Abstract
AIM
Patient populations that are prescribed antipsychotic agents have higher cardiovascular mortality rates. The risk of myocardial infarction is influenced by various factors that are more prevalent in patients with a mental illness. The aim of this review was to determine whether the use of antipsychotic agents is associated with the incidence of myocardial infarction in adults.
METHODS
Using multiple sources, all studies of antipsychotic agents using myocardial infarction as primary or secondary outcome measures were considered for inclusion. Study populations were adult subjects who had been prescribed an antipsychotic agent at least once in their medical history.
RESULTS
It total, five studies were identified. Four studies with small numbers of events reported a moderate to strong effect of typical antipsychotic agents on the risk of myocardial infarction. The largest study had a favourable internal validity compared with all other studies and reported no association between the risk of myocardial infarction and current use of either atypical (relative risk 0.98, 95% confidence interval [CI] 0.88, 1.09) or typical antipsychotic agents (relative risk 0.99, 95% CI 0.96, 1.03).
CONCLUSION
Clinical and methodological heterogeneity between the studies in this review led to an inconclusive answer to the question whether the use of antipsychotics is associated with the incidence of myocardial infarction in adults. Whilst results conflicted, the largest study did not find an association between the use of antipsychotic agents and an increased risk of myocardial infarction.
Keywords: antipsychotic agents, myocardial infarction
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
An unfavourable risk profile for myocardial infarction (MI) is associated with various lifestyle factors and co-morbidities that are more prevalent among patients with severe mental illness.
Patient populations that are prescribed antipsychotic agents have a higher cardiovascular mortality rate than the general population.
The association between the use of antipsychotic agents and any increase in the risk of MI is unclear.
WHAT THIS STUDY ADDS
The aim of this systematic literature review was to assess the relationship between antipsychotic agents and MI.
Substantial clinical and methodological heterogeneity between the five studies in this review led to an inconclusive answer to the question whether the use of antipsychotics is associated with the incidence of MI in adults.
The largest study had a favourable internal validity compared with the other studies and did not find an association between the use of antipsychotic agents and an increased risk of MI.
Objectives
Patient populations that are prescribed antipsychotic agents have higher mortality rates than those of a representative healthy comparison population [1–3]. The excess mortality in these patient groups can partly be attributed to a two-fold higher cardiovascular death rate [1, 4–6]. The observed over expected numbers of deaths from cardiovascular causes in patients with schizophrenia has increased from 1.7 for both men and women in 1976–1980 to 8.3 for men and to 5.0 for women over a 20 year period during which the use of antipsychotics has become more widespread [7]. Patients experiencing psychoses and in need of antipsychotic agents may be at a higher risk of myocardial infarction (MI) regardless of any effect of antipsychotic medication [8–11]. An unfavourable risk profile for MI is associated with various lifestyle factors and co-morbidities that are more prevalent in patients with a mental illness [12–16]. The association between the use of antipsychotic agents and any increase in cardiovascular morbidity or mortality is unclear, with some studies reporting increased risks for patients prescribed high doses of antipsychotics whilst other studies have found no such association [3, 17, 18].
This systematic review set out to perform an extensive literature search on the association between antipsychotics and MI and to summarize the findings of the individual studies. The central research question in this systematic review was: ‘Is the use of antipsychotics associated with the incidence of MI in adults?’
Methods
Data sources
Individual studies were identified using multiple sources. Bibliographies used included Pubmed, Medline, Embase, The Cochrane Library and Psychinfo. References of the identified articles were used to track relevant articles not identified in the original search. The searches were performed by an epidemiologist (RB), initially in December 2009, with the main database searches updated in October 2010 and May 2011. The search strategy applied combined the following terms: ‘Antipsychotic* AND agents’ (this term includes the following subheadings: ‘antipsychotic* AND drugs’, ‘antipsychotic*, ‘major AND tranquilizers’, ‘neuroleptic* AND agents’, ‘neuroleptic*’, ‘tranquilizing AND agent’) OR ‘Psychotropic*’ OR ‘Dopamine antagonist’ OR ‘Mental* AND ill*’ OR ‘Psychot* AND ill*’ OR ‘Schizophrenia’ AND ‘Myocardial infarction’ OR ‘Coronary heart disease’. The search was limited to studies of subjects aged 18 years or over.
Inclusion and exclusion criteria
All therapeutic and aetiologic studies of antipsychotic agents using MI as a primary or secondary outcome measure were included. Study populations were all patients who had been prescribed an antipsychotic agent at least once in their medical history, allowing for studies to measure ever, past, current and/or cumulative use of antipsychotics. If possible, depending on the studies identified, secondary study populations were patients grouped by psychiatric diagnoses. Studies had to compare therapy with antipsychotic agents vs. no therapy, placebo or alternative medication and had to be at least 12 months in duration. Studies comparing the efficacy and safety of different antipsychotic agents with each other have not been included in this review.
Procedure
Information on key variables was collected to summarize the individual studies and to make comparisons between the studies. The result of interest was the effect of antipsychotic agents on the incidence of MI, expressed as an odds ratio (OR) or relative risk (RR) with 95% confidence intervals (CIs). Information on both crude and adjusted results as well as dose–response relationships and the results of relevant sub-analyses concerning gender, age and history of cardiovascular disease were all recorded.
Results
We identified two case-control studies, one nested case-control study and two historic cohort studies using the procedure shown in Figure 1[19–23]. Three studies compared the risk of MI amongst typical antipsychotic users with the risk of MI in users of other psychotropic medications [19–21]. The two remaining studies investigated the association between MI and the use of atypical antipsychotics or typical antipsychotics or both vs. no use of antipsychotic agents [22, 23]. There was substantial clinical and methodological heterogeneity between the studies (see Table 1).
Figure 1.
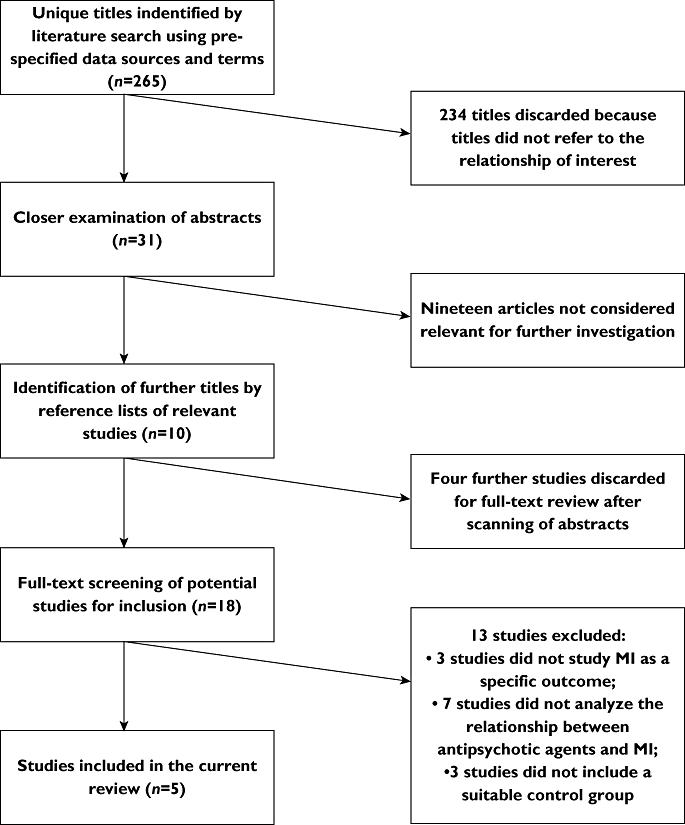
Flowchart selection studies
Table 1.
Methodological quality checklist of studies assessing the effect of antipsychotic use on the risk of myocardial infarction (MI) [31]
| Methodological feature | Pratt et al.[21] | Enger et al.[22] | Thorogood et al.[19] | Penttinen & Valonen[20] | Nakagawa et al.[23] |
|---|---|---|---|---|---|
| Sample of patients | |||||
| Inclusion criteria defined? | Yes | Yes | Yes | Yes | Yes |
| Sample selection explained? | Yes | Yes | Yes | Yes | Yes |
| Adequate description of diagnostic criteria? | Yes | Yes | Yes | Yes | Yes |
| Clinical and demographic characteristics fully described? | Yes | Yes | No | No | Yes |
| Representative? | No | No | No | No | Yes |
| Assembled at a common point in the course of their disease? | No | No | Yes | Yes | Yes |
| Complete? | No | No | Yes | Yes | Yes |
| Follow-up of patients | |||||
| Sufficiently long? | Yes | Yes | Yes | Yes | Yes |
| Outcome | |||||
| Objective? | No | Yes | Yes | Yes | Yes |
| Was the assessment blinded? | No | No | No | No | No |
| Fully defined? | No | Yes | Yes | Yes | Yes |
| Appropriate? | Yes | Yes | Yes | Yes | Yes |
| Known for all or a high proportion of patients? | No | No | Yes | Yes | Yes |
| Prognostic variable | |||||
| Fully defined, including details of method of measurement? | Yes | Yes | Yes | Yes | Yes |
| Precisely measured? | No | No | No | No | No |
| Available for all or a high proportion of patients? | No | No | Yes | Yes | Yes |
| Analysis | |||||
| Statistical adjustment for all important prognostic factors? | Yes | Yes | No | No | Yes |
| Treatment after inclusion in cohort | |||||
| Fully described? | No | No | N/A | No | N/A |
| Treatment standardized or randomized? | No | No | N/A | No | N/A |
Detailed findings by study
Details and findings of the studies are summarized in Table 2 and below.
Table 2.
Study details and findings
| First author, country and type of study | Population | Year of data collection | Follow-up time (years) | Mean age (years) at inclusion | Population size | Source of medication data | Ascertainment of MI | Comparison | Number of events/exposure in cases and control group | Details of confounders | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pratt et al. [21], USA, Historic cohort study | Patients with a history of MDE or dysphoria or no MDE or dysphoria, identified during household interviews using a validated psychiatric survey. | Exposure and mental health status assessed through interviews in 1981. Incidence of MI questioned in 1993–1994. | 13 | Age in 1981 18–29 years: 36%; 30–44 years:38.2%; 45–54 years: 10.2% 55–64 years: 9.4% >65 years: 6.2% | 71 patients treated with phenothiazines in a cohort of 1 551 participants | Interview data on self-reported drug use assessed with colour photographs of pills. | Self-reported MI | Interviewees using or having used phenothiazines vs. interviewees not using phenothiazines | MI exposed: 8 | Crude OR adjusted for sex, age, marital status, history of hypertension, and history of MDE or dysphoria | Crude OR for ever use of phenothiazines: 3.26 (95% CI 1.49, 7.12). Adjusted OR for ever use of phenothiazines: 2.92 (1.23, 6.98) |
| MI unexposed: 55 | |||||||||||
| Enger et al. [22], USA, Matched historic cohort study | Patients with schizophrenia, defined by a visit to a healthcare provider or inpatient hospital stay and an antipsychotic prescription in a general population cohort identified via a healthcare organisation | 01/04/1995 until 21/03/1999 | 1.76 | 38.2 | 1 920 patients with schizophrenia matched to 9600 healthcare members | Private Health Insurance Database | Private Health Insurance Database | Schizophrenia patients using antipsychotics vs. a 5:1 non-using sample matched by age, gender, date and health plan; class of antipsychotics received; intensity of exposure to AP | MI exposed: 12 | Crude RR adjusted for duration of follow-up, prior diabetes, prior use of antianginal medication, and prior use of hypertensive medication | Adjusted RR of MI 4.81 (2.44, 9.46) for any AP, 5.34 (1.75, 16.30) for typical only, 1.66 (0.19, 14.82) for atypical only, 5.22 (1.22, 22.40) for both. Ratio of dispensed days to total days 0.31 (0.05, 1.75) for 0.3–0.7; 0.46 (0.13, 1.69) for 0.7–1.0 |
| MI unexposed: 28 | |||||||||||
| Thorogood et al. [19], England and Wales, Population based case-control study | Women aged 16–39 years registered with a GP in England or Wales | January 1986 and December 1988 | Not applicable | Not given | 161 MI cases and 309 controls | Interviews with the general practioners of the cases and patient records | Death certificates supplied by the Office of Population Censuses and Surveys, verified by copies of post mortem reports and relevant hospital records | Cases matched to two controls by age, marital status and general practitioner. Recent use and ever use vs. never use. | Exposed cases: 25 | Confounders controlled for as matching variables. Additional variables, not matched for, not included in analysis | RR for ever use of thioxanthene: 4.6 (0.90, 24). RR for ever use of phenothiazine: 6.2 (2.0, 19.1). RR for current use of thioxanthene: 2.0 (0.3,14.2). RR for current use of phenothiazine: N/A (empty stratum). |
| Exposed control:s:13 | |||||||||||
| Penttinen & Valonen [20], Finland, Nested case-control study | Male farmers born between 1935 and 1958 in 14 Finnish municipalities who participated in a postal questionnaire | 01/02/1980 until 31/12/1992 | 11.92 | Not given | 83 MI cases and 249 controls | Patient records | Hospital discharge registries and copies of death certificates from the Finnish Statistics Bureau | Cases matched to three controls by age, smoking habit, social status and county of residence. Ever use of antipsychotics vs. non-use. | Exposed cases: 4 | Confounders controlled for as matching variables. Additional variables, not matched for, not included in analysis | OR for ever use of neuroleptics: 1.5 (0.40, 6.00) |
| Exposed controls: 6 | |||||||||||
| Nakagawa et al. [23], Denmark, Population-based case-control study | Cases and controls within the population of the North Jutland, Viborg and Aarhus counties aged 15 years and older and residents for over a year | 1 January 1992 (North Jutland County), 1 January 1999 (Viborg County) and 1 January 1997 (Aarhus County) until 31/12/2003 | Not applicable | 69.4 | 21 377 MI cases and 106 885 controls | Population-based prescription databases in the three counties | Hospital discharge registries in the three counties | Controls matched to patients by age, sex and residence using a 5:1 ratio; non-users vs. current users, new users and former users for different catagories of AP; non-users vs. users with a low cumulative dose, moderate cumulative dose or high cumulative dose | Exposed cases: 1024 | RRs were controlled for previous discharge diagnosis of hypertension, diabetes mellitus, chronic bronchitis and cardiovascular disease and prescriptions for high-dose aspirin, platelet inhibitors, insulin or oral hypoglycaemic drugs, antihypertensive drugs, lipid lowering drugs and oral anticoagulants | Adjusted RR for MI 0.98 (0.88, 1.09) for current users of atypical AP, 0.99 (0.96, 1.01) for current users of typical AP, 0.92 (0.71, 1.20) for current users of both, 1.02 (0.88, 1.18) for current female users of atypical AP, 0.94(0.81, 1.09) for current male users of atypical AP, 0.99(0.94, 1.04) for female users of typical AP, and 1(0.94, 1.06) for male users of typical AP. |
| Exposed controls: 4511 |
Pratt et al. [21] conducted a historic cohort study of United States survey data. The study population consisted of a random group of interviewed household residents divided in three categories: (i) individuals who had met the criteria for Major Depressive Episode (MDE), (ii) individuals with dysphoria and (iii) individuals without MDE or dysphoria. The 1551 subjects were originally part of a cohort of 3481 Baltimore residents. The adjusted OR for ‘ever’ use of phenothiazines was 2.92 (95% CI 1.23, 6.98).
A matched historic cohort study using data of a health care company in the United States provided information on the association between antipsychotic use and cardiovascular morbidity and mortality. Enger et al. [22] followed 11 400 cohort participants of whom 1920 were patients with schizophrenia using antipsychotics. The adjusted RR for risk of MI amongst typical antipsychotic users (RR 5.34, 95% CI 1.75, 16.30) was higher than the adjusted RR for risk of MI amongst atypical antipsychotic users (RR 1.66, 95% CI 0.19, 14.82) and the adjusted RR for risk of MI by current typical and atypical antipsychotic use (RR 5.22, 95% CI 1.22, 22.40).
Thorogood et al. [19] conducted a matched case-control study using national death certificates supplied by the Office of Population Censuses and Surveys in Wales and England to identify cases of fatal MI. One hundred and sixty-one women were matched to 309 controls by age, marital status and general practitioner. The RR for ever use of a phenothiazine was 6.2 (95% CI 2.0, 19.1). The RR could not be calculated for current use of phenothiazines (within 90 days of death) as none of the controls had experienced the outcome of interest. The RR for ever use of thioxanthene was 4.6 (95% CI 0.90, 24) and higher than the RR for current use of thioxanthene (RR 2.0, 95% CI 0.3, 14.2).
Penttinen & Valonen [20] conducted a nested case-control study in Finland using cohort data of 3172 male farmers who participated in a postal questionnaire concerning working conditions, lifestyle and various diseases and symptoms. Eighty-three men who experienced a MI were identified via hospital discharge registries. These cases were free of ischaemic heart disease at the start of follow-up and were each matched to three controls by age, smoking habit, social status and county of residence. The OR for MI amongst ever users of neuroleptic agents was 1.5 (95% CI 0.40, 6.0).
A matched case-control study using national health services registries in Denmark found no association between a first-time hospitalization for MI and current or former use of antipsychotic agents. Nakagawa et al. [23] matched 21 377 patients who were hospitalized with MI with 106 885 controls by age, gender and county of residence. The adjusted RR for current conventional antipsychotic use was 0.99 (95% CI 0.96, 1.03) and similar to the adjusted RR for current atypical antipsychotic use (RR 0.98, 95% CI 0.88, 1.09).
Subgroup analyses
Subgroup-analyses were performed by Nakagawa et al. [23]. The effect of antipsychotic agents on the risk of first-time hospitalization of MI was not influenced by gender (see Table 1), age or previous cardiovascular disease nor by the cumulative number of daily-defined dosages [23].
Enger et al. found an inverse dose-response relationship when the group with the smallest ratio of dispensed days to total days (0–0.3] was used as a reference group (see Table 2[22]).
Between study comparisons
The definitions used to identify cases of MI differed widely. MI was measured subjectively by Pratt et al. [21]. The relative validity and completeness of each objective source differed. Nakagawa et al. included first-time hospitalizations for MI only, whereas Thorogood et al. [19] included only fatal cases of MI. Penttinen & Valonen [20] used a mix of incident cases of MI that were either hospitalized or had died as a result of their MI. Enger et al. [22] included both prevalent and incident cases of MI.
All studies used heterogeneous sources of medication records and different types of medication. Thorogood et al. [19] looked specifically into two types of conventional psychotic agents (thioxanthene and phenothiazine). Pratt et al. [21] and Penttinen & Valonen [20] limited the analysis to phenothiazines only and Nakagawa et al. [23] and Enger et al. [22] grouped all typical and atypical agents together. The recording of the date of starting and stopping antipsychotic agents also differed between studies. Pratt et al. [21] and Penttinen & Valonen [20] did not measure the time of antipsychotic use and consequentially no difference between current and past use was made, nor was information given on cumulative exposure.
Almost half of all the cohort members in the study by Pratt et al. [21]had either died or were lost -to-follow-up before the study had ended. Likewise, almost 40% of the cohort members in the study by Enger et al. [22] had left the claims database before the study ended. It is not known what proportion of patients who ended their study enrolment prematurely experienced an MI.
Three studies used heterogeneous study populations. The indication for which patients received antipsychotic treatment was not shown by Thorogood et al. [19], Penttinen & Valonen [20] and Nakagawa et al. [23]. The study by Enger et al. [22] only included patients with schizophrenia who were treated with antipsychotic agents and could not control for the disease underlying the prescription of antipsychotics. Pratt et al. [21] adjusted for a history of major depressive episode.
The studies by Thorogood et al. [19] and Penttinen & Valonen [20] included only female subjects and male subjects respectively and were published without the clinical and demographic characteristics of the participants, limiting the generalizability of their results.
The size of the study populations ranged from a few hundred subjects to more than 100 000. The four studies with the smallest number of events reported moderate to strong effects of typical antipsychotic agents on the incidence of MI [19–22]. The internal validity of the largest study by Nakagawa et al. [23] compared favourably with all other studies (see Table 2). Nakagawa et al. reported no association between the use of any type of antipsychotic agent and the experience of a MI [23].
Discussion
The aim of this systematic literature review was to assess the relationship between antipsychotic agents and myocardial infarction, a drug-event pair that has not been systematically reviewed previously. The five observational studies that were the focus of this review conducted a primary or secondary analysis addressing the research question of interest.
The use of typical antipsychotics was associated with an increase in the risk of MI in four studies with a small number of events. Two studies investigated the association between MI and the use of atypical antipsychotics and found no association and a weak association respectively. These results need to be interpreted with caution as the estimates of effect size varied greatly between studies and confidence intervals for these studies finding an effect were wide. None of the studies reported a protective effect of the use of antipsychotics on the risk of MI.
The review had several limitations. The review was restricted to published studies written in English that included adult populations. No randomized controlled trials were identified. None of the studies provided information with regard to the prescription of antipsychotic agents via psychiatric services and there was a lack of information with regard to treatment compliance. The study populations in all five studies, the course of their diseases, the source and type of medication, the covariates, the study outcomes and study sizes differed substantially between studies which may have influenced the difference in results between all five studies.
Between-study comparisons of studies conducted in the early 1990s and those conducted more than a decade later are likely to have been influenced by trends in MI incidence and case-fatality as well as trends in the prescription of antipsychotic medication and of medication for the prevention and treatment of cardiovascular diseases. The studies conducted in 1992 and 1996 could not include any information on the use of atypical antipsychotic agents.
Residual confounding is likely to have influenced the results of the studies conducted in 1992 and 1996 as the adjustments of the crude associations were not as comprehensive as those made in later studies. Lifestyle factors were not accounted for or through proxy variables in all five studies. Three of the studies included in this review were conducted amongst patients using antipsychotics from the general population, whereas two studies specifically estimated a relative risk for MI in patients with schizophrenia and MDE respectively. As severe mental illness in general, and schizophrenia in particular, is associated with both the prescription of antipsychotic agents and a change in the risk of MI, the results of the individual studies are likely to have been affected by confounding by indication [3, 22]. Any difference in risk of MI that is reported in patient populations with a severe mental illness, who are also prescribed antipsychotic agents, may not be causally related to the use of the antipsychotic agent, but to factors relating to the underlying disease.
Both typical and atypical agents increase the risk of prolonged QT interval and torsades de pointes [24]. Observational studies using administrative data of patients who are prescribed antipsychotic agents have found a two-fold increase in risk of ventricular arrhythmias compared with patient populations not taking antipsychotic agents [25, 26]. It is not known through which mechanism the use of antipsychotics influences the risk of MI. Patient populations for whom antipsychotic agents are indicated differ considerably from healthy comparison populations, making it complicated to disentangle the various determinants that influence the risk of MI in users of antipsychotics [8–10, 27–29]. First time use of antipsychotics has been associated with a change in risk markers for cardiovascular events [30]. Any effect of antipsychotic agents on the risk of MI could be mediated by risk factors of MI that are more prevalent in patients suffering from psychoses.
Conclusion
Substantial clinical and methodological heterogeneity between the studies in this review led to an inconclusive answer to the question whether the use of antipsychotics is associated with the incidence of MI in adults. The largest study did not find an association between the use of antipsychotic agents and an increased risk of MI. Future studies designed to investigate the coronary side-effects of antipsychotic agents are recommended, paying attention to the strong possibility of confounding by indication. A case-only approach such as the self-controlled case series may be one solution; comparisons are made within individuals who have both the event and exposure of interest and therefore fixed confounders are completely controlled for.
Acknowledgments
In the context of the Innovative Medicine Initiative Joint Undertaking (IMI JU), Ruth Brauer's PhD is funded by Amgen Limited
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–7. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- 2.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–8. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 3.Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom's General Practice Rsearch Database. Arch Gen Psychiatry. 2007;64:242–9. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- 4.Curkendall SM, Mo J, Glasser DB, Rose Stang M, Jones JK. Cardiovascular disease in patients with schizophrenia in Saskatchewan, Canada. J Clin Psychiatry. 2004;65:715–20. doi: 10.4088/jcp.v65n0519. [DOI] [PubMed] [Google Scholar]
- 5.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000;45:21–8. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 6.Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–50. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 7.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321:483–4. doi: 10.1136/bmj.321.7259.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn T, Prud'homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry. 2004;49:753–60. doi: 10.1177/070674370404901106. [DOI] [PubMed] [Google Scholar]
- 9.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D'Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Bobes J, Arango C, Aranda P, Carmena R, Garcia-Garcia M, Rejas J. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: results of the CLAMORS Study. Schizophr Res. 2007;90:162–73. doi: 10.1016/j.schres.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–21. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Lin HC, Chen YH, Lee HC. Increased risk of acute myocardial infarction after acute episode of schizophrenia: 6 year follow-up study. Aust N Z J Psychiatry. 2010;44:273–9. doi: 10.3109/00048670903487209. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein BI, Fagiolini A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–62. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang KL, Su TP, Chen TJ, Chou YH, Bai YM. Comorbidity of cardiovascular diseases with mood and anxiety disorder: a population based 4-year study. Psychiatry Clin Neurosci. 2009;63:401–9. doi: 10.1111/j.1440-1819.2009.01974.x. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen AH, Foldager L, Parker G, Munk-Jorgensen P. Quantifying links between acute myocardial infarction and depression, anxiety and schizophrenia using case register databases. J Affect Disord. 2008;109:177–81. doi: 10.1016/j.jad.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–7. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 17.Joukamaa M, Heliovaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V. Schizophrenia, neuroleptic medication and mortality. Br J Psychiatry. 2006;188:122–7. doi: 10.1192/bjp.188.2.122. [DOI] [PubMed] [Google Scholar]
- 18.Barak Y, Baruch Y, Mazeh D, Paleacu D, Aizenberg D. Cardiac and cerebrovascular morbidity and mortality associated with antipsychotic medications in elderly psychiatric inpatients. Am J Geriatr Psychiatry. 2007;15:354–6. doi: 10.1097/JGP.0b013e318030253a. [DOI] [PubMed] [Google Scholar]
- 19.Thorogood M, Cowen P, Mann J, Murphy M, Vessey M. Fatal myocardial infarction and use of psychotropic drugs in young women. Lancet. 1992;340:1067–8. doi: 10.1016/0140-6736(92)93081-w. [DOI] [PubMed] [Google Scholar]
- 20.Penttinen J, Valonen P. Use of psychotropic drugs and risk of myocardial infarction: a case-control study in Finnish farmers. Int J Epidemiol. 1996;25:760–2. doi: 10.1093/ije/25.4.760. [DOI] [PubMed] [Google Scholar]
- 21.Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction. Prospective data from the Baltimore ECA follow-up. Circulation. 1996;94:3123–9. doi: 10.1161/01.cir.94.12.3123. [DOI] [PubMed] [Google Scholar]
- 22.Enger C, Weatherby L, Reynolds RF, Glasser DB, Walker AM. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis. 2004;192:19–27. doi: 10.1097/01.nmd.0000105996.62105.07. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa S, Pedersen L, Olsen ML, Mortensen PB, Sorensen HT, Johnsen SP. Antipsychotics and risk of first-time hospitalization for myocardial infarction: a population-based case-control study. J Intern Med. 2006;260:451–8. doi: 10.1111/j.1365-2796.2006.01708.x. [DOI] [PubMed] [Google Scholar]
- 24.Camm AJ. Cardiovascular Risk Associated with Schizophrenia and Its Treatment. London: Galliard Healthcare Communications; 2003. [Google Scholar]
- 25.Liperoti R, Gambassi G, Lapane KL, Chiang C, Pedone C, Mor V, Bernabei R. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med. 2005;165:696–701. doi: 10.1001/archinte.165.6.696. [DOI] [PubMed] [Google Scholar]
- 26.Hennessy S, Bilker WB, Knauss JS, Margolis DJ, Kimmel SE, Reynolds RF, Glasser DB, Morrison MF, Strom BL. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ. 2002;325:1070–5. doi: 10.1136/bmj.325.7372.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCreadie RG. Use of drugs, alcohol and tobacco by people with schizophrenia: case-control study. Br J Psychiatry. 2002;181:321–5. doi: 10.1192/bjp.181.4.321. [DOI] [PubMed] [Google Scholar]
- 28.McCreadie R, Macdonald E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, Wiles D, Halliday J, Paterson J. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. BMJ. 1998;317:784–5. doi: 10.1136/bmj.317.7161.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. 2006;21:1133–7. doi: 10.1111/j.1525-1497.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham KA, Cho H, Brownley KA, Harp JB. Early treatment-related changes in diabetes and cardiovascular disease risk markers in first episode psychosis subjects. Schizophr Res. 2008;101:287–94. doi: 10.1016/j.schres.2007.12.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Smith GD, Altman DG. Systematic Reviews in Healthcare: Meta Analysis in Context. 2nd edn. London: BMJ; 2000. [Google Scholar]


