Abstract
AIMS
To evaluate whether rescinding the prior authorization (PA) requirement (managerial pre-approval) for losartan in an health maintenance organization (HMO) could reduce prescribing of the more expensive angiotensin receptor blockers (ARBs).
METHODS
HMO physicians were notified that losartan would no longer require PA, and appropriate changes were made to the electronic prescribing computer program. The monthly distribution by drug of the number of prescriptions for ARBs dispensed for new patients was calculated before and after the policy change from data captured from electronic records. The proportion of patients (percentage and 95% confidence interval) treated with losartan who met the criteria for treatment with ARBs (hypertension or cardiac insufficiency in patients who have developed adverse effects in response to angiotensin-converting enzyme inhibitors or macroproteinuria) during the first month after the PA requirement was rescinded was calculated.
RESULTS
The total number of PA requests for ARBs declined by 48.6% from 961 in December 2008, the month before the policy change, to 494 the following January, rising again to 651 during January 2010. Prescription incidence changed from 121 to 255 patients treated per month (114% increase) for losartan, from 15 to 16 (6.7% increase) for candesartan, and from 89 to 71 (20.2% decrease) for valsartan. The duration of effect for decrease in ARB requests for the more expensive drugs was approximately 1 year. Only 23.3% (95% confidence interval 18.1–28.4) of patients receiving losartan met the criteria for receiving ARBs.
CONCLUSIONS
Rescinding the PA requirement for this drug alone was an effective limited-duration strategy for reduction of prescription of relatively expensive drugs.
Keywords: angiotensin receptor blocker, drug utilization analysis, managed care, managerial experiment, prior authorization
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The utility of a prior authorization (PA) requirement for curtailing the prescription of expensive drugs and improving quality of care has been well substantiated. Although studies have evaluated changes in prescribing behaviour after revocation of a PA requirement, the effectiveness of selective revocation for the first drug within a class to go off patent as an incentive to reduce prescription of the more expensive drugs has not been studied.
WHAT THIS STUDY ADDS
Rescinding the PA requirement for a generic drug alone within a pharmacological category upon its introduction into the market is a successful managerial strategy for reduction of prescription of the more expensive drugs still on patent in that class. The observed duration of effect was approximately 1 year.
Introduction
Inappropriate use of drug resources exacerbates the problem of skyrocketing drug expenditure, often without contributing to improved patient outcomes. Prior authorization (PA), the managerial technique requiring physicians to obtain pre-approval for drugs as a prerequisite for insurer or health maintenance organization (HMO) coverage, is increasingly being implemented to improve prescribing precision and to limit unnecessary utilization of drugs [1, 2]. While the PA rejection rate has been observed to be relatively small (4.4%), it has been suggested that PA may generate a ‘sentinel effect’[2], the ‘… decrease in services given by providers as a result of having a utilization reviewer keep tabs of them’[3] and/or a ‘hassle effect’, the decrease in services given by providers due to unwillingness to deal with annoying paperwork and bureaucratic nuisances [4].
Angiotensin receptor blockers (ARBs) are a relatively costly class of drugs commonly used to assist in lowering elevated blood pressure (BP). A Cochrane review has been published evaluating how much this class of drugs lowers BP and whether there is a difference between individual drugs within this class. The clinical trials included in this review evaluated the BP-lowering ability of nine different ARBs in 13 451 participants who were followed for approximately 7 weeks. No ARB appeared to be any better or worse in terms of BP-lowering ability, and most of the BP-lowering effect occurred at the starting doses of these drugs. Owing to lack of reporting and the short duration of these trials, this review did not provide a good estimate of the harm associated with this class of drugs [5].
Since the introduction of ARBs into the Israeli market in 2001, Leumit Health Services (LHS), a Managed Care Organization in Israel, like other health plans [6] has enforced a prior PA requirement for these drugs. Approval criteria are as follows: hypertension or cardiac insufficiency in patients who have developed adverse effects in response to angiotensin-converting enzyme inhibitors (ACE-Is); combination therapy with ACE-Is with proteinuria measured to be 1 g of protein in urine in 24 h; or in patients with proteinuria measured between 30 mg day−1 and 1 g day−1 in patients without hypertension or cardiac insufficiency with adverse effects or intolerance of ACE-Is.
The first ARB to present with generic products was losartan. This markedly reduced its cost, rendering the cost of the two remaining patented products available in Israel, valsartan and candesartan, to be considerably higher. This was significant to the LHS because at that time ∼50% of the PA requests for ARBs were for valsartan. Accordingly, strategies were sought to influence physicians to prefer prescription of the less expensive drug. With this goal in mind, we designed a novel experiment in which the PA requirement was rescinded for losartan while being left unchanged for valsartan and candesartan. The purpose of this study was to evaluate the effectiveness of a new managerial strategy in modifying physician preferences in a common scenario where the first drug in a therapeutic class goes off patent. Our research hypothesis was that eliminating the ‘sentinel’ and ‘hassle’ effects for only one drug within a class can incentivize physicians to prefer prescription of this product over the more expensive yet therapeutically equivalent alternatives for which these barriers were still intact. All physicians practicing in ‘Leumit’ were notified of the policy change during the month of December 2008. The new policy was implemented on 1 January 2009.
Methods
Data were electronically captured from LHS information systems. The LHS has implemented a centralized, on-line PA process, which operates under the fund's electronic patient-record (EPR) program. Information available for review includes the following: drugs prescribed and dispensed; laboratory tests ordered and results; and history of PA requests. The EPR data were retrieved in large databases tailored to facilitate analyses of trends in PA requests and utilization of ARBs before and after the policy change under evaluation and calculated via electronic database queries. To evaluate whether the change in policy alleviated the workload on PA authorization officers, the number of PA requests per month for ARBs was calculated for the 1 year period before and 15 months after the policy change. Trends in prescribing patterns for patients in LHS were evaluated by calculating the distributions of the number of new patients for whom a prescription was dispensed for each ARB each month during the 1 year period before and 15 months after the policy change. To evaluate physician adherence to criteria for prescription of losartan after PA had been revoked, an EPR chart review was conducted for all patients for whom losartan was dispensed during the first month after the PA requirement was rescinded to calculate what percentage of these patients were eligible to receive the drug and the rate of use of the drug as first-line therapy for treatment.
Results
The total number of PA requests for ARBs declined by 48.6% from 961 in December 2008, the month before the policy change, to 494 the following January, rising again to 651 during January 2010. Requests for valsartan diminished by 22.4%, from 474 to 368, while those for candesartan dropped by 7.4%, from 136 to 126 (Figure 1). The number of new patients receiving ARBs changed from 121 to 255 (114% increase) for losartan and from 15 to 16 (6.7% increase) for candesartan, while incident use of valsartan decreased by 20.2%, from 89 patients not previously treated with ARBs in December 2008 to 71 the following January (Figure 2). The chart review revealed that of the 258 new patients treated with losartan during January 2009, only 60 [23.3%, 95% confidence interval (CI) 18.1–28.4] met the criteria for receiving the drug, while all other patients would have been denied treatment had the PA requirement still been enforced. Although 199 (77.1%, 95% CI 72–82.3) of these patients had been previously treated with ACE-Is, only 56 (21.7%, 95% CI 16.6–26.8) were found to have adverse effects for the drug recorded in their EPR, while laboratory data recorded showed that an additional four patients (1.6%, 95% CI 0–3.1) had macroproteinuria. Of these 258 patients, 11 (4.3%, 95% CI 1.8–6.8) had no record of any previous treatment with antihypertensive medications in their EPR.
Figure 1.
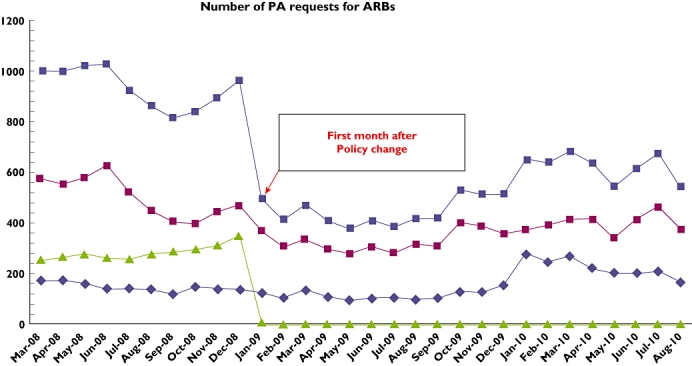
Incidence of PA requests for ARBs. Candesartan ( ); valsartan (
); valsartan ( ); losartan (
); losartan ( ); total (
); total ( )
)
Figure 2.
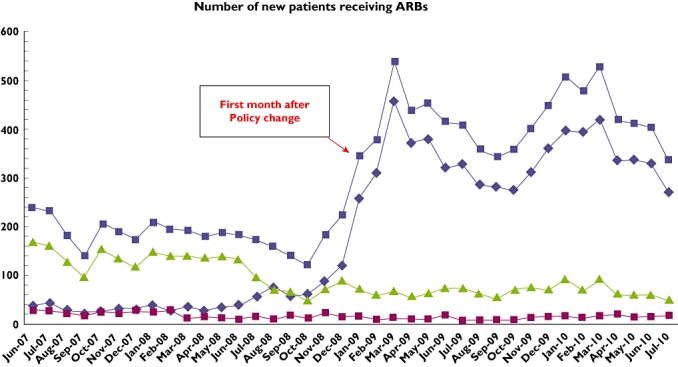
Incidence of new patients receiving ARBs. Losartan ( ); candesartan (
); candesartan ( ); valsartan (
); valsartan ( ); total (
); total ( )
)
Discussion
The 22.4% decrease in PA requests and 20.2% decrease in monthly incident utilization of valsartan observed in this managerial experiment demonstrate that rescinding the PA requirement for generic losartan was successful in partially reducing prescription of the more expensive drug in this pharmacological family. These abrupt changes in prescription patterns observed immediately after the policy change illustrate how, as hypothesized, selective implementation of the ‘sentinel’ and/or ‘hassle’ effects can incentivize physicians to prefer prescription of a less expensive yet equally effective drug. These findings are particularly significant because they indicate that the managerial incentive evaluated in this study was capable of overcoming the aggressive marketing efforts implemented by drug companies during this period to promote the two drugs still on patent for approximately 1 year. However, although these conclusions are limited to situations where evidence is lacking for preference of one agent over another within a drug class, they may be generalizable to other primary care settings internationally that implement PA restrictions.
A novel component and strength of this study is the introduction of number of PA requests and distribution of requests within a therapeutic category as new variables for physician prescribing behaviour analyses. Under PA restrictions, dispensing data will not accurately reflect physician preferences for choice of drug because the PA barrier curtails physician volition and autonomy for resource utilization. Accordingly, this new metric becomes increasingly informative as PA rejection rates rise and consequently, observed utilization patterns underestimate real physician preferences for drug use. We therefore suggest that analysis of PA request prevalence should be incorporated into routine physician prescribing surveillance.
The chart analysis performed on the patients receiving losartan during the first month after repealing the PA requirement indicates that significant knowledge gaps and misconceptions exist within this physician population regarding the proper use of ARBs. Our finding that less than one in four of these patients met the labelled indications suggests that the years of PA were ineffective in educating physicians concerning the proper place for these drugs in pharmacotherapy regimens. However, the validity of the post-PA period EMR data may be limited because physicians were no longer compelled to document adverse effects as a requirement for PA approval. We therefore cannot rule out the possibility that a proportion of these patients may have been eligible to receive the drug. These findings are noteworthy because they relate to a generic drug which had not been directly promoted to physicians for quite some time. The prescribing behaviour observed amongst these physicians may indicate residual effects of aggressive marketing campaigns for ARBs, which have instilled within physicians erroneous perceptions concerning the superiority of these products over other antihypertensive agents. The 4.3% of these patients found to be treated first line with losartan supports this premise. Accordingly, these findings substantiate that implementing the PA requirement for these drugs was indeed warranted. Furthermore, these findings demonstrate that similar policy changes in the future should be accompanied by continuing education efforts designed to reduce knowledge gaps that may exist within the target physician population concerning evidence-based therapy with the drugs in question.
Acknowledgments
Portions of the findings of this study were presented at the International Society for Pharmacoeconomics and Outcomes Research 14th International Meeting, May 16–20, 2009, Orlando, FL, USA, and The Israel National Institute for Health Policy and Health Services Research 8th Annual Conference, December 22, 2010, Tel-Aviv, Israel. The authors wish to thank Professor Robert Aumann of the Hebrew University of Jerusalem Center for the Study of Rationality for his interesting comments on the results of this study.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.MacKinnon NJ, Kumar R. Prior authorization programs: a critical review of the literature. J Manag Care Pharm. 2001;4:297–302. [Google Scholar]
- 2.LaPensee KT. Analysis of a prescription drug prior authorization program in a Medicaid health maintenance organization. J Manag Care Pharm. 2003;9:36–44. doi: 10.18553/jmcp.2003.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koike A, Klap R, Unützer J. Utilization management in a large managed behavioral health organization. Psychiatr Serv. 2000;51:621–6. doi: 10.1176/appi.ps.51.5.621. [DOI] [PubMed] [Google Scholar]
- 4.Soumerai SB. Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Aff. 2004;23:135–46. doi: 10.1377/hlthaff.23.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD003822.pub2. CD003822. DOI: 10.1002/14651858.CD003822.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer MA, Choudhry NK, Winkelmeyer WC. Impact of Medicaid prior authorization on angiotensin-receptor blockers: can policy promote rational prescribing? Health Aff. 2007;26:800–7. doi: 10.1377/hlthaff.26.3.800. [DOI] [PubMed] [Google Scholar]


