While the majority of subjects experienced mild transient symptoms such as dizziness, paresthesia, euphoria, and hypoesthesia, a detailed assessment of vital signs, electrocardiograms, physical and neurologic examination results, and laboratory test results showed that inhalation of multiple 1-L volumes of undiluted hyperpolarized 129Xe was well tolerated in healthy subjects and those with mild or moderate chronic obstructive pulmonary disease.
Abstract
Purpose:
To evaluate the safety and tolerability of inhaling multiple 1-L volumes of undiluted hyperpolarized xenon 129 (129Xe) followed by up to a 16-second breath hold and magnetic resonance (MR) imaging.
Materials and Methods:
This study was approved by the institutional review board and was HIPAA compliant. Written informed consent was obtained. Forty-four subjects (19 men, 25 women; mean age, 46.1 years ± 18.8 [standard deviation]) were enrolled, consisting of 24 healthy volunteers, 10 patients with chronic obstructive pulmonary disease (COPD), and 10 age-matched control subjects. All subjects received three or four 1-L volumes of undiluted hyperpolarized 129Xe, followed by breath-hold MR imaging. Oxygen saturation, heart rate and rhythm, and blood pressure were continuously monitored. These parameters, along with respiratory rate and subjective symptoms, were assessed after each dose. Subjects’ serum biochemistry and hematology were recorded at screening and at 24-hour follow-up. A 12-lead electrocardiogram (ECG) was obtained at these times and also within 2 hours prior to and 1 hour after 129Xe MR imaging. Xenon-related symptoms were evaluated for relationship to subject group by using a χ2 test and to subject age by using logistic regression. Changes in vital signs were tested for significance across subject group and time by using a repeated-measures multivariate analysis of variance test.
Results:
The 44 subjects tolerated all xenon inhalations, no subjects withdrew, and no serious adverse events occurred. No significant changes in vital signs (P > .27) were observed, and no subjects exhibited changes in laboratory test or ECG results at follow-up that were deemed clinically important or required intervention. Most subjects (91%) did experience transient xenon-related symptoms, most commonly dizziness (59%), paresthesia (34%), euphoria (30%), and hypoesthesia (30%). All symptoms resolved without clinical intervention in 1.6 minutes ± 0.9.
Conclusion:
Inhalation of hyperpolarized 129Xe is well tolerated in healthy subjects and in those with mild or moderate COPD. Subjects do experience mild, transient, xenon-related symptoms, consistent with its known anesthetic properties.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11102172/-/DC1
Introduction
The field of hyperpolarized gas magnetic resonance (MR) imaging was introduced by using the isotope xenon 129 (129Xe) (1), and the first human studies with hyperpolarized 129Xe were reported by Mugler et al in 1997 (2). However, the relatively low available 129Xe polarization (1%–2%) generated only modest image quality, and this, combined with early recognition that 129Xe would be regulated as a drug, diminished enthusiasm for this agent. As a result, the field transitioned to using helium 3 (3He), which offered a simpler and more mature polarization technology (3), a large magnetic moment, and absence of physiologic effect. Hyperpolarized 3He MR imaging entered clinical research in 1996 (4,5), expanded to multicenter studies (6), has shown significant correlation to conventional techniques (eg, spirometry, diffusing capacity, radiography) (7–10), and has enabled longitudinal studies by virtue of its noninvasive nature (11). Recent years have seen the addition of numerous contrast mechanisms, including the apparent diffusion coefficient as a sensitive marker of emphysema (7,12). Considerable experience at numerous institutions has proved hyperpolarized 3He MR imaging to be safe, not only in healthy subjects, but also in subjects with obstructive airflow (13). Unfortunately, dissemination of 3He MR imaging faces challenges given the constrained supply of 3He and recent increased 3He use for homeland security applications, which has driven up costs from approximately $100 per liter to approximately $500 per liter (14). Therefore, it is now recognized that development of imaging with the more readily abundant isotope 129Xe should be pursued (15,16).
Recent progress in 129Xe polarization technology (17) led Patz and co-workers (18,19) to reintroduce 129Xe MR imaging in humans with promising results. Although these studies reported no adverse events, they were not specifically designed to assess the safety and tolerability of hyperpolarized 129Xe. Xenon, unlike 3He, is soluble in blood and tissues, and a fraction of the inhaled gas leaves the lungs and can have systemic effects, including anesthesia (20). Although xenon has a long history of safe use as a contrast agent in x-ray computed tomographic (CT) studies (21), a dedicated study was deemed necessary to rigorously document the effects of inhaled xenon as used for MR imaging. Hence, the purpose of this study was to evaluate the safety and tolerability of inhaling multiple 1-L volumes of undiluted hyperpolarized 129Xe followed by up to a 16-second breath hold.
Materials and Methods
This prospective, phase I, nonrandomized study was approved by the institutional review board. Written informed consent was obtained from all participants. This study was Health Insurance Portability and Accountability Act compliant. The trial was conducted under an investigational new drug application to the U.S. Food and Drug Administration held by GE Healthcare. The study was supported by GE Healthcare through an equipment loan, supplying of 129Xe gas, and funding. Control and presentation of the data in this article remained with the authors who were not employed by GE Healthcare.
Research Subjects
Between October 2008 and February 2010, a total of 44 subjects (mean age, 46.1 years ± 18.8 [standard deviation]) were enrolled according to the inclusion and exclusion criteria detailed in Table 1. The subjects included 25 women (mean age, 42.1 years ± 17.6) and 19 men (mean age, 51.2 years ± 19.6). The first consecutive 24 subjects were healthy volunteers (16 women [mean age, 31.7 years ± 10.7] and eight men [mean age, 32.7 years ± 12.7]) who participated in the technical run-in phase of the trial. The run-in phase was deliberately conducted slowly to permit time to establish optimal technical imaging parameters for 129Xe ventilation, apparent diffusion coefficient (22), and 129Xe distribution in the dissolved phase (15). This was followed by an efficacy phase with 10 subjects with chronic obstructive pulmonary disease (COPD) (three women [mean age, 62.7 years ± 9.5] and seven men [mean age, 67.1 years ± 4.9]) and 10 age-matched control subjects (six women [mean age, 61.5 years ± 7.9] and four men [mean age, 65.0 years ± 7.9]). The subjects with COPD were characterized by Global Initiative for Obstructive Lung Disease criteria as stage I (n = 1), II (n = 8), and III (n = 1). Detailed pulmonary function data for the subjects with COPD and age-matched control subjects are provided in Kaushik et al (22). All 44 individuals were assessed identically for safety and tolerability after inhalation of hyperpolarized 129Xe, as described below. Subject demographic characteristics are summarized in Table 2.
Table 1.
Inclusion and Exclusion Criteria
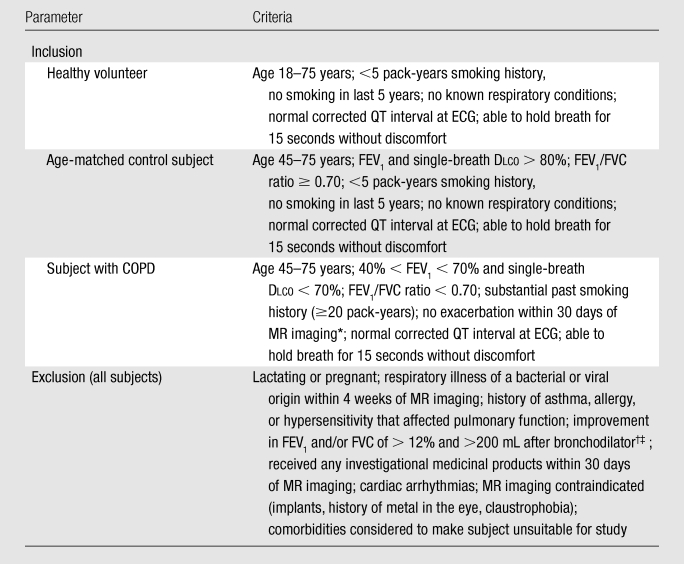
Note.—Dlco = diffusing capacity of lung for carbon monoxide, FEV1 = forced expiratory volume in the first second of expiration, FVC = forced vital capacity.
Exacerbations within 30 days of study were determined by querying subjects as to whether they were recently prescribed antibiotics, increased their use of inhaled or oral steroids, or experienced decreased capability to carry out their daily activities.
Did not apply to healthy volunteers.
Bronchodilator responders were excluded because other aims of the study sought subjects with COPD with primarily emphysematous airflow limitation.
Table 2.
Subject Demographics
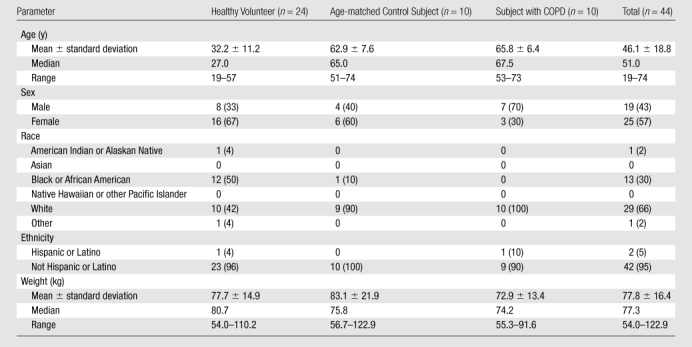
Note.—Unless otherwise indicated, data are numbers of subjects, with percentages in parentheses.
Xenon 129 Hyperpolarization
Two prototype polarizers (GE Healthcare; Durham, NC) were installed adjacent to the MR suite and were used to prepare hyperpolarized 129Xe for all studies. These devices produced 1 L of undiluted 129Xe (40.9 mmol) polarized to 6%–10% in 1 hour by using rubidium-based spin-exchange optical pumping (23). This modest polarization level relative to what is now becoming available (17) led to the choice of a 1-L dose volume to maximize image signal-to-noise ratio, while anticipating that physiologic effects would remain tolerable. The source gas used for polarization consisted of 1% xenon (isotopically enriched to 83% 129Xe), 10% N2, and 89% helium 4 supplied by Spectra Gases (Alpha, NJ) and certified to be more than 99.999% pure. Each dose was prepared by flowing the gas mixture through the heated optical pumping cell of the polarizer and cryogenically extracting the polarized xenon gas in a cold trap immersed in liquid nitrogen. Once the desired xenon volume had been accumulated, it was thawed into a dose delivery bag (Tedlar; Jensen Inert Products, Coral Springs, Fla) through 3/8-inch tubing (Tygon; Saint-Gobain, Akron, Ohio) and clamped shut with a plastic clip. Final 129Xe polarization was measured by using a prototype polarimeter (GE Healthcare, Durham, NC). The dose bag was then labeled and delivered to the adjacent MR imaging suite. The gas contents of each dose were fully traceable to the original source gases with detailed batch records. By producing doses by using cryogenic accumulation of hyperpolarized 129Xe, any possible trace rubidium flowing out of the optical cell was extracted in the cryogenic trap. As an added precaution, this trap was replaced after every 20 batches to prevent potential long-term rubidium accumulation.
MR Examination
Because the primary focus of this article was to report on hyperpolarized 129Xe safety, detailed 129Xe imaging methods and findings are reported separately (15,22). Briefly, subjects were imaged in the supine position, with their arms over their heads (except for one subject who was unable to adopt this position and held arms to the side) with a 1.5-T MR system (Excite 14m5; GE Healthcare, Waukesha, Wis). Subjects were fitted with a flexible vest coil (Clinical MR Solutions, Brookfield, Wis) designed for quadrature transmit-receive at the 17.66-MHz 129Xe resonance frequency and equipped with a blocking network to permit hydrogen 1 (1H) MR imaging by using the body coil in the MR imager. Prior to hyperpolarized 129Xe administration, subjects underwent a free-breathing three-plane localizer examination and a higher resolution 20-second breath-hold steady-state free-precession examination to highlight the pulmonary vessels (field of view = 40 cm, 128 × 128 matrix, 15-mm sections, repetition time msec/echo time msec = 2.8/1.2, flip angle = 45°, bandwidth = 125 kHz).
After 1H anatomic imaging, each subject received a calibration dose consisting of 200 mL hyperpolarized 129Xe and 800 mL N2 (99.999% pure, Airgas, Durham, NC), which was used to verify the transmit frequency and set the transmit gain. Each subject then received either three or four (healthy volunteers only) 1-L doses of hyperpolarized 129Xe, separated by at least 15 minutes. Prior to the first calibration dose, the subject received a bag of air to practice the inhalation and breath-hold maneuver. For each dose (air, calibration, or full dose), the subject was instructed by the technologist to fully inhale and exhale two times. Subjects then received the tube attached to the dose bag in their mouth and were then coached to inhale vigorously. Xenon inhalation was found to be challenging in five of the subjects with COPD as evidenced by them requiring more than 5 seconds to inhale the bag contents. For these subjects, the technologist assisted the respiratory maneuver by physically compressing the bag during inhalation. Note that xenon has a density 4.55 times greater than air, which dominates pulmonary resistance during turbulent flow (24). After inhalation, subjects held their breath until the imaging finished, at which point they were instructed to exhale. The breath-hold period was kept to less than 16 seconds for all hyperpolarized 129Xe acquisitions, which was consistent with the common practice for delivering anoxic hyperpolarized 3He for MR imaging studies (25).
Each 129Xe dose was used for either technical development or efficacy evaluation of ventilation, apparent diffusion coefficient, or dissolved-phase 129Xe imaging, the results of which have been reported elsewhere (15,22). The 129Xe ventilation images reported here were acquired with the following parameters: field of view of 40 cm, matrix of 128 × 128, 15-mm sections, 7.8/1.9, flip angle of 5°–6°, bandwidth of 8 kHz, and sequential k-space ordering.
Evaluation of Safety and Tolerability
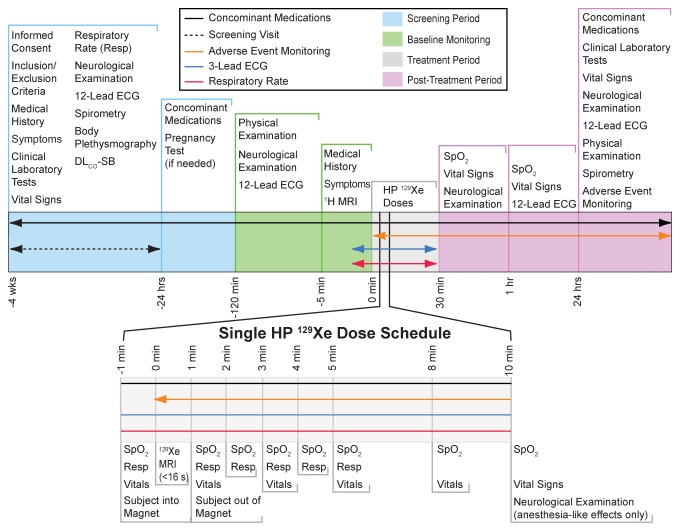
Detailed clinical parameters were recorded at screening (4 weeks to 24 hours before 129Xe MR imaging) and at 24 hours after 129Xe MR imaging, as outlined in Figure 1. At screening, the following were measured, recorded, or performed: any current symptoms, clinical laboratory tests (Appendix E1 [online]), vital signs, respiratory rate, neurologic and neurobehavioral examination, 12-lead ECG, and limited physical examination. For women of childbearing age, a pregnancy test was performed within 24 hours of MR imaging. At 120 minutes before the first 129Xe dose, the following were conducted: neurologic and neurobehavioral examination, 12-lead ECG, and limited physical examination.
Figure 1:
Schedule of study events. Healthy volunteers during the technical run-in phase received four 1-L doses of hyperpolarized (HP) 129Xe, while age-matched control subjects and subjects with COPD during the efficacy phase received three doses. Age-matched control subjects and subjects with COPD underwent pulmonary function testing, whereas healthy volunteers did not. The specific monitoring schedule for each dose is broken out at the bottom of the figure. DLCO-SB = single-breath diffusing capacity of lung for carbon monoxide, ECG = electrocardiography, Resp = respiratory rate recording, Spo2 = oxygen saturation as measured by pulse oximetry.
While in the MR imager, subjects were continuously attended to by a physician, nurse, or respiratory therapist, who recorded any reported symptoms, as well as the subject’s Spo2, three-lead ECG results, and blood pressure, which were continuously displayed by using an MR-compatible monitoring system (GE Healthcare, Helsinki, Finland).
An adverse event was defined as any unfavorable and unintended sign, symptom, or disease temporally associated with the use of hyperpolarized 129Xe, whether or not considered related to the product or expected on the basis of the known properties of xenon. Adverse events were classified (26) by the medical personnel attending to the subject as mild (tolerable), moderate (interferes with normal activity), or severe (incapacitating, unable to perform usual activity or work). A hypoxic adverse event was specifically defined as a reduction in Spo2 of 5% or more that had not resolved by 1 minute after 129Xe inhalation. Adverse events and changes in vital signs or laboratory parameters were considered clinically important if study personnel judged them to require either intervention or further medical evaluation. A serious adverse event was defined as one that resulted in death, was immediately life threatening, required hospitalization, or resulted in persistent or substantial disability or incapacity (26).
At 5 minutes prior to the 129Xe dose, subjects were queried for symptoms, and at 1 minute before dosing, vital signs and respiratory rate were recorded. During inhalation and imaging, ECG and Spo2 were monitored by the medical personnel attending to the subject in the MR imaging suite. On completion of the breath hold, the subject was moved out of the magnet and queried for symptoms. At 1, 2, 3, 4, 5, 8, and 10 minutes after inhalation of each dose, vital signs and respiratory rate measurements were repeated, and at 10 minutes, a brief neurobehavioral examination was conducted. After completion of the imaging session and 1 hour after inhalation of the last 129Xe dose, vital sign measurements were repeated, and a 12-lead ECG was obtained. The subjects returned for follow-up 24 hours after the imaging session for repeat clinical laboratory tests, measurement of vital signs, neurologic and neurobehavioral examination, 12-lead ECG, and a limited physical examination. In addition, subjects were queried for any adverse events or symptoms.
Statistical Analysis
Changes in vital signs were analyzed for significance, and the incidence of xenon-related symptoms was evaluated for relationship to subject group and age by using software (JMP, version 9.0; SAS Institute, Cary, NC). To test whether adverse event incidence was different among the subject groups, a χ2 test was used, employing the Fisher exact test as necessary. Adverse event incidence was tested for association with age by using a logistic regression analysis. Changes in vital signs after xenon inhalation were tested for statistically significant differences over the course of time and across subject groups by using a repeated-measures multivariate analysis of variance. For all tests, a P value less than .05 was considered to indicate a significant difference.
Results
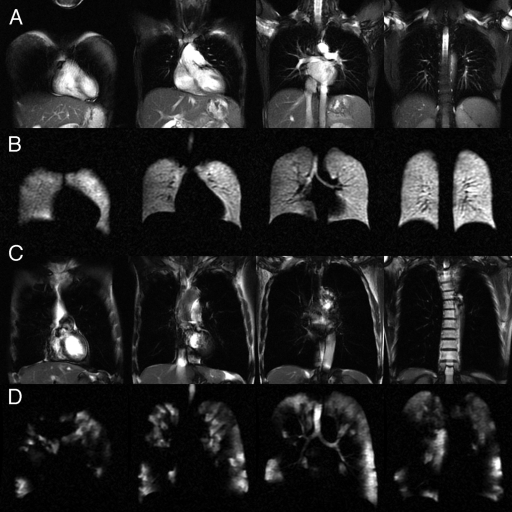
The 44 subjects tolerated all xenon inhalations (four doses during run-in, three during efficacy). There were no withdrawals, no hypoxic adverse events, no severe adverse events, and no serious adverse events. However, a large fraction of the study population (40 [91%] subjects) did experience transient symptoms associated with xenon inhalation, as detailed next. Representative 1H anatomic images and 129Xe ventilation images obtained from a healthy volunteer and a subject with COPD are shown in Figure 2. The images show relatively homogeneous ventilation in the healthy volunteer and substantial ventilation abnormalities in the subject with COPD.
Figure 2:
Selected sections from representative 129Xe ventilation and 1H MR anatomic images in individual subjects. A, Steady-state free-precession 1H MR images in a healthy volunteer. B, Corresponding 129Xe ventilation MR images in the same healthy volunteer. C, Steady-state free-precession 1H MR images in a subject with COPD. D, Corresponding 129Xe ventilation MR images in the same subject with COPD show substantial ventilation defects and regions lacking ventilation.
Transient Xenon-related Symptoms
Transient xenon-related symptoms were reported by a total of 40 (91%) subjects for at least one of the three or four xenon doses, as summarized in Table 3. Symptoms were classified according to Medical Dictionary for Regulatory Activities definitions as affecting the nervous (82%) and gastrointestinal (32%) systems, followed by psychiatric disorders (30%) and subcutaneous tissue or skin disorders (11%). These symptoms along with those in other system organ classes, which were reported by fewer than 10% of subjects, are detailed in Table 4. All symptoms were considered to be related to xenon administration, except one incidence of high blood pressure (180/83 mm Hg just prior to the third xenon dose, which decreased after xenon inhalation). The most common symptoms were dizziness (59%), followed by paresthesia (34%), euphoria (30%), and hypoesthesia (30%). All symptoms were classified by medical personnel monitoring the subjects according the criteria defined in the Materials and Methods section as mild adverse events with the exception of four, which were classified as moderate. The four moderate adverse events were experienced by two subjects—one healthy volunteer and one age-matched control subject. The healthy volunteer experienced extreme dizziness (one event) and paresthesia (one event). The age-matched control subject experienced a depressed level of consciousness (one event) and somnolence (one event). None of the events were considered a serious adverse event, and none required discontinuation from the study. Adverse events recorded for all subjects resolved within 1.6 minutes ± 0.9 without treatment or clinical intervention. The longest lasting adverse event was that of somnolence experienced by the age-matched control subject, and this resolved within 10 minutes as documented in detail in Appendix E2 (online). The healthy volunteer who experienced two moderate adverse events was the first subject in the trial, and the adverse events occurred on the first dose, which may have reflected a level of anxiety associated with initiation of the study. There was no significant difference in the incidence of adverse events according to either age (P = .45) or subject group (P = .29).
Table 3.
Summary of Adverse Event Intensity
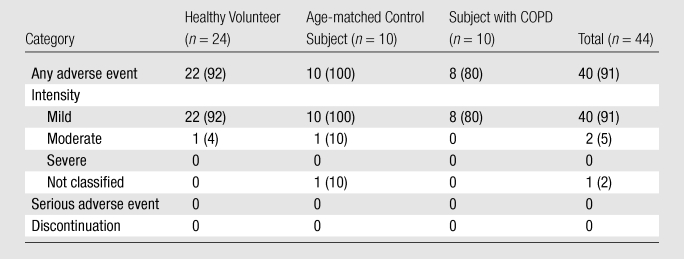
Note.—Data are numbers of subjects, with percentages in parentheses.
Table 4.
Classification of Adverse Events
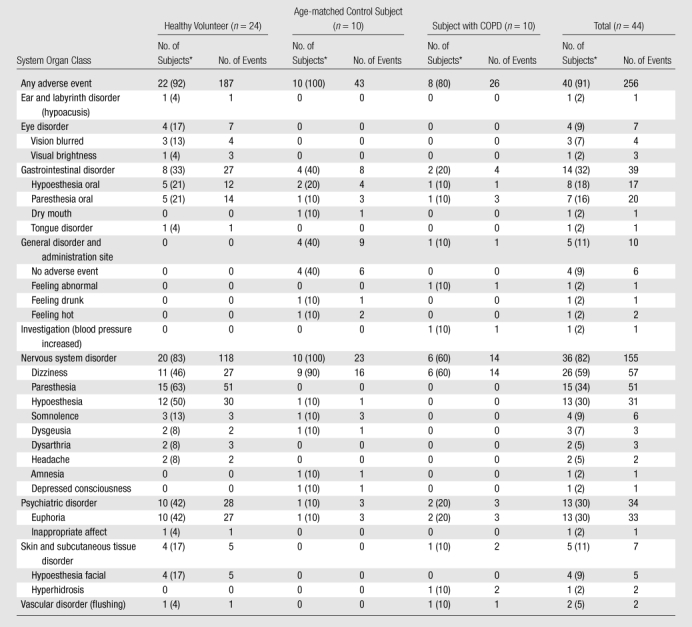
Data in parentheses are percentages.
24-hour Follow-up Safety Assessments
No subjects exhibited a change in serum biochemistry or hematology values at 24-hour follow-up relative to baseline that met the study definition of clinical importance. No subjects exhibited changes relative to screening at their 12-lead ECG at either 1 hour after the last dose or at 24-hour follow-up that met the study definition of clinical importance. No subject had an abnormal neurologic examination finding at any of the examination times. No subjects had differences in physical examination findings meeting the study definition of clinical importance. There were some fluctuations in clinical parameters consistent with expected normal variation, which are summarized in Appendix E3 (online).
Monitoring of Vital Signs during Xenon Administration
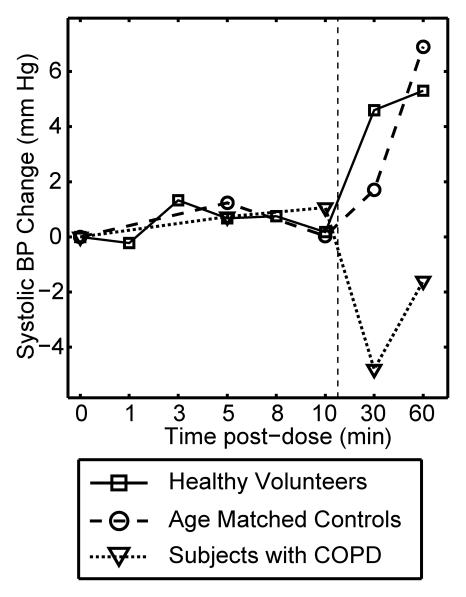
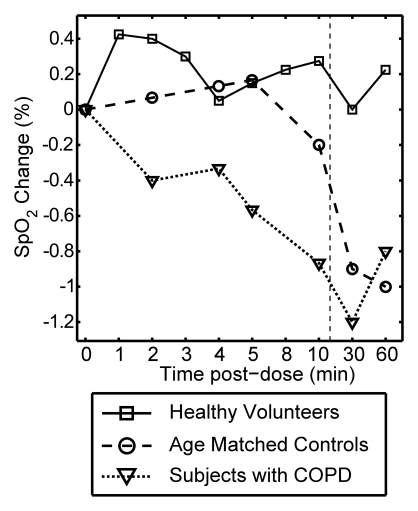
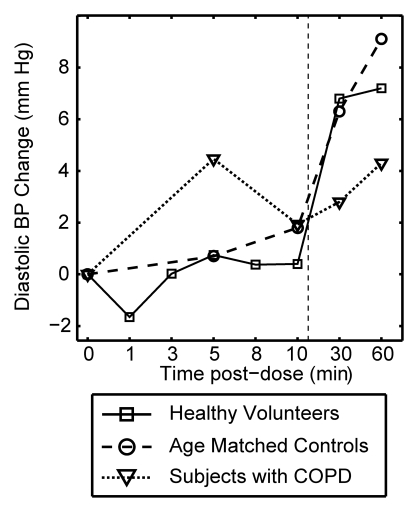
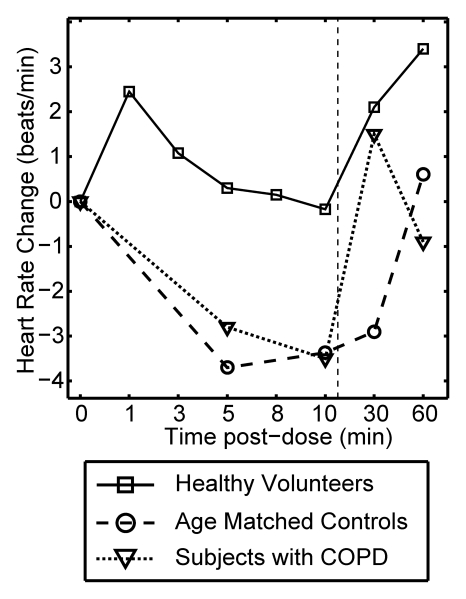
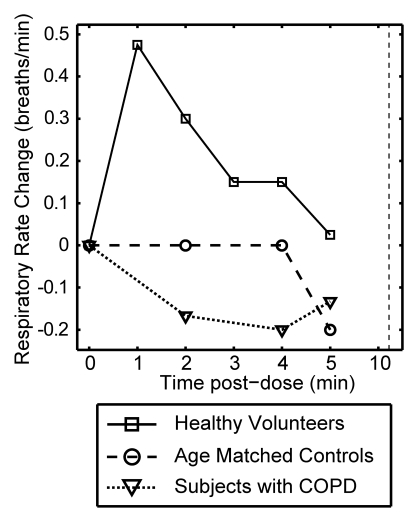
Figure 3 plots the mean change from baseline for all vital signs recorded over a period of 10 minutes after each xenon dose and at 30 and 60 minutes after the last dose. The data are delineated by subject group (healthy volunteer, age-matched control subject, COPD), and the 0–10-minute data are averaged over all doses received. Multivariate analysis of variance of the vital signs measured over the course of time and across groups showed that changes were not statistically significant, whether analyzed within a given subject group (P > .40) or over the entire population (P > .27).
Figure 3a:
Graphs show mean change in vital signs after xenon dose administration relative to baseline as a function of time. Data were acquired after inhaling the xenon dose and were averaged over all doses for a given time point (four doses for healthy volunteers, three doses for age-matched control subjects and subjects with COPD) but are separated by subject group. Dashed vertical lines = time after which subjects were permitted to move about freely. Graphs show (a) systolic blood pressure (BP), (b) diastolic blood pressure, (c) blood oxygen saturation (Spo2), (d) heart rate, and (e) respiratory rate. No clinically important changes in any of these vital signs were noted for any of the groups.
Figure 3c:
Graphs show mean change in vital signs after xenon dose administration relative to baseline as a function of time. Data were acquired after inhaling the xenon dose and were averaged over all doses for a given time point (four doses for healthy volunteers, three doses for age-matched control subjects and subjects with COPD) but are separated by subject group. Dashed vertical lines = time after which subjects were permitted to move about freely. Graphs show (a) systolic blood pressure (BP), (b) diastolic blood pressure, (c) blood oxygen saturation (Spo2), (d) heart rate, and (e) respiratory rate. No clinically important changes in any of these vital signs were noted for any of the groups.
As shown in Figure 3a and 3b, both systolic and diastolic blood pressure did not change appreciably during the 10 minutes after each xenon dose. The modest changes observed 30 and 60 minutes after the last dose can be attributed to increased physical activity because the subjects were permitted to move about freely prior to measurements taken at those time points (27).
Figure 3b:
Graphs show mean change in vital signs after xenon dose administration relative to baseline as a function of time. Data were acquired after inhaling the xenon dose and were averaged over all doses for a given time point (four doses for healthy volunteers, three doses for age-matched control subjects and subjects with COPD) but are separated by subject group. Dashed vertical lines = time after which subjects were permitted to move about freely. Graphs show (a) systolic blood pressure (BP), (b) diastolic blood pressure, (c) blood oxygen saturation (Spo2), (d) heart rate, and (e) respiratory rate. No clinically important changes in any of these vital signs were noted for any of the groups.
As shown in Figure 1c, average oxygen saturation for each subject group remained stable and within reference range for the 10 minutes after xenon administration. However, the COPD group exhibited a marginally lower Spo2 during the period from 1 to 10 minutes after xenon administration (−0.54%) than the healthy volunteer (0.26%) and age-matched control subject (0.04%) groups. This difference in Spo2 trend was significantly different among the groups (P = .01).
Figure 3d and 3e show the mean change in heart rate and respiratory rate after each xenon dose, respectively. Both remained within normal range (60 < heart rate < 80 beats per minute, 12 < respiratory rate < 20 beats per minute) at all time points for all groups. There were no abnormal three-lead ECG readings at any point for any subject. For the healthy volunteer group, both heart and respiratory rates did increase slightly from baseline to the 1-minute postdose interval, with a gradual return to near mean baseline levels. A similar trend has been reported for 3He MR imaging and attributed to a likely slight anxiety associated with imaging examination (13). There was also a trend toward slightly higher heart rate at the 30- and 60-minute time points after the last dose. These were again likely a result of increased physical activity when subjects were out of the imager. In general, vital signs changed more when subjects got up from the imager bed (time points of 30 and 60 minutes) than when they inhaled hyperpolarized 129Xe.
Figure 3d:
Graphs show mean change in vital signs after xenon dose administration relative to baseline as a function of time. Data were acquired after inhaling the xenon dose and were averaged over all doses for a given time point (four doses for healthy volunteers, three doses for age-matched control subjects and subjects with COPD) but are separated by subject group. Dashed vertical lines = time after which subjects were permitted to move about freely. Graphs show (a) systolic blood pressure (BP), (b) diastolic blood pressure, (c) blood oxygen saturation (Spo2), (d) heart rate, and (e) respiratory rate. No clinically important changes in any of these vital signs were noted for any of the groups.
Figure 3e:
Graphs show mean change in vital signs after xenon dose administration relative to baseline as a function of time. Data were acquired after inhaling the xenon dose and were averaged over all doses for a given time point (four doses for healthy volunteers, three doses for age-matched control subjects and subjects with COPD) but are separated by subject group. Dashed vertical lines = time after which subjects were permitted to move about freely. Graphs show (a) systolic blood pressure (BP), (b) diastolic blood pressure, (c) blood oxygen saturation (Spo2), (d) heart rate, and (e) respiratory rate. No clinically important changes in any of these vital signs were noted for any of the groups.
Discussion
This study was the first to evaluate the safety and tolerability of inhaling multiple 1-L volumes of hyperpolarized 129Xe without additional oxygen. The work differs from the approach of Patz et al (18,19) who administered hyperpolarized 129Xe mixed with O2. Because O2 is paramagnetic and rapidly depolarizes 129Xe (28), administering 129Xe without oxygen reduces hyperpolarized 129Xe signal loss until the gas enters the airspaces of the lungs. This delivery approach was important to preserve our relatively modest 129Xe polarization and is identical to the one used to administer hyperpolarized 3He for many years without adverse consequences (13). The present study has shown that O2 saturation after inspiration and breath hold changed, on average, by less than 1% during the 10 minutes of recording after all xenon doses.
This study of the safety and tolerability of hyperpolarized 129Xe inhalation was consistent with previous human experience by using both hyperpolarized and stable xenon. In a study specifically designed to mirror single bolus xenon administration, Liotti et al (29) administered 1-L doses of unpolarized xenon in six healthy volunteers who held their breath up to 1 minute. These subjects reported mild to moderate euphoria and mild tingling in the fingertips that resolved within a timescale of 3–4 minutes. Preliminary work by Mugler et al (2), involving administration of hyperpolarized 129Xe in three healthy subjects, reported one subject with numbness and nausea. Kilian et al (30) reported one volunteer inhaling hyperpolarized 129Xe without any adverse effects. In the largest study of hyperpolarized 129Xe prior to ours, Patz et al (18,19) reported on five subjects who had undergone at least 18 breaths of hyperpolarized 129Xe. The subjects experienced no significant changes in Spo2 measured during 129Xe inhalation or in blood pressure measured 10 minutes after 129Xe inhalation, and no other symptoms from xenon inhalation were reported. By contrast, in our studies, 91% of subjects reported symptoms after xenon inhalation for at least one dose. However, none of these symptoms were long lasting, and none interfered with the subject’s ability to complete the study.
Our findings were also consistent with previous literature reports of xenon used in medicine for other applications. Studies using stable xenon for cerebral blood flow imaging with CT require sustained alveolar concentrations of xenon that are higher (approximately 30%) and maintained for a longer time (approximately 4 minutes) than for hyperpolarized 129Xe MR imaging. Even under these conditions, Latchaw et al (21) found an acceptably low incidence of adverse reactions that included respiratory rate delay (3.6%), headache (0.4%), seizures (0.2%), nausea and vomiting (0.2%), and change in neurologic status (0.1%). (Note that, in contrast to our study, Latchaw et al did not report symptoms like dizziness and numbness as adverse events because, on the basis of the known anesthetic properties of xenon, they were expected.) In our subject population, the expected total lung capacity was 5–7 L, and thus inhaling 1 L of xenon results in an alveolar concentration of 15%–20%. This is below the 28%–33% maintained for xenon CT and well below the 70% mean alveolar concentration required to initiate anesthesia (20).
This study did have several limitations. First, the population was relatively small (44 total subjects, including only 10 with moderate COPD). It is conceivable that studies of larger populations would uncover new effects not seen in this study, although as already noted, the effects reported here were consistent with expectations based on well-known properties of xenon. Furthermore, the relatively modest 129Xe polarization led us to administer anoxic 129Xe to minimize polarization loss. It is conceivable that xenon effects could be diminished if the 129Xe were administered with oxygen (18). Moreover, each subject received 1 L of 129Xe, regardless of their lung volume, which likely caused some variability in the alveolar xenon concentrations from subject to subject. While a very detailed panel of vital signs was collected during this study, there was no effort to capture the nadir of O2 saturation that may have occurred before the first recording at 1 minute after dose (13). Finally, subjects were only observed for 24 hours. However, on the basis of a detailed understanding of the clearance of xenon from radioisotope studies (31), there was little reason to expect any xenon effects beyond this window.
In summary, while the majority of subjects experienced mild transient symptoms such as dizziness, paresthesia, euphoric mood, and hypoesthesia, a detailed assessment of vital signs, ECG, physical and neurologic examinations, and laboratory tests showed that inhalation of multiple 1-L volumes of undiluted hyperpolarized 129Xe was well tolerated in healthy subjects and in those with mild or moderate COPD. The symptoms described here are likely to be diminished by reducing the administered xenon volume, which will be possible without sacrificing signal-to-noise ratio if polarization can be commensurately increased by using improved technology (17).
Advances in Knowledge.
Inhalation of 1-L volumes of undiluted hyperpolarized 129Xe caused transient symptoms in 40 (91%) of 44 subjects, most commonly dizziness (26 [59%] of 44), paresthesia (15 [34%] of 44), euphoria (13 [30%] of 44], and hypoesthesia (13 [30%] of 44).
No hypoxic adverse events were observed, and xenon-related symptoms caused no withdrawals and no severe or serious adverse events and resolved without clinical intervention in 1.6 minutes ± 0.9.
Inhalation of multiple 1-L volumes of undiluted hyperpolarized 129Xe caused no significant changes in vital signs (P > .27) and no changes in 12-lead electrocardiographic or laboratory test results that study personnel deemed clinically important or to require intervention.
Implication for Patient Care.
Inhalation of multiple 1-L volumes of undiluted hyperpolarized 129Xe is well tolerated by adult subjects, including those with mild or moderate chronic obstructive pulmonary disease.
Disclosures of Potential Conflicts of Interest: B.D. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: author was consultant to GE Healthcare; author receives money from Princeton University because author is inventor on several patents related to hyperpolarized gas MR imaging; author and institution receive money from Duke University for several patents related to hyperpolarized gas MR imaging; author receives royalties from Princeton University for patents ($2500/year); author received one-time royalty ($10,000) for patent licensed by GE Healthcare from Duke University. Other relationships: none to disclose. S.M.J. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: author receives royalties from Amyrsis. Other relationships: none to disclose. Z.I.C. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. G.M.M. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. D.M.B. No potential conflicts of interest to disclose. J.C.N. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. S.S.K. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. R.F. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. C.W. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. K.T.K. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. J.W. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: employed at GE Healthcare. Other relationships: none to disclose. M.K. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor). Financial activities not related to the present article: institution has grants or grants pending from GSK, Merck, Eumedics, Genentech, and Asthmatx. Other relationships: none to disclose. H.P.M. Financial activities related to the present article: institution received grant from GE Healthcare (study sponsor); institution receives provision of writing assistance, medicines, equipment, or administrative support from GE Healthcare. Financial activities not related to the present article: institution receives institutional research grants from GE Healthcare. Other relationships: none to disclose.
Supplementary Material
Acknowledgments
We thank Jerry Dahlke, BS, of GE Healthcare for tireless efforts to optimize the 1.5-T clinical imager for 129Xe MR imaging and Sally Zimney, MS, for careful proofreading of this manuscript.
Received November 3, 2010; revision requested January 6, 2011; revision received May 9; accepted May 18; final version accepted August 3.
Supported by GE Healthcare and the Duke Center for In Vivo Microscopy.
Funding: This research was supported by the National Institutes of Health (grants P41 RR005959 and R01 HL105643).
Abbreviations:
- COPD
- chronic obstructive pulmonary disease
- ECG
- electrocardiogram
- Spo2
- oxygen saturation as measured by pulse oximetry
References
- 1.Albert MS, Cates GD, Driehuys B, et al. Biological magnetic resonance imaging using laser-polarized 129Xe. Nature 1994;370(6486):199–201 [DOI] [PubMed] [Google Scholar]
- 2.Mugler JP, 3rd, Driehuys B, Brookeman JR, et al. MR imaging and spectroscopy using hyperpolarized 129Xe gas: preliminary human results. Magn Reson Med 1997;37(6):809–815 [DOI] [PubMed] [Google Scholar]
- 3.Möller HE, Chen XJ, Saam B, et al. MRI of the lungs using hyperpolarized noble gases. Magn Reson Med 2002;47(6):1029–1051 [DOI] [PubMed] [Google Scholar]
- 4.Ebert M, Grossmann T, Heil W, et al. Nuclear magnetic resonance imaging with hyperpolarised helium-3. Lancet 1996;347(9011):1297–1299 [DOI] [PubMed] [Google Scholar]
- 5.MacFall JR, Charles HC, Black RD, et al. Human lung air spaces: potential for MR imaging with hyperpolarized He-3. Radiology 1996;200(2):553–558 [DOI] [PubMed] [Google Scholar]
- 6.van Beek EJR, Dahmen AM, Stavngaard T, et al. Hyperpolarised 3He MRI versus HRCT in COPD and normal volunteers: PHIL trial. Eur Respir J 2009;34(6):1311–1321 [DOI] [PubMed] [Google Scholar]
- 7.Fain SB, Panth SR, Evans MD, et al. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology 2006;239(3):875–883 [DOI] [PubMed] [Google Scholar]
- 8.McAdams HP, Palmer SM, Donnelly LF, Charles HC, Tapson VF, MacFall JR. Hyperpolarized 3He-enhanced MR imaging of lung transplant recipients: preliminary results. AJR Am J Roentgenol 1999;173(4):955–959 [DOI] [PubMed] [Google Scholar]
- 9.Mentore K, Froh DK, de Lange EE, Brookeman JR, Paget-Brown AO, Altes TA. Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: assessment at baseline and after bronchodilator and airway clearance treatment. Acad Radiol 2005;12(11):1423–1429 [DOI] [PubMed] [Google Scholar]
- 10.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP., 3rd Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes—initial experience. Radiology 2002;222(1):252–260 [DOI] [PubMed] [Google Scholar]
- 11.Kirby M, Mathew L, Wheatley A, Santyr GE, McCormack DG, Parraga G. Chronic obstructive pulmonary disease: longitudinal hyperpolarized 3He MR imaging. Radiology 2010;256(1):280–289 [DOI] [PubMed] [Google Scholar]
- 12.Yablonskiy DA, Sukstanskii AL, Woods JC, et al. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol 2009;107(4):1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutey BA, Lefrak SS, Woods JC, et al. Hyperpolarized 3He MR imaging: physiologic monitoring observations and safety considerations in 100 consecutive subjects. Radiology 2008;248(2):655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer D. DOE begins rationing helium-3. Physics Today June 10, 201022–25 [Google Scholar]
- 15.Cleveland ZI, Cofer GP, Metz G, et al. Hyperpolarized 129Xe MR imaging of alveolar gas uptake in humans. PLoS One 2010;5(8):e12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugler JP, 3rd, Altes TA, Ruset IC, et al. Simultaneous magnetic resonance imaging of ventilation distribution and gas uptake in the human lung using hyperpolarized xenon-129. Proc Natl Acad Sci U S A 2010;107(50):21707–21712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruset IC, Ketel S, Hersman FW. Optical pumping system design for large production of hyperpolarized 129Xe. Phys Rev Lett 2006;96(5):053002. [DOI] [PubMed] [Google Scholar]
- 18.Patz S, Hersman FW, Muradian I, et al. Hyperpolarized (129)Xe MRI: a viable functional lung imaging modality? Eur J Radiol 2007;64(3):335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patz S, Muradian I, Hrovat MI, et al. Human pulmonary imaging and spectroscopy with hyperpolarized 129Xe at 0.2T. Acad Radiol 2008;15(6):713–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy RR, Stokes JW, Downing P. Anaesthesia and the ‘inert’ gases with special reference to xenon. Anaesth Intensive Care 1992;20(1):66–70 [DOI] [PubMed] [Google Scholar]
- 21.Latchaw RE, Yonas H, Pentheny SL, Gur D. Adverse reactions to xenon-enhanced CT cerebral blood flow determination. Radiology 1987;163(1):251–254 [DOI] [PubMed] [Google Scholar]
- 22.Kaushik SS, Cleveland ZI, Cofer GP, et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 2011;65(4):1154–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driehuys B, Cates GD, Miron E, Sauer K, Walter DK, Happer W. High-volume production of laser-polarized 129Xe. Appl Phys Lett 1996;69(12):1668–1670 [Google Scholar]
- 24.Drazen JM, Loring SH, Ingram RH., Jr Distribution of pulmonary resistance: effects of gas density, viscosity, and flow rate. J Appl Physiol 1976;41(3):388–395 [DOI] [PubMed] [Google Scholar]
- 25.Fain SB, Korosec FR, Holmes JH, O’Halloran R, Sorkness RL, Grist TM. Functional lung imaging using hyperpolarized gas MRI. J Magn Reson Imaging 2007;25(5):910–923 [DOI] [PubMed] [Google Scholar]
- 26.Liu MB, Davis K. A clinical trials manual from the Duke Clinical Research Institute: lessons from a horse named Jim. Oxford, England: Wiley, 2010 [Google Scholar]
- 27.Højgaard MV, Holstein-Rathlou NH, Agner E, Kanters JK. Reproducibility of heart rate variability, blood pressure variability and baroreceptor sensitivity during rest and head-up tilt. Blood Press Monit 2005;10(1):19–24 [DOI] [PubMed] [Google Scholar]
- 28.Jameson CJ, Jameson AK, Hwang JK. Nuclear-spin relaxation by intermolecular magnetic dipole coupling in the gas-phase: 129Xe in oxygen. J Chem Phys 1988;89(7):4074–4081 [Google Scholar]
- 29.Liotti M, Martin CC, Gao JH, et al. Xenon effects on regional cerebral blood flow assessed by 15O-H2O positron emission tomography: implications for hyperpolarized xenon MRI. J Magn Reson Imaging 1997;7(4):761–764 [DOI] [PubMed] [Google Scholar]
- 30.Kilian W, Seifert F, Rinneberg H. Dynamic NMR spectroscopy of hyperpolarized (129)Xe in human brain analyzed by an uptake model. Magn Reson Med 2004;51(4):843–847 [DOI] [PubMed] [Google Scholar]
- 31.Susskind H, Atkins HL, Cohn SH, Ellis KJ, Richards P. Whole-body retention of radioxenon. J Nucl Med 1977;18(5):462–471 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.