Abstract
AIMS
To investigate putative associations of reports of memory disorders and suspected drugs.
METHODS
We used the case/noncase method in the French PharmacoVigilance Database (FPVD). Cases were reports of memory loss in the FPVD between January 2000 and December 2009. Noncases were all other reports during the same period. To assess the association between memory impairment and drug intake, we calculated an odds ratio with its 95% confidence interval.
RESULTS
Among the 188 284 adverse drug reactions recorded, we identified 519 cases of memory loss. The sex ratio was 0.6 and the median age was 54 years (range 4–93). The maximal number of cases occurred between 40–49 and 50–59 years. Evolution was favourable in 63% of the cases. We found significant odds ratios for benzodiazepines (alprazolam, bromazepam, prazepam, clonazepam etc.), benzodiazepine-like hypnotics (zolpidem and zopiclone), antidepressants (fluoxetine, paroxetine and venlafaxine), analgesics (morphine, nefopam and tramadol), anticonvulsants (topiramate, pregabalin, levetiracetam etc.), antipsychotics (aripiprazole and lithium) and other drugs, such as trihexyphenidyl, ciclosporin and isotretinoin.
CONCLUSIONS
Our study confirmed an association between memory disorders and some drugs, such as benzodiazepines and anticonvulsants. However, other drugs, such as benzodiazepine-like hypnotics, newer anticonvulsants, serotonin reuptake inhibitor antidepressants, isotretinoin and ciclosporin were significantly associated with memory disorders, although this was not described or poorly described in the literature.
Taking account of the limits of this study in the FPVD (under-reporting, notoriety bias etc.), the case/noncase method allows assessment and detection of associations between exposure to drugs and a specific adverse drug reaction, such as memory disorders, and could thus generate signals and orientate us to further prospective studies to confirm such associations.
Keywords: adverse drug reaction, drug, memory, pharmacoepidemiology, pharmacovigilance
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Iatrogenic amnesia is one of the main aetiologies of transient amnesia.
Benzodiazepines and anticholinergic drugs are considered to be the drugs most often responsible for iatrogenic amnesia.
The impact of drugs in memory disorders is particularly pronounced in elderly people, especially due to polymedication.
WHAT THIS STUDY ADDS
An association between memory disorders and some drugs, such as benzodiazepines, antidepressants and older anticonvulsants, was found as expected.
A strong association was found with unexpected drugs, such as benzodiazepine-like hypnotics (zopiclone and zolpidem), serotonin reuptake inhibitor antidepressants and newer anticonvulsants.
Non-neurotropic drugs, such as isotretinoin and ciclosporin, were also associated with memory disorders.
Introduction
Memory is defined as the ability to store, retain and recall information. The human memory system is divided into the following three components: short-term memory, allowing recall for a period of several seconds to 1 min without rehearsal (one form is working memory); long-term explicit memory, concerning facts taken out of context (semantic memory) and concerning information specific to a particular context, such as time and place (episodic memory); and finally, implicit memory or procedural memory, based on implicit learning.
Every stages of the process of memorization and all of the different components of memory can be affected in an isolated way or in association. Among the different patterns of memory disorders, the following two main forms of amnesia can be isolated: anterograde amnesia, when the subject cannot memorize new information; and retrograde amnesia, when the subject is unable to recall events occured before the injury.
Amnesia can also be subdivided into long-term amnesia and short-term (or transient) amnesia. Concerning transient amnesia, the main aetiologies are idiopathic transient global amnesia, epileptic, vascular, psychogenic and iatrogenic amnesia [1]. Traditionally, it is considered that benzodiazepines and anticholinergic drugs are mainly responsible for this last form [2].
The impact of drugs on memory disorders is particularly pronounced in elderly people because of polymedication. It is established that there is a decrease of mnesic abilities with ageing, but iatrogenic responsibility may be evocated when a memory alteration occurs suddenly and/or recently in an elderly person without other symptoms of dementia. The action of drugs on memory is more or less specific and serious depending on the memory system affected. Thus, analysis of the type of memory alteration can be used to incriminate more a particular drug during polymedication.
The aim of this study was to investigate the putative association of reports of memory disorders (without dementia) and suspected drugs, using the case/noncase method in the French PharmacoVigilance Database (FPVD).
Methods
Case/noncase method
The case/noncase method measures the disproportionality of combination between a drug and a particular adverse drug report (ADR) in a pharmacovigilance database [3–6]. This method can be used to generate signals from a pharmacovigilance database. Cases are defined as reports of the ADR of interest and noncases as all other reports of ADR.
For each drug of interest, the association with the ADR was assessed by calculating an ADR reporting odds ratio (ROR) with its 95% confidence interval (CI).
Selection of cases and noncases
Since 1985, all ADR reports sent spontaneously by health professionals to one of the 31 French Regional Centres of Pharmacovigilance have been entered into the FPVD.
In our study, we collected ADRs recorded in the FPVD between 1 January 2000 and 31 December 2009.
Cases were defined as HLT (High Level Term, MedDRA 11.0, Medical Dictionary for Regulatory Activities) ‘memory loss (dementia excluded)’, including ‘amnesia’, ‘anterograde amnesia’, ‘global amnesia’, ‘memory disturbance’ and ‘amnesic disorder’. Noncases, using as controls, were all the remaining ADR reports recorded in the database during the same period.
Drug exposition was defined by the presence in the report of the drug checked ‘suspect’ according to the World Health Organization criteria, whatever the level of causality assessment. We selected drugs for which four or more reports of memory disorders had been registered in the FPVD.
If available, the following data were also collected from each report: age, sex, seriousness and evolution of the ADR.
Seriousness was defined as an ADR leading to hospitalization (or prolongation of hospitalization), persistent or significant disability or incapacity, life threatening or death.
Statistical analysis
Collected data were compared between reports defined as memory disorders (cases) and all other reports in the database (noncases). We calculated an ROR to compare risk of exposure to different drugs in cases and noncases. The RORs are given with their 95% CIs [7].
Results
Of the 188 284 reports recorded in the French Pharmacovigilance database during the studied period, we identified 519 cases of memory loss (dementia excluded), representing 0.3% of all reports. Unfortunately, the type of memory affected was often not specified. Among them, 292 (57%) were observed in women (sex ratio 0.6). Median age was 54 years (range 4–93 years).
The maximal number of cases occurred between 40–49 and 50–59 years (n = 98 and n = 96, respectively; Figure 1). In addition, 127 cases were reported after 70 years (74 after 75 years; Figure 1).
Figure 1.
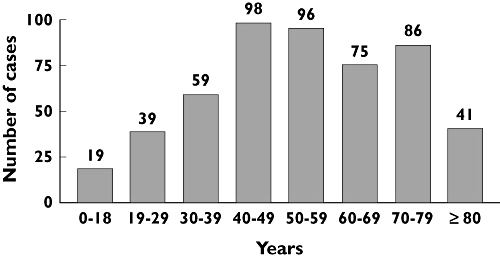
Repartition of the 519 cases of memory disorders according to age
Most cases (60%) were considered as ‘nonserious’. None of the cases induced irreversible damage. For the majority of the patients, symptoms resolved after withdrawal of the suspect drug (63% of the cases).
Among the 519 cases, 301 drugs were mentioned as suspect. The main therapeutic classes suspected were hypnotics (76 cases), anticonvulsants (68 cases), anxiolytics (66 cases), antidepressants (55 cases), analgesics (45 cases) and antipsychotic drugs (29 cases).
Significant RORs were found for 30 drugs mentioned in Table 1. Among these drugs, zolpidem [ROR 23.9, 95% CI (17.9, 31.9)], topiramate [ROR 11.6, 95% CI (6.3, 11.3)], zopiclone [ROR 8.7, 95% CI (5.2, 14.3)], alprazolam [ROR 8.0, 95% CI (4.7, 13.7)] and bromazepam [ROR 7.6, 95% CI (4.4, 13.0)] presented the most significant associations with memory disorders.
Table 1.
Risk of exposure to drugs and occurrence of memory disorders in the French PharmacoVigilance Database (FPVD)
| Drugs | Number of ADRs in the FPVD | Number of memory disorders in the FPVD | Mention in French Summary of Product Characteristics | Reporting odds ratio (95% confidence interval) | P-value |
|---|---|---|---|---|---|
| Benzodiazepines (anxiolytics or not) | 82 | ||||
| Alprazolam | 664 | 14 | Anterograde amnesia (risk increasing with dosage) | 8.0 (4.7, 13.7) | <0.001 |
| Bromazepam | 697 | 14 | 7.6 (4.4, 13.0) | <0.001 | |
| Clonazepam | 900 | 17 | 7.2 (4.4, 11.7) | <0.001 | |
| Lorazepam | 387 | 7 | 6.8 (3.2, 14.4) | <0.001 | |
| Prazepam | 305 | 6 | 7.3 (3.3, 16.5) | <0.001 | |
| Tetrazepam | 770 | 5 | 2.4 (1.0, 5.8) | <0.05 | |
| Other anxiolytics | 12 | ||||
| Hydroxyzine | 717 | 6 | No data | 3.1 (1.4, 6.9) | <0.01 |
| Meprobamate | 630 | 6 | No data | 3.5 (1.6, 7.9) | <0.01 |
| Benzodiazepine-like hypnotics | 76 | ||||
| Zolpidem | 963 | 54 | Anterograde amnesia (risk increasing with dosage) | 23.9 (17.9, 31.9) | <0.001 |
| Zopiclone | 703 | 16 | 8.7 (5.2, 14.3) | <0.001 | |
| Antidepressants | 55 | ||||
| Selective serotonin reuptake inhibitors | 30 | ||||
| Fluoxetine | 932 | 9 | No data | 3.6 (1.8, 6.9) | <0.001 |
| Paroxetine | 1831 | 16 | No data | 3.3 (2.0, 5.4) | <0.001 |
| Others | 14 | ||||
| Venlafaxine | 1145 | 7 | No data | 2.2 (1.0, 4.6) | <0.05 |
| Antipsychotic | 25 | ||||
| Aripiprazole | 234 | 4 | No data | 6.3 (2.3, 17.1) | <0.001 |
| Mood stabilizer | 4 | ||||
| Lithium | 450 | 4 | No data | 3.3 (1.2, 8.8) | <0.05 |
| Anticonvulsants | 68 | ||||
| Gabapentin | 628 | 5 | Frequent: amnesia | 2.9 (1.2, 7.1) | <0.05 |
| Lamotrigine | 658 | 5 | No data | 2.8 (1.2, 6.8) | <0.05 |
| Levetiracetam | 388 | 4 | Frequent: amnesia | 3.8 (1.4, 10.2) | <0.01 |
| Pregabalin | 567 | 7 | Frequent: memory disorders | 4.6 (2.2, 9.7) | <0.001 |
| Topiramate | 361 | 11 | Frequent: memory disorders, amnesia | 11.6 (6.3, 11.3) | <0.001 |
| Valproic acid/valproate | 1732 | 12 | Rare: cognitive disorders | 2.6 (1.4, 4.6) | <0.001 |
| Analgesics | 45 | ||||
| Morphine | 751 | 6 | No data | 2.9 (1.3, 6.9) | <0.01 |
| Nefopam | 520 | 4 | No data | 2.8 (1.1, 7.6) | <0.05 |
| Tramadol | 2463 | 12 | No data | 1.8 (1.0, 3.2) | <0.05 |
| Others | |||||
| Bupropion | 568 | 7 | Rare: memory disorders | 4.6 (2.2, 9.7) | <0.001 |
| Ciclosporin | 547 | 4 | No data | 2.7 (1.0, 7.2) | <0.05 |
| Hepatitis B vaccine | 1965 | 31 | No data | 6.1 (4.2, 8.8) | <0.001 |
| Isotretinoin | 553 | 4 | No data | 2.7 (1.0, 7.1) | <0.05 |
| Mefloquine | 247 | 4 | Memory disorders | 6.0 (2.2, 16.2) | <0.001 |
| Trihexyphenidyl | 201 | 4 | No data | 7.4 (2.7, 20.0) | <0.001 |
Discussion
The aim of this work was to assess which drugs could be implicated in the occurrence of memory disorders through a case/noncase study in the FPVD.
Preclinical data are often limited and clinical trials, although essential, are performed on a too small number of patients for detecting uncommon effects. Thus, the case/noncase method is a very useful method for assessing and detecting associations between a specific adverse drug effect and exposure to drugs in real conditions of use, because it is simple and quick and the data used are already available. Several studies have been recently published using this method on this database applying to different fields of drug safety [3, 8] but also to pharmacodependence [9].
The limits of this methodology in the FPVD lie especially in the under-reporting of ADRs in general, and particularly in elderly people, and notoriety bias. Indeed, the association between a drug and an adverse drug effect could be decreased if another effect is more often reported by physicians, and vice versa. Despite these inherent limits of this type of study, case/noncase methodology is very useful to generate signals, especially in pharmacovigilance.
In the FPVD, 519 cases of memory disorders were reported in the last 10 years. The type of memory affected could not be analysed because this information was often not described. The median age was 54 years and, surprisingly, only 17% of cases were older than 75 years, indicating that age is not the most important associated factor in drug-induced amnesia. However, in elderly people, the iatrogenic responsibility for the occurrence of amnesia is difficult to determine because of the presence of other aetiologies, such as incipient dementia and depression. Consequently, this ADR is probably under-reported in this age category.
Memory disorders were reported mostly in women in the FPVD between 1 January 2000 and 31 December 2009 (57%). However, we also observed that women presented ADRs more often in the FPVD during the same period (54%).
Concerning drugs involved in memory disturbance, positive associations were found with some expected drugs, such as benzodiazepines, tricyclic antidepressants or antipsychotics, but also with unexpected drugs, such as benzodiazepine-like hypnotics, serotonin reuptake inhibitors (SRIs) antidepressants and second-generation anticonvulsants. Overall, six main therapeutic classes were mainly associated with memory alterations.
The most represented class was the benzodiazepines (82 cases), in particular, alprazolam, bromazepam, clonazepam, lorazepam, prazepam and tetrazepam. In these cases, after analysis of the clinical description in the database, we frequently retrieved anterograde amnesia, which is a well-known effect with this pharmacological class; indeed, it was mentioned in the summary of product characteristics as a potentially dose-dependent effect. Moreover, in the literature, these effects are often described with short-acting benzodiazepines [10], and the mechanism underlying the effects involves the ω1 subtype of GABAA receptors [11, 12].
We also observed a possible association with the benzodiazepine-like hypnotics, zolpidem and zopiclone. Interestingly, zolpidem presented the most important association with memory disorders [ROR 23.9, 95% CI (17.2, 31.9)]. The effects of zolpidem on memory are at the moment controversial. Some authors consider that this molecule can induce similar amnesic effects to benzodiazepines [13], whereas others [14] consider it to be safer than benzodiazepines. The fact that zolpidem had the most important association with amnesia could be explained by its mechanism of action. Indeed, zolpidem has a great affinity for ω1 subtype receptors which, as mentioned earlier, are implicated in memory defects. In contrast, zopiclone presents affinity not only for the ω1 subtype receptors but also for the ω2 subtype, underlying its anxiolytic properties in humans [15, 16] and anticonvulsant, myorelaxant and anxiolytic properties in animals [17]. Thus, zopiclone induces memory disorders to the same extent as benzodiazepines [18, 19].
Antidepressants were also well represented in our study. Although we did not show significant associations with memory disorders, and memory disorders were not mentioned in the summary of product characteristics of these drugs, tricyclic antidepressants are well described as able to induce these disturbances, at least in part through their atropinic properties [20]. It is now clear that some of the adverse effects produced by many tricyclic antidepressants are a consequence of their blockade of muscarinic cholinergic receptors, which are the dominant cholinergic receptors in the brain and seem to be involved in learning and memory. Among tricyclic antidepressants, amitriptyline tends to produce atropinic adverse effects more frequently [21–24]. However, surprisingly, in our study, no tricyclic antidepressant was found to be significantly associated with a memory disorder. One hypothesis is that this could be linked to a notoriety bias. Indeed, as effects on memory are well known with these drugs owing to their atropinic properties, physicians do not report them to their regional centres of pharmacovigilance. Moreover, these drugs are now less prescribed, especially in elderly people.
Conversely, in this class of antidepressants we identified associations between memory disorders and some SRIs, such as fluoxetine and paroxetine. These antidepressants share a common characteristic, their selective blockade of the reuptake of serotonin (5-HT) [21, 25, 26]. The principal advantages of these drugs are that they are safer and better tolerated than tricyclic antidepressants [27]. However, SRI antidepressants have several other properties, including inhibition of noradrenaline reuptake, inhibition of dopamine reuptake, a 5HT2c receptor agonist effect, a muscarinic antagonist effect, effects on sigma receptors, inhibition of nitric oxide synthase and inhibition of cytochromes P450 2D6, 3A4 and 1A2. For example, paroxetine has a muscarinic antagonist effect [27], which could explain its amnesic effects observed in this study. Moreover, memory disorders could be due to their serotoninergic effects, because several studies indicate that serotoninergic neurons could play a significant role in learning and memory [28–30]. Injections of drugs with serotoninergic properties in animals induce disturbances in memory functions [31–33], but the precise nature of this regulation remains unclear. Some authors suggest that serotoninergic and cholinergic systems interact in a complex manner in the regulation of learning and memory functions [34, 35]. Conversely, although associated with memory dysfunctions in the present study, it has been shown that fluoxetine, which has no atropinic effects, could lead to positive effects on memory by counteracting the memory impairment produced by other agents, especially in rats [36–38].
In the same way, another antidepressant, venlafaxine, a noradrenaline and serotonin reuptake inhibitor, was also associated with memory disorders in our study. However, this drug has not been shown to have a significant affinity for muscarinic cholinergic receptors and does not seem to exert the stimulant effect on memory seen with fluoxetine [21].
In the pharmacological class of antipsychotics, only one drug, aripiprazole, was identified as being associated with memory disorders, although there are many drugs in this class with atropinic properties. As for tricyclic antidepressants, we hypothesized an under-reporting owing to the knowledge by the physicians of the atropinic properties of these drugs and thus their ability to induce memory disorders. For aripiprazole, we did not find any report of memory trouble mentioned in the literature.
Lithium was also associated with memory disorders in this study. The effects of this drug on cognition have been well described, in particular, delirium and impaired memory and psychomotor performance [39, 40].
Anticonvulsants were also reported in our study to be associated with amnesic effects. Six drugs were identified; one ‘older’ anticonvulsant (valproate/valproic acid) and five newer drugs (lamotrigine, levetiracetam, gabapentin, pregabalin and topiramate). In the context of epilepsy, it is difficult to assess the involvement of drugs in the occurrence of mnesic effects, because of the multifactorial pattern of the pathology. Cognitive disorders induced by anticonvulsants seem to be different in children, adults and elderly people. Polymedication with several anticonvulsants could also increase the risk of memory disorders [41]. Some authors reported that valproic acid does not alter memory function [42]. In other studies conducted in non-elderly people, phenobarbital and primidone appeared to have more cognitive effects than carbamazepine, phenytoin and valproic acid [43, 44]. Newer anticonvulsants seem to have less cognitive effects than older ones [45], but only a few studies have been conducted in monotherapy. Among them, topiramate presented a strong association with memory disorders [ROR 11.6, CI (6.3, 11.3)]. These effects are well known with this drug [46, 47], which especially alters short-term memory [48]. Levetiracetam could also induce memory disorders, because it is reported in the summary of product characteristics. Conversely, in the literature, levetiracetam is considered as inducing minor effects on cognition [46, 49]. However, manufacturing data reported that 2% of levetiracetam-treated patients experienced amnesia compared with 1% of placebo-treated patients in randomized, double-blind, placebo-controlled studies.
An increase of the incidence of memory disorders was also associated with pregabalin use. This drug is mentioned in particular in a publication reporting cases of treated patients which manifested partial significant impairments in episodic memory of verbal and visual information [50].
We found mention of amnesia with gabapentin and lamotrigine only in clinical trials (manufacturing data), although it is mentioned in the summary of product characteristics for gabapentin.
The last main pharmacological class of drugs associated with memory disorders in our study is analgesics, including morphine, nefopam and tramadol. In contrast to our results, in a study assessing cognitive effects of different analgesics, it has been shown that morphine could increase cognitive performances of healthy subjects aged from 25 to 40 years [51]. Concerning nefopam and tramadol, no data are available in the literature about their potentially amnesic properties. However, it is known that nefopam presented atropinic properties (summary of product characteristics). Thus, these properties could explain the memory disorders observed.
Among the other drugs with a positive association, we found mefloquine, an antimalarial drug for which one case of memory disorders has been described, but the causative role of the drug is discussed; moreover, the importance of a differential diagnosis of neuropsychiatric disorders from toxic or parasitic origins was underlined [52].
For isotretinoin, hepatitis B vaccine and ciclosporin, no previous cases of memory disturbance have been reported to our knowledge. In our study, for all the cases associated with isotretinoin administration, imputability, a causality assessment score, was evaluated as possible (I1), according to the official French methodology [53], excepted for one in which imputability was plausible (I2). However, for two cases, after isotretoin discontinuation, patients completely recovered.
In the same way, the responsibility of ciclosporin for memory disorders was judged as possible in three of the four cases reported. For the fourth case, imputability was plausible; the subject developed paraesthesia, dysarthria, tremor, amnesia and faintness nearly 7 months after initiation of treatment with ciclosporin. These effects resolved spontaneously 2 months after drug withdrawal.
Finally, for hepatitis B vaccine, most of the cases of memory disorders were accompanied by other adverse effects, and the responsibility of the vaccine in the occurrence of memory disorders was often discussed.
In conclusion, taking into account the limits of the methodology, this study confirmed the association of memory disorders with drugs such as benzodiazepine and older anticonvulsants and, interestingly, showed a strong association with other drugs, such as benzodiazepine-like hypnotics, SRI antidepressants and newer anticonvulsants. Moreover, we found positive associations with non-neurotropic drugs, such as isotretinoin and ciclosporin, which merit exploration. All these data should be confirmed with prospective studies, and we encourage physicians to notify memory disorders, especially in elderly people.
Acknowledgments
The authors would like to thank the 31 Centers of the French Regional PharmacoVigilance System for providing data.
Competing Interests
There are no competing interests to declare.
One part of this study was performed as a pharmacy thesis by F. Chavant.
REFERENCES
- 1.Sellal F. [Transient amnesia in the elderly] Psychol Neuropsychiatr Vieil. 2006;4:31–8. [PubMed] [Google Scholar]
- 2.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455–9. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favreliere S, Lafay-Chebassier C, Alkhidir F, Merlet I, Perault Pochat MC. [Drug-induced dementia: a case/non-case study in the French Pharmacovigilance database] Therapie. 2007;62:507–11. doi: 10.2515/therapie:2007070. [DOI] [PubMed] [Google Scholar]
- 4.Moore N, Kreft-Jais C, Haramburu F, Noblet C, Andrejak M, Ollagnier M, Begaud B. Reports of hypoglycaemia associated with the use of ACE inhibitors and other drugs: a case/non-case study in the French pharmacovigilance system database. Br J Clin Pharmacol. 1997;44:513–8. doi: 10.1046/j.1365-2125.1997.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore N, Noblet C, Kreft-Jais C, Lagier G, Ollagnier M, Imbs JL. [French pharmacovigilance database system: examples of utilisation] Therapie. 1995;50:557–62. [PubMed] [Google Scholar]
- 6.Lugardon S, Lapeyre-Mestre M, Montastruc JL. Upper gastrointestinal adverse drug reactions and cyclo-oxygenase-2 inhibitors (celecoxib and rofecoxib): a case/non-case study from the French Pharmacovigilance Database. Eur J Clin Pharmacol. 2004;60:673–7. doi: 10.1007/s00228-004-0813-5. [DOI] [PubMed] [Google Scholar]
- 7.Ewans SJW. Statistics: analysis and presentation of safety data. In: Talbot J, Waller P, editors. Stephen's Detection of New Adverse Drug Reactions. 5th edn. Chichester: Wiley; 2004. pp. 301–28. [Google Scholar]
- 8.Montastruc G, Favreliere S, Sommet A, Pathak A, Lapeyre-Mestre M, Perault-Pochat MC, Montastruc JL. Drugs and dilated cardiomyopathies: a case/noncase study in the French PharmacoVigilance Database. Br J Clin Pharmacol. 2010;69:287–94. doi: 10.1111/j.1365-2125.2009.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beau-Salinas F, Jonville-Bera AP, Cissoko H, Bensouda-Grimaldi L, Autret-Leca E. Drug dependence associated with triptans and ergot derivatives: a case/non-case study. Eur J Clin Pharmacol. 2010;66:413–7. doi: 10.1007/s00228-009-0769-6. [DOI] [PubMed] [Google Scholar]
- 10.Roth T, Roehrs T, Wittig R, Zorick F. Benzodiazepines and memory. Br J Clin Pharmacol. 1984;18(Suppl 1):45S–49S. doi: 10.1111/j.1365-2125.1984.tb02581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Savic MM, Obradovic DI, Ugresic ND, Bokonjic DR. Memory effects of benzodiazepines: memory stages and types versus binding-site subtypes. Neural Plast. 2005;12:289–98. doi: 10.1155/NP.2005.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo BL, Greene WL. Zolpidem: distinct from triazolam? Ann Pharmacother. 1997;31:625–32. doi: 10.1177/106002809703100518. [DOI] [PubMed] [Google Scholar]
- 14.Declerk A, Bisserbe J. Short-term safety profile of zolpidem: objective measures of cognitive effects. Eur Psychiatry. 1997;12(Suppl 1):15–20. doi: 10.1016/s0924-9338(97)80016-8. [DOI] [PubMed] [Google Scholar]
- 15.Agnoli A, Manna V, Martucci N. Double-blind study on the hypnotic and antianxiety effects of zopiclone compared with nitrazepam in the treatment of insomnia. Int J Clin Pharmacol Res. 1989;9:277–81. [PubMed] [Google Scholar]
- 16.Fontaine R, Beaudry P, Le Morvan P, Beauclair L, Chouinard G. Zopiclone and triazolam in insomnia associated with generalized anxiety disorder: a placebo-controlled evaluation of efficacy and daytime anxiety. Int Clin Psychopharmacol. 1990;5:173–83. doi: 10.1097/00004850-199007000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Julou L, Blanchard JC, Dreyfus JF. Pharmacological and clinical studies of cyclopyrrolones: zopiclone and suriclone. Pharmacol Biochem Behav. 1985;23:653–9. doi: 10.1016/0091-3057(85)90433-2. [DOI] [PubMed] [Google Scholar]
- 18.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–33. [PMC free article] [PubMed] [Google Scholar]
- 19.Warot D, Bensimon G, Danjou P, Puech AJ. Comparative effects of zopiclone, triazolam and placebo on memory and psychomotor performance in healthy volunteers. Fundam Clin Pharmacol. 1987;1:145–52. doi: 10.1111/j.1472-8206.1987.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 20.Amado-Boccara I, Gougoulis N, Poirier Littre MF, Galinowski A, Loo H. Effects of antidepressants on cognitive functions: a review. Neurosci Biobehav Rev. 1995;19:479–93. doi: 10.1016/0149-7634(94)00068-c. [DOI] [PubMed] [Google Scholar]
- 21.Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;17(Suppl 1):2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 22.Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–22. [PubMed] [Google Scholar]
- 23.Richelson E. Pharmacology of antidepressants. Mayo Clin Proc. 2001;76:511–27. doi: 10.4065/76.5.511. [DOI] [PubMed] [Google Scholar]
- 24.Stahl SM. Basic psychopharmacology of antidepressants, part 1: antidepressants have seven distinct mechanisms of action. J Clin Psychiatry. 1998;59(Suppl 4):5–14. [PubMed] [Google Scholar]
- 25.Fuller RW. Serotonin uptake inhibitors: uses in clinical therapy and in laboratory research. Prog Drug Res. 1995;45:167–204. doi: 10.1007/978-3-0348-7164-8_5. [DOI] [PubMed] [Google Scholar]
- 26.Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- 27.Stahl SM. [Psychopharmacologie essentielle, 1ère édition française] Paris: Ed Médecine-Sciences. 1998 [Google Scholar]
- 28.Altman HJ, Nordy DA, Ogren SO. Role of serotonin in memory: facilitation by alaproclate and zimeldine. Psychopharmacology (Berl) 1984;84:496–502. doi: 10.1007/BF00431456. [DOI] [PubMed] [Google Scholar]
- 29.Hong E, Meneses A. The activation of serotonergic 5-HT1A presynaptic receptors or an enhancement of 5-HT postsynaptic activity increase learning. Proc West Pharmacol Soc. 1995;38:85–6. [PubMed] [Google Scholar]
- 30.Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–25. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 31.Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol Learn Mem. 1998;69:219–24. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- 32.Lawlor BA, Sunderland T, Mellow AM, Hill JL, Molchan SE, Murphy DL. Hyperresponsivity to the serotonin agonist m-chlorophenylpiperazine in Alzheimer's disease. A controlled study. Arch Gen Psychiatry. 1989;46:542–9. doi: 10.1001/archpsyc.1989.01810060064010. [DOI] [PubMed] [Google Scholar]
- 33.Mongeau R, Blier P, de Montigny C. The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res Brain Res Rev. 1997;23:145–95. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 34.Vanderwolf CH. Near-total loss of ‘learning’ and ‘memory’ as a result of combined cholinergic and serotonergic blockade in the rat. Behav Brain Res. 1987;23:43–57. doi: 10.1016/0166-4328(87)90241-5. [DOI] [PubMed] [Google Scholar]
- 35.Ögren SO, Stone WS, Altman HJ. Evidence for a functional interaction between serotoninergic and cholinergic mechanisms in memory retrieval. Soc Neurosc Abstract. 1985;256:11. doi: 10.1016/s0163-1047(87)90574-7. [DOI] [PubMed] [Google Scholar]
- 36.Garrigou D, Broekkamp CL, Lloyd KG. Involvement of the amygdala in the effect of antidepressants on the passive avoidance deficit in bulbectomised rats. Psychopharmacology (Berl) 1981;74:66–70. doi: 10.1007/BF00431759. [DOI] [PubMed] [Google Scholar]
- 37.Strek KF, Spencer KR, DeNoble VJ. Manipulation of serotonin protects against an hypoxia-induced deficit of a passive avoidance response in rats. Pharmacol Biochem Behav. 1989;33:241–4. doi: 10.1016/0091-3057(89)90456-5. [DOI] [PubMed] [Google Scholar]
- 38.Nowakowska E, Chodera A, Kus K. Anxiolytic and memory improving activity of fluoxetine. Pol J Pharmacol. 1996;48:255–60. [PubMed] [Google Scholar]
- 39.Smith SJ, Kocen RS. A Creutzfeldt-Jakob like syndrome due to lithium toxicity. J Neurol Neurosurg Psychiatry. 1988;51:120–3. doi: 10.1136/jnnp.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin LS, Arana GW, Melvin JA. Toxicity resulting from lithium augmentation of antidepressant treatment in elderly patients. J Clin Psychiatry. 1990;51:344–5. [PubMed] [Google Scholar]
- 41.Caer-Frouard M, Weill-Engerer S, Piette F. [Medication and memory: interactions among the elderly] Presse Med. 2006;35:91–6. doi: 10.1016/s0755-4982(06)74529-8. [DOI] [PubMed] [Google Scholar]
- 42.Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35:381–90. doi: 10.1111/j.1528-1157.1994.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 43.Devinsky O. Cognitive and behavioral effects of antiepileptic drugs. Epilepsia. 1995;36(Suppl 2):S46–65. doi: 10.1111/j.1528-1157.1995.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 44.Nichols ME, Meador KJ, Loring DW. Neuropsychological effects of antiepileptic drugs: a current perspective. Clin Neuropharmacol. 1993;16:471–84. [PubMed] [Google Scholar]
- 45.Lamberty Y, Margineanu DG, Klitgaard H. Absence of negative impact of levetiracetam on cognitive function and memory in normal and amygdala-kindled rats. Epilepsy Behav. 2000;1:333–42. doi: 10.1006/ebeh.2000.0098. [DOI] [PubMed] [Google Scholar]
- 46.Gomer B, Wagner K, Frings L, Saar J, Carius A, Harle M, Steinhoff BJ, Schulze-Bonhage A. The influence of antiepileptic drugs on cognition: a comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10:486–94. doi: 10.1016/j.yebeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Privitera M, Fincham R, Penry J, Reife R, Kramer L, Pledger G, Karim R. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 600-, 800-, and 1,000-mg daily dosages. Topiramate YE Study Group. Neurology. 1996;46:1678–83. doi: 10.1212/wnl.46.6.1678. [DOI] [PubMed] [Google Scholar]
- 48.Thompson PJ, Baxendale SA, Duncan JS, Sander JW. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69:636–41. doi: 10.1136/jnnp.69.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neyens LG, Alpherts WC, Aldenkamp AP. Cognitive effects of a new pyrrolidine derivative (levetiracetam) in patients with epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:411–9. doi: 10.1016/0278-5846(95)00022-n. [DOI] [PubMed] [Google Scholar]
- 50.Ciesielski AS, Samson S, Steinhoff BJ. Neuropsychological and psychiatric impact of add-on titration of pregabalin versus levetiracetam: a comparative short-term study. Epilepsy Behav. 2006;9:424–31. doi: 10.1016/j.yebeh.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 51.O'Neill WM, Hanks GW, Simpson P, Fallon MT, Jenkins E, Wesnes K. The cognitive and psychomotor effects of morphine in healthy subjects: a randomized controlled trial of repeated (four) oral doses of dextropropoxyphene, morphine, lorazepam and placebo. Pain. 2000;85:209–15. doi: 10.1016/s0304-3959(99)00274-2. [DOI] [PubMed] [Google Scholar]
- 52.Marsepoil T, Petithory J, Faucher JM, Ho P, Viriot E, Benaiche F. [Encephalopathy and memory disorders during treatments with mefloquine] Rev Med Interne. 1993;14:788–91. doi: 10.1016/s0248-8663(05)81426-2. [DOI] [PubMed] [Google Scholar]
- 53.Begaud B, Evreux JC, Jouglard J, Lagier G. [Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France] Therapie. 1985;40:111–8. [PubMed] [Google Scholar]


