Abstract
Objective
A review of the peri-operative risk associated with hepatic resection in patients with metabolic syndrome (MetS) and identification of measures for the improvement of cardiometabolic disturbances and liver-related mortality.
Background
MetS and its hepatic manifestation non-alcoholic fatty liver disease (NAFLD) are associated with an increased operative mortality in spite of a significant improvement in peri-operative outcome after hepatic resection.
Methods
A review of the English literature on MetS, liver resection and steatosis was performed from 1980 to 2011 using the MEDLINE and PubMed databases.
Results
MetS is a predictor of NAFLD and patients with multiple metabolic risk factors may harbour non-alcoholic steatohepatitis (NASH) predictive of operative and cardiovascular mortality. Pre-operative diagnosis of unsuspected NASH with the selective use of a liver biopsy can modify the operative strategy by limiting the extent of hepatic resection, avoiding or altering the pre-operative chemotherapy regimen and the utilization of portal vein embolization. Thiazolidinediones are therapeutic for MetS and NASH and Vitamin E for active NASH; however, their utility in improving the peri-operative outcome after hepatic resection is unknown. A short-term regimen for weight loss improves post-operative patient and liver-related outcomes in patients with >30% steatosis. Cardiovascular disease associated with MetS or NAFLD should be managed aggressively. Peri-operative measures to minimize thrombotic events and acute renal injury secondary to the pro-inflammatory, prothrombotic state of MetS may further improve the outcome.
Conclusion
Potential candidates for hepatic resection should be screened for MetS as the pre-operative identification of NASH, short-term treatment of significant steatosis, cardiovascular risk assessment and optimization of each component of MetS may improve the peri-operative outcome in this high-risk subset of patients.
Keywords: liver resection, steatohepatitis, cardiovascular risk, operative mortality
Introduction
Metabolic syndrome (MetS) is a constellation of inter-related risk factors of metabolic origin including abdominal obesity, atherogenic dyslipidemia, hypertension, insulin resistance and a pro-inflammatory prothrombotic state which has an adverse impact on the peri-operative outcome.1–9 Patients with MetS have a two-fold risk of operative mortality and are at an increased risk for cardiac events, acute kidney injury, stroke and infectious complications. MetS is prevalent in 23.7% of adults and 43.5% of adults aged >60 years in the United States and non-alcoholic fatty liver disease (NAFLD) is widely accepted as the hepatic manifestation of MetS.10,11 MetS is prevalent in 36% NAFLD, 67% obese NAFLD and 88% patients with NASH.12,13 It is important to identify the presence of MetS in NAFLD patients as they frequently have advanced histologic injury of the liver and an elevated risk of mortality from cardiovascular disease (CVD) and liver-related causes.13–15 Over the past two decades, high-volume centres have reported a significant decline in operative mortality after hepatic resection; however, the presence of ≥30% hepatic steatosis is associated with a three-fold risk of mortality and a two-fold risk of post-operative morbidity.16–23
Optimization of peri-operative outcome in this high-risk subset of patients requires treatment of the individual components of MetS, NAFLD and CVD. Insulin sensitizers including thiazolidinediones (TZD) are beneficial in the treatment of MetS and NASH as insulin resistance is central to their development.24,25 Vitamin E has demonstrated therapeutic efficacy in the reduction of the histologic severity of steatosis and NASH.26 A short-term regimen for pre-operative weight loss improves patient and liver-related outcome in patients with >30% steatosis.27 Patients with multiple metabolic risk factors may harbour unsuspected NASH identifiable by a liver biopsy which will determine the extent of hepatectomy and the use of pre-operative chemotherapy or portal vein embolization (PVE). Peri-operative measures to minimize cardiovascular risk, thrombotic events and acute renal injury associated with MetS may further improve the outcome.5–9 The present study is a review of the peri-operative risk associated with hepatic resection in patients with MetS with the objective of identifying measures for the improvement of cardiometabolic risk and liver-related mortality.
Methods
A search of the English literature for articles published between 1980 and 2011 was performed using the MEDLINE database with PubMed and Ovid as search engines. The keywords included metabolic syndrome, liver resection and steatosis. All titles and abstracts of the publications were screened and relevant articles were retrieved excluding case reports. The reference lists of the retrieved articles were reviewed for potentially related studies and relevant articles were selected.
Metabolic syndrome
MetS has existed in various forms and definitions for over eight decades; however, it is only recently that its significance and the need for a unified definition have emerged. The National Cholesterol Education Program's Adult Treatment Panel III report (NCEP-ATP III) identified MetS as a multiplex risk factor which doubles the risk for atherosclerotic CVD.15,28 In a meta-analysis of MetS and the risk of CVD, MetS significantly increased all-cause and CVD-related mortality as well as the incidence of CVD and stroke.29 In 1988, Reaven noted that several risk factors cluster together and called it Syndrome X or insulin resistance syndrome as insulin resistance is a key component conferring increased risk for type 2 diabetes and CVD.30 ATP III prefers the term metabolic syndrome and has identified six components: abdominal obesity, atherogenic dyslipidemia, hypertension, insulin resistance, pro-inflammatory and prothrombotic state of which the last five components are termed metabolic risk factors.1
Diagnostic criteria for MetS
Several diagnostic criteria have been proposed by different organizations and most recently they have come from the International Diabetes Federation (IDF) and the American Heart Association/National Heart, Lung and Blood Institute (AHA/NHLBI). The main difference concerns waist measurement, the measure for central obesity, which is an obligatory component of the IDF criteria. In a consensus meeting of several major organizations attempting to unify the diagnostic criteria it was agreed upon that there should not be an obligatory component, however, waist measurement would continue to be a useful preliminary screening tool.31 Three out of five abnormal findings would qualify a person for the diagnosis of MetS as shown in Table 1. Measurement of waist circumference is ethnic-specific and the use of national or regional cutpoints was recommended. The recommended waist circumference threshold for abdominal obesity in United States is ≥102 cm and ≥88 cm in men and women, respectively.32
Table 1.
Criteria for the clinical diagnosis of metabolic syndrome [Alberti KG et al. Circulation 2009; 120 (16):1640–1645)31
| Measure | Categorical Cut Points |
|---|---|
| Increased waist circumference | Population-specific and country-specific definitions |
| Increased triglycerides (drug treatment for elevated TG is alternate indicator) | ≥150 mg/dl |
| Reduced HDL cholesterol (drug treatment for reduced HDL is alternate indicator) | <40 mg/dl in males |
| <50 mg/dl in females | |
| Increased blood pressure (antihypertensive drug treatment in patient with history of hypertension is alternate indicator) | Systolic ≥130 and/or |
| Diastolic ≥85 mm Hg | |
| Increased fasting glucose (drug treatment of increased glucose is alternate indicator) | >100 mg/dl |
The diagnosis of metabolic syndrome requires three out of five abnormal findings. HDL, high-density lipoprotein; TG, triglycerides.
Pathogenesis of MetS
The AHA/NHLBI identified three potential aetiologic categories: obesity and disorders of adipose tissue, insulin resistance and a constellation of independent factors of hepatic, vascular and immunologic origin.33
Abdominal obesity
The NCEP-ATP III recommendation to measure waist circumference in the diagnosis of MetS rather than body mass index (BMI) recognizes its greater accuracy in the assessment of cardiometabolic risk and the close correlation with visceral adiposity.34 Impaired metabolism of non-esterified fatty acids (NEFA) contributes to the insulin-resistant state in visceral obesity and the excess energy instead of being channelled into the insulin-sensitive subcutaneous adipose tissue and protecting against the development of MetS is deposited at undesirable sites such as the liver, heart, skeletal muscle and visceral adipose tissue – a phenomenon described as ectopic fat deposition as shown in Fig. 1.34 The dysfunctional visceral adipose tissue is characterized by a hyperlipolytic state resistant to the anti-lipolytic effect of insulin leading to NEFA flux to the liver promoting a fatty liver and atherogenic dyslipidemia.
Figure 1.
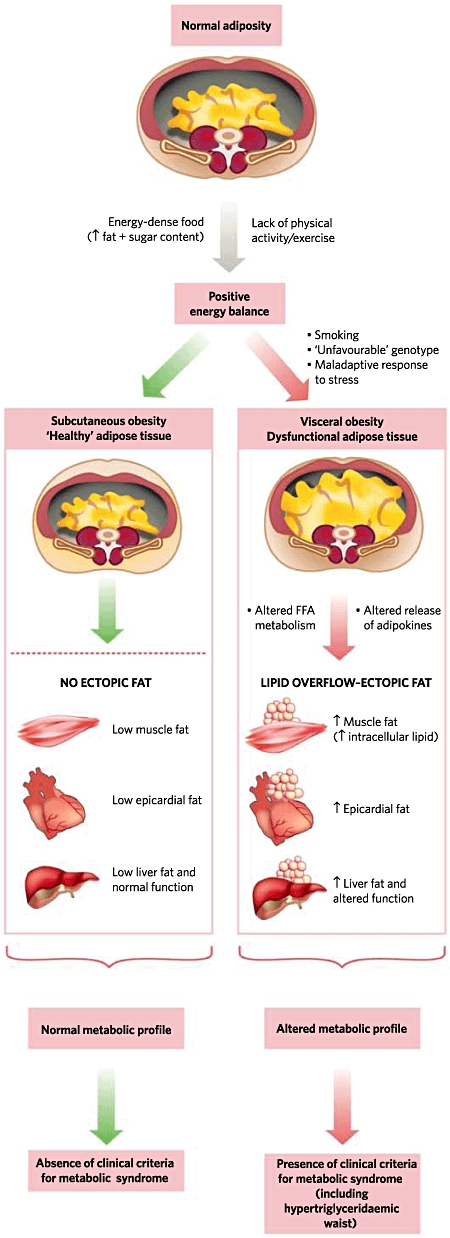
The channelling of surplus calories from excess dietary consumption and sedentary lifestyle into insulin-sensitive subcutaneous adipose tissue will protect against the development of metabolic syndrome. However, in the presence of dysfunctional adipose tissue, genetic predisposition and a neuroendocrine profile related to a maladaptive response to stress, the triacylglycerol surplus will be deposited at undesirable sites such as the liver, heart, skeletal muscle and visceral adipose tissue – a phenomenon known as ectopic fat deposition. Metabolic consequences of this defect in energy partitioning include visceral obesity, insulin resistance, atherogenic dyslipidemia and a prothrombotic proinflammatory profile the defining features of metabolic syndrome. Reprinted by permission from Macmillan Publishers Ltd: Després & Lemieux34, copyright 2006.
Insulin resistance
Several investigators consider insulin resistance and its accomplice hyperinsulinemia as the key mechanism in the development and manifestations of MetS.33 Homeostasis Model Assessment (HOMA) is used for the quantitative assessment of insulin resistance and deficient β-cell function utilizing the patient's fasting plasma insulin and glucose concentrations.35 Insulin resistance rises with increasing body fat content and most people with a BMI ≥30 kg/m2 have postprandial hyperinsulinemia with relatively low insulin sensitivity.36 Insulin resistance in muscle predisposes to glucose intolerance which is worsened by increased hepatic gluconeogenesis in an insulin-resistant liver.1
Pro-inflammatory state
Adipose tissue specializing in the storage of lipids is also an important endocrine organ releasing numerous hormones, adipocytokines and pro-inflammatory molecules such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) contributing to the inflammatory profile of the abdominally obese patients.37,38 A pro-inflammatory state recognized clinically by the elevation of C-reactive protein (CRP) is commonly present in MetS and CRP ≥3 mg/l is a risk factor for CVD.28,39,40 Low levels of adiponectin in blood is a salient feature of visceral obesity responsible for the atherogenic, diabetogenic and proinflammatory profile of MetS and is associated with an increased risk of a myocardial infarction.41–43
Prothrombotic state
A prothrombotic state characterized by increased plasma plasminogen activator inhibitor (PAI)-1 and fibrinogen is associated with MetS.44,45 Fibrinogen and acute-phase reactants such as CRP rise in response to a high-cytokine state suggesting that the prothrombotic and proinflammatory states may be metabolically interconnected.46–48
Non-alcoholic fatty liver disease
NAFLD is a spectrum of fatty liver infiltration from simple steatosis (NAFL) to necroinflammation and fibrosis (NASH). The prevalence of NAFLD in asymptomatic US adults estimated on the basis of unexplained elevation of amoinotransferases on the third National Health and Nutrition Examination Survey (NHANES-III) was 7.9% and strongly correlated with the presence of MetS.49,50 Unlike simple steatosis, NASH is a progressive liver disorder and it is estimated that 10–25% of those with NAFLD will develop NASH and 5% will progress to cirrhosis and liver failure.51–54 NAFLD is an important cause of liver-related morbidity and mortality and increases CVD-related mortality independent of MetS.55–57 Analysis of the NHANES-III participants including 980 with NAFLD and 6594 without NAFLD demonstrated that the former had a significantly higher all-cause [hazard ratio (HR) 4.10, 95% confidence interval (CI) 1.27–13.23] and cardiovascular mortality (HR 8.15, 95% CI 2.00–33.2) in the 45–54 years age group after adjusting for conventional cardiovascular risk factors.55 The authors concluded that NAFLD is a strong independent risk factor for cardiovascular death and warrants modification of CVD risk management guidelines.
The precise pathogenesis of NASH is unclear; however, the two ‘hit’ theory is most widely accepted wherein the first hit is hepatic steatosis secondary to obesity or MetS and the second hit includes insulin resistance, oxidative stress and abnormal cytokine production which plays a key role in hepatocellular injury, inflammation and fibrosis.58–60 Insulin resistance is nearly universal in NASH and plays an important role in its pathogenesis by promoting peripheral lipolysis and de novo lipogenesis.61,62 Circulating levels of IL-6 a proinflammatory cytokine elevated in MetS demonstrated a positive correlation with hepatic IL-6 expression, degree of inflammation, fibrosis and systemic insulin resistance in patients with NASH.63
Radiologic assessment of NAFLD
The adverse impact of steatosis and chemotherapy-associated liver injury on peri-operative outcome after liver resection is well recognized emphasizing the importance of its pre-operative recognition.17–23 Liver biopsy the reference for diagnosis and quantification of steatosis provides an accurate diagnosis in 90% patients; however, it is limited by its invasive nature, mortality rate of 1 in 10 000 and sampling variability.64–70 Radiologic investigations, the non-invasive alternative to liver biopsy, have demonstrated wide variability in their sensitivity and specificity for the detection and quantification of steatosis.71
More recently, in vivo proton magnetic resonance spectroscopy (MRS) [hydrogen 1 (1H) MR spectroscopy] has emerged as the reference standard in several clinical studies.72–74 However, 1H MR spectroscopy is time-consuming and has limited availability. Several modifications of the MR technique have been used with results similar to MRS.75–80 In a comparative study of the diagnostic performance of ultrasonography (US), computed tomography (CT), T1-weighted MR imaging and 1H MR spectroscopy in the preoperative assessment of steatosis in patients undergoing liver resection, MR imaging and MR spectroscopic measurements of hepatic fat had a significantly stronger correlation with histologic assessment and were able to differentiate between the 3 grades of steatosis: none, mild, moderate and severe unlike US and CT.81 The sensitivities of US, CT, T1-weighted MR imaging and MR spectroscopy were 65%, 74%, 90% and 91% and specificities were 77%, 70%, 91% and 87%, respectively. This led the authors to conclude that T1-weighted MR imaging and 1H MR spectroscopy had the best diagnostic accuracy and strongly correlate with the histologic assessment and grade of steatosis. MR elastography measuring liver stiffness demonstrated high accuracy in distinguishing simple steatosis from NASH even before the onset of fibrosis with a sensitivity of 94% and specificity of 73% using a threshold of 2.74 kPa.82 NAFLD patients with inflammation and no fibrosis had greater liver stiffness than those with simple steatosis and lower mean stiffness than those with fibrosis.
Pre-operative planning for a hepatectomy is usually based on CT findings and MR imaging is not performed routinely. In a prospective determination of the diagnostic performance of unenhanced CT in the assessment of ≥30% macrovesicular steatosis in potential donors for living donor liver transplantation using same-day biopsy as a reference standard, Park et al. reported that a liver-to-spleen (L/S ratio) attenuation ratio of 0.8 and a difference between liver and splenic attenuation of −9 yielded a 100% specificity and 82% sensitivity for both indices.83–85 The authors concluded that unenhanced CT is not clinically acceptable for quantitative assessment, however, provides high performance in the qualitative diagnosis of macrovesicular steatosis ≥30%. In a randomized placebo-controlled study of healthy individuals diagnosed with radiographically defined NAFLD based on a L/S ratio <1 on CT scan indicative of >30% hepatic steatosis, antioxidant and statin therapy significantly reduced the odds of having NAFLD at the end of follow-up.54 While these results will require validation in a trial with a liver biopsy as an end point, they demonstrate the utility of a CT scan in the radiographic diagnosis of NAFLD.
Pathologic assessment of NAFLD
The Pathology Committee of the NASH Clinical Research Network designed and validated a histologic scoring system based on the grading proposal of Brunt et al. that addresses the full spectrum of lesions of NAFLD and proposed a NAFLD activity score (NAS) for use in clinical trials.86,87 NAS specifically includes only features of active injury that are potentially reversible in the short term. The score is defined as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3) and ballooning (0–2) thus ranging from 0–8 as shown in Table 2. Fibrosis is not included in the scoring system as it is less reversible and considered a consequence of disease activity which is an accepted paradigm for staging and grading of NASH.87 NAS ≥5 correlated with a diagnosis of NASH and biopsies with scores <3 were diagnosed as ‘not NASH.’ The inter-observer agreement was 0.84 for fibrosis, 0.79 for steatosis, 0.56 for injury and 0.45 for lobular inflammation. The authors concluded that NAS was a strong scoring system with reasonable inter-observer variability that should be useful for clinical studies in both adults and children with any degree of NAFLD.
Table 2.
NAFLD activity score (NAS) [Kleiner DE et al. Hepatology 2005; 41 (6): 1313–1321]86
| Pathologic feature | Definition | Score (0–8) |
|---|---|---|
| Steatosis | Extent of parenchymal involvement | |
| <5% | 0 | |
| 5–33% | 1 | |
| >33%–66% | 2 | |
| >66% | 3 | |
| Lobular inflammation | Assessment of inflammatory foci | |
| No foci | 0 | |
| <2 foci per 200× field | 1 | |
| 2–4 foci per 200× field | 2 | |
| >4 foci per 200× field | 3 | |
| Ballooning | Liver cell injury | |
| None | 0 | |
| Few balloon cells | 1 | |
| Many cells/ prominent ballooning | 2 | |
NAS ≥5, definite NASH; NAS 3–4, borderline NASH; NAS <3, not NASH; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Impact of MetS on surgical outcome
MetS adversely impacts peri-operative outcome in patients undergoing cardiac and non-cardiac operations.2–7 In a retrospective review of 5304 consecutive patients who underwent coronary artery bypass graft (CABG) surgery, 46% patients met the diagnostic criteria for MetS and it was a strong independent predictor of operative mortality (2.4% vs. 0.9%).4 Patients with MetS had a significantly higher incidence of post-operative stroke, renal failure and infectious complications attributable to an exacerbation of the low-grade inflammatory and prothrombotic state of MetS by the systemic inflammatory response to cardiopulmonary bypass and operative stress.88 Moreover, patients with MetS have systemic oxidative stress secondary to the increased oxidative transformation of low-density lipoprotein.89 The authors recommend modification of the components of MetS with pre-operative dietary changes, an increase in physical activity and peri-operative glucose homeostasis for an improvement in outcome. CRP and albumin levels are considered as parameters of systemic inflammation and a combination of CRP ≥10 mg/l and albumin level ≤35 g/l in the Glasgow Prognostic Score for cancer is an independent predictor of peri-operative morbidity and mortality in patients with oesophageal and colorectal cancer.90,91 Other authors have identified MetS as a risk factor for post-operative atrial fibrillation, acute kidney injury, myocardial infarction and stroke.5–7
In a review of 310 208 patients with modified MetS based on obesity as the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database does not include information on abdominal circumference, presence of MetS was associated with a two-fold risk of death, 2.5-fold risk of cardiac adverse events and a three- to seven-fold higher risk of acute kidney injury leading the authors to conclude that ‘metabolically obese’ patients have a dramatically higher risk of complications after non-cardiac surgery.2 Other authors have also reported a higher rate of complications and longer hospital stay in patients with MetS undergoing elective surgery for colorectal cancer.3
Several authors have reported a significantly higher prevalence of MetS in patients with idiopathic venous thromboembolism (VTE).9,92–94 In a case–control study to investigate the presence of MetS in patients with confirmed VTE, 116 patients were compared with 129 healthy controls and the prevalence of MetS was significantly higher in patients with VTE, 35% vs. 20%.92 Individuals with MetS had significantly higher levels of high-sensitivity CRP, fibrinogen and factor VIII activity compared with those without MetS. The authors concluded that MetS is associated with a two-fold increased risk of VTE and may contribute to its development.
Impact of MetS on outcome after hepatic resection
There are several studies which evaluate the impact of steatosis on peri-operative outcome after hepatic resection, however, none specifically evaluate the outcome in patients with MetS.17–22 Furthermore, most of these studies have classified the degree of steatosis into mild, moderate and severe based on the presence of fat vacuoles in the cytoplasm of <30%, 30–60% and >60% hepatocytes without application of the NAS system for distinguishing the presence of steatohepatitis associated with a higher risk of liver failure and death after a major hepatectomy.23,86,95 An important element of the NAS system is that NAFLD can manifest with steatohepatitis in the presence of minimal steatosis.96
Transplantation of liver with severe steatosis is associated with a high risk of primary non-function in contrast to mildly steatotic grafts which yield results similar to non-steatotic livers.97,98 Fatty hepatocytes have a reduced tolerance to ischemia/reperfusion injury demonstrating a predominantly necrotic form of cell death owing to microcirculatory failure as opposed to apoptosis a key feature of ischaemic injury in the normal liver.99 Hepatocellular necrosis leads to the release of cytoplasmic contents which further increase the inflammatory response and liver injury. In addition, the ability of hepatocytes to regenerate after major tissue loss is impaired in the steatotic liver further contributing to the morbidity and mortality of hepatic resection.100
A meta-analysis of 1000 patients demonstrated a significantly increased risk of post-operative morbidity and mortality with moderate or greater steatosis after major hepatic resection.18 Several authors have confirmed steatosis as an independent predictor of complications after hepatic resection with a positive correlation between the degree of steatosis and post-operative hepatic insufficiency and an insignificant trend towards increased mortality with lobe or greater resections as shown in Table 3. Data on the influence of alcohol-related liver injury on peri-operative outcome after hepatic resection are limited with Kooby et al. reporting a similar incidence of heavy alcohol use in the steatotic and control groups.21 Little et al. reported a significantly greater operative mortality in diabetic patients undergoing hepatic resection compared with non-diabetic patients (8% vs. 2%) and the long-term survival was identical highlighting the increased peri-operative risk in these patients.101 Four of the five deaths were as a result of liver failure of which three patients had steatosis. McCormack et al. in a matched case–control study reported a mortality of 8.5% with steatotic livers compared with 1.7% in patients with lean livers [P = non-significant (NS)]. Mortality was not liver related and attributed to mesenteric infarction (n = 1), sepsis and multi-organ failure (n = 3) and cardiac failure (n = 1).19 They also reported a significantly higher incidence of major complications in the steatotic group (27% vs. 5%) and all four patients with renal failure and VTE were in the steatotic group. These data suggest that patients undergoing liver resection in the presence of steatosis are at an increased peri-operative risk not only from liver-related causes but also the systemic manifestations of MetS.
Table 3.
Impact of hepatic steatosis on peri-operative outcome after hepatic resection
| Author | Year | N | Steatosis | N | Mortality (%) | Morbidity (%) |
|---|---|---|---|---|---|---|
| Behrns22 | 1998 | 135 | None | 72 | 3 | 4 |
| Mild | 56 | 7 | 9 | |||
| Moderate-severe | 7 | 14 | 14 | |||
| Belghiti17 | 2000 | 478 | Absent | 441 | 1 | 8 |
| Present | 37 | 0 | 22 | |||
| Little101 | 2002 | 727 | Absent | 505 | 2.0 | 37 |
| Present | 222 | 4.9 | 45 | |||
| Kooby21 | 2003 | 485 | None | 160 | 5 | 35 |
| Mild | 223 | 5 | 48 | |||
| Moderate-severe | 102 | 9.4 | 62 | |||
| Gomez20 | 2007 | 386 | None | 192 | 1 | 22 |
| Mild | 122 | 2.5 | 43 | |||
| Moderate | 60 | 3 | 62 | |||
| Severe | 12 | 0 | 58 | |||
| McCormack19 | 2007 | 116 | Absent | 58 | 1.7 | 25 |
| Present | 58 | 8.6 | 50 | |||
Mild steatosis, <30% hepatocytes involved; moderate steatosis, 30–60% hepatocytes involved; severe steatosis, >60% hepatocytes involved.
Impact of pre-operative chemotherapy on NAFLD
Chemotherapy-associated steatohepatitis (CASH) based on NAS ≥4 criteria is associated with a significant increase in 90-day mortality after hepatic resection (14.7% vs. 1.6%).23 Use of irinotecan was associated with an increased risk of steatohepatitis and the effect was more pronounced in patients with a higher BMI (12.1% in BMI <25 kg/m2 and 24.6% in BMI ≥25 kg/m2) suggesting that obesity and pre-existing steatosis are risk factors for the development of CASH.23,102,103 The authors also reported a direct correlation between increasing BMI and the severity of chemotherapy-associated liver injury.102 In contrast, the use of 5-fluorouracil (5-FU) was associated with the development of steatosis without steatohepatitis in 47% of patients.104
The key molecular event underlying the development of CASH is the production of reactive oxygen species (ROS) resulting in oxidative stress in the hepatocytes.105,106 A two-hit theory for the development of CASH proposes that obesity rendering the liver susceptible to oxidative stress is the first hit and chemotherapy results in the second toxic hit.107 The generally higher BMI in the North American population compared with the European population may explain why the US study reported steatohepatitis and European studies reported vascular changes as the dominant pattern of chemotherapy-associated liver injury.102,103,108–110 These data suggest that irinotecan and oxaliplatin probably affect progression but not the development of steatosis.
Therapeutic strategies to improve outcome after hepatic resection in MetS
MetS increases the risk for advanced NAFLD and CVD suggesting that potential candidates for hepatic resection should be screened for the presence of MetS and the optimization of surgical outcome in this particularly high-risk subset of patients will require a multidisciplinary effort to treat individual components of MetS and NAFLD in consultation with a cardiologist and hepatologist.111–113
Management of MetS
The primary goal in the management of MetS is the reduction in the risk of clinical atherosclerotic disease.114 First line therapy is directed towards the major risk factors of elevated LDL cholesterol, hypertension and diabetes with the emphasis on lifestyle interventions including weight reduction, dietary modification, increased physical activity and drug therapy should be considered based on the individual cardiovascular risk.115 TZDs a peroxisome proliferator-activated receptor-γ (PPARγ) agonist class of drugs ameliorate insulin resistance and the pro-sinflammatory state of MetS by modifying the secretion of free fatty acids and adipocytokines.24 Weight reduction and drug treatment of the metabolic risk factors will reduce CRP levels and the underlying inflammatory stimulus in MetS.40,115 Aspirin prophylaxis maybe considered to counter the prothrombotic state of MetS predisposing to vascular events.114
Treatment of NAFLD
NAFLD is the most common cause of chronic liver disease in the Western world and the primary goal of treatment is to prevent associated cardiac and liver-related morbidity and mortality.116
Weight loss
The current standard of care for NAFLD is weight loss which improves liver histology, inflammatory markers and insulin resistance a key aetiologic factor in NASH.115,117–122 A randomized controlled trial to examine the effects of diet and exercise with a goal of 7–10% weight reduction demonstrated a significant improvement in serum aminotransferases and a reduction in NAS ≥3 points compared with the control group (72% vs. 30%).117 Long-term efficacy of lifestyle interventions is generally disappointing because of poor compliance; however, biochemical and histologic improvements have been reported with short-term interventions of 2–4 weeks.120–122 Significant histologic improvements reported in prospective studies evaluating the effects of lifestyle intervention in patients with NAFLD are shown in Table 4. In an effort to improve utilization of steatotic livers in living–donor liver transplantation, Nakamuta et al. treated 11 moderately steatotic donors with a short intensive treatment of a protein-rich diet (1000 kcal/day), exercise (600 kcal/day) and bezafibrate (400 mg/day) for 2–8 weeks.27 There was a significant reduction in body weight, BMI, normalization of liver function tests, lipid profile, biopsy-proven macrovesicular steatosis (30 ± 4% vs. 12 ± 2%) in the donors and the post-operative graft function was similar to donor livers without steatosis. The treated donors showed good liver function post-operatively and there was no difference in functional parameters between the treated and control groups. The best responders in the study in terms of improvement of steatosis were patients with a higher BMI. The authors concluded that the short-term intensive treatment effectively reduced steatosis and contributed to safer living-donor liver transplantation suggesting that even severely steatotic livers can be used after their intensive regimen. In a similar study of 23 potential living donors with fatty livers using a diet and exercise regimen for weight loss over 1–3 months, the authors reported a reduction in the hepatic fat content of 16.9% to 6.6% on biopsy.122 These data suggest that a pre-operative weight reduction before hepatic resection in the presence of clinically significant steatosis is feasible and useful in optimizing the peri-operative outcome.
Table 4.
Prospective studies on the effects of diet and exercise in NAFLD
| Author | Year | N | Indication | Additional therapy | Duration (months) | Histologic Improvement | ||
|---|---|---|---|---|---|---|---|---|
| NAS | Steatosis | Fibrosis | ||||||
| Promrat117 RCT | 2010 | 31 | NASH | 12 | + | + | − | |
| Hickman118 | 2004 | 14 | NAFLD | 6 | + | + | ||
| Nakamuta27 | 2005 | 11 | Pre-transplant | Bezafibrate | ½–2 | + | ||
| Chen122 | 2008 | 23 | Pre-transplant | 1–3 | + | |||
NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NAS, NAFLD activity score; RCT, randomized controlled trial.
Pharmacological treatment
A liver biopsy is required for histologic confirmation of NASH before pharmacologic therapy. As the entire histological spectrum of NAFLD including NASH and bridging fibrosis can be seen in individuals with normal alanine aminotransferase (ALT) levels and low normal ALT does not guarantee freedom from underlying steatohepatitis, serum transaminase levels cannot be used for screening patients for NASH to enhance the yield of a liver biopsy.123 In contrast, the risk of developing NASH and advanced fibrosis is related to increasing age, obesity and type 2 diabetes mellitus and the yield of a liver biopsy can be increased by targeting patients with these risk factors.124 Pharmacologic therapy should be considered in patients with active NASH to reverse fibrosis and prevent cirrhosis; however, its role in improving surgical outcome after hepatic resection is unknown.125
Insulin sensitizers
Insulin sensitizers improve insulin resistance and promote fat redistribution from ectopic tissues, liver and muscle to adipose tissue.126 Of the five randomized clinical trials evaluating the efficacy of TZDs in NASH other than PIVENS (Pioglitazone or Vitamin E for NASH Study) none were adequately powered; however, they demonstrated some consistent findings including an improvement in steatosis and liver enzymes as shown in Table 5.125 Pioglitazone is preferred because of the increased cardiovascular risk with rosiglitazone and it is reserved for the second line treatment of NASH except in select patients with diabetes and NASH where it is therapeutic for both conditions.125,128,130
Table 5.
Randomized controlled trials evaluating the therapeutic efficacy of thiazolidinediones (insulin sensitizers) in NASH
| Author | Year | N | Intervention | Comparator | Duration (months) | Histologic improvement | |||
|---|---|---|---|---|---|---|---|---|---|
| Steatosis | Inflammation | Fibrosis | Hepatocyte injury | ||||||
| Sanyal26 | 2010 | 247 | Pioglitazone or Vitamin E | Placebo | 24 | + | + | − | |
| + | + | − | |||||||
| Aithal127 | 2008 | 74 | Pioglitazone | Placebo | 12 | − | − | + | + |
| Ratziu128 | 2008 | 63 | Rosiglitazone | Placebo | 12 | + | − | − | |
| Belfort25 | 2006 | 55 | Pioglitazone | Placebo | 6 | + | + | − | + |
| Sanyal129 | 2004 | 20 | Pioglitazone + Vitamin E | Vitamin E | 6 | + | + | + | |
NASH, non-alcoholic steatohepatitis.
Antioxidants
Oxidative stress and abnormal cytokine production are ‘second hits’ in the development of NASH providing the basis for antioxidant therapy with Vitamin E.131–136 Several randomized controlled trials including PIVENS have demonstrated the therapeutic efficacy of vitamin E in NASH as shown in Table 6. Vitamin E therapy should not be considered without pathologic confirmation of the nature of the liver disease and α-tocopherol (800 IU/day) is recommended in patients with active NASH (NAS ≥4) without diabetes.125 Weight loss, TZDs and antioxidants are the most extensively evaluated therapeutic strategies in the treatment of NAFL; however, the clinical utility of these drugs in the treatment of chemotherapy-associated steatohepatitis (CASH) is unknown.137
Table 6.
Randomized controlled trials evaluating the therapeutic efficacy of Vitamin E in NASH
| Author | Year | N | Intervention | Comparator | Duration (months) | Histologic improvement | ||
|---|---|---|---|---|---|---|---|---|
| Steatosis | Inflammation | Fibrosis | ||||||
| Sanyal26 | 2010 | 247 | Vitamin E or Pioglitazone | Placebo | 24 | + | + | − |
| + | + | − | ||||||
| Dufour136 | 2006 | 48 | Vitamin E + UDCA | UDCA + Placebo or Placebo only | 24 | + | − | − |
| Sanyal129 | 2004 | 20 | Vitamin E + Pioglitazone | Vitamin E | 6 | + | + | |
| Harrison134 | 2003 | 49 | Vitamin E + Vitamin C | Placebo | 6 | − | + | |
UDCA, ursodeoxycholic acid; NASH, non-alcoholic steatohepatitis.
Peri-operative strategies
MetS is a predictor of underlying steatosis or steatohepatitis suggesting that patients who are potential candidates for a hepatic resection should be screened for MetS. The selective use of a liver biopsy in patients with multiple metabolic risk factors can identify unsuspected NASH, a predictor of post-operative mortality.124 This will permit modification of the operative strategy by limiting the extent of hepatic resection, avoiding or altering pre-operative chemotherapy regimens and the use of PVE to optimize the post-operative outcome. A standardized future liver remnant (sFLR) > 20% is adequate for the safe resection of a normal liver; however, PVE should be considered in patients with sFLR ≤ 30% in the presence of liver injury identified on the basis of clinical, laboratory, imaging or histologic data (≥30% steatosis or severe fibrosis).138–140 PVE is also recommended in patients who have received ≥6 cycles of chemotherapy as they are at an increased risk for post-operative complications.141 Further clarification is required on the issues of routine versus selective PVE in the presence of significant steatosis and the optimal sFLR based on the severity of steatosis.142
Irinotecan should be used with caution in patients with a BMI ≥25 kg/m2 and in patients with multiple metabolic risk factors. As a result of the risk of pre-existing steatosis or steatohepatitis, consideration should be given to the avoidance of pre-operative chemotherapy in favour of initial surgery for resectable colorectal liver metastases and chemotherapy should be limited with short-interval evaluations to proceed with surgery when resectability is confirmed in borderline or unresectable tumours.110 A pre-operative liver biopsy should be considered in patients at risk for CASH and major hepatic resection should be avoided in those with confirmed CASH. As imaging cannot accurately identify steatohepatitis or sinusoidal injury and liver injury is not uniform, laparoscopy with direct inspection and core biopsy before a laparotomy is preferred over image-guided percutaneous biopsy.106 Sinusoidal injury results in blue liver syndrome, steatosis in a yellow liver and non-specific findings of steatohepatitis include hepatomegaly, fatty accumulation in the round ligament and white or yellow markings or depressions on the surface of the liver.
Earlier studies have demonstrated that ischaemic preconditioning before continuous hepatic pedicle clamping reduces reperfusion injury particularly in steatotic livers.143,144 Subsequent randomized clinical trials have not confirmed a protective effect of ischaemic preconditioning with no significant difference in the mortality, liver failure, morbidity, blood loss, haemodynamic stability or hospital stay.145–147 Patients with MetS are at an increased peri-operative risk of acute kidney injury which may be aggravated by the low central venous pressure used for hepatic resection to minimize blood loss. Higher peri-operative glucose levels and glucose variability have been shown to be associated with increased complications after various types of surgical procedures including general, vascular, hepatobiliary and pancreatic surgery.148–150 These patients may benefit from a tight glucose control; however, MetS is characterized by insulin resistance and glucose homeostasis will have to be balanced against the risk of hypoglycaemia.151–153 MetS is associated with a prothrombotic state and an increased risk for VTE; however, currently there are no recommendations to modify standard prophylaxis for deep vein thrombosis.154
In conclusion, patients who are potential candidates for liver resection should be screened for the presence of MetS as they are at a high risk for an adverse peri-operative outcome. CVD should be managed aggressively in patients with MetS and NAFLD. Pre-operative identification of NASH with the selective use of a liver biopsy should prompt consideration of pharmacological therapy. The extent of the hepatic resection, the use of chemotherapy and PVE are influenced by the extent of parenchymal injury. Short-term regimens for pre-operative weight loss may be considered in patients with significant steatosis. Peri-operative measures to minimize acute renal injury, thrombotic events and glucose homeostasis may further improve the outcome. Prospective data evaluating the peri-operative outcome after hepatic resection in patients systematically screened for MetS will provide further insight into the peri-operative management of these patients. Additional research is required to identify the mechanisms responsible for the increased operative mortality associated with MetS and establish strategies to acutely modify the operative risk.
Conflicts of interest
None declared.
References
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. American Heart Association; National Heart, Lung, and Blood Institute. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Glance LG, Wissler R, Mukamel DB, Li Y, Diachun CA, Salloum R, et al. Perioperative outcomes among patients with the modified metabolic syndrome who are undergoing noncardiac surgery. Anesthesiology. 2010;113:859–872. doi: 10.1097/ALN.0b013e3181eff32e. [DOI] [PubMed] [Google Scholar]
- 3.Lohsiriwat V, Pongsanguansuk W, Lertakyamanee N, Lohsiriwat D. Impact of metabolic syndrome on the short-term outcomes of colorectal cancer surgery. Dis Colon Rectum. 2010;53:186–191. doi: 10.1007/DCR.0b013e3181bdbc32. [DOI] [PubMed] [Google Scholar]
- 4.Echahidi N, Pibarot P, Després JP, Daigle JM, Mohty D, Voisine P, et al. Metabolic syndrome increases operative mortality in patients undergoing coronary artery bypass grafting surgery. J Am Coll Cardiol. 2007;50:843–851. doi: 10.1016/j.jacc.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 5.Hong S, Youn YN, Yoo KJ. Metabolic syndrome as a risk factor for postoperative kidney injury after off-pump coronary artery bypass surgery. Circ J. 2010;74:1121–1126. doi: 10.1253/circj.cj-09-0842. [DOI] [PubMed] [Google Scholar]
- 6.Echahidi N, Mohty D, Pibarot P, Després JP, O'Hara G, Champagne J, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116(11) Suppl:I213–I219. doi: 10.1161/CIRCULATIONAHA.106.681304. [DOI] [PubMed] [Google Scholar]
- 7.Protack CD, Bakken AM, Xu J, Saad WA, Lumsden AB, Davies MG. Metabolic syndrome: a predictor of adverse outcomes after carotid revascularization. J Vasc Surg. 2009;49:1172–1180. doi: 10.1016/j.jvs.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Koren-Morag N, Goldbourt U, Tanne D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: a prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke. 2005;36:1366–1371. doi: 10.1161/01.STR.0000169945.75911.33. [DOI] [PubMed] [Google Scholar]
- 9.Dentali F, Squizzato A, Caprioli M, Fiore V, Bernasconi M, Paganini E, et al. Prevalence of arterial and venous thromboembolic events in diabetic patients with and without the metabolic syndrome: a cross sectional study. Thromb Res. 2011;127:299–302. doi: 10.1016/j.thromres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 13.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 14.Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, et al. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247–2253. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 16.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 18.de Meijer VE, Kalish BT, Puder M, Ijzermans JN. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331–1339. doi: 10.1002/bjs.7194. [DOI] [PubMed] [Google Scholar]
- 19.McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923–930. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, et al. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 21.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 23.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 24.Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 25.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. NASH CRN. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi S, et al. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 2005;80:608–612. doi: 10.1097/01.tp.0000166009.77444.f3. [DOI] [PubMed] [Google Scholar]
- 28.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 29.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 31.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 32.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 33.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA. American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 34.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 35.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 36.Bogardus C, Lillioja S, Mott DM, Hollenbeck C, Reaven G. Relationship between degree of obesity and in vivo insulin action in man. Am J Physiol. 1985;248(3)(Pt 1):E286–E291. doi: 10.1152/ajpendo.1985.248.3.E286. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Takefuji S, et al. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol. 2006;26:871–876. doi: 10.1161/01.ATV.0000208363.85388.8f. [DOI] [PubMed] [Google Scholar]
- 38.McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–47. doi: 10.1016/s0140-6736(02)07281-1. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 40.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Centers for Disease Control and Prevention; American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 41.Goldfine AB, Kahn CR. Adiponectin: linking the fat cell to insulin sensitivity. Lancet. 2003;362:1431–1432. doi: 10.1016/S0140-6736(03)14727-7. [DOI] [PubMed] [Google Scholar]
- 42.Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 43.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 44.Sjöholm A, Nyström T. Endothelial inflammation in insulin resistance. Lancet. 2005;365:610–612. doi: 10.1016/S0140-6736(05)17912-4. [DOI] [PubMed] [Google Scholar]
- 45.Anand SS, Yi Q, Gerstein H, Lonn E, Jacobs R, Vuksan V, et al. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Study of Health Assessment and Risk in Ethnic Groups; Study of Health Assessment and Risk Evaluation in Aboriginal Peoples Investigators. Circulation. 2003;108:420–425. doi: 10.1161/01.CIR.0000080884.27358.49. [DOI] [PubMed] [Google Scholar]
- 46.Aso Y, Wakabayashi S, Yamamoto R, Matsutomo R, Takebayashi K, Inukai T, et al. Metabolic syndrome accompanied by hypercholesterolemia is strongly associated with proinflammatory state and impairment of fibrinolysis in patients with type 2 diabetes: synergistic effects of plasminogen activator inhibitor-1 and thrombin-activatable fibrinolysis inhibitor. Diabetes Care. 2005;28:2211–2216. doi: 10.2337/diacare.28.9.2211. [DOI] [PubMed] [Google Scholar]
- 47.Arenillas JF, Sandoval P, Pérez de la Ossa N, Millán M, Guerrero C, Escudero D, et al. The metabolic syndrome is associated with a higher resistance to intravenous thrombolysis for acute ischemic stroke in women than in men. Stroke. 2009;40:344–349. doi: 10.1161/STROKEAHA.108.531079. [DOI] [PubMed] [Google Scholar]
- 48.Vaduganathan M, Alviar CL, Arikan ME, Tellez A, Guthikonda S, DeLao T, et al. Platelet reactivity and response to aspirin in subjects with the metabolic syndrome. Am Heart J. 2008;156:1002.e1–1002.e7. doi: 10.1016/j.ahj.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 50.Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III) Am J Med Sci. 2005;329:111–116. doi: 10.1097/00000441-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2) Suppl 1:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 52.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 54.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–77. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 55.Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology. 2006;43:1145–1151. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 58.Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 59.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 60.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 61.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 62.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 63.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 64.Hultcrantz R, Gabrielsson N. Patients with persistent elevation of aminotransferases: investigation with ultrasonography, radionuclide imaging and liver biopsy. J Intern Med. 1993;233:7–12. doi: 10.1111/j.1365-2796.1993.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 65.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 66.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 67.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. LIDO Study Group. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 68.Vuppalanchi R, Unalp A, Van Natta ML, Cummings OW, Sandrasegaran KE, Hameed T, et al. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic Fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:481–486. doi: 10.1016/j.cgh.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 70.El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, et al. Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg. 2009;250:691–697. doi: 10.1097/SLA.0b013e3181bcd6dd. [DOI] [PubMed] [Google Scholar]
- 71.Cho CS, Curran S, Schwartz LH, Kooby DA, Klimstra DS, Shia J, et al. Preoperative radiographic assessment of hepatic steatosis with histologic correlation. J Am Coll Surg. 2008;206:480–488. doi: 10.1016/j.jamcollsurg.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 72.Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, et al. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55:913–917. doi: 10.1002/mrm.20825. [DOI] [PubMed] [Google Scholar]
- 73.Irwan R, Edens MA, Sijens PE. Assessment of the variations in fat content in normal liver using a fast MR imaging method in comparison with results obtained by spectroscopic imaging. Eur Radiol. 2008;18:806–813. doi: 10.1007/s00330-007-0801-0. [DOI] [PubMed] [Google Scholar]
- 74.Thomas EL, Hamilton G, Patel N, O'Dwyer R, Doré CJ, Goldin RD, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–127. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borra RJ, Salo S, Dean K, Lautamäki R, Nuutila P, Komu M, et al. Nonalcoholic fatty liver disease: rapid evaluation of liver fat content with in-phase and out-of-phase MR imaging. Radiology. 2009;250:130–136. doi: 10.1148/radiol.2501071934. [DOI] [PubMed] [Google Scholar]
- 77.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qayyum A, Chen DM, Breiman RS, Westphalen AC, Yeh BM, Jones KD, et al. Evaluation of diffuse liver steatosis by ultrasound, computed tomography, and magnetic resonance imaging: which modality is best? Clin Imaging. 2009;33:110–115. doi: 10.1016/j.clinimag.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guiu B, Petit JM, Loffroy R, Ben Salem D, Aho S, Masson D, et al. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250:95–102. doi: 10.1148/radiol.2493080217. [DOI] [PubMed] [Google Scholar]
- 80.Bahl M, Qayyum A, Westphalen AC, Noworolski SM, Chu PW, Ferrell L, et al. Liver steatosis: investigation of opposed-phase T1-weighted liver MR signal intensity loss and visceral fat measurement as biomarkers. Radiology. 2008;249:160–166. doi: 10.1148/radiol.2491071375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256:159–168. doi: 10.1148/radiol.10091790. [DOI] [PubMed] [Google Scholar]
- 82.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–756. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 84.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 85.Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 86.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Nonalcoholic Steatohepatitis Clinical Research Network. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 87.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 88.Edmunds LH., Jr Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998;66(5) Suppl:S12–S16. doi: 10.1016/s0003-4975(98)00967-9. [DOI] [PubMed] [Google Scholar]
- 89.Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 90.Vashist YK, Loos J, Dedow J, Tachezy M, Uzunoglu G, Kutup A, et al. Glasgow prognostic score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011;18:1130–1138. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 91.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 92.Ay C, Tengler T, Vormittag R, Simanek R, Dorda W, Vukovich T, et al. Venous thromboembolism–a manifestation of the metabolic syndrome. Haematologica. 2007;92:374–380. doi: 10.3324/haematol.10828. [DOI] [PubMed] [Google Scholar]
- 93.Jang MJ, Choi WI, Bang SM, Lee T, Kim YK, Ageno W, et al. Metabolic syndrome is associated with venous thromboembolism in the Korean population. Arterioscler Thromb Vasc Biol. 2009;29:311–315. doi: 10.1161/ATVBAHA.109.184085. [DOI] [PubMed] [Google Scholar]
- 94.Ageno W, Prandoni P, Romualdi E, Ghirarduzzi A, Dentali F, Pesavento R, et al. The metabolic syndrome and the risk of venous thrombosis: a case-control study. J Thromb Haemost. 2006;4:1914–1918. doi: 10.1111/j.1538-7836.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 95.D'Alessandro AM, Kalayoglu M, Sollinger HW, Hoffmann RM, Reed A, Knechtle SJ, et al. The predictive value of donor liver biopsies on the development of primary nonfunction after orthotopic liver transplantation. Transplant Proc. 1991;23(1)(Pt 2):1536–1537. [PubMed] [Google Scholar]
- 96.Abdalla EK, Vauthey JN. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;246:340–341. doi: 10.1097/SLA.0b013e31811ea9d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation–a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 98.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 99.Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20–30. doi: 10.1097/01.sla.0000225113.88433.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagai S, Fujimoto Y, Kamei H, Nakamura T, Kiuchi T. Mild hepatic macrovesicular steatosis may be a risk factor for hyperbilirubinaemia in living liver donors following right hepatectomy. Br J Surg. 2009;96:437–444. doi: 10.1002/bjs.6479. [DOI] [PubMed] [Google Scholar]
- 101.Little SA, Jarnagin WR, DeMatteo RP, Blumgart LH, Fong Y. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6:88–94. doi: 10.1016/s1091-255x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 102.Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 103.Brouquet A, Benoist S, Julie C, Penna C, Beauchet A, Rougier P, et al. Risk factors for chemotherapy-associated liver injuries: a multivariate analysis of a group of 146 patients with colorectal metastases. Surgery. 2009;145:362–371. doi: 10.1016/j.surg.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Peppercorn PD, Reznek RH, Wilson P, Slevin ML, Gupta RK. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008–2011. doi: 10.1038/bjc.1998.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- 106.Chun YS, Laurent A, Maru D, Vauthey JN. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 2009;10:278–286. doi: 10.1016/S1470-2045(09)70064-6. [DOI] [PubMed] [Google Scholar]
- 107.Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–144. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 108.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 109.Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 110.Bilchik AJ, Poston G, Curley SA, Strasberg S, Saltz L, Adam R, et al. Neoadjuvant chemotherapy for metastatic colon cancer: a cautionary note. J Clin Oncol. 2005;23:9073–9078. doi: 10.1200/JCO.2005.03.2334. [DOI] [PubMed] [Google Scholar]
- 111.Khashab MA, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease as a component of the metabolic syndrome. Curr Gastroenterol Rep. 2008;10:73–80. doi: 10.1007/s11894-008-0012-0. [DOI] [PubMed] [Google Scholar]
- 112.Grundy SM. Cardiovascular and metabolic risk factors: how can we improve outcomes in the high-risk patient? Am J Med. 2007;120(9) Suppl 1:S3–S8. doi: 10.1016/j.amjmed.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 113.Misra VL, Khashab M, Chalasani N. Nonalcoholic fatty liver disease and cardiovascular risk. Curr Gastroenterol Rep. 2009;11:50–55. doi: 10.1007/s11894-009-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. American Heart Association; National Heart, Lung, and Blood Institute. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 115.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 116.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373–375. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 120.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 121.Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–1602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen TY, Chen CL, Tsang LL, Huang TL, Wang CC, Concejero AM, et al. Correlation between hepatic steatosis, hepatic volume, and spleen volume in live liver donors. Transplant Proc. 2008;40:2481–2483. doi: 10.1016/j.transproceed.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 123.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 124.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 125.Satapathy SK, Sanyal AJ. Novel treatment modalities for nonalcoholic steatohepatitis. Trends Endocrinol Metab. 2010;21:668–675. doi: 10.1016/j.tem.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 126.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 127.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 128.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. LIDO Study Group. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 129.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 130.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 131.Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, et al. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014–1021. doi: 10.1002/hep.1840160426. [DOI] [PubMed] [Google Scholar]
- 132.Parola M, Muraca R, Dianzani I, Barrera G, Leonarduzzi G, Bendinelli P, et al. Vitamin E dietary supplementation inhibits transforming growth factor beta 1 gene expression in the rat liver. FEBS Lett. 1992;308:267–270. doi: 10.1016/0014-5793(92)81290-3. [DOI] [PubMed] [Google Scholar]
- 133.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 134.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 135.Yakaryilmaz F, Guliter S, Savas B, Erdem O, Ersoy R, Erden E, et al. Effects of vitamin E treatment on peroxisome proliferator-activated receptor-alpha expression and insulin resistance in patients with non-alcoholic steatohepatitis: results of a pilot study. Intern Med J. 2007;37:229–235. doi: 10.1111/j.1445-5994.2006.01295.x. [DOI] [PubMed] [Google Scholar]
- 136.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Swiss Association for the Study of the Liver. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 137.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 138.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 139.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 140.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 141.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anderson CD, Meranze S, Bream P, Jr, Gorden DL, Wright JK, Pinson CW, et al. Contralateral portal vein embolization for hepatectomy in the setting of hepatic steatosis. Am Surg. 2004;70:609–612. [PubMed] [Google Scholar]
- 143.Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choukèr A, Schachtner T, Schauer R, Dugas M, Löhe F, Martignoni A, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204–211. doi: 10.1093/bja/aeh195. [DOI] [PubMed] [Google Scholar]
- 145.Azoulay D, Lucidi V, Andreani P, Maggi U, Sebagh M, Ichai P, et al. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg. 2006;202:203–211. doi: 10.1016/j.jamcollsurg.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 146.Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921–928. doi: 10.1097/01.sla.0000246834.07130.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gurusamy KS, Kumar Y, Pamecha V, Sharma D, Davidson BR. Ischaemic pre-conditioning for elective liver resections performed under vascular occlusion. Cochrane Database Syst Rev. 2009;(1):CD007629. doi: 10.1002/14651858.CD007629. [DOI] [PubMed] [Google Scholar]
- 148.Eshuis WJ, Hermanides J, van Dalen JW, van Samkar G, Busch OR, van Gulik TM, et al. Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann Surg. 2011;253:739–744. doi: 10.1097/SLA.0b013e31820b4bfc. [DOI] [PubMed] [Google Scholar]
- 149.Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248:585–591. doi: 10.1097/SLA.0b013e31818990d1. [DOI] [PubMed] [Google Scholar]
- 150.Ambiru S, Kato A, Kimura F, Shimizu H, Yoshidome H, Otsuka M, et al. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68:230–233. doi: 10.1016/j.jhin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 151.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 152.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. NICE-SUGAR Study. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 153.Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology. 2008;108:506–523. doi: 10.1097/ALN.0b013e3181649314. [DOI] [PubMed] [Google Scholar]
- 154.Tung A. Anaesthetic considerations with the metabolic syndrome. Br J Anaesth. 2010;105(Suppl. 1):i24–i33. doi: 10.1093/bja/aeq293. [DOI] [PubMed] [Google Scholar]


