Abstract
AIMS
To determine whether electronic prescribing facilitates the uptake of clinical pharmacologists' recommendations for improving drug safety in medical inpatients.
METHODS
Electronic case records and prescription charts (either electronic or paper) of 502 patients hospitalized on medical wards in a large Swiss teaching hospital between January 2009 and January 2010 were studied by four junior and four senior clinical pharmacologists. Drug-related problems were identified and interventions proposed. The implementation and time delays of these proposed interventions were compared between the patients for whom paper drug charts were used and the patients for whom electronic drug charts were used.
RESULTS
One hundred and fifty-eight drug-related problems in 109 hospital admissions were identified and 145 recommendations were made, of which 51% were implemented. Admissions with an electronic prescription chart (n = 90) were found to have 2.74 times higher odds for implementation of the change than those with a paper prescription chart (n = 53) (95% confidence interval 1.2, 6.3, P = 0.018, adjusted for any dependency introduced by patient, ward or clinical team; follow-up for two cases missing). The time delay between recommendations being made and their implementation (if any) was minimal (median 1 day) and did not differ between the two groups.
CONCLUSIONS
Electronic prescribing in this hospital setting was associated with increased implementation of clinical pharmacologists' recommendations for improving drug safety when compared with handwritten prescribing on paper.
Keywords: clinical pharmacology, electronic prescribing, hospital
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Electronic prescribing reduces drug prescription errors and therefore improves patient safety.
Whether electronic prescribing compared with prescribing on paper facilitates the implementation of clinical pharmacologists' recommendations in the care of patients hospitalized with medical problems is not known.
WHAT THIS STUDY ADDS
The use of electronic prescriptions when compared with handwritten prescriptions increased the uptake of clinical pharmacologists' recommendations for improving drug safety in hospitalized patients.
Introduction
Electronic prescribing has been shown to reduce systematically drug prescription errors [1–3]. In a large pre- and post-intervention study performed in two Dutch hospitals, the percentage of prescriptions containing at least one medication error was reduced from 55% to 17% after the introduction of electronic prescribing with clinical decision support (absolute reduction 40.3%) [1]. The reduction in errors was primarily due to improved prescription legibility and reduction in administrative errors. A reduction in errors related to therapeutics (drug choice), however, was not achieved [1]. In a similar pre- and post-intervention study of electronic prescribing in community-based office practices, Kaushal and colleagues found a nearly seven-fold reduction in errors, primarily related to illegibility errors or the inappropriate use of abbreviations, after the introduction of electronic prescribing [2]. A study of nearly 4000 paediatric inpatient, discharge and outpatient prescriptions before and nearly 5000 prescriptions after the introduction of electronic prescribing showed a significant reduction in drug dosing errors (55% relative reduction in dosing error, 1% absolute reduction), with an abolition of severe dose errors for outpatients and patients at discharge [3].
Whether electronic prescribing can additionally facilitate the uptake of clinical pharmacologists' suggestions for optimizing drug therapy where drug-related problems (DRPs) are found, however, is not known. We therefore set out to study the impact of electronic prescribing on the uptake of clinical pharmacologists' suggestions in a hospital inpatient setting.
Methods
Electronic case records and prescription charts (electronic or paper) of patients hospitalized on three medical wards in a large Swiss teaching hospital between January 2009 and January 2010 were examined on a once weekly basis for DRPs by four junior and four senior clinical pharmacologists. The patients were under the care of 10 different medical clinics (angiology, endocrinology, gastroenterology, haematology, internal medicine, immunology, infectious diseases, cardiology, nephrology and respiratory medicine). The study period represented the ‘roll-in’ phase of electronic prescribing, which occurred in a staggered ward-based fashion over a 9-month period from January until October 2009. DRPs were recorded and suggestions for improved drug prescribing or patient monitoring were given to the treating physicians by telephone and by electronic entry in the case record (in both the medical and nursing charts) on the same day. Comments were limited to regularly administered medication only; drugs prescribed on a ‘pro re nata’ basis (i.e. those not regularly administered) were not assessed.
For the purposes of further analysis, ‘proposed interventions’ were defined as DRPs for which interventions were proposed by the clinical pharmacologists. The implementation of these proposed interventions were compared between patients for whom electronic prescription charts were used and patients for whom paper handwritten prescription charts were used. The implementation of proposed interventions was the primary outcome measure. In order to investigate whether electronic prescribing shortens the time between receiving the proposed intervention and implementing it, the time between the appearance of the proposed intervention as an electronic note in the patient's case record and its implementation (if any) was additionally recorded.
The DRPs were classified according to the Pharmaceutical Care Network Europe (PCNE) Classification for drug-related problems version 6.2 (revised 14 January 2010), which defines a DRP as ‘an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes’[4]. A previous version of the PCNE classification system was evaluated as a tool in another Swiss teaching hospital and found to be applicable [5].
Proposed interventions could broadly be categorized into either drug level interventions or patient or drug- monitoring level interventions (e.g. ECG monitoring or phenytoin measurement). The current PCNE classification system records whether the proposed intervention was approved or not approved by the prescriber, but does not classify the proposed intervention itself. The outcome of a proposed intervention which has been taken up by the treating clinician can be categorized if the proposal was at the drug level (I3).
The medical records (case histories, follow-up, laboratory results) and the process of requesting diagnostic tests (e.g. blood tests and imaging) were electronic in all cases. An additional feature of the electronic prescription chart was the electronic drug interactions check programme supplied by Pharmavista®[6]. This programme assessed potential drug–drug interactions only when requested to do so by the prescriber. It did not flag up potential problems automatically. It was not possible to determine whether or how often the interactions programme was used by the prescribing physicians. Other than the voluntary drug interactions check, no electronic clinical decision support (e.g. regarding dosing) was embodied in the electronic prescription chart.
Frequency tables were used to display the different types of DRP detected. Nominal variables were compared between groups using chi-squared test and odds ratios were computed to quantify the effect. DRPs were treated as independent events because they involved individual drugs, had individual underlying causes and generated individual, specific proposed interventions. Display of the classification of DRPs was therefore not adjusted. The primary outcome measure was the implementation of clinical pharmacologists' proposed interventions for improving drug safety. To adjust for the dependency of the outcome measure induced by multiple DRPs per patient, ward and clinic, a generalized estimating equations (GEE) model was computed where we included clinic and ward as explanatory variables. Each patient within each clinic and ward was treated as a separate cluster and we included clinic and ward as explanatory variables. Clinics with less than five identified DRPs were pooled in one group. Robust standard errors from the GEE model were then extracted to compute a confidence interval for the resulting odds ratio of interest. Of note, standard errors from a GEE are also valid if the underlying correlation structure is not correctly specified by our model assumptions. All analyses were performed using R (R Development Core Team, 2010) using the package ‘geepack’[7].
Results
The number of hospital admissions screened for DRPs, the number of DRPs and the number of interventions proposed by clinical pharmacologists is shown in Figure 1. Of the 502 hospital admissions assessed for the presence of DRPs, 267 were managed with electronic prescription charts and 235 with paper prescription charts. Due to patient rehospitalizations, the number of admissions exceeded the number of individual patients assessed (Figure 1). The median age was 68.1 years (interquartile range 58.7–76 years) and 68% were male. The total number of DRPs identified was 158, representing 0.31 DRPs per admission. The maximum number of DRPs per admission was 4 (n = 2 admissions). Significantly more DRPs were identified among admissions for whom electronic prescription charts were employed (99 DRPs detected in 267 admissions) than among those for whom paper prescription charts were employed (59 DRPs detected in 235 admissions) (37% vs. 25% respectively, P = 0.005). A total of 261 drug prescriptions involving 101 different medications were implicated in the DRPs. The 10 most frequently implicated drugs and their drug classes are shown in Table 1.
Figure 1.
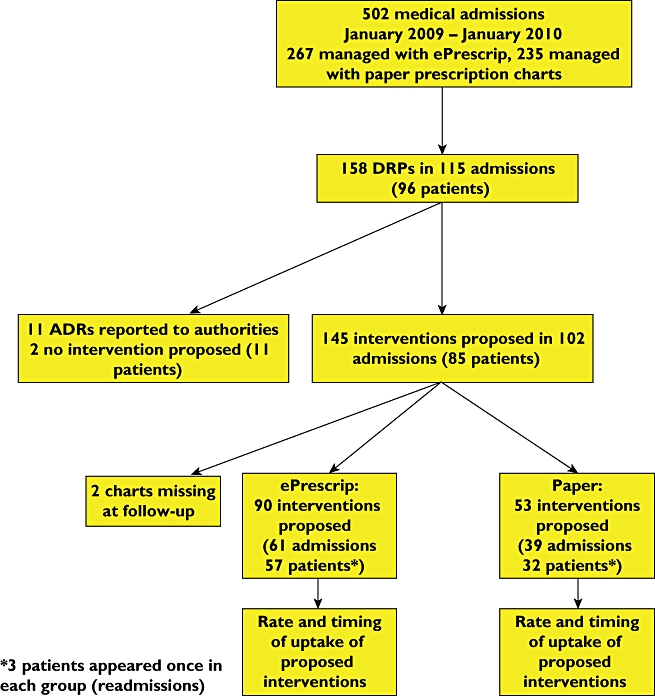
Study flowchart. ePrescrip, electronic prescription chart; DRP, drug-related problem; ADR, adverse drug reaction
Table 1.
Top 10 implicated drugs and their drug classes
| Drug | Class | Total number | ePrescrip chart | Paper prescription chart | Percentage (%) of all implicated drugs |
|---|---|---|---|---|---|
| Esomeprazole | Proton pump inhibitor | 13 | 12 | 1 | 5 |
| Amiodarone | Class III antiarrhythmic | 12 | 4 | 8 | 5 |
| Clarithromycin | Macrolide antiobiotic | 11 | 4 | 7 | 4 |
| Clopidogrel | Antiplatelet agent | 9 | 9 | 0 | 3 |
| Ciprofloxacin | Quinolone antibiotic | 9 | 6 | 3 | 3 |
| Atorvastatin | Lipid-lowering agent | 8 | 5 | 3 | 3 |
| Sulphamethoxazole/trimethoprim | Antibiotic | 7 | 0 | 7 | 3 |
| Metamizole | Antipyretic/analgesic | 6 | 6 | 0 | 2 |
| Paracetamol | Antipyretic/analgesic | 6 | 6 | 0 | 2 |
| Phenprocoumon | Oral anticoagulant | 6 | 1 | 5 | 2 |
| Total % | 32 |
ePrescrip, electronic prescription.
The DRPs classified according to PCNE version 6.2 are shown in Table 2. The commonest DRPs were 104 potential and four actual toxic adverse reactions (P2.3). The distribution of these potential and actual toxic adverse drug reactions was not significantly different between the electronic and paper prescription groups (potential toxic adverse drug events = 71 in the electronic group and 34 in the paper group, chi-square P = 0.41).
Table 2.
Classification of the drug-related problems according to PCNE V6.2. Two drug charts were missing at follow-up so were excluded from the ePrescrip and Paper prescription groups
| Characteristic | Entire group | ePrescrip | Paper prescription | Comparison test statistic P |
|---|---|---|---|---|
| Number of comments | 158 | 98 | 58 | |
| The problem | ||||
| P1: Treatment effectiveness: There is a (potential) problem with the (lack of) effect of the pharmacotherapy. | 36 | 18 | 17 | 0.16 |
| P2: Adverse reactions: Patient suffers, or will possibly suffer, from an adverse drug event. | ||||
| P2.1 Non-allergic | 11 | 6 | 5 | |
| P2.2 Allergic | 1 | 0 | 1 | |
| P2.3 Toxic | 110 | 74 | 35 | |
| The cause | ||||
| C1: Drug selection | ||||
| C1.1 Inappropriate drug (including contraindicated) | 21 | 14 | 7 | 0.73 |
| C1.3 Inappropriate combination of drugs | 52 | 34 | 18 | |
| C1.4 Inappropriate duplication of therapeutic group or active ingredients | 2 | 1 | 1 | |
| C1.8 Synergistic/preventive drug required and not given | 2 | 2 | 0 | |
| C2: Drug form | ||||
| C2.1 Inappropriate drug form | 2 | 2 | 0 | – |
| C3: Dose selection | ||||
| C3.2 Drug dose too high | 1 | 1 | 0 | 0.2 |
| C3.5 No therapeutic drug monitoring | 7 | 2 | 4 | |
| C3.6 Pharmacokinetic problem requiring dose adjustment | 43 | 29 | 14 | |
| C5: Drug use process | ||||
| C5.1 Inappropriate timing of administration and/or dosing intervals | 11 | 5 | 5 | 0.8 |
| C8: Other | ||||
| C8.1 Other cause* | 17 | 8 | 9 | |
| The intervention | ||||
| I0.0: No intervention | 2 | 2 | 0 | |
| I1: At prescriber level | ||||
| I1.3 Intervention proposed, approved by prescriber | 74 | 56 | 18 | 0.001 |
| I1.4 Intervention proposed, not approved by prescriber | 69 | 34 | 35 | 0.001 |
| I1.5 Intervention proposed, outcome unknown | 2 | 0 | 0 | – |
| I3: At drug level | ||||
| I3.1 Drug changed | 5 | 4 | 1 | 0.32 |
| I3.2 Dose changed | 24 | 20 | 4 | |
| I3.4 Instructions for use changed | 8 | 4 | 4 | |
| I3.5 Drug stopped | 20 | 16 | 4 | |
| I3.6 New drug started | 2 | 2 | 0 | |
| I4: Other intervention or activity | ||||
| I4.2 Side effect reported to authorities | 11 | 6 | 5 | – |
ePrescrip, electronic prescription.
Predictable adverse drug reactions were classified under this heading and formed this entire ‘other’ group.
There were no DRPs in the following categories: P3 Treatment costs: P3.1 Drug treatment more costly than necessary, P3.2 Unnecessary drug treatment; P4 Other problems: P4.1 Patient dissatisfied with therapy, P4.2 Unclear problem; C1 Drug selection: C1.2 No indication for drug, C1.5 Indication for drug treatment not noticed, C1.6 Too many drugs prescribed for indication, C1.7 More cost-effective drug available, C1.9 New indication for drug treatment presented; C3: Dose selection: C3.1 Drug dose too low, C3.4 Dosage regimen too frequent, C3.7 Deterioration/improvement of disease state requiring dose adjustment, C4: Treatment duration: C4.1 Duration of treatment too short, C4.2 Duration of treatment too long; C5: Drug use process: C5.2 Drug underused/under-administered (deliberately), C5.3 Drug overused/over-administered (deliberately), C5.4 Drug not taken/administered at all, C5.5 Wrong drug taken/administered, C5.6 Drug abused (unregulated overuse), C5.7 Patient unable to use drug/form as directed; C6. Logistics: C6.1 Prescribed drug not available, C6.2 Prescribing error (necessary information missing), C6.3 Dispensing error (wrong dose or drug dispensed); C7: The patient: C7.1 Patient forgets to use/take drug, C7.2 Patient uses unnecessary drug, C7.3 Patient takes food that interacts, C7.4 Patient stored drug inappropriately; C8 Other: C8.2 No obvious cause; I1. At prescriber level: I1.1 Prescriber informed only, I1.2 Prescriber asked for information; I2: At patient/carer level I2.1 Patient (medication) counselling, I2.2 Written information provided only, I2.3 Patient referred to prescriber, I2.4 Spoken to family member/caregiver; I3: At drug level: I3.3 Formulation changed; I4: Other intervention or activity: I4.1 Other intervention.
The underlying causes of the DRPs were related to inappropriate combinations of drugs (33%), dose selection (28%), contraindications (13%), adverse drug reactions (11%), inappropriate timing of drug administration (7%), the need for therapeutic drug monitoring (4%) and other causes (4% – two cases each of inappropriate duplication of therapeutic group or active ingredient, synergistic/preventive drug required and not given and inappropriate drug form). DRPs which in our opinion can be classified as ‘prescribing errors’ are those which the treating clinicians should have been able to avoid on the basis of their knowledge of the patient and the drug. Specialist pharmacological knowledge (e.g. about drug–drug interactions or pharmacokinetics) was not required to avoid such errors. In this study these were where a contraindicated drug had been prescribed (PCNE code C1.1) or where the licensed dose had been exceeded (C3.2). In total 22 such prescribing errors were detected, eight in the paper group and 14 in the electronic group (15% and 15.5% respectively). No handwriting or transcription errors were detected in either group.
The proposed interventions were: change of drug, change of drug formulation or drug cessation (34%), dose adjustment (32%), patient monitoring (17%), performance of therapeutic drug monitoring (9%) or change of timing of drug administration (8%). Overall, of the 145 proposed interventions, 51% were carried out. In two cases (1%) the outcome was unknown as the records were lost to follow-up. Exact numbers are given in Table 2. Seventeen percent of interventions proposed involved monitoring for drug side effects. These interventions could not be categorized by the current PCNE classification system.
The uptake of clinical pharmacologists' recommendations differed significantly between patients for whom an electronic prescription chart compared with a paper prescription chart was employed (62% vs. 34% respectively, Pearson's chi-square P = 0.001, Table 2). There were no differences between the two groups in terms of distribution of the other PCNE classifications.
Table 3 shows the types of proposed intervention and the number carried out by the treating physicians. The adjusted odds ratio for implementation of the proposed interventions in the electronic prescription compared with the paper prescription group was 2.74 (95% confidence interval 1.2, 6.3, P = 0.018, after adjustment for clustering by patient, clinic and ward). This difference seen in the implementation of the proposed interventions between the electronic and paper prescription groups was largely due to reduced implementation of proposed interventions at the drug-level and not at the patient or drug monitoring level in the paper prescription group (Table 3). For proposed interventions which were implemented, the median time delay was 1 day for all groups (Table 3).
Table 3.
Types of proposed interventions and their implementation
| Proposed intervention | Entire group | ePrescrip | Paper prescription | Unadjusted odds ratio ePrescrip compared with paper | 95% CI for OR | Adjusted odds ratio ePrescrip compared with paper | 95% CI for adjusted OR |
|---|---|---|---|---|---|---|---|
| Interventions implemented/total proposed interventions (%) | 74/143 (52%) | 56/90 (62%) | 18/53 (34%) | 3.2 | 1.57, 6.51 | 2.74 | 1.2, 6.3 |
| Patient and drug monitoring interventions implemented/total proposed (%) | 17/37 (46%) | 12/24 (50%) | 5/13 (38%) | 1.6 | 0.4, 6.3 | 1.07* | 0.36, 3.2 |
| Interventions proposed at drug level implemented/total proposed (%) | 57/106 (54%) | 44/66 (67%) | 13/40 (33%) | 4.2 | 1.8, 9.6 | 3.28 | 1.13, 9.48 |
| Median time (interquartile range) between proposed intervention and implementation (days) (data available for 74) | 1 (0:1) | 1 (0:1) | 1 (0:1) |
Due to the low numbers of observations this model could only adjust for multiple DRPs per patient (and not additionally for ward and clinic). ePrescrip, electronic prescription chart.
Discussion
The use of electronic prescribing was associated with an increase in the implementation of clinical pharmacologists' proposed interventions to improve drug safety among medical inpatients.
Reasons why the use of paper prescription charts might prevent the treating physicians from implementing drug safety recommendations are the physical separation of the patient notes where the recommendation is read and the prescription chart itself (held in the nurses' office or on the drug trolley), the need for manually changing a prescription or physicians being interrupted and forgetting the proposed intervention between receiving the information and finding the paper prescription chart. Our observation that interventions which did not require alteration of the paper prescription chart per se (such as patient monitoring or therapeutic drug monitoring) were implemented much more frequently than drug level interventions in patients managed with paper prescription charts supports these hypotheses. Once a decision was made to implement a change, this was done at the same speed in the two groups, implying a willingness to implement changes, but perhaps insufficient attempts to find the paper prescription chart being made.
The introduction of electronic prescribing during the time period of this study is likely to have contributed to the higher rate of DRPs identified in admissions managed with the electronic prescription chart compared with admissions managed with a paper prescription chart for a number of reasons. On the one hand clinicians may have made more errors in prescribing due to being unfamiliar with the new routine. However we did not detect a higher rate of prescribing errors in the electronic compared with the paper prescription chart groups, so this theory is not supported. A more likely explanation for the increased rate of DRPs in the electronic prescription chart group is an increased rate of detection of DRPs by the clinical pharmacologists. It is likely that the clinical pharmacologists had more time to study the electronic prescription charts compared with the paper prescription charts and were thereby able to identify more DRPs. The time allowed for a clinical pharmacologist to examine an electronic prescription chart is unrestricted, whereas the time available for study of a paper prescription chart is clearly limited, particularly when the prescription chart is urgently needed in active patient care. A further advantage of electronic prescription charts is that several users can examine the chart simultaneously at different workstations. An additional reason for the higher DRP detection rate among patients cared for with an electronic prescription chart may have been the clinical pharmacologists' use of the embedded drug interactions checking software (Pharmavista). The data from this study therefore suggest that electronic prescribing facilitates both clinical pharmacologists' detection of DRPs and treating clinicians' subsequent uptake of clinical pharmacologists' proposed interventions for improving drug safety in hospitalized medical patients.
Studies have shown that up to 91% of computer generated messages are ignored by prescribing physicians [8, 9], implying a 9% implementation rate of suggested interventions. The overall implementation rate of 51% in the study presented here (34% for the group of patients in whom paper prescription charts were used) compares very favourably with this, and supports the role of clinical pharmacologists rather than interaction check programmes in improving drug safety in the hospital setting. However future improvements in computerized clinical decision support may make such tools more widely acceptable and applicable. The relative cost-effectiveness of ‘manual’ prescription chart reviews such as those provided by a clinical pharmacologist and electronic interaction checking or decision support programmes is difficult to assess as currently the two processes provide a different ‘service’ to the treating clinician. In future, clinical decision support programmes are likely to become more sophisticated and user-friendly, at which point a prospective comparative assessment of the cost-effectiveness of electronic and manual prescription chart reviews would be valuable.
Limitations of the study
The study was carried out over 12 consecutive months and a learning effect of the hospital doctors involved cannot be excluded. However we do not think this played a significant role as the junior physicians (who have the main prescribing role) change positions at different times in 6 month cycles. This study was carried out using cases from three (out of a total of nine) medical wards and so is not representative of all the medical cases hospitalized at this hospital during 2009. A further limitation is the lack of randomization.
In conclusion, the use of electronic prescription charts increased the implementation of clinical pharmacologists' proposed interventions to improve drug safety among hospitalized medical inpatients in this large, Swiss teaching hospital.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.van Doormaal JE, van den Bemt PM, Zaal RJ, Egberts AC, Lenderink BW, Kosterink JG, Haaijer-Ruskamp FM, Mol PG. The influence that electronic prescribing has on medication errors and preventable adverse drug events: an interrupted time-series study. J Am Med Inform Assoc. 2009;16:816–25. doi: 10.1197/jamia.M3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushal R, Kern LM, Barron Y, Quaresimo J, Abramson EL. Electronic prescribing improves medication safety in community-based office practices. J Gen Intern Med. 2010;25:530–6. doi: 10.1007/s11606-009-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jani YH, Barber N, Wong IC. Paediatric dosing errors before and after electronic prescribing. Qual Saf Health Care. 2010;19:337–40. doi: 10.1136/qshc.2009.033068. [DOI] [PubMed] [Google Scholar]
- 4.Pharmaceutical Care Network Europe Foundation 2010 Classification for drug-related problems. Available at http://www.pcne.org/sig/drp/documents/drp/PCNE%20classification%20V6-2.pdf (last accessed 17 May 2011)
- 5.Lampert ML, Kraehenbuehl S, Hug BL. Drug-related problems: evaluation of a classification system in the daily practice of a Swiss University Hospital. Pharm World Sci. 2008;30:768–76. doi: 10.1007/s11096-008-9213-8. [DOI] [PubMed] [Google Scholar]
- 6.Pharmavista 2011 Information for Healthcare Providers, powered by e-mediat. Available at http://www.pharmavista.ch/content/ (last accessed 17 May 2011)
- 7.Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2005;15:1–11. [Google Scholar]
- 8.Isaac T, Weissman JS, Davis RB, Massagli M, Cyrulik A, Sands DZ, Weingart SN. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305–11. doi: 10.1001/archinternmed.2008.551. [DOI] [PubMed] [Google Scholar]
- 9.Mille F, Schwartz C, Brion F, Fontan JE, Bourdon O, Degoulet P, Jaulent MC. Analysis of overridden alerts in a drug-drug interaction detection system. Int J Qual Health Care. 2008;20:400–5. doi: 10.1093/intqhc/mzn038. [DOI] [PubMed] [Google Scholar]


