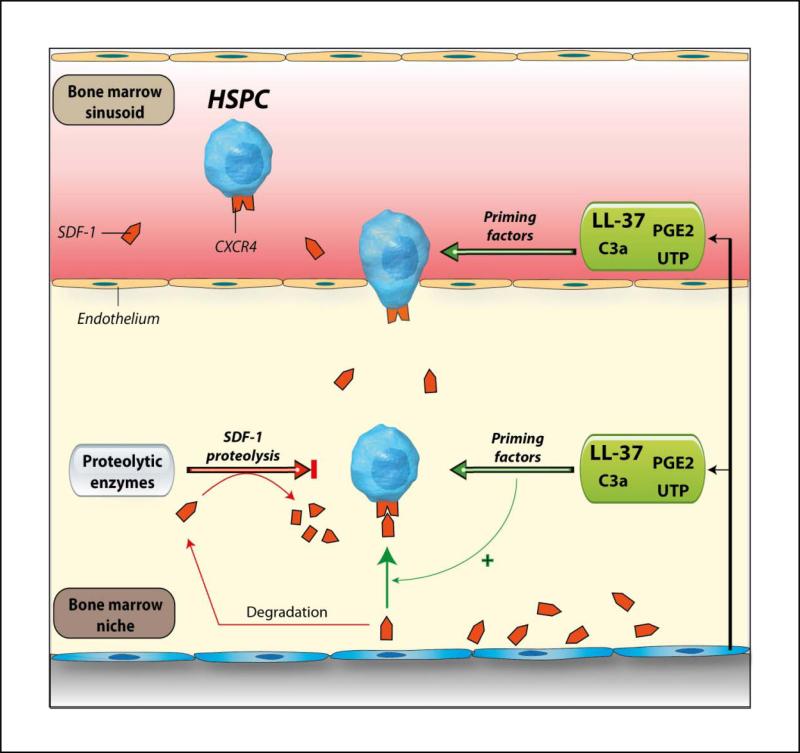
Based on these findings, we propose antimicrobial peptide LL-37 as a potential ex vivo priming factor for HSPCs that may accelerate their engraftment. This strategy would be particularly important for accelerating engraftment of umbilical cord blood HSPCs. We also propose a new paradigm in which LL-37 secreted by BM stromal cells together with other priming factors (C3a, UTP and PGE2) may convert a very low level of SDF-1 to a potent chemotactic gradient (Figure 7). Since the SDF-1 gradient decreases in BM after conditioning for transplantation due to the induction of a proteolytic microenvironment 11 this is an important mechanism promoting HSPCs homing.
Figure 7. The involvement of LL-37 in homing and engraftment of HSPCs.
Conditioning for transplantation by radio-chemotherapy induces a proteolytic microenvironment in BM and several proteolytic enzymes are released that decrease the SDF-1 level in BM. We report here that LL-37 released from BM stroma cells and/or granulocytes present in leucophoresis products enhance/sensitize responsiveness of HSPCs to decreasing SDF-1 gradient. It has been also reported that the complement cascade cleavage fragment anaphylatoxin C3a 3, uridine triphosphate (UTP) 6, and prostaglandin E2 (PGE2) 7 enhance the responsiveness of HSPCs to an SDF-1 gradient. These observations collectively suggest that the homing responsiveness of HSPCs is positively modulated by several factors related to inflammation or tissue injury.
Acknowledgments
This work was supported by NIH R01 CA106281-01, NIH R01 DK074720, EU structural funds, KBN grant (N N401 024536), Innovative Economy Operational Program POIG.01.01.01-00-109/09-01, and the Henry M. and Stella M. Hoenig Endowment (to MZR), and the Abraham J. and Phyllis Katz Foundation and the Dr. Donald and Ruth Weber Goodman Philanthropic Fund (to MJL). The authors acknowledge the Cleveland Cord Blood Center and the Cleveland Volunteer Donating Community as the source of the experimental material and the efforts of the staff at the Cleveland Cord Blood Center.
References
- 1.Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Review of Hematology. 2010;3:273–283. doi: 10.1586/ehm.10.24. 2010/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001 Nov 15;98:3143–3149. doi: 10.1182/blood.v98.10.3143. 2001. [DOI] [PubMed] [Google Scholar]
- 3.Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003 May 15;101:3784–3793. doi: 10.1182/blood-2002-10-3233. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Shirvaikar N, Marquez-Curtis LA, Ratajczak MZ, Janowska-Wieczorek A. Hyaluronic Acid and Thrombin Upregulate MT1-MMP Through PI3K and Rac-1 Signaling and Prime the Homing-Related Responses of Cord Blood Hematopoietic Stem/Progenitor Cells. Stem Cells and Development. 2011;20:19–30. doi: 10.1089/scd.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Kimura T, Boehmler AM, Seitz G, Kuçi S, Wiesner T, Brinkmann V, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004 Jun 15;103:4478–4486. doi: 10.1182/blood-2003-03-0875. 2004. [DOI] [PubMed] [Google Scholar]
- 6.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007 Jan 15;109:533–542. doi: 10.1182/blood-2006-01-035634. 2007. [DOI] [PubMed] [Google Scholar]
- 7.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009 May 28;113:5444–5455. doi: 10.1182/blood-2009-01-201335. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucki R, Leszczyńska K, Namiot A, Sokołowski W. Cathelicidin LL-37: A Multitask Antimicrobial Peptide. Archivum Immunologiae et Therapiae Experimentalis. 2010;58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 10.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-Plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, M S, Morris A, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow- a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2011 doi: 10.1038/leu.2011.185. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005 Jan 1;105:40–48. doi: 10.1182/blood-2004-04-1430. 2005. [DOI] [PubMed] [Google Scholar]
- 13.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 14.Wysoczynski M, Kucia M, Ratajczak J, Ratajczak MZ. Cleavage fragments of the third complement component (C3) enhance stromal derived factor-1 (SDF-1)-mediated platelet production during reactive postbleeding thrombocytosis. Leukemia. 2007;21:973–982. doi: 10.1038/sj.leu.2404629. [DOI] [PubMed] [Google Scholar]
- 15.Méndez-Samperio P. The human cathelicidin hCAP18/LL-37: A multifunctional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–1798. doi: 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Le Y, Yang Y, Cui Y, Yazawa H, Gong W, Qiu C, et al. Receptors for chemotactic formyl peptides as pharmacological targets. International Immunopharmacology. 2002;2:1–13. doi: 10.1016/s1567-5769(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Ratajczak M. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Archivum Immunologiae et Therapiae Experimentalis. 2009;57:269–278. doi: 10.1007/s00005-009-0037-6. [DOI] [PubMed] [Google Scholar]
- 18.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)--Implications for trafficking of CXCR4+ stem cells. Experimental Hematology. 2006;34:986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of Normal Stem Cells and Metastasis of Cancer Stem Cells Involve Similar Mechanisms: Pivotal Role of the SDF-1–CXCR4 Axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 20.Sharma M, Afrin F, Satija N, Tripathi RP, Gangenahalli GU. Stromal-Derived Factor-1/CXCR4 Signaling: Indispensable Role in Homing and Engraftment of Hematopoietic Stem Cells in Bone Marrow. Stem Cells and Development. 2011 Jun;20:933–946. doi: 10.1089/scd.2010.0263. [DOI] [PubMed] [Google Scholar]
- 21.Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004 Mar 15;103:2071–2078. doi: 10.1182/blood-2003-06-2099. 2004. [DOI] [PubMed] [Google Scholar]
- 22.Honczarenko M, Lu B, Nicholson-Weller A, Gerard NP, Silberstein LE, Gerard C. C5L2 receptor is not involved in C3a // C3a-desArg-mediated enhancement of bone marrow hematopoietic cell migration to CXCL12. Leukemia. 2005;19:1682–1683. doi: 10.1038/sj.leu.2403862. [DOI] [PubMed] [Google Scholar]
- 23.Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proceedings of the National Academy of Sciences. 2009 Mar 10;106:3806–3811. doi: 10.1073/pnas.0900244106. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber G, Chamorro C, Granath F, Liljegren A, Zreika S, Saidak Z, et al. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Research. 2009;11:R6. doi: 10.1186/bcr2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hensel JA, Chanda D, Kumar S, Sawant A, Grizzle WE, Siegal GP, et al. LL-37 as a therapeutic target for late stage prostate cancer. The Prostate. 2011;71:659–670. doi: 10.1002/pros.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciornei CD, Sigurdardottir T, Schmidtchen A, Bodelsson M. Antimicrobial and Chemoattractant Activity, Lipopolysaccharide Neutralization, Cytotoxicity, and Inhibition by Serum of Analogs of Human Cathelicidin LL-37. Antimicrob Agents Chemother. 2005 Jul 1;49:2845–2850. doi: 10.1128/AAC.49.7.2845-2850.2005. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zughaier SM, Svoboda P, Pohl J, Stephens DS, Shafer WM. The Human Host Defense Peptide LL-37 Interacts with Neisseria meningitidis Capsular Polysaccharides and Inhibits Inflammatory Mediators Release. PLoS ONE. 2010;5:e13627. doi: 10.1371/journal.pone.0013627. [DOI] [PMC free article] [PubMed] [Google Scholar]