Abstract
AIMS
XEN-D0501, a novel TRPV1 antagonist, is being developed to treat overactive bladder. This study investigated the safety and pharmacokinetics of repeat-dose XEN-D0501 in healthy subjects.
METHODS
The study was conducted in two parts. Part 1 was a double-blind, randomized, placebo-controlled, two-way crossover study in three cohorts of 12 young male subjects. Each subject received XEN-D0501 and placebo (in random order) twice daily for 13 days, with a final single dose on day 14. Doses of 1, 2.5 and 5 mg XEN-D0501 were investigated. Part 2 was an open-label, randomized, two-way crossover study in male and female subjects (45 to 65 years). Subjects received single doses of 5 mg XEN-D0501 under fasted and fed conditions in random order. Blood sampling and safety assessments were conducted throughout the study.
RESULTS
XEN-D0501 was rapidly absorbed (tmax generally 0.5–4 h post dose). XEN-D0501 exposure increased less than proportionally to dose over the range studied and exhibited minimal accumulation with twice daily dosing. Food had no clinically relevant effects on the pharmacokinetics of XEN-D0501. There were no severe or serious adverse events and all doses were well tolerated. A dose-related increase in body temperature was seen with XEN-D0501 which attenuated over time. Differences from placebo in mean maximum core body temperatures were 0.22°C, 0.5°C and 0.74°C following 1 mg, 2.5 mg and 5 mg twice daily XEN-D0501. The observed increase in body temperature was not considered to be of clinical concern.
CONCLUSIONS
XEN-D0501 appeared safe and well tolerated at doses up to 5 mg twice daily for 14 days in healthy subjects.
Keywords: overactive bladder, TRPV1 antagonist, XEN-D0501
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Transient receptor potential vanilloid 1(TRPV1) receptor antagonists have the potential to be used in a wide variety of indications. Whilst many compounds have been evaluated pre-clinically, published clinical data are sparse. A possible dose-limiting effect of TRPV1 antagonists is their potential to cause hyperthermia.
WHAT THIS STUDY ADDS
This study reports the safety and pharmacokinetic data for XEN-D0501, a novel TRPV1 antagonist, in healthy subjects including effects on body temperature.
Introduction
XEN-D0501 is a potent and selective transient receptor potential vanilloid 1 (TRPV1) receptor antagonist [1] currently being investigated for the treatment of overactive bladder (OAB). OAB afflicts around 200 million people worldwide and is characterized by the International Continence Society (ICS) as a syndrome encompassing urinary urgency (with or without urge incontinence), usually with frequency and nocturia in the absence of obvious pathological or metabolic disease [2]. The symptoms are suggestive of detrusor overactivity but can occur due to other forms of voiding or urinary dysfunction [3].
Currently available medications are usually antimuscarinic agents (oxybutynin, tolterodine and solifenacin [4–6], etc.), with varying degrees of muscarinic receptor subtype and/or organ selectivity [4, 5, 7–9]. Antimuscarinics hold a virtual monopoly in the therapy of urinary incontinence in the western world [8]. Nevertheless, there is considerable interest in developing alternative non-antimuscarinic treatments, with the objective of avoiding the significant side effects associated with this class of drug (including dry-mouth, nausea and constipation) which are believed to contribute to poor long term compliance.
TRPV1 receptors are found in small myelinated Aδ-fibres and sensory unmyelinated C-fibres [10]. These types of fibres are located in the pelvic nerve afferents which monitor the volume of the bladder and the amplitude of bladder contractions. In a healthy bladder, these C-fibres are ‘silent’ and do not respond to bladder contractions. However, in the case of a person with OAB, spinal cord injury or in situations where noxious stimuli are involved, these C-fibres become active and will convey a signal to the spinal cord resulting in a feeling of urgency to urinate [11]. To combat this overactivity, one possible strategy is to desensitize the C-fibre afferents, so as to reduce or eliminate the overactivity of the bladder without affecting the voluntary bladder function. Hence, modulating the TRPV1 receptors in the C-fibres could be a viable treatment for OAB.
Clinical evidence shows that instillation of the neurotoxic TRPV1 agonist capsaicin, into the bladder of subjects with OAB, alleviates the OAB symptoms associated with spinal cord injury and multiple sclerosis [12–15]. This may be explained by the observation that in some of these subjects the micturition reflex was transduced mainly by C-fibres carrying the TRPV1 receptor at its nerve ending. Capsaicin activates the TRPV1 receptor causing depolarization and release of neurotransmitters from the nerve terminals [10]. Sustained activation of the TRPV1 receptor leads to desensitization, resulting in reduced neurotransmitter release. This in turn alleviates the symptoms of overactive bladder, a component of which is excessive release of neurotransmitters in C-fibres [16–18]. Consequently, capsaicin instillation directly into the bladder, and the resulting desensitization, is a useful treatment for OAB [13–15]. It has therefore been postulated that a TRPV1 antagonist such as XEN-D0501 may be of clinical benefit to subjects with OAB of various origins, since an antagonist would by-pass the need for desensitization (required for efficacy with an agonist such as capsaicin) and hence result in an immediate, beneficial effect on the bladder overactivity.
XEN-D0501 has demonstrated subnanomolar potency for TRPV1 receptors in human and rat recombinant cellular systems and rat cultured dorsal root ganglion neurons [unpublished data]. Selectivity of XEN-D0501 for TRPV1 has been confirmed using panels of enzymes, ion channels and G-protein coupled receptors. Further, XEN-D0501 has demonstrated good efficacy in disease models of OAB in anaesthetized and conscious rats when administered by both the intravenous and oral routes [manuscript in preparation].
Two placebo-controlled clinical studies had been conducted prior to the study discussed here [unpublished data]. XEN-D0501 was administered to healthy male subjects as an oral solution in both studies. The first in man study was initially planned to investigate single ascending doses up to 160 mg XEN-D0501. Ultimately, only 5 and 10 mg XEN-D0501 were investigated. The 5 mg dose appeared safe and well tolerated, but the 10 mg dose was not considered to be well tolerated due to mild hyperthermia and a high incidence of oral adverse events (which were thought to be due to TRPV1 antagonism by XEN-D0501 at sensory nerve endings in the mouth). The study was terminated without further dose escalation. The second study investigated multiple ascending doses of 5 and 10 mg twice daily XEN-D0501 for 12 days. The decision to include the 10 mg dose despite the apparent poor toleration in the single dose study, was made on the basis that the body temperature changes observed in the previous study were not considered to be of clinical concern in a trial setting and inclusion of this dose would enable assessment of any attenuation of body temperature effects with time (as had been observed in the literature [19]). Eight subjects received the 5 mg twice daily dose, which appeared safe and well tolerated. However the 10 mg twice daily dose (administered to three subjects) was not considered to be tolerated and the study was terminated on the fourth day of dosing due to emerging adverse events, notably one subject who had two episodes of transient urinary retention, both of which resolved spontaneously and without medical intervention. The two other subjects who also received 10 mg twice daily XEN-D0501 were noted to have high post voiding residual volumes (as assessed by non-invasive ultrasound) but did not experience any difficulties with voiding. Aural temperature was assessed throughout the study and there was some evidence that body temperature was higher following XEN-D0501 compared with placebo. The magnitude of the increase appeared to be 0.5°C to 1.5°C following XEN-D0501 compared with placebo. However technical issues with the recording devices led to significant concerns over the validity of the data and this, coupled with small subject numbers and limited dosing duration at the higher dose, prevented any meaningful conclusions being drawn on the effect of XEN-D0501 on body temperature. There were no individual temperature measurements that were considered to be clinically significant and none was reported as an adverse event.
The primary objective of the study described herein was to assess the safety, tolerability and pharmacokinetics of multiple oral doses of XEN-D0501 given as a tablet formulation to healthy male subjects aged 18 to 45 years. Core body temperature (assessed using a validated telemetric physiological monitoring system) was a key safety endpoint. Secondary objectives of the study were 1) to investigate the effect of food on the pharmacokinetics of a single oral dose of XEN-D0501 and 2) to investigate the safety, tolerability and pharmacokinetics of a single oral dose of XEN-D0501 in healthy male and female subjects aged 46 to 65 years.
Methods
Subjects
The study population consisted of healthy male subjects aged 18 to 45 years inclusive in part 1, and healthy male or female subjects aged 46 to 65 years inclusive in part 2. Female subjects were of non-childbearing potential (i.e. post menopausal, or had undergone surgical sterilization by hysterectomy or bilateral oophorectomy). All subjects weighed between 50 and 100 kg, had a body mass index between 18 and 32 kg m−2 and were willing and able to comply with study requirements. Exclusion criteria included clinically significant abnormal medical history or physical examination including febrile illness within 1 week prior to the first dose, clinically significant history of recurrent urinary tract infections, known prostatic hyperplasia or clinically significant bladder outlet obstruction or heart disease. There were also other standard exclusion criteria concerning allergies, hypersensitivity, QTc interval, blood pressure, laboratory abnormalities, pregnant or nursing females, alcohol, nicotine and drug use. All subjects gave written informed consent prior to participation in the study and the study was conducted in accordance with good clinical practice and with the ethical principles outlined in the Declaration of Helsinki. The study was conducted at LCG Bioscience (Bourn, UK) and the protocol was approved by Welwyn Research Ethics Committee appointed by AAPEC, the Appointing Authority for Phase 1 Ethics Committees in the UK.
Study design
The study was conducted in two parts. Part 1 was a double-blind, randomized, placebo-controlled, two-way crossover study involving three cohorts of 12 subjects. Each subject received either XEN-D0501 or placebo in study period 1 followed by the alternative treatment in study period 2. A washout period of at least 48 h was required between the two study periods. Subjects remained resident at the study site throughout each study period. XEN-D0501 or placebo was administered twice daily for 13 days with a final dose on the morning of day 14 in each study period. The doses of XEN-D0501 investigated were 1 mg twice daily (cohort 1), 2.5 mg twice daily (cohort 2) and 5 mg twice daily (cohort 3). The cohorts were started sequentially. However there was no formal safety review between cohorts and they were allowed to overlap as the highest dose (5 mg twice daily) had been studied previously and shown to be well tolerated. Subjects were fasted for 10 h prior to each morning dose and remained fasted until 4 h post dose on days 1 and 14. On all other days, subjects were allowed breakfast at least 1 h post dose. Standard meals were allowed after at least 4 h post dose. Part 2 of the study was an open-label, randomized, two-way crossover study involving 16 subjects (eight male and eight female). Each subject received a single dose of 5 mg XEN-D0501 on two occasions separated by a minimum of 48 h. XEN-D0501 was administered either after a 10 h fast or within 30 min after a high-fat breakfast. The breakfast was in accordance with the FDA guidance on food–effect bioavailability studies [20].
Pharmacokinetic methods
Blood samples for determination of XEN-D0501 plasma concentrations were collected in part 1, prior to dosing on each study day and at intervals up to 12 and 24 h post dose on days 1 and 14 respectively. In part 2, blood samples were collected pre dose and up to 24 h post dose in each study period. Plasma samples were analyzed using a validated LC-MS/MS procedure. The overall method imprecision values for the analysis of plasma quality control (QC) samples were 3.8%, 3.8%, 2.5% and 3.5% at XEN-D0501 concentrations of 1.00, 3.00, 50.0 and 800 ng ml−1 respectively. The accuracy (relative error) of the assay ranged from −1.4% to +1.0% over the QC range. The calibration range was 1 to 1000 ng ml−1 XEN-D0501. Pharmacokinetic parameters were calculated using noncompartmental analysis (WinNonlin™ version 5.2, model 200). Pharmacokinetic parameters calculated on days 1 and 14 in part 1 included maximum plasma concentration (Cmax), time to Cmax (tmax), area under the concentration vs. time curve (AUC) over the dosing interval τ (AUC(0,τ)). Terminal elimination half-life (t1/2) and accumulation ratio (Rac; assessed as ratio of AUC(0,τ) on day 14 : day 1) were calculated on day 14. Pharmacokinetic parameters calculated in part 2 included Cmax, tmax, AUC from zero to the last measured time point (AUC(0,tlast), AUC from zero to infinity (AUC(0,∞)) and t1/2.
Safety
Safety assessments including adverse event (AE) monitoring, physical examinations, 12-lead ECGs, vital signs (including blood pressure, pulse rate and aural temperature), core body temperature and laboratory safety tests, were conducted at intervals throughout the study. All observed or reported AEs were recorded for all subjects throughout the study. AEs were classified as mild, moderate or severe and the relationship to study drug was determined.
Aural temperature was measured using an appropriately calibrated tympanic infrared thermometer (Braun ThermoScan®). Single measurements were taken in the same ear at each time point by trained study personnel using a standardized technique. Subjects were not allowed to use ear plugs or cover the ear within 20 min of each measurement.
Core body temperature was measured continuously for 24 h on days 1, 2, 7 and 14 in part 1 using a Respironics telemetric physiological monitoring system: Mini Mitter with a Jonah™ ingestible core temperature capsule (Vitalsense®) [21, 22]. Capsules were ingested 3 h prior to the morning and evening doses (or equivalent time on day 14) to ensure continuous availability of temperature measurements throughout the dosing period from capsules that had already passed through the stomach. Data handing rules were defined and included exclusion of data transmitted during the first 3.5 h after capsule ingestion or more than 24 h after ingestion. In addition, data collected within the last hour prior to excretion of each capsule were excluded. Any data points below 30°C were excluded as these were considered to be erroneous. Valid data from the morning capsule were used in the first instance for each day. However if the morning capsule was excreted before the end of the 24 h window or if there were significant gaps in data transmission, then valid data from a subsequent capsule were used instead. On days 1, 2, 7 and 14 when core body temperature was measured, subjects were not allowed to consume hot or chilled/frozen food or drinks and all drinks and meals were served at room temperature.
Statistical analysis
No formal sample size calculations were performed for this study and the subject numbers were considered to be sufficient to assess the safety, tolerability and pharmacokinetics of XEN-D0501 at different dose levels. In addition, a cohort size of 12 subjects in part 1 was expected to provide 80% statistical power to confirm a treatment difference of 0.5°C in body temperature (assuming a standard deviation of 0.4). No assumptions were made prospectively on the effect of food on the pharmacokinetics of XEN-D0501. The sample size of 16 subjects in part 2 was estimated to provide 90% confidence intervals (CI) of ± 0.131 and 0.189 on a natural log scale for AUC(0,tlast) and Cmax respectively with 80% coverage probability. If no change in AUC(0,tlast) and Cmax was observed, it was estimated that the 90% CIs would be (88%, 114%) and (83%, 121%) respectively.
In part 1, the area under the core body temperature (effect) time curve up to 12 h post-morning dosing (AUEC(0,12 h)) was calculated for each subject and treatment period, on days 1, 2, 7 and 14 using the trapezoidal rule. AUEC(0,12 h) was evaluated as this was consistent with the dosing interval and was the longest duration that allowed direct comparison between treatment days, given that an evening dose was administered on all days except day 14. Minimum and maximum core body temperature measurements over the same time period were also assessed. These parameters were only calculated if there were at least 10 h of valid data available during the 12 h period. If the duration of valid recording was less than 10 h then the data from that subject were excluded for that day. If the duration of valid recording was between 10 and 12 h then AUEC(0,12 h) was normalized to 12 h by dividing the subject's AUEC by the duration of valid recording then multiplying by 12 h.
AUEC(0,12 h) was analyzed by cohort using a repeated measures mixed effects model with treatment, period, sequence and study day as fixed effects and subject as a random effect.
In part 2, an analysis of variance (anova) was performed on the log-transformed AUC(0,∞), AUC(0,tlast) and Cmax fitting sequence and fed/fasting status as fixed effects and subject nested within sequence as a random effect. Ratios between the means and 90% CIs for the differences are presented.
Results
Subjects
A total of 52 subjects were enrolled and 50 subjects completed the study. Thirty-six subjects entered part 1 of the study, but two subjects were withdrawn having completed study period 1, but prior to dosing in period 2, due to having tested positive for drugs of abuse. One of the subjects received placebo in period 1 and was withdrawn prior to receiving 2.5 mg XEN-D0501, whilst the other received 5 mg XEN-D0501 in period 1 and was withdrawn prior to receiving placebo in period 2. Sixteen subjects entered part 2 of the study and all completed. All subjects in part 1 were males aged 20 to 44 years (mean 31.7 years). Of the 16 subjects who entered part 2 of the study, eight were male and eight were female and the subjects were aged 46 to 64 years (mean 55.0 years).
PK results
XEN-D0501 was rapidly absorbed with tmax occurring within 0.5 to 4 h following single and multiple dosing in part 1 (Table 1, Figure 1). XEN-D0501 appeared to exhibit non-proportional pharmacokinetics, with exposure (geometric mean Cmax and AUC(0,τ)) increasing 3.5-fold (day 1) and 2.8-fold (day 14) over the five-fold dose range. The pharmacokinetics of XEN-D0501 exhibited moderate inter-subject variability with geometric mean coefficients of variation (CV) for Cmax and AUC parameters ranging from 30 to 50%. Plasma XEN-D0501 concentrations generally declined in a monoexponential manner with t1/2values of approximately 3–4 h. Accumulation ratios based on Cmax and AUC(0,τ) were comparable across the dose range and suggested minimal accumulation of XEN-D0501 with twice daily dosing. Visual assessment of individual trough concentrations (data not shown) suggested steady-state was achieved within 1 to 2 days of dosing.
Table 1.
Summary of XEN-D0501 pharmacokinetic parameters
| XEN-D0501 dose group | ||||
|---|---|---|---|---|
| Parameter | Day | 1 mg twice daily (n = 12) | 2.5 mg twice daily (n = 11) | 5 mg twice daily (n = 12) |
| tmax (h) median (range) | 1 | 1.27 (0.50–3.00) | 1.50 (0.60–4.00) | 1.50 (1.00–2.00) |
| 14 | 1.00 (0.50–3.00) | 1.00 (0.50–3.00) | 2.02 (0.72–4.00) | |
| Cmax (ng ml−1) mean (CV%)† | 1 | 14.9 (36%) | 26.8 (31%) | 51.6 (34%) |
| 14 | 17.6 (34%) | 31.4 (36%) | 49.8 (38%) | |
| AUC(0,τ) (ng ml−1 h) mean (CV%)† | 1 | 56.0 (46%) | 112 (43%) | 198 (47%) |
| 14 | 73.2 (47%) | 136 (48%) | 248 (52%) | |
| t1/2 (h) mean (CV%)† | 14 | 3.18 (44%) | 2.89 (63%) | 4.12 (51%) |
| Rac (AUC(0,τ)) mean (CV%)† | 14 | 1.31 (19%) | 1.22 (18%) | 1.25 (16%) |
| Rac (Cmax) mean (CV%)† | 14 | 1.18 (37%) | 1.17 (20%) | 0.97 (20%) |
Unadjusted geometric mean.
Figure 1.
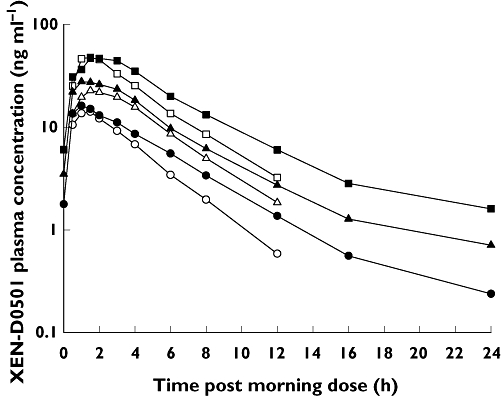
Mean XEN-D0501 plasma concentration vs. time. 1 mg XEN-D0501 – day 1 (○), 1 mg XEN-D0501 – day 14 (•), 2.5 mg XEN-D0501 – day 1 (▵), 2.5 mg XEN-D0501 – day 14 (▴), 5 mg XEN-D0501 – day 1 (□), 5 mg XEN-D0501 – day 14 ( )
)
Mean Cmax was lower and occurred later when XEN-D0501 was administered in the fed compared with the fasted state. The statistical analysis (Table 2) shows a food effect (mainly on Cmax) but this effect was considered to be small and unlikely to be clinically relevant. In addition, the Cmax and AUC(0,tlast) data were affected by one subject who had notably lower XEN-D0501 plasma concentrations in the fed state compared with all of the other subjects. It was also not possible to calculate AUC(0,∞) in this subject during the fed period. Re-running the statistical analysis without this outlying subject had no relevant effect on the ratio and 90% CIs for AUC(0,∞), but changed the ratio and 90% CIs for AUC(0,tlast) to 102.06 (95.40, 109.19) and for Cmax to 91.65 (76.23, 110.19).
Table 2.
Summary of XEN-D0501 pharmacokinetic parameters in fasted and fed conditions
| Parameter | 5 mg XEN-D0501 fasted (n = 16) | 5 mg XEN-D0501 fed (n = 16) | Ratio of adjusted least squares means (90% CI) |
|---|---|---|---|
| tmax (h) median (range) | 1.50 (0.50–3.00) | 3.00 (1.00–6.00) | NA |
| Cmax (ng ml−1) mean (CV%)† | 77.9 (34%) | 62.8 (68%) | 80.62 (58.39, 111.31) |
| AUC(0,tlast) (ng ml−1 h) mean (CV%)† | 347 (45%) | 316 (67%) | 91.08 (70.89, 117.02) |
| AUC(0,∞) (ng ml−1 h) mean (CV%)† | 361 (47%) | 328 (70%) | 100.98 (93.99, 108.48) |
| t1/2 (h) mean (CV%)† | 4.57 (39%) | 3.70 (42%) | NA |
Unadjusted geometric mean.
Safety
There were no serious or severe AEs in this study and the majority of AEs were classed as mild in nature. A summary of the most common AEs, defined as any AE that occurred in more than one subject in any treatment group, is presented in Table 3. The most common AEs in part 1 were headache, feeling cold, dizziness, oropharyngeal pain and feeling hot. With the exception of oropharyngeal pain, the other most common AEs were all reported in cohorts 2 and 3 (2.5 and 5 mg BID XEN-D0501), and predominantly following XEN-D0501 rather than placebo. They were also predominantly considered treatment-related. The incidence of other individual AEs was generally low, although some AEs could be grouped into similar or related terms. In particular there were a variety of AEs associated with perception of, or response to, body temperature, e.g. hot flush, burning sensation, feeling hot, pyrexia, feeling of body temperature change, feeling cold, chills, peripheral coldness, night sweats, hyperhydrosis and cold sweat. All of these terms were described as mild, with the exception of burning sensation (in head) which was described as moderate. Taken as a group, there appeared to be a dose related trend to increased reporting with increased XEN-D0501 dose. None of the subjects reported any difficulties or changes in their ability to urinate.
Table 3.
Summary of the most common AEs†
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| XEN-D0501 | XEN-D0501 | XEN-D0501 | ||||
| 1 mg twice daily | Placebo | 2.5 mg twice daily | Placebo | 5 mg twice daily | Placebo | |
| Total subjects | 12 | 12 | 11 | 12 | 12 | 11 |
| Subjects with AEs | 8 | 6 | 10 | 8 | 11 | 7 |
| AE (Preferred term) | ||||||
| Headache | 0 | 0 | 4 (4) | 1 (1) | 7 (7) | 4 (3) |
| Feeling cold | 0 | 0 | 3 (3) | 0 | 5 (5) | 0 |
| Dizziness | 0 | 0 | 1 (1) | 0 | 4 (4) | 2 (0) |
| Oropharyngeal pain | 1 (0) | 1 (0) | 1 (0) | 2 (0) | 0 | 2 (0) |
| Feeling hot | 0 | 0 | 3 (3) | 0 | 1 (1) | 1 (1) |
| Abnormal dreams | 0 | 0 | 1 (0) | 2 (0) | 0 | 1 (0) |
| Nasal congestion | 0 | 1 (0) | 1 (0) | 2 (0) | 0 | 0 |
| Nausea | 0 | 0 | 0 | 0 | 2 (2) | 2 (1) |
| Night sweats | 1 (1) | 0 | 1 (1) | 0 | 2 (2) | 0 |
| Paraesthesia | 0 | 0 | 1 (1) | 0 | 3 (3) | 0 |
| Dry throat | 0 | 0 | 3 (2) | 0 | 0 | 0 |
| Dysgeusia | 0 | 0 | 0 | 0 | 3 (3) | 0 |
| Hot flush | 0 | 0 | 0 | 0 | 3 (3) | 0 |
| Nasal pharyngitis | 0 | 2 (0) | 1 (0) | 0 | 0 | 0 |
| Burning sensation | 0 | 0 | 0 | 0 | 2 (2) | 0 |
| Erythema | 0 | 0 | 0 | 0 | 2 (0) | 0 |
| Hyperhidrosis | 0 | 0 | 0 | 0 | 2 (2) | 0 |
Most common is defined as any adverse event that occurred in more than one subject in any treatment group. Values are the number of subjects with all causality AEs (treatment-related AEs).
The AE profile in part 2 was similar to part 1 with the most common AEs being dysgeusia, feeling cold, headache and oral paraesthesia.
A diurnal variation in mean core body temperature was observed in all subjects with all treatments, with temperatures generally peaking at approximately 6 h after the morning dose (Figure 2). Visual inspection of mean core body temperatures, suggested that they were generally higher following XEN-D0501 compared with placebo at all time points, with the greatest differences generally observed over the 0 to 8 h following each dose (morning and evening). There was a dose-dependent increase in mean core body temperature; the maximum core body temperature values following placebo ranged from 37.03 to 37.99°C, whilst those following XEN-D0501 ranged from 37.23 to 39.07°C (1 mg twice daily), 37.43 to 38.35°C (2.5 mg twice daily) and 37.23 to 38.82°C (5 mg twice daily). No formal statistical analyses were conducted. However the differences from placebo in mean maximum core body temperature (Table 4) following XEN-D0501 on day 1 were 0.22°C (1 mg twice daily), 0.50°C (2.5 mg twice daily) and 0.74°C (5 mg twice daily). By day 14, these differences had reduced to 0.15°C (1 mg twice daily), 0.11°C (2.5 mg twice daily) and 0.30°C (5 mg twice daily) (Table 4). Visual assessment of the core body temperature profiles suggests that there was no residual effect of XEN-D0501 on core body temperature by approximately 12 h after the last dose on day 14. Maximum core body temperature over the 12 h after the morning dose for each subject was assessed on each measurement day and for each treatment. The statistical analysis of the AUEC(0,12 h) data demonstrated a statistically significant difference (P < 0.05) between XEN-D0501 and placebo on all days except for day 7 following 1 mg twice daily XEN-D0501; the magnitude of the difference decreased with time (Table 5).
Figure 2.
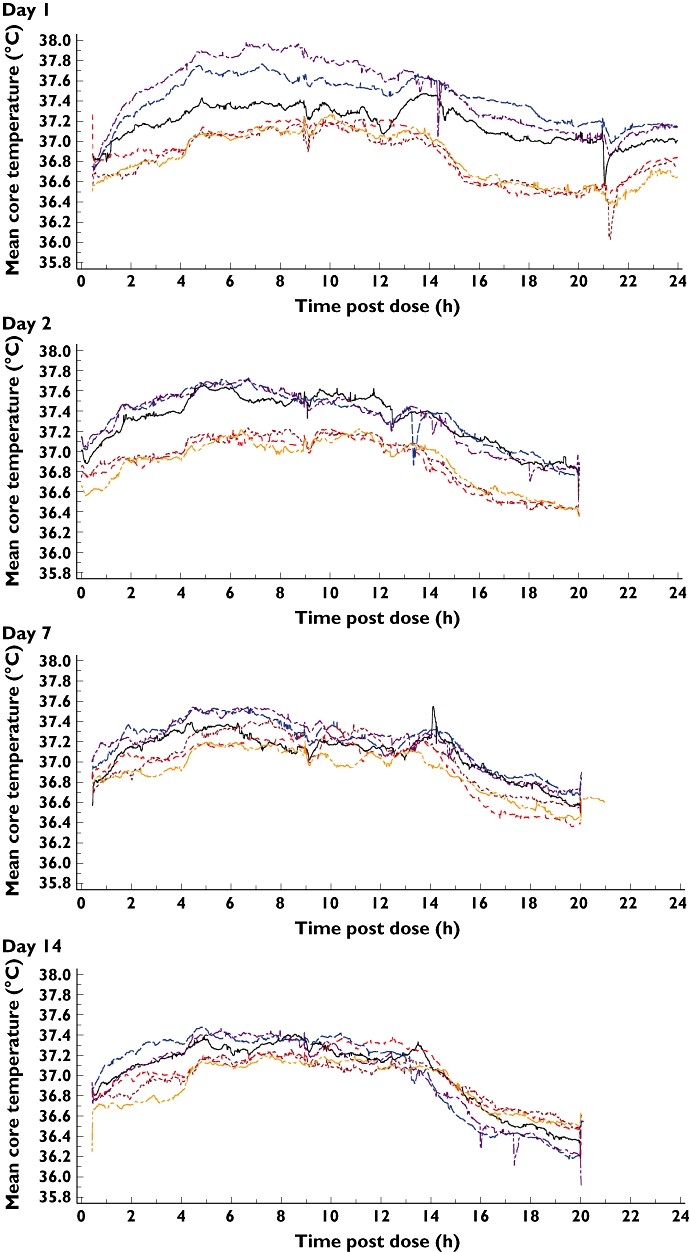
Mean core body temperature. 1 mg twice daily XEN-D0501 ( ), 2.5 mg twice daily XEN-D0501 (
), 2.5 mg twice daily XEN-D0501 ( ), 5 mg twice daily XEN-D0501 (
), 5 mg twice daily XEN-D0501 ( ), Cohort 1 placebo (
), Cohort 1 placebo ( ), Cohort 2 placebo (
), Cohort 2 placebo ( ), Cohort 3 placebo (
), Cohort 3 placebo ( )
)
Table 4.
Summary of core body temperature parameters
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| n = 12 | n = 12 | n = 12 | ||||
| 1 mg twice daily | 2.5 mg twice daily | 5 mg twice daily | ||||
| Parameter† | XEN-D0501 | Placebo | XEN-D0501 | Placebo | XEN-D0501 | Placebo |
| AUEC(0,12 h) (°C h) | ||||||
| Day 1 | ||||||
| n | 11 | 10 | 11 | 11 | 10 | 10 |
| Mean | 447 | 443 | 450 | 445 | 453 | 444 |
| SD | 2.1 | 2.0 | 2.4 | 1.5 | 3.7 | 1.5 |
| Min, max | 444, 451 | 440, 447 | 447, 454 | 442, 446 | 446, 461 | 440, 446 |
| Day 2 | ||||||
| n | 7 | 11 | 8 | 11 | 10 | 9 |
| Mean | 450 | 445 | 450 | 444 | 450 | 444 |
| SD | 4.8 | 1.0 | 1.6 | 1.5 | 2.5 | 1.3 |
| Min, max | 446, 460 | 444, 446 | 448, 452 | 442, 447 | 447, 456 | 442, 446 |
| Day 7 | ||||||
| n | 8 | 9 | 11 | 10 | 11 | 8 |
| Mean | 446 | 446 | 448 | 446 | 448 | 444 |
| SD | 1.4 | 1.8 | 2.1 | 1.4 | 1.7 | 2.0 |
| Min, max | 444, 448 | 444, 450 | 444, 452 | 443, 448 | 446, 451 | 440, 447 |
| Day 14 | ||||||
| n | 10 | 9 | 10 | 11 | 10 | 10 |
| Mean | 447 | 445 | 448 | 445 | 447 | 444 |
| SD | 1.6 | 1.9 | 1.8 | 1.9 | 2.1 | 1.4 |
| Min, max | 444, 449 | 443, 448 | 445, 451 | 441, 447 | 443, 450 | 441, 446 |
| Maximum temperature (°C) | ||||||
| Day 1 | ||||||
| n | 11 | 10 | 11 | 11 | 10 | 10 |
| Mean | 37.64 | 37.42 | 37.90 | 37.40 | 38.16 | 37.42 |
| SD | 0.21 | 0.13 | 0.19 | 0.14 | 0.32 | 0.16 |
| Min, max | 37.23, 37.98 | 37.21, 37.58 | 37.66, 38.16 | 37.06, 37.55 | 37.60, 38.82 | 37.16, 37.73 |
| Day 2 | ||||||
| n | 7 | 11 | 8 | 11 | 10 | 9 |
| Mean | 37.95 | 37.44 | 37.91 | 37.43 | 37.91 | 37.46 |
| SD | 0.51 | 0.13 | 0.23 | 0.15 | 0.27 | 0.19 |
| Min, max | 37.61, 39.07 | 37.23, 37.70 | 37.59, 38.27 | 37.24, 37.71 | 37.58, 38.49 | 37.16, 37.72 |
| Day 7 | ||||||
| n | 8 | 9 | 11 | 10 | 11 | 8 |
| Mean | 37.54 | 37.60 | 37.77 | 37.56 | 37.74 | 37.45 |
| SD | 0.18 | 0.14 | 0.29 | 0.21 | 0.18 | 0.22 |
| Min, max | 37.29, 37.85 | 37.40, 37.90 | 37.46, 38.35 | 37.24, 37.88 | 37.44, 38.13 | 37.19, 37.82 |
| Day 14 | ||||||
| n | 10 | 9 | 10 | 11 | 10 | 10 |
| Mean | 37.59 | 37.44 | 37.70 | 37.59 | 37.69 | 37.39 |
| SD | 0.20 | 0.23 | 0.26 | 0.25 | 0.25 | 0.18 |
| Min, max | 37.32, 37.90 | 37.21, 37.91 | 37.43, 38.12 | 37.13, 37.99 | 37.23, 38.03 | 37.03, 37.64 |
Measured over the 0–12 h after the morning dose of XEN-D0501 or placebo.
Table 5.
Summary of repeated measures analysis of core body temperature AUEC(0,12 h)
| XEN-D501 vs. placebo | ||||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 7 | Day 14 | |
| Cohort 1: 1 mg twice daily vs. placebo | ||||
| Treatment difference | 3.39 | 4.68 | −0.29 | 1.80 |
| 95% CI | 1.80, 4.98 | 2.90, 6.45 | −2.08, 1.51 | 0.10, 3.50 |
| P value | <0.0001 | <0.0001 | 0.75 | 0.04 |
| Cohort 2: 2.5 mg twice daily vs. placebo | ||||
| Treatment difference | 5.70 | 5.65 | 2.29 | 2.22 |
| 95% CI | 4.45, 6.95 | 4.27, 7.04 | 1.01, 3.56 | 0.94, 3.51 |
| P value | <0.0001 | <0.0001 | 0.0006 | 0.0010 |
| Cohort 3: 5 mg twice daily vs. placebo | ||||
| Treatment difference | 8.86 | 6.12 | 3.75 | 2.92 |
| 95% CI | 7.50, 10.22 | 4.73, 7.51 | 2.35, 5.15 | 1.56, 4.28 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Aural temperatures were also assessed throughout the study and no subjects had any measurements that were deemed to be clinically significant. Aural temperatures following placebo ranged from 35.2°C to 38.0°C, whilst those following XEN-D0501 ranged from 35.7°C to 38.4 (1 mg twice daily), 35.6°C to 37.7°C (2.5 mg twice daily) and 35.5°C to 38.2°C (5 mg twice daily). In general, the aural temperature measurements were slightly lower than the core body temperature measurements, although the trends over time and differences between XEN-D0501 doses and placebo were similar for both measurements (Figure 3).
Figure 3.
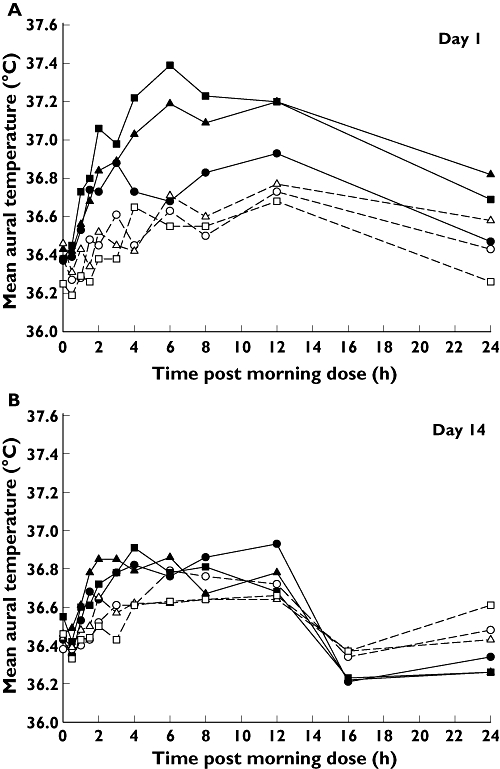
Mean aural temperature vs. time. (A) 1 mg twice daily XEN-D0501 – day 1 (•), Cohort 1 placebo – day 1 (○), 2.5 mg twice daily XEN-D0501 – day 1 (▴), Cohort 2 placebo – day 1 (▵), 5 mg twice daily XEN-D0501 – day 1 ( ), Cohort 3 placebo – day 1 (□). (B) 1 mg twice daily XEN-D0501 – day 14 (•), Cohort 1 placebo – day 14 (○), 2.5 mg twice daily XEN-D0501 – day 14 (▴), Cohort 2 placebo – day 14 (▵), 5 mg twice daily XEN-D0501 – day 14 (
), Cohort 3 placebo – day 1 (□). (B) 1 mg twice daily XEN-D0501 – day 14 (•), Cohort 1 placebo – day 14 (○), 2.5 mg twice daily XEN-D0501 – day 14 (▴), Cohort 2 placebo – day 14 (▵), 5 mg twice daily XEN-D0501 – day 14 ( ), Cohort 3 placebo – day 14 (□)
), Cohort 3 placebo – day 14 (□)
There were no clinically relevant findings or changes in any of the other safety parameters.
Discussion
XEN-D0501 had previously been administered to healthy male subjects in two phase 1 studies, as single and multiple (twice daily) doses (5 and 10 mg). In each study the 5 mg dose appeared to be safe and was well tolerated, but the 10 mg dose was found to induce mild hyperthermia and oral hypoaesthesia (thought to be associated with the solution formulation) in several subjects, and transient urinary retention following repeated dosing in one subject. Whilst urinary retention in healthy subjects is clearly undesirable, coupled with the raised residual urine volumes observed in the other two subjects who received 10 mg twice daily XEN-D0501, it does suggest that XEN-D0501 is capable of having an appropriate effect on the bladder for the proposed indication of OAB.
The involvement of TRPV1 in thermoregulation has been known for many years [18, 23, 24] and the more recent interest in TRPV1 antagonists for various therapeutic indications has led to the testing of many compounds (mainly for pain indications) some of which have been noted to cause increases in body temperature either in animals and/or man [25, 26]. This effect has also been shown to attenuate with multiple dosing of TRPV1 antagonists [19]. The magnitude of the changes in temperature also appears to be compound dependent with some compounds producing marked hyperthermia [19, 26], whereas others, such as capsazepine, SB-366791 and AS1928370, have been reported to have no apparent effect on body temperature (in rats) [27, 28].
In the light of the potential for XEN-D0501 to increase body temperature, particular attention was paid to monitoring body temperature in this study. The ingestion of telemetric VitalSense® capsules at specified intervals on days 1, 2, 7, and 14 allowed continuous monitoring of core body temperature for 24–48 h. The two-way crossover design of the study also allowed for within-subject comparisons of core body temperature. The data suggested that XEN-D0501 caused a dose-dependent increase in core body temperature which was greatest on the first day of dosing but rapidly attenuated thereafter. Despite the attenuation of effect, core body temperatures did remain higher following XEN-D0501 compared with placebo throughout the dosing period. There was no discernable effect of XEN-D0501 on core body temperature by approximately 12 h after the final dose, presumably reflecting the declining plasma XEN-D0501 concentrations. The effect of XEN-D0501 on body temperature was also apparent and adequately characterized using aural (tympanic) measurements, confirming that this method is appropriate for ongoing safety assessments in the clinic.
The mechanism for TRPV1-mediated hyperthermia is still to be fully proven, but an increasing body of evidence suggests that these effects are driven by block of afferent fibres innervating the abdominal viscera [29]. Tonic activation of TRPV1 channels in the viscera is believed to regulate thermogenesis and skin vasoconstriction processes which, if disturbed through visceral and/or peripheral inhibition of TRPV1, could give rise to a hyperthermic response. It is quite clear that the hyperthermic effects of TRPV1 antagonists are not a central effect through regulation of hypothalamic TRPV1 channels involved in body temperature control, and nor are they a thermosensor response through inhibition of heat-activated TRPV1 [29]. There was no evidence from review of standard laboratory safety parameters of concomitant inflammatory processes associated with the changes in body temperature following XEN-D0501.
This study demonstrated that repeat dosing of XEN-D0501 up to 5 mg twice daily appeared safe and well tolerated. XEN-D0501 was also administered as single doses (5 mg) to males and females aged 46 to 65 years (a population considered to more closely reflect the target OAB population), and whilst no placebo control was available for this population, there was no evidence of any significant differences in the safety and tolerability profile compared with the younger males.
The pharmacokinetic data suggest that XEN-D0501 exposure (Cmax and AUC) increases less than dose proportionally. Whilst it should be noted that this study was not designed nor formally analyzed to assess dose proportionality, the data are consistent with previous data from the single dose first-in-human study and data observed in rats and dogs [unpublished data]. The subproportionality is considered most likely to be due to limited absorption, probably caused by the low solubility of the compound (2 mg l−1 in pH 7 buffer [unpublished data]). In vitro data from studies using recombinant cytochrome P450 (CYP) isoforms, human liver microsomes and human hepatocytes, suggest that XEN-D0501 is primarily metabolized by CYP3A4, and does not inhibit or induce this enzyme at clinically relevant concentrations [unpublished data]. The terminal half-life of XEN-D0501 did not appear to change with dose. Data from part 2 of the study suggest that XEN-D0501 can be given without regard for food, although there was one outlier who had very low XEN-D0501 exposures following administration with food. The reason for this apparent discrepancy with the other subjects' data and its clinical relevance is not known.
Overall the data suggest that XEN-D0501 is a promising TRPV1 antagonist that has potential for the treatment of OAB.
Acknowledgments
The authors would like to thank the staff and subjects who participated in this research as well as Syne Qua Non Ltd (SQN) who were responsible for the data management and statistics, and Nicola Hewson (SQN) in particular who provided statistical support. The authors would also like to thank Samantha Abel (Valley Writing Solutions Ltd) who provided clinical pharmacology and medical writing support.
Competing Interests
PR is an employee of Xention Ltd and holds stock in the company. JR was a paid consultant for Xention Ltd to manage the clinical conduct of the study. AP was the Principal Investigator who was employed at LCG Bioscience who were responsible for the conduct of the study. The study was supported by Xention Ltd.
REFERENCES
- 1.Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 3.Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urology. 2002;60(Suppl 1):7–12. doi: 10.1016/s0090-4295(02)01784-3. [DOI] [PubMed] [Google Scholar]
- 4.Abrams P, Jackson S, Mattiasson A, Krishnan K, Haendler L. A randomized, double-blind, placebo controlled, dose ranging study of the safety and efficacy of tolterodine in patients with hyperreflexia. 1996. pp. 276–7. Proceedings of the International Continence Society 26th Annual Meeting.
- 5.Jonas U, Hofner K, Madersbacher H. Efficacy and safety of two doses of tolterodine versus placebo in patients with detrusor overactivity and symptoms of frequency, urge incontinence, and urgency: urodynamic evaluation. World J Urol. 1997;15:144–51. doi: 10.1007/BF02201987. [DOI] [PubMed] [Google Scholar]
- 6.Chapple CR, Arano P, Bosch JLHR, De Ridder D, Kramer AEJL, Ridder AM. Solifenacin appears effective and well tolerated in patients with symptomatic idiopathic detrusor overactivity in a placebo- and tolterodine-controlled phase 2 dose-finding study. Br J Urol. 2003;93:71–7. doi: 10.1111/j.1464-410x.2004.04561.x. [DOI] [PubMed] [Google Scholar]
- 7.Cardozo L, Chapple CR, Toozs-Hobson P, Grosse-Freese M, Bulitta M, Lehmacher W, Strosser W, Ballering-Bruhl B, Schafer M. Efficacy of trospium chloride in patients with detrusor instability: a placebo-controlled, randomized, double-blind, multicentre clinical trial. BJU Int. 2000;85:659–64. doi: 10.1046/j.1464-410x.2000.00575.x. [DOI] [PubMed] [Google Scholar]
- 8.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–6. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 9.Stewart WF, Corey R, Herzog AR, Wein A, Norton PA, Payne C, Versi E, on behalf of the NO-BLE Program Research Team Prevalence of overactive bladder in women: results from the Noble Program. Int Urogynecol J. 2001;12(Suppl 3):S66. [Google Scholar]
- 10.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;50:159–211. [PubMed] [Google Scholar]
- 11.de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010;29:63–76. doi: 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geirsson G, Fall M, Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J Urol. 1995;154:1825–9. [PubMed] [Google Scholar]
- 13.Lazzeri M, Beneforti P, Benaim G, Maggi CA, Lecci A, Turini D. Intravesical capsaicin for treatment of severe bladder pain: a randomized placebo controlled study. J Urol. 1996;156:947–52. [PubMed] [Google Scholar]
- 14.De Seze M, Wiart L, Joseph PA, Dosque JP, Mazaux JM, Barat M. Capsaicin and neurogenic detrusor hyperreflexia: a double-blind placebo-controlled study in 20 patients with spinal cord lesions. Neurourol Urodyn. 1998;17:513–23. doi: 10.1002/(sici)1520-6777(1998)17:5<513::aid-nau7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Lazzeri M, Spinelli M, Zanollo A, Turini D. Intravesical vanilloids and neurogenic incontinence: ten years experience. Urol Int. 2004;72:145–9. doi: 10.1159/000075969. [DOI] [PubMed] [Google Scholar]
- 16.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 17.Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 19.Gavva NR, Bannon AW, Hovland DN, Lehto SG, Klionsky L, Surapaneni S, Immke DC, Henley C, Arik L, Bak A, Davis J, Ernst N, Hever G, Kuang R, Shi L, Tamir R, Wang J, Wang W, Zajic G, Shu D, Norman MH, Louis J-C, Magal E, Tranor JS. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther. 2007;323:128–37. doi: 10.1124/jpet.107.125674. [DOI] [PubMed] [Google Scholar]
- 20.FDA Guidance for Industry. Food-effect bioavailability and fed bioequivalence studies. 2002. December.
- 21.Byrne C, Lim CL. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med. 2007;41:126–33. doi: 10.1136/bjsm.2006.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie JE, Osgood DW. Validation of a new telemetric core temperature monitor. International Thermal Physiology Symposium: Physiology and Pharmacology of Temperature Regulation, October–December 2004. J Thermal Biol. 2004;29:605–11. [Google Scholar]
- 23.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 24.Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JS, Louis J-C. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–74. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis J-C, Treanor JJS, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–68. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavva NR, Treanor JJS, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–10. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci. 2010;30:1435–40. doi: 10.1523/JNEUROSCI.5150-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watabiki T, Kiso T, Kuramochi T, Yonezawa K, Tsuji N, Kohara A, Kakimoto S, Aoki T, Matuoka N. Amelioration of neuropathic pain by novel TRPV1 antagonist (AS1928370) in rats without hypothermic effect. J Pharmacol Exp Ther. 2011;336:743–50. doi: 10.1124/jpet.110.175570. [DOI] [PubMed] [Google Scholar]
- 29.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228–61. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]


