Abstract
Background
Pancreatitis-induced splenic vein thrombosis (PISVT) is an acquired anatomic abnormality that impacts decision making in pancreatic surgery. Despite this influence, its incidence and the rate of associated gastrointestinal (GI) bleeding are imprecisely known.
Methods
The MEDLINE, EMBASE, Cochrane Central Register of Clinical Trials and Cochrane Database of Systematic Reviews databases were searched from their inception to June 2010 for abstracts documenting PISVT in acute (AP) or chronic pancreatitis (CP). Two reviewers independently graded abstracts for inclusion in this review. Heterogeneity in combining data was assumed prior to pooling. Random-effects meta-analyses were performed to estimate percentages and 95% confidence intervals.
Results
After review of 241 abstracts, 47 studies and 52 case reports were graded as relevant. These represent a cohort of 805 patients with PISVT reported in the literature. A meta-analysis of studies meeting inclusion criteria shows mean incidences of PISVT of 14.1% in all patients, 22.6% in patients with AP and 12.4% in patients with CP. The incidence of associated splenomegaly was only 51.9% in these patients. Varices were identified in 53.0% of patients and were gastric in 77.3% of cases. The overall rate of GI bleeding was 12.3%.
Conclusions
Although reported incidences of PISVT vary widely across studies, an overall incidence of 14.1% is reported. Splenomegaly is an unreliable sign of PISVT. Although the true natural history of PISVT remains unknown, the collective reported rate of associated GI bleeding is 12.3%.
Keywords: acute pancreatitis, chronic pancreatitis, splenic vein thrombosis, left-sided portal hypertension, sinistral hypertension, gastric varices, gastrointestinal bleeding
Introduction
Pancreatitis-induced splenic vein thrombosis (PISVT) is an acquired disorder that occurs as a sequel to both acute (AP) and chronic pancreatitis (CP). Although as many as 37 different specific aetiologies for splenic vein thrombosis (SVT) have been reported,1 the majority are related to diseases of the pancreas.2 Although PISVT has an historical association with neoplasms,3 recent data incriminate AP and CP as its principal causes.4–8 Regardless of its aetiology, SVT generates a localized form of portal hypertension commonly referred to as ‘sinistral’, ‘left-sided’ or ‘linear’. Collateral blood flow develops through the splenoportal or gastroepiploic systems and the resulting localized venous hypertension may produce gastric, oesophageal or colonic varices. These varices are a potential source of significant gastrointestinal (GI) bleeding (Figs 1 and 2).9,10 In relation to operative management, it has been suggested that patients with PISVT and a prior history of upper GI tract bleeding or symptomatic hypersplenism may represent a high-risk subgroup in whom the risk : benefit profile is altered in favour of splenectomy.11–13 By contrast, patients in a small cohort without this history of bleeding, in whom PISVT was identified through imaging, were found to have an incidence of bleeding of only 3.8% (two of 53 patients) over a median follow-up of 34 months.14
Figure 1.
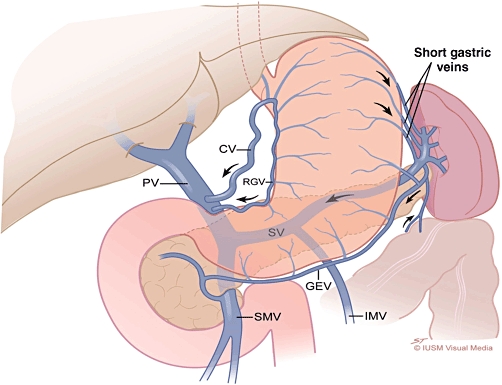
Normal venous anatomy. Arrows indicate the directional flow of venous blood. CV, coronary vein; PV, portal vein; RGV, right gastric vein; SV, splenic vein; GEV, gastroepiploic vein; SMV, superior mesenteric vein; IMV, inferior mesenteric vein
Figure 2.
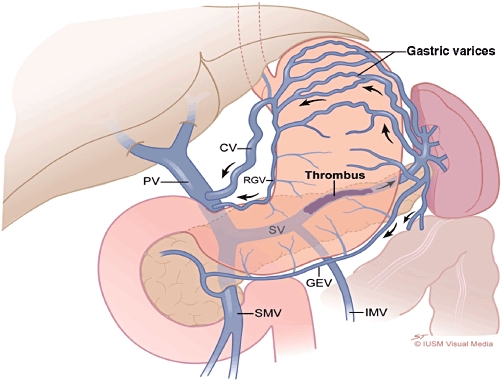
Splenic vein thrombosis causing left-sided portal hypertension. Note the gastric varices, dilatation of short gastric, gastroepiploic (GEV) and coronary (CV) veins. The portal vein (PV), superior mesenteric vein (SMV) and inferior mesenteric vein (IMV) are patent. RGV, right gastric vein; SV, splenic vein
Although PISVT is an important anatomic abnormality that impacts operative decision making in pancreatic surgery, no consensus has been reached on either its incidence in patients with pancreatitis or the rate of associated GI bleeding.1 The aim of this study was to conduct a systematic review of the published literature on PISVT, with appropriate filters and controls, to determine both its incidence and the rate of associated GI tract bleeding.
Materials and methods
Data sources
The MEDLINE, EMBASE, Cochrane Database of Systematic Reviews (CDSR) and Cochrane Central Register of Clinical Trials (CCRCT) databases were searched from their inception up to and including June 2010. Search terms were used in both isolation and combination to identify all published evidence. Reference lists from identified review articles and published studies were queried to identify articles not found through our initial database search.
Data extraction
Two investigators (JRB and TJH) independently reviewed the titles and abstracts of all returned references regardless of language or publication status to identify studies for inclusion in the analysis. All identified articles were examined using a predesigned proforma and the data collected were entered into a database for analysis. A list of gathered data is detailed in Table 1. The methodological quality of studies was assessed for a minimum Oxford Centre for Evidence-Based Medicine (CEBM) level of 2B.15 When appropriate, studies were allocated to separate quantitative cohorts for independent meta-analyses of variables. Results of studies yielding a heterogeneous dataset of isolated and non-isolated PISVT were corrected for if results were reported in a manner that allowed the exclusion of outcomes specific for an isolated PISVT patient cohort. An analogous process was rarely employable for datasets confounded by malignancy. References reporting datasets with incomplete or inconsistent isolation of a non-confounded PISVT cohort were excluded (Table 2). A schematic diagram depicting reference flow through the systematic review process is shown in Fig. 3.
Table 1.
Data collected according to the proforma for articles to be included in this review of reported cases of pancreatitis-induced splenic vein thrombosis (PISVT)
| Incidence of reported PISVT |
| Isolated splenic vein thrombosis identified, n |
| Pancreatitis patients studied, n |
| Diagnostic medium of splenic vein thrombosis |
| Type of pancreatitis |
| Incidence of bleed |
| Patients with documented gastrointestinal bleed, n |
| Patients with isolated PISVT, n |
| Incidence of varices |
| Varices, n/patients with isolated PISVT, n |
| Gastric varix |
| Oesophageal varix |
| Gastro-oesophageal varix |
| Varix diagnostic media |
| Computed tomography-diagnosed varices bleed rate |
| Oesophagogastroduodenoscopy-diagnosed varix bleed rate |
| Incidence of PISVT-associated gastrointestinal bleeding |
| Splenomegaly |
| Patients demonstrating splenomegaly, n |
| Patients with isolated PISVT, n |
| Patients with PISVT–splenomegaly with bleed, n |
| Splenectomy cohort bleed in follow-up |
| Non-splenectomy cohort bleed in follow-up |
| Mean follow-up in patients involved |
| Type of study |
Table 2.
Exclusion criteria and numbers of papers excluded by these criteria
| CEBM evidence level < 2B, n = 6a |
| Studies enrolling fewer than six patients, n = 2 |
| Dataset previously published, n = 4 |
| Failure to identify isolated PISVT cohort, n = 41 |
| Failure to identify pancreatitis, n = 10 |
| Confounding presence of malignancy, n = 13 |
| Confounding presence of cirrhosis, n = 5 |
| Data derived from animal model, n = 2 |
| Data limited to post-splenectomy outcomes, n = 18 |
| Data derived from autopsy alone, n = 2 |
| Inconsistent radiologic analysis for PISVT, n = 10 |
| Irrelevant title with no abstract, n = 9 |
| Unacceptable publication format, n = 5 |
| Article irretrievable, n = 4 |
| References meeting global exclusion criteria, n = 130b |
| Exclusion criteria for individual meta-analyses |
| Data pre-1995 with unreliable PISVT identification strategy |
| Mean follow-up time of <3 months |
| Data not limited to identified acute pancreatitis patients |
| Data not limited to identified chronic pancreatitis patients |
After removing case reports and references meeting other exclusion criteria.
After removing duplicates and case reports.
CEBM, Oxford Centre for Evidence-Based Medicine; PISVT, pancreatitis-induced splenic vein thrombosis.
Figure 3.
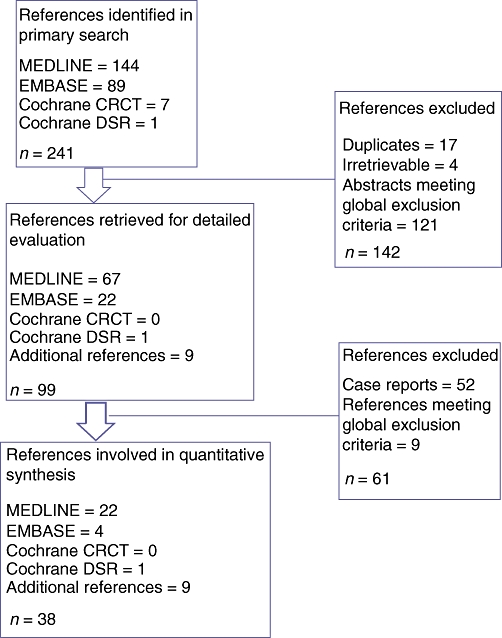
Flow of references through the systematic review. Cochrane CRCT, Cochrane Central Register of Clinical Trials; Cochrane DSR, Cochrane Database of Systematic Reviews
Statistical analysis
All included references were assigned to appropriate cohorts for individual meta-analyses of variables. A schematic diagram depicting the flow of references through the quantitative arm of this study is shown in Fig. 4. When combining data from the trials, we assumed the presence of heterogeneity existed prior to pooling and used the random effects model developed by DerSimonian.16 This model allows for adjustment for variability between trials by providing a more conservative estimate of the range of an effect. Individualized random effects meta-analyses were performed to estimate percentages and 95% confidence intervals (CIs) for all endpoints queried.
Figure 4.
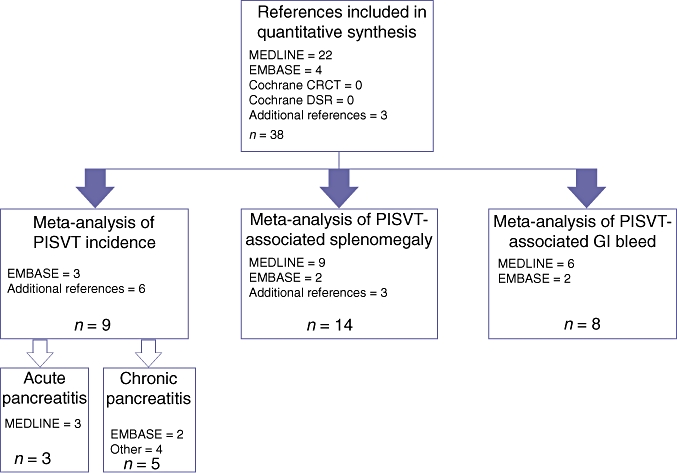
Flow of references through the meta-analysis. Cochrane CRCT, Cochrane Central Register of Clinical Trials; Cochrane DSR, Cochrane Database of Systematic Reviews; PISVT, pancreatitis-induced splenic vein thrombosis; GI, gastrointestinal
Results
In the initial search, a total of 241 articles were retrieved; nine additional unique references were found through a review of reference lists. After duplicates had been removed and the exclusion criteria noted in Table 2 applied, 47 clinical studies and 52 case reports were graded as relevant; these referred to a cohort of 805 distinct patients with PISVT. Thirty-eight references were assessed as eligible for quantitative inclusion by the reviewers.2,3,6,7,9,11–14,17–45 The median CEBM level of included studies was 2A.15 A meta-analysis of contemporary studies meeting inclusion criteria showed an overall mean reported incidence of PISVT of 14.1% (Figure 5).11,13,17,25–29,36 Mean incidences varied from 22.6% in patients with AP to 12.4% in patients with CP. The incidence of associated splenomegaly was only 51.9%.3,6,7,9,11,13,18,19,24,26,28,36,42,46 Overall, 53.0% of patients in this cohort were found to have varices, 77.3% of which were gastric. The aggregate rate of associated GI bleeding in this series was 12.3% (Figure 6).11,13,14,17,20,21,27,29 All reported rates with associated 95% CIs are shown in Table 3.
Figure 5.
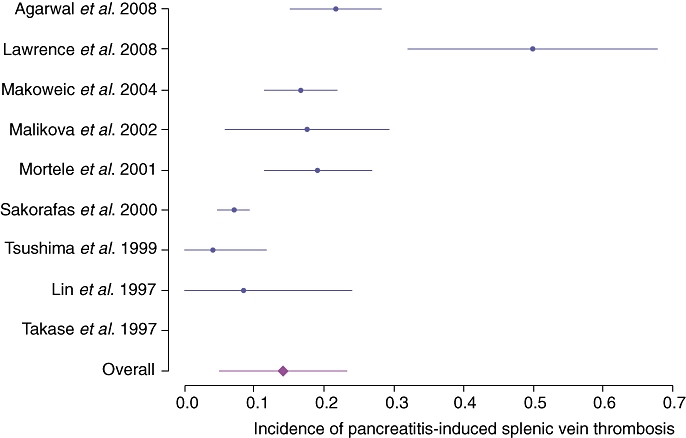
Incidences of pancreatitis-induced splenic vein thrombosis reported from 1997
Figure 6.
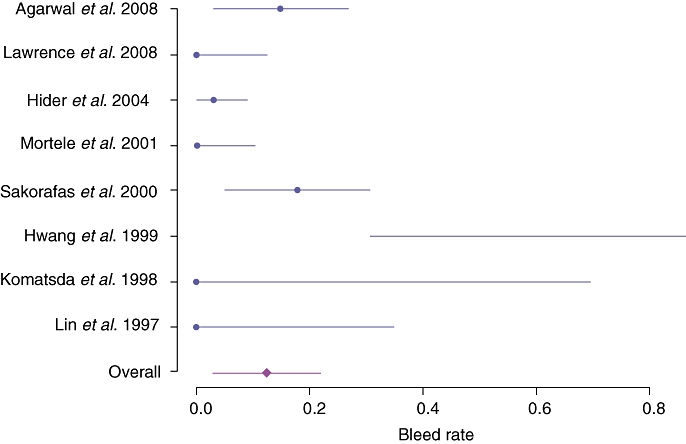
Bleed rates in patients with pancreatitis-induced splenic vein thrombosis reported from 1995
Table 3.
Meta-analysis of incidence of pancreatitis-induced splenic vein thrombosis (PISVT) and associated morbidity
| Proportion | 95% confidence interval | |
|---|---|---|
| PISVT incidence | 0.141 | 0.049–0.233 |
| PISVT incidence in acute pancreatitis | 0.226 | 0.006–0.455 |
| PISVT Incidence in chronic pancreatitis | 0.124 | 0.023–0.266 |
| Incidence of varices | 0.530 | 0.264–0.796 |
| Incidence of gastric varices | 0.410 | 0.116–0.703 |
| Incidence of oesophageal varices | 0.033 | 0–0.068 |
| Incidence of gastro-oesophageal varices | 0.200 | 0.026–0.374 |
| Bleed rate | 0.124 | 0.029–0.216 |
| Bleed rate in acute pancreatitis | 0.067 | 0–0.186 |
| Bleed rate in chronic pancreatitis | 0.069 | 0–0.149 |
| Splenomegaly | 0.519 | 0.262–0.776 |
Discussion
Thrombosis and occlusion of the splenic vein following inflammation of the pancreas are principally related to location (Figs 1 and 2). The consequence of this altered venous anatomy is isolated hypertensive short gastric veins that can develop into varices in the gastric fundus submucosa. These ‘isolated gastric varices’, without evidence of oesophageal varices, represent the sine qua non of SVT. Although it has been postulated that splenomegaly might also be a consistent finding in all patients with SVT,47 explicit studies of splenomegaly in SVT report actual incidences in the range of 42–54%.42,48 The 51.9% incidence of splenomegaly in patients in this collective review supports these observations. This lack of consistent compensatory hypersplenism makes the diagnosis of SVT dependent on imaging either the obstructed splenic vein directly or the associated gastro-oesophageal varices. To this end, contrast-enhanced computed tomography (CT) or direct endoscopic examination [oesophagogastroduodenoscopy (OGD)] are the modalities most widely used to identify SVT.29 In this study, oesophagogastric varices were identified in only 53.0% of patients with PISVT and 77.3% of these cases involved isolated gastric varices. These troubling inconsistencies in accurately identifying the hallmark sequelae of PISVT can be at least partially explained by the heterogeneity across studies of the imaging techniques used to identify varices (CT vs. venous portography vs. upper GI endoscopy). Although OGD is the reference standard for the diagnosis of gastro-oesophageal varices, adherence to an optimal technique is important in maximizing its sensitivity.49 Perri and colleagues50 prospectively studied 102 patients with liver disease in a screening programme using OGD and multi-detector CT scans of the abdomen. In this study, CT had sensitivity of 90% and specificity of 50% for diagnosing oesophageal varices and sensitivity of 87% for the detection of gastric varices. In addition, CT identified a significant number of gastric varices, peri-oesophageal varices and extra-luminal pathology that were important for establishing optimal patient care. These authors concluded that contrast-enhanced CT appeared to be a reasonable screening tool for the identification of varices relative to the reference standard OGD.50
Another possible explanation for the lack of isolated gastric varices in PISVT is the variation in coronary vein drainage described by Little and Moossa.9 Coronary veins in up to 17% of the patients studied by these authors were found to drain directly into the splenic vein rather than the portal vein. In these patients, adequate venous decompression of splenoportal venous hypertension is established through gastro-oesophageal collaterals (oesophageal varices). Although the current results support the notion that the most common pathway to venous decompression is through isolated gastric varices, this review also identified gastro-oesophageal varices in patients with SVT at ranges that support the observations of Little and Moossa.9
Lastly, further confounding the interpretation of many studies in this field is the failure of investigators to isolate PISVT from confounding variables such as hepatic cirrhosis or generalized portal hypertension. This lack of precision was the main reason why 41 reports (17%) were excluded from this analysis. It is well established that a third of cirrhosis patients with documented oesophageal varices will bleed within 2 years of diagnosis and that the mortality rate in the first episode of upper GI haemorrhage is 20–35% despite aggressive management.49,51 Although the incidence and severity of GI bleeding from isolated SVT with sinistral portal hypertension remain poorly defined, it is clear that neither is of the magnitude reported in patients with hepatic cirrhosis and generalized portal hypertension.
All forms of pancreatitis have been implicated as risk factors for SVT. Targeted studies report its incidence in hereditary pancreatitis,31 autoimmune pancreatitis,52,53 AP and CP.11,13,14,32 Splenic vein thrombosis is most commonly associated with CP, although a single attack of AP appears sufficient to cause this disorder.5,9,54 It is possible that the current data on AP may overestimate the incidence of SVT in patients with AP as a result of selection bias towards a higher severity in reported studies. Unfortunately, the severity of AP was inconsistently indexed in most studies. Moreover, debate continues on whether or not the severity of pancreatitis has any bearing on the incidence of SVT.9,29,38 Individual studies have incriminated high peak serum amylase levels, pancreatic pseudocyst, splenomegaly, documented right upper quadrant varices and the decision to manage complications in AP operatively as correlated to higher rates of PISVT.7,13,32
In either AP or CP, the incidence of splenic vein abnormalities has ranged from 0.9% to 54% in surgical series35,55 and up to 89% in radiographic series.56 Complete venous occlusion has been most widely studied in the context of CP, with reported rates ranging from 1.5% to 41.6% in surgical series28,55 and from 4.5% to 45% in radiographic series.18,57 Historically, patients with SVT most commonly presented clinically with an episode of GI bleeding or abdominal pain.3 With improvement in both the availability and quality of cross-sectional imaging, the majority of patients with SVT are currently diagnosed while they are asymptomatic.11,13,14,58 Despite this trend towards increasing asymptomatic diagnosis, the literature may retain an overall sampling bias towards symptomatic PISVT; it is for this reason that the current meta-analysis was limited to post-1995 data. For example, between 1969 and 1984, 45% of patients reported with PISVT initially presented with GI bleeding.7 Similarly, removing the limit on study age in the literature included in this analysis yields an overall PISVT incidence of 14.1% (22.6% in AP and 12.4% in CP) and a bleed rate of 19.0%.2,3,6,7,9,11–14,17–45 A comparison of these data with results reported in the current review indicates both an increasing awareness of PISVT and a decreasing concern for GI bleed in the era of universal cross-sectional imaging.
In summary, despite the heterogeneity of available data, this systematic analysis is the first study to attempt to quantify the incidence and rate of associated GI bleeding in patients with PISVT. Reported incidences of PISVT were found to vary widely among individual studies, but an overall incidence of 14.1% was reported. Splenomegaly was found to be an unreliable sign of PISVT. Although the true natural history of PISVT remains controversial, the collective reported rate of associated GI bleeding was 12.3%. Although routine prophylactic splenectomy at the time of pancreatic surgery in patients with PISVT has been recommended by some authors, these relatively low rates of reported GI bleeding seem to support the safety of observational management in asymptomatic patients.
Conflicts of interest
None declared.
References
- 1.Koklu S. Left-sided portal hypertension. Dig Dis Sci. 2007;52:1141–1149. doi: 10.1007/s10620-006-9307-x. [DOI] [PubMed] [Google Scholar]
- 2.Lareo J, Gea F, Abreu C, Barrios A, Garrido A, Albillos C, et al. Isolated splenic vein thrombosis. J Clin Nutr Gastroenterol. 1986;1:221–224. [Google Scholar]
- 3.Sutton JP, Yarborough DY, Richards JT. Isolated splenic vein occlusion. Arch Surg. 1970;100:623–630. doi: 10.1001/archsurg.1970.01340230089024. [DOI] [PubMed] [Google Scholar]
- 4.Belli AM, Jennings CM, Nakienly RA. Splenic and portal venous thrombosis: a vascular complication of pancreatic disease demonstrated on computed tomography. Clin Radiol. 1990;41:13–16. doi: 10.1016/s0009-9260(05)80924-9. [DOI] [PubMed] [Google Scholar]
- 5.Lillemoe KD, Yeo CJ. Management of complications of pancreatitis. Curr Probl Surg. 1998;35:1–98. [PubMed] [Google Scholar]
- 6.Madsen MS, Peterson TH, Sommer H. Segmental portal hypertension. Ann Surg. 1986;204:72–77. doi: 10.1097/00000658-198607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moossa AR, Gadd MA. Isolated splenic vein thrombosis. World J Surg. 1985;9:384–390. doi: 10.1007/BF01655272. [DOI] [PubMed] [Google Scholar]
- 8.Tsan-Long H, Yi-Yin J, Long-Bin J. The different manifestations and outcome between pancreatitis and pancreatic malignancy with left-sided portal hypertension. Int Surg. 1999;84:209–212. [PubMed] [Google Scholar]
- 9.Little AG, Moossa AR. Gastrointestinal haemorrhage from left-sided portal hypertension. Am J Surg. 1981;141:153–157. doi: 10.1016/0002-9610(81)90029-5. [DOI] [PubMed] [Google Scholar]
- 10.Turrill FL, Mikkelesen WP. Sinistral (left-sided) extrahepatic portal hypertension. Arch Surg. 1969;99:365–368. doi: 10.1001/archsurg.1969.01340150073014. [DOI] [PubMed] [Google Scholar]
- 11.Sakorafas GH, Sarr MG, Farley DR. The significance of sinistral portal hypertension complicating chronic pancreatitis. Am J Surg. 2000;179:129–133. doi: 10.1016/s0002-9610(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 12.Bradley EL. The natural history of splenic vein thrombosis due to chronic pancreatitis: indication for surgery. Int J Pancreatol. 1987;2:87–92. doi: 10.1007/BF03015001. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal AK, Kumar R, Agarwal S, Singh S. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg. 2008;196:149–154. doi: 10.1016/j.amjsurg.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 14.Hider RT, Azeem SA, Galanko JA, Behrns KE. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004;239:876–882. doi: 10.1097/01.sla.0000128685.74686.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heneghan C. EBM resources on the new CEBM website. Evid Based Med. 2009;14:67–68. doi: 10.1136/ebm.14.3.67. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R. Combining evidence from clinical trials. Anesth Analg. 1990;70:475–476. doi: 10.1213/00000539-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence C, Howell DA, Stefan AM, Conklin DE, Lukens FJ, Martin RF, et al. Disconnected pancreatic tail syndrome: potential for endoscopic therapy and results of longterm follow-up. Gastrointest Endosc. 2008;67:673–679. doi: 10.1016/j.gie.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Arner O, Fernstrom I. Obstruction of the splenic vein. Acta Chir Scand. 1961;122:263–272. [PubMed] [Google Scholar]
- 19.Evans GR, Yellin AE, Weaver FA, Stain SC. Sinistral (left-sided) portal hypertension. Am Surg. 1990;56:758–763. [PubMed] [Google Scholar]
- 20.Hwang TL, Jan YY, Jeng LB, Chen MF, Hung CF, Chiu CT. The different manifestation and outcome between pancreatitis and pancreatic malignancy with left-sided portal hypertension. Int Surg. 1999;84:209–212. [PubMed] [Google Scholar]
- 21.Komatsuda T, Ishida H, Konno K, Hamashima Y, Ohnami Y, Naganuma H. Colour Doppler findings of gastrointestinal varices. Abdom Imaging. 1998;23:45–50. doi: 10.1007/s002619900283. [DOI] [PubMed] [Google Scholar]
- 22.Morel P, Rohner A. Thromboses de la veine splenique. Notre experience de trente-quatre cas opérés. Ann Chir. 1988;42:442–447. [PubMed] [Google Scholar]
- 23.Salam AA, Warren WD, Tyras DH. Splenic vein thrombosis: a diagnosable and curable form of portal hypertension. Surg. 1973;74:961–972. [PubMed] [Google Scholar]
- 24.Simpson WG, Schwartz RW, Strodel WE. Splenic vein thrombosis. South Med J. 1990;83:417–421. doi: 10.1097/00007611-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Makowiec F, Riediger H, Emmrich J, Kroger J, Hopt UT, Adam U. Prophylaktische Splenektomie wahrend Resektion bei chronischer Pankreatitis. Zentralbl Chir. 2004;129:191–195. doi: 10.1055/s-2004-822782. [DOI] [PubMed] [Google Scholar]
- 26.Tsushima Y, Tamura T, Tomioka K, Okada C, Kusano S, Endo K. Transient splenomegaly in acute pancreatitis. Br J Radiol. 1999;72:637–643. doi: 10.1259/bjr.72.859.10624319. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Wang HP, Chen MF, Soon MS, Mo LR, Lin XZ. Chronic calcifying pancreatitis in Taiwan: a multicentric study and comparison with western countries. Hepatogastroenterology. 1997;44:842–848. [PubMed] [Google Scholar]
- 28.Takase M, Suda K, Suzuki F. A histopathologic study of localized portal hypertension as a consequence of chronic pancreatitis. Arch Pathol Lab Med. 1997;121:612–614. [PubMed] [Google Scholar]
- 29.Mortele KJ, Mergo PJ, Taylor HM, Ernst MD. Splenic and perisplenic involvement in acute pancreatitis: determination of prevalence and morphologic helical CT features. J Comput Assist Tomogr. 2001;25:50–54. doi: 10.1097/00004728-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Mauro MA, Schiebler ML, Parker LA, Jacques PF. The spleen and its vasculature in pancreatitis: CT findings. Am Surg. 1993;59:155–159. [PubMed] [Google Scholar]
- 31.Miller AR, Nagorney DM, Sarr MG. The surgical spectrum of hereditary pancreatitis. Ann Surg. 1991;215:39–43. doi: 10.1097/00000658-199201000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers C, Klatt EC. Splenic vein thrombosis in patients with acute pancreatitis. Int J Pancreatol. 1989;5:117–121. doi: 10.1007/BF02924412. [DOI] [PubMed] [Google Scholar]
- 33.Hofer BO, Ryan JA, Jr, Freeny PC. Surgical significance of vascular changes in chronic pancreatitis. Surg Gynecol Obstet. 1987;164:499–505. [PubMed] [Google Scholar]
- 34.Rosch J, Herfort K. Contribution of splenoportography to the diagnosis of diseases of the pancreas. II. Inflammatory diseases. Acta Med Scand. 1962;171:263–272. doi: 10.1111/j.0954-6820.1962.tb04188.x. [DOI] [PubMed] [Google Scholar]
- 35.Leger L, Lenroit JP, Lemaigre G. Hypertension and segmental portal stasis in chronic pancreatitis. Apropos of 126 cases examined by splenoportography and splenomanometry. J Chir. 1968;95:599–608. [PubMed] [Google Scholar]
- 36.Malikova H, Kaspar M, Weichet J, Sobotovicova A, Drechslerova J. Vascular complications of acute pancreatitis. Ceska Radiologie. 2002;56:275–279. [Google Scholar]
- 37.Leverat M, Croisille M, Pasquier J. Pancreatic lithiasis. Rev Lyon Med. 1965;14:415–425. [PubMed] [Google Scholar]
- 38.Bernades P, Baetz A, Levy P, Belghiti J. Splenic and portal venous obstruction in chronic pancreatitis: a prospective study. Dig Dis Sci. 1992;37:340–346. doi: 10.1007/BF01307725. [DOI] [PubMed] [Google Scholar]
- 39.Renner IG, Savage WT, Pantoja JL, Renner VJ. Death due to acute pancreatitis: a retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- 40.Grunert R. Pathogenic classification of portal hypertension. Ann Surg. 1965;161:350–352. doi: 10.1097/00000658-196503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemaitre G, L'Hermine C, Maillard JP, Toison FL. Hypertension portale segmentaire des pancreatities. Aspect angiographiques. Lille Med. 1971;16:928–932. [PubMed] [Google Scholar]
- 42.Koklu SE, Yuksel OS, Arhan ME. Report of 24 left-sided portal hypertension cases: a single-centre prospective study. Dig Dis Sci. 2005;50:976–982. doi: 10.1007/s10620-005-2674-x. [DOI] [PubMed] [Google Scholar]
- 43.Bloechle C, Busch C, Tesch C, Nicolas V, Binmoeller KF, Soehendra N, et al. Prospective randomized study of drainage and resection on non-occlusive segmental portal hypertension in chronic pancreatitis. Br J Surg. 1997;84:477–482. [PubMed] [Google Scholar]
- 44.Leger L, Lenroit JP, Chiche B, Lemaigre G. Sectorized portal hypertension. Aetiology; clinical aspects and complication; treatment. Ann Gastroenterol Hepatol (Paris) 1974;10:497–519. [Google Scholar]
- 45.Rosch W, Lux G, Riemann JF, Hoh L. Chronic pancreatitis and the neighbouring organs. Fortschr Med. 1981;99:1118–1121. [PubMed] [Google Scholar]
- 46.Loftus JP, Nagorney DM, Ilstrup D. Sinistral portal hypertension; splenectomy or expectant management. Ann Surg. 1992;217:35–40. doi: 10.1097/00000658-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakorafas GH, Tsiotou AG. Splenic vein thrombosis complicating chronic pancreatitis. Scand J Gastroenterol. 1999;34:1171–1177. doi: 10.1080/003655299750024661. [DOI] [PubMed] [Google Scholar]
- 48.Itschak YA, Glickman MG. Splenic vein thrombosis in patients with a normal sized spleen. Invest Radiol. 1977;12:158–163. doi: 10.1097/00004424-197703000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastro-oesophageal varices and variceal haemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 50.Perri RE, Chiorean MV, Fidler JL, Fletcher JG. A prospective evaluation of computerized tomographic (CT) scanning as a screening modality for oesophageal varices. Hepatology. 2008;47:1587–1594. doi: 10.1002/hep.22219. [DOI] [PubMed] [Google Scholar]
- 51.The North Italian Endoscopic Club for the Study and Treatment of Oesophageal Varices. Prediction of the first variceal haemorrhage in patients with cirrhosis of the liver and oesophageal varices. A prospective-multicentre study. N Engl J Med. 1988;319:983–989. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 52.Kobiashi GO, Fujita N, Noda Y. Autoimmune pancreatitis: with special reference to a localized variant. J Med Ultrason. 2008;35:41–50. doi: 10.1007/s10396-008-0177-z. [DOI] [PubMed] [Google Scholar]
- 53.Suda K, Takase M, Fukumura Y, Ogura K. Histopathologic characteristics of autoimmune pancreatitis based on comparison with chronic pancreatitis. Pancreas. 2005;30:355–358. doi: 10.1097/01.mpa.0000160283.41580.88. [DOI] [PubMed] [Google Scholar]
- 54.Johnston FR, Myers RT. Aetiologic factors and consequences of splenic vein obstruction. Ann Surg. 1973;177:736–739. doi: 10.1097/00000658-197306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nordback I, Sisto T. Peripancreatic vascular occlusions as a complication of pancreatitis. Int Surg. 1989;74:36–39. [PubMed] [Google Scholar]
- 56.Rosch J, Herfort K. Contribution of splenoportography to the diagnosis of disease of the pancreas. Acta Med Scand. 1962;171:251–261. doi: 10.1111/j.0954-6820.1962.tb04187.x. [DOI] [PubMed] [Google Scholar]
- 57.Rignault D, Mine J, Moine D. Splenoportographic changes in chronic pancreatitis. Surg. 1968;63:571–575. [PubMed] [Google Scholar]
- 58.Weber SM, Rikkers LF. Splenic vein thrombosis and gastrointestinal bleeding in chronic pancreatitis. World J Surg. 2003;27:1271–1274. doi: 10.1007/s00268-003-7247-6. [DOI] [PubMed] [Google Scholar]


