Abstract
AIM
To assess the effect of AeroChamber Plus™ on lung deposition and systemic exposure to extra-fine beclometasone dipropionate (BDP)/formoterol (100/6 µg) pMDI (Foster®). The lung deposition of the components of the combination given with the pMDI was also evaluated using the charcoal block technique.
METHODS
Twelve healthy male volunteers received four inhalations of extra-fine BDP/formoterol (100/6 µg) using (i) pMDI alone, (ii) pMDI and AeroChamber Plus™ and (iii) pMDI and charcoal ingestion.
RESULTS
Compared with pMDI alone, use of AeroChamber Plus™ increased the peak plasma concentrations (Cmax) of BDP (2822.3 ± 1449.9 vs. 5454.9 ± 3197.1 pg ml−1), its active metabolite beclometasone 17-monopropionate (17-BMP) (771.6 ± 288.7 vs. 1138.9 ± 495.6 pg ml−1) and formoterol (38.4 ± 17.8 vs. 54.7 ± 20.0 pg ml−1). For 17-BMP and formoterol, the AUC(0,30 min), indicative of lung deposition, was increased in the AeroChamber Plus™ group by 41% and 45%, respectively. This increase was mainly observed in subjects with inadequate inhalation technique. However, use of AeroChamber Plus™ did not increase the total systemic exposure to 17-BMP and formoterol. Results after ingestion of charcoal confirmed that AUC(0,30 min) can be taken as an index of lung bioavailability and that more than 30% of the inhaled dose of extra-fine BDP/formoterol 100/6 µg was delivered to the lung using the pMDI alone.
CONCLUSIONS
The use of AeroChamber Plus™ optimizes the delivery of BDP and formoterol to the lung in subjects with inadequate inhalation technique. The total systemic exposure was not increased, supporting the safety of extra-fine BDP/formoterol pMDI with AeroChamber Plus™.
Keywords: beclometasone 17-monopropionate, beclometasone dipropionate, extra-fine, fixed combination, formoterol, spacer
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Use of a spacer minimizes oropharyngeal deposition and optimizes drug targeting to the airways in subjects with coordination difficulties. However, the increase in pulmonary deposition often observed with spacer devices, could potentially lead to an increase in overall systemic exposure.
EMA guidelines recommend that the development of a pMDI should always include testing of at least one specific spacer for use with a particular pMDI.
The aim of this study was to examine the effect of AeroChamber Plus™ on the lung bioavailability and total systemic exposure of a hydrofluoroalkane (HFA) pMDI fixed combination of extra-fine beclometasone dipropionate/formoterol (100/6 µg) (Foster®).
WHAT THIS STUDY ADDS
The use of AeroChamber Plus™ optimizes the lung delivery of beclometasone and formoterol in subjects that find it difficult to synchronize aerosol actuation with the inspiration of breath.
The total systemic exposure of beclometasone 17-monopropionate and formoterol was not significantly increased by the use of the AeroChamber Plus™ spacer.
Use of the AeroChamber Plus™ spacer device with the extra-fine beclometasone dipropionate/formoterol (100/6 µg) fixed combination pMDI can be a valuable option for certain patients groups, such as subjects with difficulties in achieving an adequate inhalation technique.
Introduction
Long-acting β2-adrenoceptor agonists (LABAs) and inhaled corticosteroids (ICS) are recommended for the treatment of both asthma and chronic obstructive pulmonary disease (COPD) [1, 2]. Inhaled corticosteroids and LABAs can be administered in combination to obtain additive clinical benefits, and there are also molecular interactions between these drugs that can lead to synergistic effects [3]. In asthmatic patients, LABA/ICS combinations produce greater improvements in lung function, symptom control and exacerbation rate reduction compared with doubling the dose of ICS [4–8]. Unfortunately, despite the widespread availability of these combination therapies, many patients with asthma remain poorly controlled [9].
A fixed ICS/LABA combination pressurized metered dose inhaler (pMDI) containing extrafine beclometasone dipropionate (BDP 100 µg) and formoterol fumarate (6 µg) (Foster®, Chiesi Farmaceutici, Italy) has been recently developed using proprietary Modulite® (Chiesi Farmaceutici, Italy) technology [10]. This technology allows the drug particle size to be tailored, thus enabling the development of extra-fine formulations with an improved lung deposition [11]. A recent study [12], demonstrated that the extrafine beclometasone dipropionate/formoterol fixed dose combination was superior to separate components in asthma control.
It is well recognized that pMDIs require good coordination of patient inspiration and inhaler activation to ensure correct inhalation and deposition of drugs in the lung [13, 14]. Poor inhalation technique can result in a decrease in pulmonary deposition [15], increase in oropharyngeal deposition and a concomitant reduction in the therapeutic effect [14, 16]. The use of spacer and holding chamber devices can eliminate the issues associated with inadequate inhalation technique by minimizing coordination difficulties, reducing oropharyngeal deposition and increasing lung deposition [17, 18].
This study investigates how the use of AeroChamber Plus™ Valved Holding Chamber (Trudell Medical International, Canada) affects the lung deposition and systemic exposure to BDP, its active metabolite beclometasone 17-monopropionate (17-BMP) and formoterol after administration of the extra-fine pMDI BDP/formoterol (100/6 µg) fixed combination. The contribution of the swallowed portion of the dose to the systemic exposure was also assessed using the charcoal block technique.
Methods
In vitro study design
An Andersen Cascade Impactor (ACI) (Copley Instruments, Nottingham, UK) operated at 28.3 l min−1 was used to determine the particle size distribution of BDP and formoterol from the pMDI alone and via AeroChamber Plus™. Pre-primed devices were actuated directly into the induction port of the impactor and the amount of drug collected at each stage was determined using high performance liquid chromatography with U.V. detector (HPLC-UV) method. Six independent replicates were performed for each condition (with and without spacer). The dose delivered was the amount of drug deposited in the induction port as well as in all stages of the impactor (S0-Filter). The fine particle dose (FPD; particles ≤ 5 µm) and the coarse dose (induction port plus ACI stages 0–2; particles > 5 µm) were evaluated.
In vivo study design
This was an open, randomized, single dose, three way, crossover study, consisting of one single treatment of four puffs of extra-fine BDP/formoterol (100/6 µg) combination delivered by pMDI with or without the AeroChamber Plus™, yielding a total dose of 400 µg BDP and 24 µg formoterol. The lung deposition of the combination was evaluated using the charcoal block technique without AeroChamber Plus™. There was a 7-day wash-out between treatment periods. Pharmacokinetic (PK) and lung bioavailability parameters were assessed for BDP, 17-BMP and formoterol. Safety was assessed by documenting all adverse events which occurred during the study. The study was carried out in accordance with the Declaration of Helsinki (1996), the ICH Harmonized Tripartite Guideline for Good Clinical Practice (GCP) and with applicable regulatory requirements. The study protocol was approved by an Independent Ethics Committee (Comitato Etico Cantonale, Canton Ticino, Switzerland).
The study was published on clinicaltrials.gov (NCT01280175).
Study participants
To be eligible for randomization, participants were required to be healthy, male, non-smokers, aged 18–45 years, and have no history of respiratory or other disease with a body mass index (BMI) of 18–28 kg m−2. Volunteers were required to be able to use correctly a pMDI. All subjects gave their written informed consent.
Subjects were excluded from the study if they had a clinically relevant electrocardiogram (ECG) abnormality, physical or laboratory analysis findings indicative of a clinically significant condition which could interfere with the objectives of the study. Volunteers were also excluded if they were known to be hypersensitive to the active substances and/or any component of the formulation, or had used over the counter medications within 2 weeks or an enzyme inducing/inhibiting drug within 2 months before the start of the study.
Test drug inhalation
At screening and on each administration day (pre-dose), volunteers were trained in the correct inhalation technique using the Vitalograph® aerosol inhalation monitor (AIM) with placebo chloro-fluorocarbon (CFC)-free inhalers. The AIM allowed the correct inhalation technique to be evaluated and reinforced by monitoring correct timing of the actuation of the pMDI as well as the correct inhalation time, inspiratory flow and breath holding. Volunteers were trained using placebo inhalers at an inspiratory flow rate of 30 l min−1, and were asked to use the same technique and inspiratory flow rate during the test drug inhalation.
During each treatment period, the inhalers (for use with or without the AeroChamber Plus™) were primed. Volunteers held their breath for 10 s following each inhalation, and waited 30 s before taking the next inhalation. The AeroChamber Plus™ was cleaned in accordance with the instructions in the manufacturer's leaflet.
Charcoal block
Volunteers ingested 5 g of activated charcoal (Carbo Activatus, Switzerland) suspended in 50 ml of water 10 min before treatment. Moreover, after each puff and at the end of breath holding, volunteers rinsed their mouths and gargled for approximately 10 s with 30 ml of activated charcoal suspension. Immediately following inhalation treatment, and at 1, 2 and 4 h post treatment, volunteers ingested an additional 5 g of activated charcoal suspended in 50 ml of water.
Pharmacokinetic measurements
Patients were required to fast from the evening before day 1 of each study period and remain fasted until 2 h post-dose. Blood samples were obtained at pre-dose (within 10 min before inhalation), 5, 15, 30 and 45 min post-dose followed by 1, 2, 3, 4, 6, 8 and 12 h. Plasma was separated by refrigerated centrifugation and stored at −80°C for formoterol assay and at −20°C for BDP/17-BMP assay. The pharmacokinetic assays were performed using validated liquid chromatography-mass spectrometry (LC-MS) methods (SGS Life Sciences Services, Belgium) with limit of quantification (LOQ) of 2 pg ml−1 and maximum CV% of 10% for formoterol and LOQ of 20 pg ml−1 and maximum CV% of 10% for BDP and 17-BMP. The following PK parameters were calculated from the individual plasma drug concentration vs. time profiles: maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax), area under the plasma concentration vs. time curve observed from 0 to 30 min (AUC(0,30 min)) and from 0 to the last observed concentration time t (AUC(0,tlast)), calculated using the linear trapezoidal rule. The elimination half-life (t1/2) was calculated as 0.693/λz, where λz is the first order terminal rate constant. AUC(0,tlast) was used to measure the extent of absorption. The AUC(0,30 min) was evaluated as an index of lung absorption, whilst Cmax and tmax are indicators of the rate of absorption.
Data analyses
The statistical analysis was performed using SAS® software (version 9.12). PK calculations and statistical comparisons on PK data were performed according to a non-compartmental kinetic model with validated software (Kinetica™ version 4.41, Thermo Electron Corporation, USA). Log-transformed values for Cmax, AUC(0,30 min), AUC(0,tlast) were analysed using a Latin-square analysis of variance (anova) for a crossover design with ‘treatment’, ‘period’, ‘sequence’ and ‘subject nested to sequence’ as variables. The tmax was analyzed using the non-parametric Friedman test.
Differences between treatments were considered not significant, and hence the treatments were considered bioequivalent, if the 90% confidence intervals (CIs) of the treatment ratios were within the range 67–150%. In a previous study [19], the largest intra-individual coefficient of variation for 17-BMP and formoterol AUC was 30% for formoterol. Given such variability, in the present study it was estimated that 12 subjects were required to reach an 80% power to detect a more than 1.5 fold difference in 17-BMP and formoterol systemic exposure at the 0.05 α-level.
Results
In vitro results
Results obtained with the extra-fine BDP/formoterol fixed dose 100/6 µg are presented in Table 1. Use of the AeroChamber Plus™ resulted in a reduced delivered dose of both BDP (36% reduction) and formoterol (39% reduction). This reduction was mainly due to the fall in coarse fraction, which was reduced by 97% and 100% for BDP and formoterol respectively with the use of AeroChamber Plus™ compared with pMDI alone (Table 1). Conversely the fine particle dose (FPD) for both BDP and formoterol increased with AeroChamber Plus™ use compared with pMDI alone (53.0 ± 6.3 µg vs. 33.3 ± 1.4 µg for BDP; 3.1 ± 0.4 µg vs. 2.1 ± 0.1 µg for formoterol).
Table 1.
In vitro deposition parameters of beclometasone dipropionate (BDP) and formoterol (mean ± SD, n = 6) following one actuation of extra-fine BDP/formoterol fixed combination (100/6 µg) with and without the AeroChamber Plus™
| BDP | Formoterol | |||
|---|---|---|---|---|
| pMDI | pMDI + AeroChamber Plus™ | pMDI | pMDI + AeroChamber Plus™ | |
| Delivered dose (µg) | 85.4 ± 1.7 | 54.6 ± 5.9 | 5.1 ± 0.2 | 3.1 ± 0.4 |
| Coarse dose (µg) | 52.1 ± 2.7 | 1.7 ± 0.7 | 3.0 ± 0.2 | 0.0 ± 0.0 |
| FPD (µg) | 33.3 ± 1.4 | 53.0 ± 6.3 | 2.1 ± 0.1 | 3.1 ± 0.4 |
| Fine particle fraction (%)* | 39.0 ± 2.1 | 96.9 ± 1.6 | 40.7 ± 2.6 | 98.8 ± 1.3 |
Expressed as % of total delivered dose. Coarse dose (particles > 5 µm) = induction port plus Anderson cascade impactor (ACI) stages 0–2. Fine particle dose (FPD, particles < 5 µm) = ACI Stage S3 – F.
In vivo results in healthy volunteers
Use of the AeroChamber Plus™
A total of 12 male subjects were recruited (mean age 29.2 years, range 18–41 years), with a mean height of 176 cm (range 164–190 cm) and a mean BMI of 24.3 kg m–2 (range 20.4–27.9 kg m–2).
Compared with the pMDI alone, use of AeroChamber Plus™ increased the Cmax of BDP and formoterol with the 90% CI for the treatment ratios being greater than the bioequivalence range. Following Cmax, BDP concentrations promptly declined as it was metabolized to 17-BMP (Figure 1).
Figure 1.
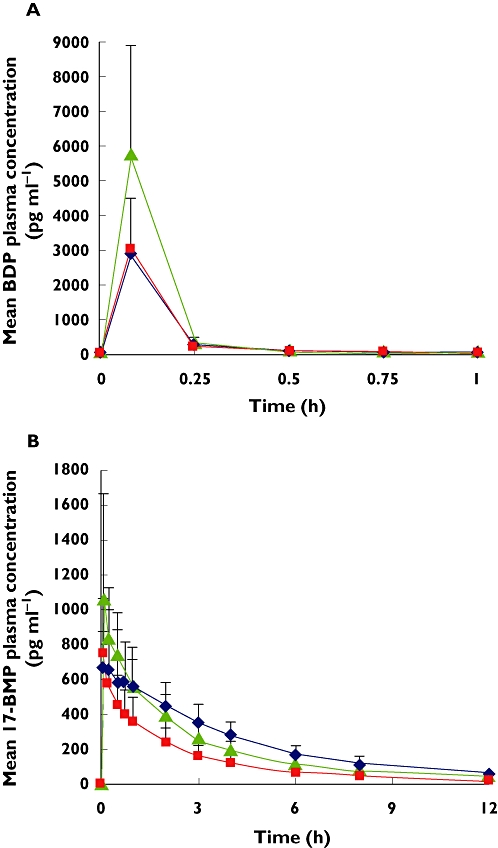
(A) Beclometasone dipropionate (BDP) and (B) beclometasone 17-monopropionate (17-BMP) mean plasma profiles (standard deviation) after inhalation of four puffs extra-fine BDP/formoterol (100/6 µg) using pMDI alone ( ); pMDI + charcoal block (
); pMDI + charcoal block ( ); pMDI + Aerochamber Plus (
); pMDI + Aerochamber Plus ( )
)
The AUC(0,30 min), indicative of lung deposition, was increased with AeroChamber Plus™ for BDP, 17-BMP and formoterol. In each case the 90% CI for the treatment ratios was outside the specified equivalence range (Table 2).
Table 2.
BDP, 17-BMP and formoterol pharmacokinetic parameters in healthy volunteers following inhalation extra-fine BDP/formoterol (100/6 µg) using pMDI alone (R), pMDI and charcoal ingestion (T1) and pMDI and AeroChamber Plus™ (T2)
| pMDI (R) | Charcoal block (T1) | AeroChamber Plus™ (T2) | % PE T1 vs. R (90% CI) | % PE T2 vs. R (90% CI) | |
|---|---|---|---|---|---|
| BDP | |||||
| Cmax (pg ml−1) | 2822.3 ± 1449.9 | 3033.1 ± 1448.4 | 5454.9 ± 3197.1 | 109 | 181 |
| (83, 142) | (138, 237) | ||||
| tmax* (h) | 0.08 | 0.08 | 0.08 | NC | NC |
| (0.08–0.12) | (0.08–0.08) | (0.08–0.12) | |||
| AUC(0,30 min) (pg ml−1 h) | 399.5 ± 199.4 | 435.2 ± 204.2 | 781 ± 439.8 | 110 | 185 |
| (85, 142) | (144, 239) | ||||
| AUC(0,tlast) (pg ml−1 h) | 407.8 ± 204.9 | 445.0 ± 213.3 | 803 ± 449.6 | 110 | 188 |
| (86, 141) | (146, 241) | ||||
| 17-BMP | |||||
| Cmax (pg ml−1) | 771.6 ± 288.7 | 775.0 ± 292.7 | 1138.9 ± 495.6 | 100 | 144 |
| (83, 122) | (119, 175) | ||||
| tmax (h) | 0.08 | 0.08 | 0.10 | NC | NC |
| (0.08–0.75) | (0.08–0.28) | (0.08–0.5) | |||
| AUC(0,30 min) (pg ml−1 h) | 292.3 ± 126.3 | 271.3 ± 87.9 | 402.2 ± 135.2 | 96 | 141 |
| (82, 112) | (121, 164) | ||||
| AUC(0,tlast) (pg ml−1 h) | 2900.8 ± 893.4 | 1559.2 ± 565.3 | 2539.6 ± 726.9 | 53 | 88 |
| (48, 59)* | (79, 98) | ||||
| Formoterol | |||||
| Cmax (pg ml−1) | 38.4 ± 17.8 | 37.2 ± 15.8* | 54.7 ± 20.0 | 97 | 147 |
| (83, 114) | (126, 173) | ||||
| tmax (h) | 0.08 | 0.08 | 0.08 | NC | NC |
| (0.08–0.27) | (0.08–0.08)* | (0.08–0.25) | |||
| AUC(0,30 min) (pg ml−1 h) | 12.7 ± 5.7 | 11.9 ± 5.2* | 17.6 ± 5.9 | 94 | 145 |
| (79, 110) | (123, 171) | ||||
| AUC(0,tlast) (pg ml−1 h) | 75.7 ± 32.4 | 46.9 ± 24.7* | 77.4 ± 29.8 | 55 | 105 |
| (46, 66)* | (88, 125) |
Values are arithmetic means ± SD, except tmax, median (range); AUC(0,30 min), area under the plasma concentration vs. time curve observed from 0 to 30 min; AUC(0,tlast), area under the plasma concentration vs. time curve observed from 0 to the last observed concentration time; 17-BMP, beclometasone 17-monopropionate; BDP, beclometasone dipropionate; CI, confidence interval; Cmax, maximum plasma concentration; tmax, time to maximum plasma concentration. PE, point estimate, calculated as ratio of geometric means test/reference × 100
n = 11.
Even though the total systemic exposure (AUC(0,tlast)) for BDP was increased (90% CI 146, 241%) with the use of AeroChamber Plus™, the AUC(0,tlast) for 17-BMP and formoterol were not affected using AeroChamber Plus™, since the CIs of the changes were almost within the specified equivalence range.
The individual data using AeroChamber Plus™ showed that the increase in AUC(0,30 min) was more evident in those subjects with a lower AUC(0,30 min) with the pMDI. A statistically significant inverse correlation (P < 0.001) between the % change AUC(0,30 min) using AeroChamber Plus™ and AUC(0,30 min) with pMDI alone was observed for both formoterol and 17-BMP. This suggests that low AUC(0,30 min) may be related to subjects with limited inhalation ability.
An additional analysis was performed by stratifying subjects into two groups on the basis of their AUC(0,30 min) values with pMDI alone. Subjects with a lower AUC(0,30 min) with pMDI alone (below the 50th percentile) showed an AUC(0,30 min) increase of 69% for 17-BMP and of 89% for formoterol when using the AeroChamber Plus™ compared with the pMDI alone. A limited effect of the AeroChamber Plus™ was noted in the subjects with higher AUC(0,30 min) with pMDI alone (above the 50th percentile). In both subgroups the AUC(0,tlast) was not affected by the use of AeroChamber™ (Figure 2).
Figure 2.
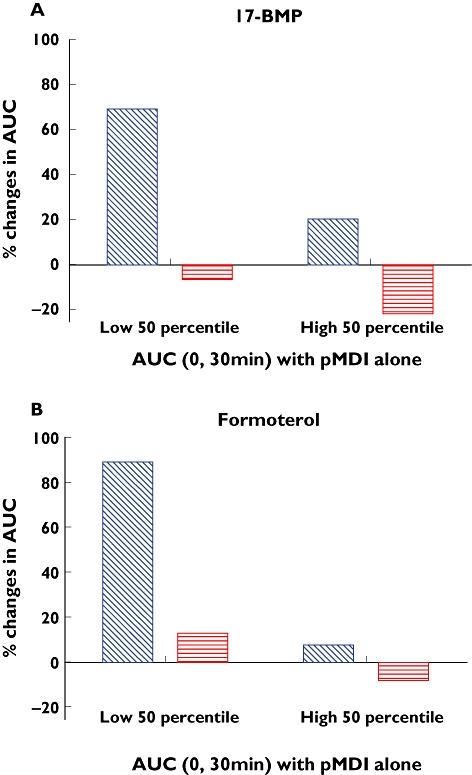
% Changes (median values) in the AUC(0,30 min) and AUC(0,tlast) for 17-BMP (A) and formoterol (B) after administration using the AeroChamber Plus™ compared with the pMDI alone in two groups of subjects: subjects with poor inhalation technique (low 50th percentile) and subjects with good inhalation technique (high 50th percentile). AUC (0, 30 min) ( ); AUC (0, tlast) (
); AUC (0, tlast) ( )
)
Charcoal block effect
The charcoal block reduced the total systemic exposure to formoterol (90% CI 46, 66%) and 17-BMP (90% CI 48, 59%) when compared with the pMDI alone (Table 2).
The BDP plasma concentrations were not influenced by charcoal ingestion, confirming that systemic BDP arises from lung absorption (Figure 1). The 17-BMP plasma concentrations were comparable in the initial absorption phase with or without charcoal block, while they decreased quickly after charcoal ingestion, as it prevents the absorption of BDP from the gut (Figure 1).
Formoterol peak concentrations and AUC(0,30 min) values were comparable with or without charcoal block (Table 2). Plasma concentrations of formoterol declined rapidly soon after 30 min with the charcoal block as the gastrointestinal absorption of the drug was prevented (Figure 3).
Figure 3.
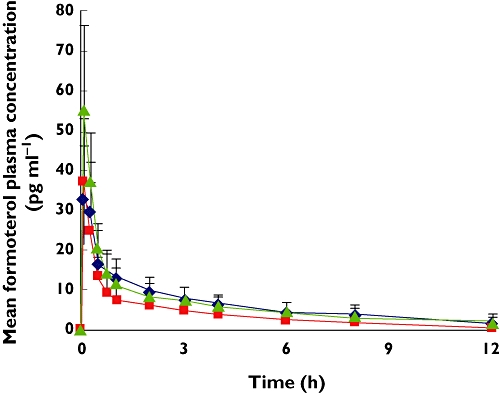
Formoterol mean plasma profile (standard deviation) after inhalation of four puffs extrafine BDP/formoterol (100/6 µg) using pMDI alone ( ); pMDI + charcoal block (
); pMDI + charcoal block ( ); pMDI + Aerochamber Plus (
); pMDI + Aerochamber Plus ( )
)
Discussion
Spacer and holding chamber devices facilitate optimal drug delivery to the lungs by making co-ordination of inhalation easier and decreasing the amount of drug deposited in the oropharynx which is subsequently swallowed. The performance of specific devices may vary according to the inhaled drug and specific pMDI used. With this in mind, the recently published European Medicines Agency guidelines recommend that the development of a pMDI should always include the testing of at least one specific named spacer [20]. The present study therefore investigated the effect of the Aerochamber Plus™ on lung deposition and systemic exposure to BDP/formoterol 100/6 µg pMDI.
It has been demonstrated that BDP given orally has minimal systemic absorption, and that inhaled BDP concentrations are not affected by charcoal block [21, 22]. These findings show that systemic concentrations of inhaled BDP are derived predominantly from pulmonary rather than gastro-intestinal absorption. In contrast, 17-BMP can be absorbed from both the pulmonary and gastro-intestinal route. 17-BMP absorption after oral administration is known to be relatively slow, with a tmax at 4 h. Furthermore, systemic 17-BMP concentrations from 0 to 30 min after inhalation are not affected by charcoal block, while there is a reduction at later time points, reflecting the effect of charcoal on the orally swallowed fraction and supporting the concept that the AUC(0,30 min) for 17-BMP derives predominantly from pulmonary absorption. This has also been proved for salbutamol [23]. We therefore used the AUC(0,30 min) as an indicator of pulmonary absorption in this study. We observed a reduction in 17-BMP and formoterol AUC(0,tlast) caused by charcoal block without the use of Aerochamber Plus™. The AUC(0,30 min) results, used as an index of pulmonary absorption, did not change with charcoal block treatment, showing that the reduction in AUC(0,tlast) was due to the gastro-intestinal component. In contrast, there was an increase in AUC(0,30 min) for BDP, 17-BMP and formoterol when the Aerochamber Plus™ was used. This is indicative of increased pulmonary absorption. We would expect charcoal block to have minimal effect on AUC(0,30 min) after inhalation using the Aerochamber Plus™, as it had little effect on this parameter without the spacer. Furthermore, our in vitro results indicate that the coarse fraction is almost completely retained by the Aerochamber Plus™, with the amount of drug available for gastro-intestinal absorption when using this spacer being approximately 3%.
Post hoc analysis showed that the use of AeroChamber Plus™ optimizes the lung deposition to a greater extent in subjects with lower AUC(0,30 min) values. These lower AUC(0,30 min) values could be due to an inadequate inhalation technique suggesting that the AeroChamber Plus™ overcomes low lung deposition due to poor inhalation technique.
The AeroChamber Plus™ did not increase the total systemic exposure to 17-BMP and formoterol measured by AUC(0,tlast). This was observed both in the whole population and also in the subgroup of subjects with the greatest increase in AUC(0,30 min) using the AeroChamber Plus™. This indicates that the increase in lung deposition by AeroChamber Plus™ is balanced by the decrease in the amount of drug swallowed and subsequently absorbed from the gut leading to no change in overall systemic exposure.
Mulrennan et al. [24] showed that differences in peak plasma concentration of ICS did not translate into differential hypothalamic-pituitary-adrenal (HPA) axis suppression. It would therefore be expected that the observed increases in plasma peak concentrations of 17-BMP with AeroChamber Plus™ would have little clinical relevance. A short duration of the rise of 17-BMP plasma concentrations in the first 30 min after inhalation would interfere only on the ‘fast reaction’ of the HPA-axis feedback mechanism giving a limited contribution to the overall suppression [25]. Additionally it is known that formoterol absorbed via the lungs in the systemic circulation is three-fold less potent, as regards to systemic effects, than orally absorbed formoterol [26] and that side effects are likely to be reduced with repeated dosing due to β2-adrenoceptor down-regulation [27, 28]. Again this evidence suggests that the increase in peak plasma formoterol concentrations due to the use of AeroChamber Plus™ are of limited clinical relevance.
It is interesting to note the consistency between the in vivo data for lung absorption and the in vitro fine particle fraction obtained using the AeroChamber Plus™. The AUC(0,30 min) increase of approximately 40% for both 17-BMP and formoterol when using the AeroChamber Plus™ matched the in vitro results, which showed an increase in the FPD of BDP and formoterol.
Interestingly, by comparing the AUC(0,tlast) values obtained with and without charcoal block following correction for the bioavailability factor (F = 41%) [21], this study confirmed that when using the pMDI alone more than 30% of the extra-fine BDP/formoterol dose is delivered to the lung [29, 30], in agreement with previously published scintigraphic data [31, 32]. Given this relatively high delivered dose using the pMDI, it is worth considering which patients might benefit most from using the AeroChamber Plus™. This approach may be most beneficial for subjects with inadequate inhalation technique and when higher doses of ICS are indicated.
The results of the present study are particularly relevant as previous studies with a different combination (i.e. fluticasone/salmeterol) showed a significant increase in total systemic exposure for the ICS administered with the spacer when compared with the pMDI alone [33]. This does not appear to be the case for BDP/formoterol delivered using the Modulite® pMDI.
The power calculation for this study used a within-subject CV% assumption of 30%. The CV% that we actually observed was lower: 25% and 15% for AUC(0,tlast) for formoterol and 17-BMP, respectively. At the time the study was designed, no specific guideline for inhalation products was available and we therefore considered that an appropriate equivalence criteria for the total systemic exposure (AUC(0,tlast)) was a 90% CI of 67%, 150%. Current guidelines [34] now state that 80, 125% should be used. Our results for AUC(0,tlast) for the comparison with and without Aerochamber Plus™ show that the 90% CIs were fully within the 80, 125% region for formoterol and slightly outside (79, 98%) for 17-BMP. To address these newer guidelines, a revised power calculation for a larger sample size would have been preferable, as the number that we used (n = 12) is regarded as the minimum required. The inclusion of only male subjects can be considered as another limitation of the present study, although it does not impact on the study outcome due to the crossover design.
In conclusion, use of the AeroChamber Plus™ with the extra-fine BDP/formoterol (100/6 µg) fixed combination pMDI optimizes BDP/formoterol lung deposition. The total systemic exposure is not increased, suggesting that the use of extra-fine BDP/formoterol with AeroChamber Plus™ does not affect the safety profile of the product.
Acknowledgments
Thank you to Dr Ruth Murray for assistance in writing this document. Thank you also to the Analytical Chemistry Department (Chiesi Farmaceutici) for sharing the in vitro results presented in this article and to CROSS Research S.A and SGS Life Science Services for the clinical and analytical work, respectively. This study was sponsored by Chiesi Farmaceutici.
Competing Interests
Dave Singh has received lectures fees, support for conference attendance, advisory board fees and research grants from a range of pharmaceutical companies including GSK, Chiesi Farmaceutici, AstraZeneca, CIPLA, Novartis, Forest, MSD, Boehringer and Allmiral. Sara Collarini, Gianluigi Poli, Daniela Acerbi, Alessio Amadasi and Antonio Rusca have no conflict of interest to disclose.
REFERENCES
- 1.Global Initiative for Asthma (GINA) Available at http://www.ginasthma.org/ (last accessed 18 January 2011)
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Available at http://www.goldcopd.org (last accessed 18 January 2011)
- 3.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonist and corticosteroids. Eur Respir J. 2002;19:182–91. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 4.Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 5.Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Salmeterol Study Group. Ann Allergy Asthma Immunol. 1999;82:383–9. doi: 10.1016/s1081-1206(10)63288-7. [DOI] [PubMed] [Google Scholar]
- 6.Van Noord JA, Schreurs AJ, Mol SJ, Mulder PG. Addition of salmeterol versus doubling the dose of fluticasone propionate in patients with mild to moderate asthma. Thorax. 1999;54:207–12. doi: 10.1136/thx.54.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse W, Koenig SM, Oppenheimer J, Sahn SA, Yancey SW, Reilly D, Edwards LD, Dorinsky PM. Steroid-sparing effects of fluticasone propionate 100 µg and salmeterol 50 µg administered twice daily in a single product in patients previously controlled with fluticasone propionate 250 µg administered twice daily. J Allergy Clin Immunol. 2003;111:57–65. doi: 10.1067/mai.2003.38. [DOI] [PubMed] [Google Scholar]
- 8.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJH, Pauwels RA, Pedersen SE. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 9.Rabe K, Vermeire P, Soriano J, Maier W. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16:802–7. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- 10.Ganderton D, Lewis DA, Davies R, Meakin B, Brambilla G, Church T. Modulite: a means of designing the aerosols generated by pressurized metered dose inhalers. Respir Med. 2002;96(Suppl 1):S3–S8. doi: 10.1016/s0954-6111(02)80018-x. [DOI] [PubMed] [Google Scholar]
- 11.Acerbi D, Brambilla G, Kottakis I. Advances in asthma and COPD management: delivering CFC-free inhaled therapy using Modulite technology. Pulm Pharmacol Ther. 2007;20:290–303. doi: 10.1016/j.pupt.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103:41–9. doi: 10.1016/j.rmed.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Crompton GK. Problems patients have using pressurized aerosol inhalers. Eur J Respir Dis Suppl. 1982;119:101–4. [PubMed] [Google Scholar]
- 14.Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104:1332–7. doi: 10.1378/chest.104.5.1332. [DOI] [PubMed] [Google Scholar]
- 15.Newman SP, Weisz AW, Talee N, Clarke SW. Improvement of drug delivery with a breath-activated aerosol for patients with poor inhaler technique. Thorax. 1991;46:712–6. doi: 10.1136/thx.46.10.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindgren S, Bake B, Larsson S. Clinical consequences of inadequate inhalation technique in asthma therapy. Eur J Respir Dis. 1987;70:93–8. [PubMed] [Google Scholar]
- 17.Newman SP. Spacer devices for metered dose inhalers. Clin Pharmacokinet. 2004;43:349–60. doi: 10.2165/00003088-200443060-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lavorini F, Fontana GA. Targeting drugs to the airways: the role of spacer devices. Expert Opin Drug Deliv. 2009;6:91–102. doi: 10.1517/17425240802637862. [DOI] [PubMed] [Google Scholar]
- 19.Bousquet J, Poli G, Acerbi D, Monno R, Ramael S, Nollevaux F. Systemic exposure and implications for lung deposition with an extra-fine HFA beclometasone dipropionate/formoterol fixed combination. Clin Pharmacokinet. 2009;48:347–58. doi: 10.2165/00003088-200948060-00001. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma or chronic obstructive pulmonary disease (COPD) in adults, and for use in the treatment of asthma in children and adolescents. 2009. Doc Ref. CPMP/EWP/4151/OO Rev.1 Available at http://www.emea.europa.eu/ (last accessed 18 January 2011)
- 21.Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N. Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol. 2001;51:400–9. doi: 10.1046/j.0306-5251.2001.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boobis AR. Comparative physicochemical and pharmacokinetic profiles of inhaled beclometasone dipropionate and budesonide. Respir Med. 1998;92(Suppl)(B):2–6. doi: 10.1016/s0954-6111(98)90434-6. [DOI] [PubMed] [Google Scholar]
- 23.Girodet PO, Molimard M. Pharmacological approach to evaluate aerosol pulmonary deposition. J Aerosol Med. 2005;18:183–92. doi: 10.1089/jam.2005.18.183. [DOI] [PubMed] [Google Scholar]
- 24.Mulrennan S, Hogg JS, Teoh RC, Morice AH. Adrenal axis suppression unrelated to the dynamics of dosing with beclomethasone dipropionate. Br J Clin Pharmacol. 2007;63:1365–2125. doi: 10.1111/j.1365-2125.2006.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 26.Derks MGM, van den Berg BTJ, van der Zee JS, Braat MCP, van Boxtel CJ. Biphasic effect-time courses in man after formoterol inhalation: eosinopenic and hypokalemic effects and inhibition of allergic skin reactions. J Pharmacol Exp Ther. 1997;283:824–32. [PubMed] [Google Scholar]
- 27.Grove A, Lipworth BJ. Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet. 1995;346:201–6. doi: 10.1016/s0140-6736(95)91265-7. [DOI] [PubMed] [Google Scholar]
- 28.Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. Am J Med. 1994;97:29–37. doi: 10.1016/0002-9343(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 29.Mobley C, Hochhaus G. Methods used to assess pulmonary deposition and absorption of drugs. Drug Discov Today. 2001;6:367–75. doi: 10.1016/s1359-6446(01)01691-9. [DOI] [PubMed] [Google Scholar]
- 30.Agertoft L, Laulund LW, Harrison LI, Pedersen S. Influence of particle size on lung deposition and pharmacokinetics of beclomethasone dipropionate in children. Pediatr Pulmonol. 2003;35:192–9. doi: 10.1002/ppul.10238. [DOI] [PubMed] [Google Scholar]
- 31.De Backer W, De Volder A, Poli G, Acerbi D, Monno R, Herpich C, Brand P, Meyer T, Mariotti F. Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic and COPD patients. J Aerosol Med Pulm. Drug Deliv. 2010;23:137–48. doi: 10.1089/jamp.2009.0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA-beclomethasone from a metered dose inhaler. J Aerosol Med. 2005;18:379–85. doi: 10.1089/jam.2005.18.379. [DOI] [PubMed] [Google Scholar]
- 33.Dempsey OJ, Wilson AM, Coutie WJR, Lipworth BJ. Evaluation of the effect of a large volume spacer on the systemic bioactivity of fluticasone propionate metered-dose inhaler. Chest. 1999;116:935–40. doi: 10.1378/chest.116.4.935. [DOI] [PubMed] [Google Scholar]
- 34.EMA guideline ‘Guideline on Investigation of Bioequivalence’, CPMP/EWP/QWP/1401/98 Rev. 1/ Corr, 20 January 2010


