Abstract
AIMS
The aim of this study was to compare the prevalence of diabetes in children across seven European countries, when using prescribing of anti-diabetics as a proxy for diabetes. A secondary aim was to assess the potential for collaboration between countries using different databases in diabetes research.
METHODS
Data were obtained from population-based clinical databases in seven European countries. The study population comprised children aged 0–18 years. Prescriptions were categorized using the Anatomic Therapeutic Chemical (ATC) classification. The one-year user prevalence in 2008 was calculated for each country and stratified by age and sex.
RESULTS
We studied a total of 5.8 million children and adolescents. The prevalence of insulin prescribing varied between 1.1 and 3.5 per 1000 population, being highest in Sweden and lowest in Italy. In all countries, novel insulin analogues were the most commonly used insulins. The prevalence of oral anti-diabetic prescribing ranged from 0.08 per 1000 individuals in Sweden and Germany to 0.21 per 1000 population in the UK. Overall, the absolute number of oral anti-diabetic users was very low.
CONCLUSION
This study shows that there is a varying frequency of type 1 diabetes in children and adolescents across Europe. We also demonstrated that it is possible to obtain similar information from different clinical databases within Europe, which would allow continuous monitoring of type 1 diabetes. Owing to the lack of indications in most of the databases, this approach is less suitable for type 2 diabetes.
Keywords: adolescents, children, insulin, oral anti-diabetics, type 1 diabetes, type 2 diabetes
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The incidence of both type 1 and type 2 diabetes is rising among children and adolescents.
Data on the prevalence of type 1 and type 2 diabetes are limited.
Routine clinical databases can be used to study the prevalence and epidemiology of treatment, depending on the completeness of data capture and their representativeness of the whole population.
WHAT THIS STUDY ADDS
The prevalence of insulin prescribing appears to vary among countries, being highest in Sweden (3.5 per 1000 population) and lowest in Italy (1.1 per 1000 population).
The prevalence of oral anti-diabetic prescribing ranges from 0.08 per 1000 population in Sweden and Germany to 0.21 per 1000 population in the UK; however, the total number of patients receiving oral anti-diabetics is low.
It is possible to use the same study protocol across clinical databases in Europe to study the prevalence of type 1 diabetes in children.
Routine clinical databases can identify the prevalence of type 1 diabetes prescribing and could be used to study the secular changes in disease prevalence in children.
Introduction
The number of people with diabetes is increasing due to several reasons [1], so the treatment and management of diabetes poses a major burden for healthcare systems worldwide [2]. The incidence of both type 1 and type 2 diabetes is also rising among children and adolescents [3, 4].
A recent analysis of 20 population-based diabetes registers in 17 European countries showed a yearly increase in the incidence of type 1 childhood diabetes, ranging from 0.6 to 9.3%, in all but two countries. The overall annual increase (age 0–14 years) was 3.9% [95% confidence interval (CI) 3.6–4.2]. The predicted annual number of new cases for 2020 is 24 400, with a doubling of numbers in children younger than 5 years and a more even distribution across age groups than at present [5].
However, data on the prevalence of type 1 and 2 diabetes in children and adolescents are limited. This information is particularly important from a health policy point of view because it will enable the monitoring of disease burden, which is directly related to the planning of healthcare resources.
Routine clinical databases are powerful instruments to study the prevalence and epidemiology of treatments. The data collection process is embedded into routine clinical practice, no additional ad hoc database has to be maintained and no additional human resources are required to keep records up to date [6].
Hsia et al. have previously studied the prevalence of type 1 and type 2 diabetes in children and adolescents in the UK using prescribing data in a routine clinical database [7]. The authors showed that the prevalence of children receiving insulin and oral anti-diabetic drugs for diabetes has increased two- and eightfold, respectively, between 1998 and 2005. The study by Hsia et al. demonstrated the potential of using existing routine clinical databases in studying the prevalence of diabetes. Overall, there is a considerable potential in using these routine databases in diabetes epidemiological studies across Europe.
The present study applied the same methodology used by Hsia et al. [7] to investigate the prescribing of anti-diabetics and thereby the prevalence of diabetes in children across seven European countries. It also serves as a proof-of-concept study to demonstrate the potential for collaboration between countries using different databases in diabetes research.
The aim of this study was to compare the one-year prevalence in 2008 of anti-diabetic drug prescribing (insulin and oral anti-diabetics) in children and adolescents aged 0–18 years using population-based clinical databases from seven European countries. Also, we wanted to investigate the potential of obtaining similar information from different clinical databases for paediatric diabetes research across countries.
Methods
Data source
We conducted a prevalence study using the following databases: IADB.nl database (The Netherlands), Gmuender ErsatzKasse (GEK; Germany), IMS Disease Analyser (UK), Norwegian Prescription Database (NorPD), National Prescription Register (Finland), the Lombardy region prescription database (Italy), prescription databases of Central and North Denmark regions and the Swedish Prescribed Drug Register (PDR, Sweden). All databases except the IMS Disease Analyser are population-based prescription databases. The IMS Disease Analyser is a patient record database.
A common study protocol was developed, considering the information available in each database. A standardized data specification was sent to each participating centre. Medications were classified according to the Anatomical Therapeutic Chemical (ATC) classification system [8].
From each database, the requested information comprised patient demographics and the number of patients receiving insulin and oral anti-diabetic drug prescriptions by ATC subtherapeutic level, sex and age group.
The following details describe each database at the time of study commencement.
UK: IMS Disease Analyser
The data from the UK were derived from the IMS Disease Analyser (IMS DA) [7, 9]. This database contains approximately 3 million anonymous patient records from about 570 general practitioners (GPs), representing about 5% of the UK population [6, 10]. Information held on the IMS DA includes patient demographics, indications for treatment and details of prescriptions issued in primary care.
The Netherlands
The IADB.nl database is a pharmacy prescription database from community pharmacies located in The Netherlands (http://www.iadb.nl).
Dutch patients usually register at a single pharmacy, and therefore an almost complete overview of drug history, excluding over-the-counter medications and in-hospital prescriptions, can be achieved. The database records longitudinal drug prescriptions records from more than 50 community pharmacies in The Netherlands, covering a population of 500 000 people since 1999. The number of children aged 0–18 years in this population was approximately 123 000 in 2008 [11, 12].
Germany
Data from Germany were derived from individual-level prescription data from the Gmuender ErsatzKasse (GEK), one of about 270 different statutory health insurance companies in Germany. More than 85% (70.2 million) of all German inhabitants (82 million) are enrolled in a statutory health insurance company. The GEK comprises 1.75 million members located in all regions of Germany. The data from the GEK are especially representative for children and youths [13, 14]. The data file for this analysis comprised 341 544 enrollees aged 0–18 years in 2008.
Sweden
Swedish data were derived from the Swedish Prescribed Drug Register (PDR, Sweden), which was established in 2005. The register contains data with unique patient identifiers for all dispensed prescriptions for the whole population of Sweden (9 million inhabitants). Patient-specific information, such as age, sex and place of living, is included in the register, which also contains information concerning dispensed substance, brand name, formulation, package size, amount, expenditure, reimbursement and prescriber's profession and practice. The register is complete for the entire population of the country (patient identity data are missing for <0.3% of all items) [15].
Norway
Norwegian data were retrieved from the Norwegian Prescription Database (NorPD) managed by the Norwegian Institute of Public Health (NIPH). The NorPD is a prescription database established in 2004, covering the entire nation of Norway (4.8 million inhabitants). The population aged 0–18 years covered was 1 133 758 in 2008.
The database contains information from all drugs prescribed (reimbursed or not) and dispensed at pharmacies to individual patients living outside institutions, i.e. ambulant care. All pharmacies in Norway (∼650) are obliged by law to report their data electronically every month to NIPH. Data include patient demographic data, drug information and details of the dispensing pharmacy and the prescriber. To date there are over 4.6 million individuals and more than 208 million prescriptions in the database [16].
Denmark
Danish data came from the regions of Central and North Denmark, with a combined population of 1.8 million people (approximately 30% of the entire Danish population). These regions are served by pharmacies equipped with electronic accounting systems used primarily to secure reimbursement from the Danish National Health Service, which refunds part of the cost of most prescribed medicines, including anti-diabetics. Prescription information, including the customer's civil registration number, the type and amount of drug prescribed and the date the drug was dispensed, is transferred from the pharmacies to a regional prescription database at Aarhus University. We used this database to identify all prescriptions for insulin or oral anti-diabetics for all patients aged 0–18 years.
A more detailed description of the prescription databases in the Nordic (Scandinavian) countries is published elsewhere [17]. All drugs in the Nordic Countries are classified according to the ATC classification system [8].
Italy
The data from Italy were retrieved from the Lombardy region prescription database, which stores all community (i.e. outside hospitals) prescriptions, reimbursed by the National Health Service (NHS) and issued to individuals living in the Lombardy region, in northern Italy. Information held on the Lombardy region prescription database includes patient demographics and prescription details.
Data were managed and analysed using an anonymous patient code. The study population was composed of 1 702 851 children and adolescents aged 0–18 years, male/female ratio 1.06, living in the Lombardy region. The study sample represented 15% of the Italian paediatric population [18–20].
Study population
The study population comprised all individuals aged 0–18 years who had received at least one prescription for insulin or an oral anti-diabetic drug between 1 January 2008 and 31 December 2008.
Anti-diabetic drugs were classified based on the WHO ATC [8] therapeutic level A10 (drugs used in diabetes) and stratified according to the subsequent levels as follows: A10A (insulins and analogues); and A10B (blood-glucose-lowering drugs, excluding insulins). In addition, the proportion of insulin analogues within the different insulin groups was examined. Table 1 shows the ATC codes used for individual drug classes. The age classification of the International Conference of Harmonization was modified in this study, and the age bands were stratified as follows: 0–1, 2–5, 6–11 and 12–18 years.
Table 1.
Anti-diabetic drugs by Anatomical Therapeutic Chemical (ATC) code and drug class
| ATC code | Drug name |
|---|---|
| Insulin | |
| A10AB | Insulins and analogues for injection, fast acting |
| A10AB01–A10AB03 | Insulin (human/beef/pork) |
| A10AB04–A10AB06 | Insulin analogues (lispro/aspart/glulisine) |
| A10AC | Insulins and analogues for injection, intermediate acting |
| A10AC01–A10AC03 | Insulin human/beef/pork |
| A10AC04 | Insulin analogue (lispro) |
| A10AD | Insulins and analogues for injection, intermediate acting combined with fast acting |
| A10AD01–A10AD03 | Insulin (human/beef/pork) |
| A10AD04–A10AD05 | Insulin analogues (lispro/aspart) |
| A10AE | Insulins and analogues for injection, long acting |
| A10AE01–A10AE03 | Insulin (human/beef/pork) |
| A10AE04–A10AE05 | Insulin glargine/detemir |
| Oral anti-diabetic drugs | |
| A10BA | Biguanides |
| A10BB | Sulfonamides, urea derivatives |
| A10BC | Sulfonamides (heterocyclic) |
| A10BD | Combinations of oral blood-glucose-lowering drugs |
| A10BF | α-Glucosidase inhibitors |
| A10BG | Thiazolidinediones |
| A10BH | Dipeptidyl peptidase 4 (DPP-4) inhibitors |
| A10BX | Other blood-glucose-lowering drugs, excluding insulins |
Data analysis
Prevalence was defined as the number of individuals with at least one prescription of anti-diabetic drugs divided by the total number of individuals aged 0–18 years within the study period. In the Scandinavian and Dutch prescription databases, prevalence was defined as the number of individuals with at least one prescription of anti-diabetic drugs divided by the total number of inhabitants aged 0–18 years as provided by the national statistics institutes. In the Italian database, the total number of individuals equalled the number of inhabitants in the Lombardi region of Italy. In the databases from Germany and the UK, the denominator was the total number of patients aged 0–18 years in the database. Data were stratified by age groups and country using the Poisson distribution with a 95% confidence interval. Statistical analyses were conducted using STATA/SE version 11.0 (Stata Corp., College Station, TX, USA).
Results
The total population comprised 5 893 657 children and adolescents aged 0–18 years in seven databases in 2008. Table 2 shows the distribution of anti-diabetic drug use in children and adolescents by anti-diabetic drug type, sex and country.
Table 2.
Characteristics of study subjects aged 0–18 years by anti-diabetic drug type and country in 2008
| UK | The Netherlands | Germany | Sweden | Denmark | Norway | Italy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | |
| Population (2008) | 102 168 | 98 412 | 57 749 | 56 685 | 175 006 | 166 538 | 1 047 446 | 992 407 | 184 819 | 175 818 | 581 074 | 552 684 | 877 389 | 825 462 |
| Insulin | 230 | 203 | 109 | 101 | 333 | 328 | 3 844 | 3 331 | 401 | 384 | 1 557 | 1 388 | 1 019 | 896 |
| Number of patients | ||||||||||||||
| A10AB | ||||||||||||||
| Fast-acting insulins and analogues for injection, n (%) | 186 (80.8) | 181 (89.1) | 104 (95.4) | 96 (95.0) | 327 (98.2) | 324 (98.7) | 3 804 (98.9) | 3 293 (98.8) | 392 (97.7) | 381 (99.2) | 1 549 (99.4) | 1 384 (99.7) | 978 (95.9) | 858 (95.7) |
| Proportion of insulin analogues, n (%) | 180 (96.7) | 175 (96.6) | 94 (90.3) | 83 (86.4) | 246 (75.2) | 262 (80.8) | 3 704 (99.7) | 3 279 (99.5) | 222 (56.6) | 216 (56.6) | 1 549 (100) | 1 384 (100) | 909 (92.9) | 798 (93.0) |
| A10AC | ||||||||||||||
| Intermediate-acting insulins and analogues for injection, n (%) | 24 (10.4) | 13 (6.4) | 0 (0) | 3 (3.1) | 145 (44.3) | 104 (32.1) | 252 (6.6) | 224 (6.7) | 62 (15.4) | 49 (12.7) | 712 (45.7) | 651 (46.9) | 243 (23.8) | 208 (23.2) |
| Proportion of insulin analogues, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 17 (27.4) | 14 (28.5) | 0 (0) | 0 (0) | 13 (5.3) | 9 (4.3) |
| A10AD | ||||||||||||||
| Intermediate-acting combined with fast-acting insulins and analogues for injection, n (%) | 94 (40.8) | 69 (33.9) | 4 (3.6) | 7 (6.9) | 8 (2.4) | 6 (1.8) | 59 (1.5) | 45 (1.3) | 130 (32.4) | 116 (30.2) | 27 (1.7) | 29 (2.1) | 234 (22.9) | 194 (21.6) |
| Proportion of insulin analogous, n (%) | 64 68.0 | 42 60.8 | 2 (50.0) | 4 (57.1) | 1 (12.5) | 0 (0) | 47 (80.0) | 43 (95.5) | 31 (23.8) | 19 (16.3) | 27 (100) | 29 (100) | 218 (93.1) | 183 (94.3) |
| A10AE | ||||||||||||||
| Long-acting insulins and analogues for injection, n (%) | 157 (68.2) | 151 (74.3) | 77 (70.6) | 56 (55.4) | 140 (42.0) | 153 (46.6) | 2 995 (77.9) | 2 532 (76.0) | 288 (71.8) | 322 (83.8) | 543 (34.8) | 504 (36.3) | 664 (65.1) | 607 (67.7) |
| Proportion of insulin analogues, n (%) | 157 (100) | 151 (100) | 77 (100) | 56 (100) | 140 (100) | 153 (100) | 2 995 (100) | 2 532 (100) | 131 (45.4) | 135 (41.9) | 543 (100) | 504 (100) | 664 (100) | 607 (100) |
| Oral anti-diabetic drugs | 8 | 34 | 5 | 8 | 8 | 18 | 60 | 101 | 7 | 28 | 25 | 81 | 145 | 194 |
| Number of patients | ||||||||||||||
| A10BA | ||||||||||||||
| Biguanides | 8 (100) | 34 (100) | 3 (60) | 5 (62.5) | 6 (75.0) | 17 (94.4) | 59 (98.3) | 99 (98.0) | 5 (71.4) | 24 (85.7) | 17 (68.0) | 73 (90.1) | 72 (49.6) | 127 (65.4) |
| A10BB and A10BC | ||||||||||||||
| Sulfonamides, urea derivatives and sulfonamides (heterocyclic) | 0 (0) | 0 (0) | 4 (80) | 3 (37.5) | 1 (12.5) | 0 (0) | 0 (0) | 5 (4.9) | 2 (28.5) | 6 (21.4) | 9 (36) | 12 (14.8) | 35 (24.1) | 36 (18.5) |
| Other oral anti-diabetic drugs* | 0 (0) | 0 (0) | 0 (0) | 1 (12.5) | 3 (37.5) | 2 (11.1) | 1 (1.6) | 4 (3.9) | 0 (0) | 1 (3.5) | 0 (0) | 2 (2.4) | 47 (32.4) | 44 (22.6) |
Other oral anti-diabetic drugs includes A10BD (combinations of oral blood-glucose-lowering drugs), A10BF (α-glucosidase inhibitors), A10BG (thiazolidinediones), A10BH (dipeptidyl peptidase 4 inhibitors) and A10BX (other blood-glucose-lowering drugs, excluding insulins).
Insulin prescribing
The overall prevalence of insulin prescribing varied between 1.1 per 1000 population (95% CI 1.1–1.2) and 3.5 per 1000 population (95% CI 3.4–3.6). In 2008 the prevalence of insulin prescribing in children and adolescents aged 0–18 years was highest in Sweden (3.5 per 1000 population), followed by Norway (2.6 per 1000 population) and Denmark (2.2 per 1000 population), and was lowest in Italy (1.1 per 1000 population; Figure 1). The prevalence of insulin prescribing in Sweden was about three times that in Italy. The prescribing of insulin was slightly higher in boys than in girls in five countries (The Netherlands, Norway, UK, Italy and Sweden), but not in Denmark and Germany.
Figure 1.
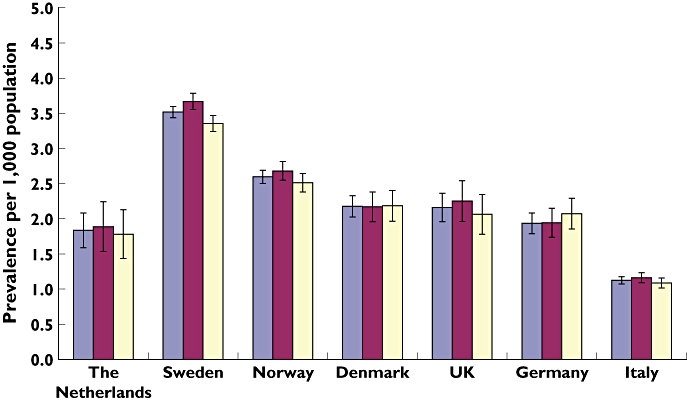
Prevalence of insulin prescribing in children and adolescents aged 0–18 years with 95% confidence interval from seven European countries. Overall ( ); boys (
); boys ( ); girls (
); girls ( )
)
In all countries apart from the UK, between 95 and 100% of patients received fast-acting insulin. In the UK, 80% of boys and 89% of girls received fast-acting insulin, respectively. Long-acting insulins were received by more than 50% of patients in all countries except Germany and Norway, where only 42 and 35% of boys and 47 and 36% of girls received long-acting insulins, respectively.
More than 90% of fast-acting insulin prescriptions were analogues in all countries except in Germany (75% in boys and 80% in girls) and in Denmark (56% in girls and boys). One hundred per cent of long-acting insulin prescriptions were analogues in all countries except Denmark, 45.4% in boys and 41.9% in girls, respectively (Table 2).
With respect to intermediate-acting insulins, fixed combinations with fast-acting insulin (A10AD) were prescribed more commonly in Italy, Denmark and the UK, whereas in Norway more single intermediate-acting insulins were prescribed (Table 2).
Oral anti-diabetics
The prevalence of oral anti-diabetic prescribing ranged from 0.08 per 1000 population in Sweden (95% CI 0.06–0.09) and Germany (95% CI 0.04–0.10) to 0.21 per 1000 population (95% CI 0.15–0.27) in the UK (Figure 2). Biguanides (A10BA) were favoured over other oral anti-diabetic drug types; metformin was the most commonly prescribed oral anti-diabetic drug in all countries (Table 2). The prevalence of oral anti-diabetic prescribing was higher in girls in all seven countries, in particular the UK (0.36 per 1000 population) and Italy (0.24 per 1000 population; Figure 2).
Figure 2.
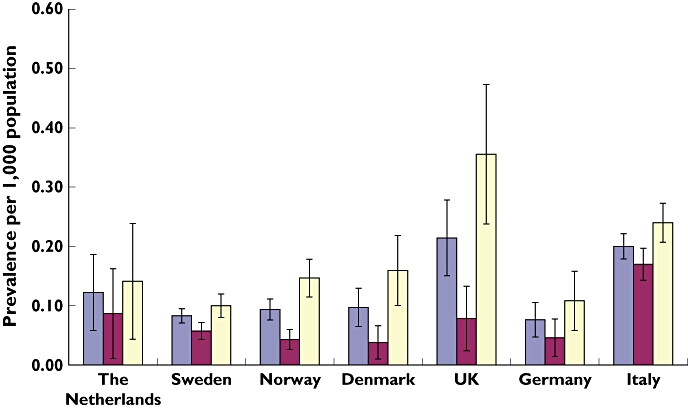
Prevalence of oral anti-diabetic drug prescribing in children and adolescents aged 0–18 years by sex and country with 95% confidence interval from seven European countries. Overall ( ); boys (
); boys ( ); girls (
); girls ( )
)
With respect to age, the prevalence of oral anti-diabetic prescribing was highest in those aged 12–18 years in six countries (Sweden, Norway, Denmark, UK, Italy and Germany), with the UK being the country with the highest prevalence (0.47 per 1000 population).
In The Netherlands, the prescribing of oral anti-diabetics was highest in those aged 6–11 years. The use of oral anti-diabetics in children aged less than 6 years was extremely low in all countries except Italy (Figure 3).
Figure 3.
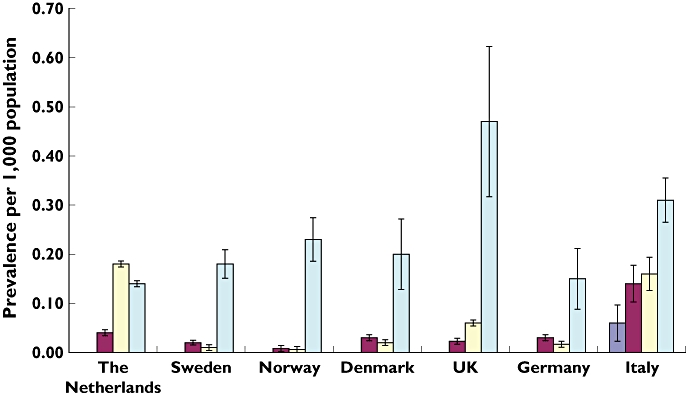
Prevalence of oral anti-diabetic drug prescribing by age groups and country with 95% confidence interval in seven European countries. Aged 0–1 ( ); aged 2–5 (
); aged 2–5 ( ); aged 6–11 (
); aged 6–11 ( ); aged 12–18 (
); aged 12–18 ( )
)
Discussion
In this study, among 5.8 million children and adolescents in seven different European countries, we found that there is a variation in the prescribing of anti-diabetic drugs among countries, indicating differences in the prevalence of type 1 diabetes and variations in the use of oral anti-diabetics.
We were unable to identify data on prevalence from the literature to compare our results. However, the EURODIAB registers previously reported substantial variations in incidence (new cases) of childhood type 1 diabetes among countries ranging from 10.3 to 52.6 per 100 000 persons [5].
One limitation of this study is the variation in the denominators used for the different databases. While the Scandinavian databases (Denmark, Sweden and Norway) used prescriptions issued to the entire population and the number of inhabitants as provided by their national institutes of statistics, the prescription databases from Germany and Italy used the number of individuals captured in their database. Likewise, the clinical patient database from the UK used all individuals registered with the GPs contributing to the database. This means the latter databases only looked at subpopulations, whereas the Scandinavian databases looked at their entire population, which may have caused a slight shift of the data from the non-Scandinavian databases.
For the IMS DA, a very good correlation between the database population and the UK population in terms of age and male-to-female ratio has been reported. The panel of GPs is broadly representative of the UK population, although there is under-representation of smaller practices and of practices in Scotland and Northern Ireland, and there is a slight over-representation of younger doctors [10]. The health insurance data from Germany have been found to be particularly representative for children and youths [14].
Differences in the source of data among databases may also have an impact on the comparability of the data.
A limitation of data from health insurance claim databases (e.g. from Germany and Italy) is that only prescriptions reimbursed by the national health systems are included. Private prescriptions and over-the-counter drugs are not included. Nevertheless, the proportion of privately insured patients in Germany, for instance, is only 9%. The same applies for Italy. With respect to prescribing status, insulin and oral antidiabetics are ‘prescription only medicine’ in all countries. Therefore, the exclusion of over-the-counter products in claim databases is not relevant.
Nevertheless, all databases have previously been used in research and represented data from their country of origin [6, 7, 11–13, 18, 20].
Our study showed the highest prevalence of insulin prescribing in Sweden (3.52 per 1000 individuals), followed by Norway (2.6 per 1000 population), Denmark (2.2 per 1000 population) and the UK (2.1 per 1000 population). Prevalences in the latter two countries were similar, whereas in Germany (1.9 per 1000 population) it was somewhat lower and in Italy it was lowest (1.1 per 1000 population).
The pattern of these findings is similar to the results reported by Patterson et al. [5]. Although Patterson et al. reported on the incidence, highest numbers were seen in Sweden, followed by Norway, Denmark, UK and Germany. Furthermore, the data reported by Patterson et al. indicated a north–south gradient, i.e. incidences were highest in the northern coutnries and lower in southern countries. This is in line with our data, where highest rates were found in Scandinavia and lowest in Italy.
Another explanation for these between-country differences is a relationship between genetic disposition and environmental factors and the occurrence of type 1 diabetes [21].
Incidence rates for type 1 diabetes in Italy vary between 6.1 and 34.0 per 100 000 per year, which has been attributed to the genetic heterogeneity in the Mediterranean area [21, 22]. The Sardinia region has one of the highest incidence rates of type 1 diabetes in the world, with incidence rates about threefold higher than in the Liguria region, which has the second highest overall incidence in Italy [22]. However, we used data from one of the regions with the lowest incidence of type 1 diabetes [22, 23]. This may explain why Italy presents with the lowest prevalence of anti-diabetic prescribing in our study population.
In addition, different prescribing practices and different diagnostic criteria before treatment initiation may cause variations in anti-diabetes drug prescribing between countries. However, with respect to treatment of type 1 diabetes, the impact of these factors is rather small because the only treatment option for type 1 diabetes is substitution therapy with insulin. There may be different criteria when treatment is initiated, but this is mainly a question of time because these patients always will need insulin at some point.
To our knowledge, this is the first study to investigate anti-diabetic drug use in the paediatric population in seven different countries across Europe. We have shown that there is a high use of insulin analogues in the paediatric population. Analogues are relatively new to the market and have been deemed to be particularly suitable for the paediatric and adolescent population. Our data show that this seems to have been put into practice. However, analogues have also been heavily marketed, which may have influenced prescribing practices [24].
This study has further demonstrated the potential to use information from several European clinical databases for paediatric research, as proposed by Neubert et al. [6]. We have shown that routine clinical databases can be used to monitor the prevalence of diabetes. Despite the differences in prevalences which we observed and in line with previously published data, at least in children insulin prescribing is a good surrogate for type 1 diabetes [7]. The results of the present study clearly demonstrate that it would be feasible to utilize these databases as an automated system to study the prevalence of type 1 diabetes in children.
This is not so much the case with respect to the use of oral anti-diabetic drugs. The number of patients receiving oral anti-diabetics is very low, i.e. 722 of 5.8 million children (0.012%). The UK stood out with the highest prevalence of oral anti-diabetics (0.36 per 1000), followed by Italy (0.24 per 1000). The most frequently prescribed oral anti-diabetic in all countries was metformin. Metformin is currently licensed in most countries for the treatment of type 2 diabetes in children above 10 years of age; however, it is also increasingly used off label for the treatment of polycystic ovarian syndrome and obesity [25, 26]. Hsia et al. were able to estimate the prevalence of type 2 diabetes in children based on the indication for the prescriptions and found a high proportion of oral anti-diabetics used for PCOS [7]. However, in our present study, not all databases record the indications for the prescription; hence, we were unable to estimate the prevalence of children receiving oral anti-diabetics for the treatment of type 2 diabetes. Consequently, some of our routine prescribing databases are less suitable for the assessment of type 2 diabetes.
Conclusion and future research
Our study confirms previous findings on varying frequency of type 1 diabetes in children and adolescents using prescribed insulin as a proxy for type 1 diabetes.
We demonstrated that it is possible to use the same study protocol across clinical databases in seven different EU countries to study type 1 diabetes in children. Owing to a lack of indications in some of the databases and as oral anti-diabetics can be used off label for other indications, using them to study prevalence of type 2 diabetes is less appropriate because they are not such a good proxy as insulin is for type 1 diabetes.
As the next step, an automated long-term tracking system to monitor the prevalence of type 1 diabetes in children could be developed; however, further research is still needed for healthcare systems to benefit from this. Surely, another step would be to refine the techniques in order to identify annual incidence as well as prevalence.
Acknowledgments
The authors would like to thank Andrejs Leimanis at the National Board of Health and Welfare in Sweden and Ulrika Undén at the Centre for Pharmacoepidemiology, Karolinska Institutet, Sweden for their support. We also thank the biostatistician Rikke Bech Nielsen at the Department of Clinical Epidemiology, Aarhus University Hospital, Denmark for retrieving all Danish data and Nienke van Rein for doing the Dutch analyses. Also, the authors would like to acknowledge Dr Angela Bortolotti, Dr Luca Merlino and Dr Ida Fortino, of the Regional Health Ministry, Lombardy Region, Milan, for providing data (EPIFARM project) and Dr Marco Sequi for helping with the data extraction. Finally, we would like to thank Peter Stephens, who provided us with the IMS data.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–61. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 4.Haines L, Wan KC, Lynn R, Barrett TG, Shield JP. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30:1097–101. doi: 10.2337/dc06-1813. [DOI] [PubMed] [Google Scholar]
- 5.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 6.Neubert A, Sturkenboom MC, Murray ML, Verhamme KM, Nicolosi A, Giaquinto C, Ceci A, Wong IC. Databases for pediatric medicine research in Europe – assessment and critical appraisal. Pharmacoepidemiol Drug Saf. 2008;17:1155–67. doi: 10.1002/pds.1661. [DOI] [PubMed] [Google Scholar]
- 7.Hsia Y, Neubert AC, Rani F, Viner RM, Hindmarsh PC, Wong IC. An increase in the prevalence of type 1 and 2 diabetes in children and adolescents: results from prescription data from a UK general practice database. Br J Clin Pharmacol. 2009;67:242–9. doi: 10.1111/j.1365-2125.2008.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. Norwegian Institute of Public Health. Oslo, Norway: Norwegian Institute of Public Health; 2009. Internet. [Google Scholar]
- 9.Sturkenboom MC, Verhamme KM, Nicolosi A, Murray ML, Neubert A, Caudri D, Picelli G, Sen EF, Giaquinto C, Cantarutti L, Baiardi P, Felisi MG, Ceci A, Wong IC. Drug use in children: cohort study in three European countries. BMJ. 2008;337:a2245. doi: 10.1136/bmj.a2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong IC, Murray ML. The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol. 2005;59:750–5. doi: 10.1111/j.1365-2125.2005.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT. Pharmacy data in epidemiological studies: an easy to obtain and reliable tool. Pharmacoepidemiol Drug Saf. 2002;11:379–84. doi: 10.1002/pds.722. [DOI] [PubMed] [Google Scholar]
- 12.Schirm E, Monster TB, de Vries R, van den Berg PB, de Jong-van den Berg LT, Tobi H. How to estimate the population that is covered by community pharmacies? An evaluation of two methods using drug utilisation information. Pharmacoepidemiol Drug Saf. 2004;13:173–9. doi: 10.1002/pds.882. [DOI] [PubMed] [Google Scholar]
- 13.Fegert JM, Kolch M, Zito JM, Glaeske G, Janhsen K. Antidepressant use in children and adolescents in Germany. J Child Adolesc Psychopharmacol. 2006;16:197–206. doi: 10.1089/cap.2006.16.197. [DOI] [PubMed] [Google Scholar]
- 14.Janhsen K. [Public health research with statutory health insurance drug data] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004;47:521–5. doi: 10.1007/s00103-004-0836-1. [DOI] [PubMed] [Google Scholar]
- 15.Wettermark B, Hammar N, Fored CM, Leimanis A, Olausson P, Bergman U, Persson I, Sundstrom A, Westerholm B, Rosen M. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months 4. Pharmacoepidemiol Drug Saf. 2007;16:726–35. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 16.Furu K. Establishment of the nationwide Norwegian Prescription Database (NorPD) – New opportunities for research in pharmacoepidemiology in Norway. Norsk Epidemiol. 2008;18:129–36. [Google Scholar]
- 17.Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106:86–94. doi: 10.1111/j.1742-7843.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 18.Clavenna A, Sequi M, Bortolotti A, Merlino L, Fortino I, Bonati M. Determinants of the drug utilization profile in the paediatric population in Italy's Lombardy Region. Br J Clin Pharmacol. 2009;67:565–71. doi: 10.1111/j.1365-2125.2009.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavenna A, Sequi M, Bonati M. Drug prescribing by Italian family paediatricians: an exception? Acta Paediatr. 2010;99:754–7. doi: 10.1111/j.1651-2227.2010.01691.x. [DOI] [PubMed] [Google Scholar]
- 20.Clavenna A, Sequi M, Bonati M. Differences in the drug prescriptions to children by Italian paediatricians and general practitioners. Eur J Clin Pharmacol. 2010;66:519–24. doi: 10.1007/s00228-010-0786-5. [DOI] [PubMed] [Google Scholar]
- 21.Ehehalt S, Popovic P, Muntoni S, Willasch A, Hub R, Ranke MB, Neu A, DIARY Group Baden Wuerttemberg Incidence of diabetes mellitus among children of Italian migrants substantiates the role of genetic factors in the pathogenesis of type 1 diabetes. Eur J Pediatr. 2009;168:613–7. doi: 10.1007/s00431-008-0808-9. [DOI] [PubMed] [Google Scholar]
- 22.Muntoni S, Muntoni S. New Insights into the Epidemiology of Type 1 Diabetes in Mediterranean Countries. Diabtes Metab Res Rev. 1999;15:133–40. doi: 10.1002/(sici)1520-7560(199903/04)15:2<133::aid-dmrr20>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Green A, Gale EA, Patterson CC. Incidence of childhood-onset insulin-dependent diabetes mellitus: the EURODIAB ACE Study. Lancet. 1992;339:905–9. doi: 10.1016/0140-6736(92)90938-y. [DOI] [PubMed] [Google Scholar]
- 24.Owens DR, Zinman B, Bolli GB. Insulins today and beyond. Lancet. 2001;358:739–46. doi: 10.1016/S0140-6736(01)05842-1. [DOI] [PubMed] [Google Scholar]
- 25.Mastorakos G, Lambrinoudaki I, Creatsas G. Polycystic ovary syndrome in adolescents: current and future treatment options. Paediatr Drugs. 2006;8:311–8. doi: 10.2165/00148581-200608050-00004. [DOI] [PubMed] [Google Scholar]
- 26.Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care. 2009;32:1743–5. doi: 10.2337/dc09-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]


