Abstract
Background
Traditional survival estimates after resection for pancreatic cancer are based on clinicopathological variables at the time of diagnosis. Estimates have not reflected time survived after resection, as investigated for other malignancies. The aim of the present study was to understand how survival estimates change after pancreatic resection for cancer based on time already survived (conditional survival).
Methods
Pancreatectomies performed for pancreatic ductal adenocarcinoma (PDAC) between 2001 and 2010 were reviewed. Clinicopathological variables were evaluated to identify predictors of survival. Expected survival according to a validated nomogram for pancreatic cancer as well as conditional survival estimates and actual survival were calculated.
Results
In all, 186 patients underwent pancreatic resection for PDAC [154 (82.8%) Whipple, 26 (14.0%) distal and 6 (3.2%) total]. Median (range) survival was 22 (3.4–107.3) months. Predictors of overall survival were: absence of nodal disease [odds ratio (OR) 8.8], age <67 years (OR 8.4) and lower stage (OR 4.3). Expected survival according to the nomogram was 70% (1 year), 39.5% (2 years) and 24% (3 years). As time passed, and overall and expected survival decreased, conditional survival increased.
Discussion
The available prognostic system for PDAC underestimated survival compared with actual survival in the present study. Conditional survival estimates, based on accrued lifespan, were better than either predicted or actual survival, suggesting that survival is a dynamic, rather than static, concept. Conditional survival may, therefore, be a useful tool to allow patients and clinicians to project subsequent survival based on time accrued since resection.
Keywords: adenocarcinoma, pancreatic neoplasia, outcomes, pancreatic neoplasia
Introduction
After the diagnosis and operative management of pancreatic adenocarcinoma (PDAC), the most common question patients ask is what their anticipated survival may be. Traditional survival estimates after resection for PDAC are based on clinicopathological variables established at the time of diagnosis and surgery, and are often grim1. Thus far, estimates have not reflected time survived after resection and so, in follow-up, survival estimates remain unchanged, based on pre-operative and operative data alone.
However, conditional survival has been investigated in other malignancies including colorectal cancer liver metastases,2 where it has been found to provide more accurate prognostic information, particularly over time and for patients thought to be high risk but who survived longer than expected. Conditional survival (CS) is defined as the probability of surviving an additional number of years/months, based on a specific length of time already survived. Conditional survival estimates account for time already survived when determining ongoing survival estimates, i.e. from that point onwards. The aim of the present study was to understand whether survival estimates after pancreatectomy for PDAC change with time survived post-operatively, in order to be able to better explain post-operative survival to patients.
Methods
Under Institutional Review Board (IRB) approval, data on all pancreatectomies performed with curative intent for pancreatic ductal adenocarcinoma (PDAC) by three pancreatic surgical specialists between 2001 and 2010 were reviewed from a prospectively maintained database. Resection was classified as a pancreaticoduodenectomy, distal or total pancreatectomy. All major resections were incorporated in accordance with inclusion criteria for the Memorial Sloan Kettering Cancer Center (MSKCC) Pancreatic Cancer Nomogram (Brennan), where required data points include: location of the lesion and splenectomy, indicative of type of operation performed. Peri-operative care followed the standardized post-operative Carepath for Pancreatic Resection.3,4 Date of the last follow-up, adjuvant therapy, survival status and clinicopathological variables were tabulated. During the timeframe of the study, annual volume increased. Additionally, over this time, cyberknife radiotherapy was incorporated into the adjuvant therapy tools, but neoadjuvant therapy was not routinely employed.
Statistical analysis was performed using SAS 9.2 (SAS Inc., Cary, NC, USA). Clinicopathological variables were evaluated to identify predictors of survival in univariate and multivariate regression analysis. As is standard, statistical significance was established as P = 0.05. Expected survival was calculated and defined according to a validated nomogram for pancreatic cancer.5 CS was defined as the probability of surviving an additional number of years/months, based on time already survived. CS was calculated according to the formula:2 CSy = S(x+y)/Sx, where x = years survived and y = additional years. Previous papers have referred to the ‘5-year CS5’, as the probability of surviving a second 5-year period (10 years total) based on initial 5-year survival.2,6 This paper uses the same formula to calculate shorter and variable CS, given the more aggressive natural history of PDAC and its shorter overall survival compared with other previously investigated cancers. Results are presented and discussed as the probability of surviving a total period of time, i.e. 3 years, based on initial survival at 1 and 2 years. Conditional survival probability is presented with actual survival and nomogram-based survival estimates serving as reference points. Actual survival was calculated individually and directly, based on months survived compared with eligibility for analysis at that point. No estimation of additional survival was undertaken for censoring; thus, the actual, rather than actuarial, survival is incorporated, according to previously reported definition and methodology.7
Results
In all, 188 patients underwent pancreatic resection for PDAC (156 Whipple, 26 distal and 6 total) between 2001 and 2010. Two patients who had undergone a Whipple procedure were excluded because their pathology could not be utilized for staging per the nomogram: in one patient, the primary tumour origin could not be confirmed as PDAC, and in the other patient, no residual tumour was identified after neoadjuvant treatment. Between 2001 and 2010, 154 (82.8%), 26 (14.0%) and 6 (3.2%) patients underwent a Whipple, distal or a total pancreatectomy, respectively.
Overall median survival was 22 months (3.4–107.3 m) and 5-year survival was 20%. Figure 1 demonstrates the percentage of patients surviving at each time point, accounting for eligibility, i.e. length of follow-up. The slope of this curve lessens as time passes, again reflecting the concept of conditional survival, in which the likelihood of further survival increases the further the patient is from the time of diagnosis. In univariate analysis, negative nodes (P < 0.001) and lower stage (P < 0.001) predicted 5-year survival. In multivariate regression analysis, predictors of 5-year survival included: age < 67 years [odds ratio (OR) 8.4, 1.0–69], lower stage (OR 4.3, 1.1–17.1) and negative nodes (OR 8.8, 1.3–60.3). Patient characteristics in terms of the required input for the MSKCC nomogram are listed in Table 1, in comparison to the MSKCC patient characteristics.8 According to the nomogram, expected survival was 70% at 1 year, 39.5% at 2 years and 24% at 3 years.
Figure 1.
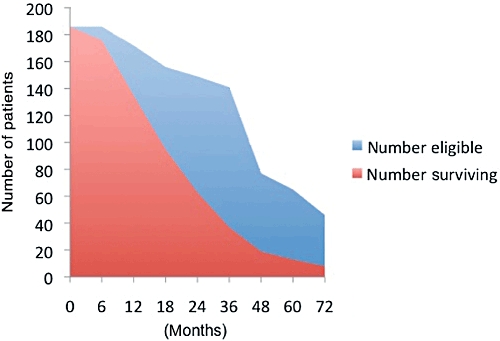
Patients surviving compared to patients eligible for survival analysis at that time point, based on date of procedure and length of follow up
Table 1.
Patient characteristics (required for nomogram)
| Current patients | MSKCC patients | ||
|---|---|---|---|
| n (186) | % | % | |
| Age (mean) | 62.4 | N/A | 65 |
| Female gender | 97 | 51.6 | 50% |
| Portal vein resection/reconstruction | 6 | 3.2 | 14% |
| Back pain | 30 | 16.1 | 14% |
| Weight loss | 87 | 46.8 | 54% |
| Splenectomy | 30 | 16.1 | 10% |
| Margin positive | 69 | 37.1 | 21% |
| Posterior margin positive | 17 | 9.1 | 14% |
| HOP mass | 160 | 86 | 89% |
| T-stage | |||
| T1 | 14 | 7.5 | 4% |
| T2 | 39 | 21 | 12% |
| T3 | 130 | 69.9 | 80% |
| T4 | 2 | 1.1 | 1% |
| Unknown | 1 | 0.5 | 3% |
| Lymph nodes positive | 117 | 62.2 | |
| Differentiation | |||
| Well | 31 | 16.8 | 14% |
| Moderate | 101 | 54.9 | 54% |
| Poor | 52 | 28.3 | 28% |
HOP, head of the pancreas; MSKCC, Memorial Sloan Kettering Cancer Center.
Conditional survival results are detailed in Table 2. The reader will note that these conditional survival calculations begin at some time (6 months, in the present study) after the initial entry into the system. This reflects both the purpose of conditional survival calculations, for modulation of survival estimates in longer-term follow-up, and also the equation itself, by which calculation results would equal actual survival at time zero. As a reference point, expected survival based on the nomogram and actual survival are included. At each total survival time, the conditional survival estimates increased per length of time already survived, even as overall and predicted survival decreased. For example, at 5 years, actual survival is 20%. Given the patient survived 6 months, the probability of surviving an additional 4.5 years (to a total of 5) is 0.255 (or 25.5%). The probability increases with additional time survived from the initial time, such that of patients surviving to the 2-year mark, 47.3% would survive an additional 3 years to a total of 5 years, and of those surviving to 4 years, 81% would survive the additional 1 year, to a total of 5 years.
Table 2.
Expected, actual, and conditional survival for resected pancreatic ductal adenocarcinoma (PDAC) (%)
| Total survival | Expected survival | Actual survival | Survival conditional on initial: | |||||
|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 18 months | 24 months | 36 months | 48 months | |||
| 6 months | – | 93.6 | ||||||
| 12 months | 70 | 78.5 | 83.6 | |||||
| 18 months | – | 60.9 | 65.1 | 77.6 | ||||
| 2 years | 39.5 | 42.3 | 45.2 | 53.9 | 69.4 | |||
| 3 years | 24 | 26.2 | 28 | 33.4 | 43.1 | 62.1 | ||
| 4 years | – | 24.7 | 26.4 | 31.4 | 40.5 | 58.4 | 94.1 | |
| 5 years | – | 20 | 21.4 | 25.5 | 32.8 | 47.3 | 76.2 | 81 |
Conditional survival was then stratified by age groups, nodal status and T-stage (Fig. 2). Overall, younger age was predictive of 5-year survival in the multivariate regression analysis. When stratified by age and by year(s) already survived, the younger patients initially had a higher conditional survival, but those older patients who did survive to the 3-year mark, were more likely than the younger patients to survive to 5 years. Stratifying the patients by lower (T1, T2) and higher (T3, T4) T-stage demonstrates the better conditional survival for the lower stage patients. However, all T3/4 patients who survived to 4 years and were 5 or more years out from their surgical date did survive to 5 years (n = 6). Lymph node negative patients had better conditional survival than lymph node positive patients at each time point. In all, 123 patients were known to undergo chemotherapy (65.4% of all patients) and 92 patients underwent radiation therapy (48.9%), usually in combination with chemotherapy. Adjuvant therapy data were unable to be determined in 45 (24.2%) patients who stated a preference for treatment closer to home but did not return for tertiary institutional follow-up. These patients were thus lost to follow-up other than for survival data, given the constraints of our IRB approval for the database.
Figure 2.
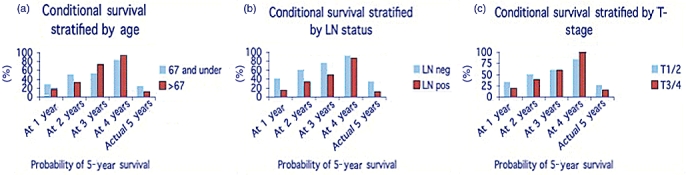
Conditional survival stratified by age, T-stage, and lymph node status. Conditional survival was better for the younger patients overall, lower T-stage, and negative lymph node status
Discussion
Pancreatic cancer is commonly understood to be an aggressive disease. Patients who receive this diagnosis are largely ineligible for resection.9 Even when patients are found to have resectable disease, survival is fairly dismal, certainly in comparison to other cancers, such as cancer of the breast, prostate, or colon.1 The study of survival, therefore, takes on a different, and often more limited, scope.
Brennan et al. have published a prognostic nomogram5 for up to 3 years, to assist in pre-operative and immediate post-operative survival estimations. The nomogram provides more specific estimates than survival based on TNM staging alone, as it accounts for patient and tumour features known at the time of surgery.10 It has been validated by other groups.8,10 Using this nomogram as it applies to our practice of pancreatectomy for PDAC, expected 1, 2, and 3-year survival was 70, 39.5, and 24%, respectively. Actual 5-year survival was 20%. As discussed by Ferrone et al., the post-resection nomogram is an important tool for short-term stratification, potentially for defining entry criteria for adjuvant therapy trials.8 They also comment that the nomogram can provide a more realistic survival estimate for patients, based on the unique features of the patient and the tumour.
This point is worth considering further as time goes on, yet survival estimates are not usually adjusted for time passed. Therein lies the relevance of the conditional survival concept: to be able to adjust our survival estimates in an ongoing manner as time passes, in order to inform patient discussions and impact quality of life for the pancreatic cancer patient. The present study demonstrates that conditional survival, in which time survived is accounted for in subsequent survival estimates, exceeds expected and actual survival. This concept has been investigated previously on a database-wide level, where improvements in survival estimates accounting for time already survived was greatest for the most lethal cancers, including lung and pancreas.6 In fact, 5-year survival for pancreas cancer patients who had survived the first 5 years, was greater than 90%.6 Practice-level data provide a different perspective than Surveillance Epidemiology and End Results (SEER) data, as it has the capacity to account for standardization within a singular practice. An additional goal was to investigate conditional survival calculations using variable time points, in contrast to the previous papers2,6 in which the concept has been framed as a 5-year re-evaluation of survival. Given the often grim prognosis and fairly short typical survival even for resected patients, the authors felt it would be useful to look at conditional survival at shorter time intervals. Conditional survival estimates here were further stratified according to those factors found to be significant in regression analysis. Similarly conditional survival continued to improve for each group in the stratified analyses but with some variation. Overall, younger age predicted 5-year survival. Younger patients had better shorter-term conditional survival but those older patients who survived to 3 and 4 years, were more likely than the younger patients to survive to 5 years. Perhaps this finding reflects some self-selection, in terms of those patients who may have been strong enough to complete adjuvant therapy. In the present study, lymph node negativity was the strongest predictor of long-term survival and afforded better conditional survival at each time point.
For this concept to be best applied in a fairly lethal disease, a large patient cohort is required,2 given the expected death rates, and a long follow-up allows longer projection in the future. The present study incorporates the pancreatectomies for cancer in a single practice with standard peri-operative care,3 and commonalities of medical oncology practice, and we feel, represents a starting point for ongoing analysis of this concept. Nonetheless, the lack of complete information about disease-free survival given local versus institutional follow-up, and the small sample size here still clearly limit the interpretation of results, and longer follow-up will be required to project survival out to 10 years and longer.
The reason for the difference in survival estimates may be, at least in part, as a result of features of the tumour biology that are not yet fully understood. For example, standard staging for pancreatic cancer falls under the TNM staging system. However, a recent paper demonstrates the importance of tumour grade on prognostication and purports that it should be included in the staging of pancreatic cancer.11 Even in the absence of further knowledge about these individual tumour biology features, the use of conditional survival allows for the adjustment of survival estimates as time goes on.
Conditional survival estimates are not intended to have pre-operative utility. Certainly the nomogram and TNM staging currently provide some starting survival estimates, but they matter less as time goes by. Conditional survival allows the physician to adjust these survival estimates as the patient continues to survive. This concept has been discussed with respect to less lethal malignancies, including in the recent paper on colorectal liver metastases survival by Nathan et al.,2 as particularly useful because patients are surviving longer after liver resection.
However, the application of the same concept to more lethal cancers such as pancreatic cancer still provides the patient with additional confidence in future survival if he/she has already survived up to or beyond what was initially forecasted. Previous work on the transition from active cancer patient to survivor with the conclusion of adjuvant treatment identifies an association between uncertainty and both functional and physical impairment.12 Literature primarily from the nursing perspective on other cancers such as breast and colon demonstrates the negative impact of uncertainty of recurrent disease and survival on the patient's quality of life after cancer treatment,13–15 which suggests that relieving some of that uncertainty may potentially improve the patient's quality of life.16
Conclusions
The available prognostic scoring system for PDAC underestimated survival compared with actual survival in the present study. Conditional survival estimates, based on accrued lifespan, were better than either predicted or actual survival at multiple time points. This finding suggests that survival is a dynamic, rather than static, concept. Patient quality of life is often impacted by uncertainty regarding survival. While that uncertainty cannot currently be eliminated, conditional survival estimates may be able to predict survival more appropriately based on time survived as a marker for currently undefined tumour biological factors. Conditional survival may therefore be a useful tool in the post-operative setting to allow patients and clinicians to project subsequent survival based on time accrued since resection. Ongoing study of this concept is required to make these estimations more robust.
Conflicts of interest
None declared.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, et al. Conditional survival after surgical resection of colorectal liver metastases: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–766. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer CM, Pratt W, Vanounou T, Maithel SK, Callery MP. Quality assessment in high acuity surgery: volume and mortality are not enough. Arch Surg. 2007;142:371–380. doi: 10.1001/archsurg.142.4.371. [DOI] [PubMed] [Google Scholar]
- 4.Vanounou T, Pratt W, Fischer JE, Vollmer CM, Callery MP. Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg. 2007;204:570–579. doi: 10.1016/j.jamcollsurg.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merrill RM, Hunter BD. Conditional survival among cancer patients in the United States. Oncologist. 2010;15:873–882. doi: 10.1634/theoncologist.2009-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540–547. doi: 10.1097/01.sla.0000184190.20289.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23:7529–7535. doi: 10.1200/JCO.2005.01.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark EJ, Taylor MA, Connor S, O'Neill R, Brennan MF, Garden OJ, et al. Validation of a prognostic nomogram in patients undergoing resection for pancreatic ductal adenocarcinoma in a UK tertiary referral centre. HPB. 2008;10:501–505. doi: 10.1080/13651820802356606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Reber H, et al. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17:2312–2320. doi: 10.1245/s10434-010-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garofalo JP, Choppala S, Hamann HA, Gjerde J. Uncertainty during the transition from cancer patient to survivor. Cancer Nurs. 2009;32:E8–E14. doi: 10.1097/NCC.0b013e31819f1aab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wonghongkul T, Dechaprom N, Phumivichuvate L, Losawatkul S. Uncertainty appraisal coping and quality of life in breast cancer survivors. Cancer Nurs. 2006;29:250–257. doi: 10.1097/00002820-200605000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Elmir R, Jackson D, Beale B, Schmied V. Against all odds: Australian women's experiences of recovery from breast cancer. J Clin Nurs. 2010;19:2531–2538. doi: 10.1111/j.1365-2702.2010.03196.x. [DOI] [PubMed] [Google Scholar]
- 15.Worster B, Holmes S. The preoperative experience of patients undergoing surgery for colorectal cancer: a phenomenological study. Eur J Oncol Nurs. 2008;12:418–424. doi: 10.1016/j.ejon.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat. 1996;39:261–273. doi: 10.1007/BF01806154. [DOI] [PubMed] [Google Scholar]


