Myeloperoxidase is important in the development of Kawasaki vasculitis and can be a key functional imaging biomarker for assessing vasculitis at high anatomic resolution afforded by MR molecular imaging.
Abstract
Purpose:
To determine if a molecular imaging approach targeting the highly oxidative enzyme myeloperoxidase (MPO) can help noninvasively identify and confirm sites of vascular wall inflammation in a murine model of vasculitis.
Materials and Methods:
Animal experiments were approved by the institutional animal care committee. Twenty-six mice were studied, including eight MPO-deficient and six sham-operated mice as controls. Vasculitis was induced with intraperitoneal injection of Candida albicans water-soluble fraction (CAWS). Aortic root magnetic resonance imaging was performed after intravenous injection of the activatable MPO sensor (bis-5-hydroxytryptamide-diethylenetriaminepentatacetate gadolinium) (n = 23), referred to as MPO-Gd, or gadopentetate dimeglumine (n = 10). Seven mice were randomly assigned to receive either MPO-Gd or gadopentetate dimeglumine first. Aortic root specimens were collected for biochemical and histopathologic analyses to validate imaging findings. Statistical significance was calculated for contrast-to-noise ratios (CNRs) by using the paired t test.
Results:
In the aortic root, the mean MPO-Gd CNRs after agent injection (CNR = 28.1) were more than 2.5-fold higher than those of sham-operated mice imaged with MPO-Gd and vasculitis mice imaged with gadopentetate dimeglumine (CNR = 10.6) (P < .05). MPO-Gd MR imaging helped identify areas of vasculitis that were not seen at unenhanced and contrast material–enhanced imaging with gadopentetate dimeglumine. Histopathologic and biochemical analyses for MPO and myeloid cells confirmed imaging findings. In MPO-deficient mice, injection of CAWS did not result in a vasculitis phenotype, implying a key role of the imaging target in disease cause.
Conclusion:
Molecular imaging targeting MPO can be a useful biomarker to noninvasively detect and confirm inflammation in vasculitis by using a murine model of Kawasaki disease.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11110040/-/DC1
Introduction
The vasculitides, a heterogeneous group of disorders that can affect multiple organ systems, are characterized by perivascular inflammation and the influx of immune cells into the vascular wall. Vasculitides are subdivided clinically according to vessel caliber size and functionally according to cause (1–3). The diagnosis of vasculitis is often challenging because of nonspecific symptoms, deficiency of strict diagnostic criteria, and lack of good diagnostic tests (4). Vascular imaging (typically computed tomography [CT], magnetic resonance [MR] imaging, or conventional angiography) is often performed. Although contrast material–enhanced CT and MR images can occasionally show some vessel wall enhancement, the main criterion for the imaging diagnosis of vasculitis is determined by assessment of changes in luminal diameter, which can be limited by spatial resolution—especially for small-vessel vasculitides.
The pathophysiology of vasculitis is not well understood, but the ensuing inflammatory response has been generally well described (5,6). Myeloperoxidase (MPO), a highly oxidative enzyme produced by proinflammatory myeloid cells, is emerging to be a key biomarker of vascular inflammation (7,8). Although the exact role of MPO in the pathogenesis of vasculitis remains unresolved, studies have suggested a multifactorial scenario involving direct production of reactive oxygen species, facilitation of neutrophil priming or activation, elevation of extracellular proteolytic activity, or contribution to endothelial dysfunction (9). Antibodies directed against MPO—such as antineutrophil cytoplasmic antibodies—can directly induce certain forms of small-vessel vasculitis in mouse models and are used clinically as a diagnostic marker in serum (10). Elevated serum antineutrophil cytoplasmic antibody levels have been detected in many forms of small-, medium-, and large-vessel vasculitides, including Kawasaki disease which afflicts medium-sized vessels (11).
Kawasaki disease is noted for its occurrence in Japanese pediatric populations and production of coronary angiitis (12). The cause of Kawasaki disease has not yet been identified but is thought to relate to a number of infectious agents (13). A murine Kawasaki disease model with histopathologic features resembling the human disease can be induced with intraperitoneal injections of purified fractions of the yeast Candida albicans (14–16). Several groups have performed retrospective studies (17,18) or developed imaging techniques (19) that utilized contrast-enhanced MR imaging to detect areas of vessel wall abnormality in patients with vasculitis, which suggests that contrast-enhanced MR imaging can be a useful imaging tool for evaluating vasculitis. However, because the conventional imaging agents used in these studies are nonspecific, confirmation of the imaging diagnosis might still require biopsy. Therefore, we hypothesized that molecular contrast-enhanced MR imaging targeting MPO activity by using the gadolinium-based MPO-specific agent MPO-Gd (20,21) can be used to better detect and confirm vasculitis noninvasively.
Materials and Methods
Murine Model of Vasculitis
The Table is a study flowchart. Animal experiments were approved by the institutional animal care committee. IFO 1385 strain of C albicans was obtained (ATCC, Manassas, Va). Purification of C albicans water-soluble fraction (CAWS) was adapted from previously published methods (22). Briefly, C albicans was cultured in 1 L of restricted media for 5 days at 37°C. Equal volume of ethanol was added to the culture, and CAWS was precipitated overnight at room temperature. The precipitate was collected and resuspended in 250 mL of distilled water before reprecipitation of the soluble fraction with equal volume of ethanol. The precipitate was again collected and allowed to dry before resuspending in phosphate-buffered saline. DBA/2 mice (Charles River Laboratories, Wilmington, Mass) (n = 13), C57BL/6 mice (Jackson Laboratory, Bar Harbor, Me) (n = 5), and MPO-deficient mice (Jackson Laboratory) (n = 8) between the ages of 4–5 weeks were used. Intraperitoneal injection for 5 consecutive days of phosphate-buffered saline alone as a control or 4 mg of CAWS in phosphate-buffered saline was performed.
Study Flowchart
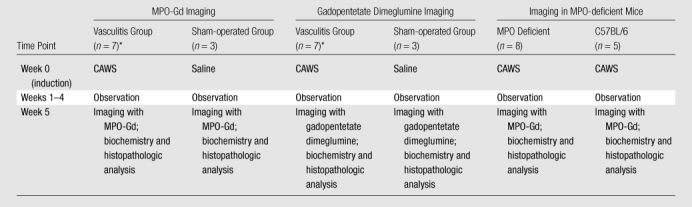
Same mice, randomly assigned to receive MPO-Gd or gadopentetate dimeglumine first. Total number of mice was 26.
MPO Genotyping
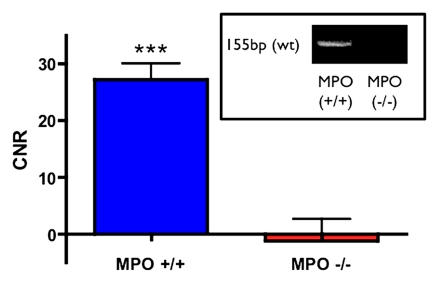
Confirmation of genotype was performed in all MPO-deficient mice. Genomic DNA was isolated from overnight proteinase K (50 μg) digestion of tail clips at 55°C. Primers flanking the insertion sites of neomycin cassette were used to generate a 155-bp product band corresponding to the wild-type allele in identical Taq polymerase reactions containing equal concentrations of genomic DNA in the reactions. Protocol was provided by the supplier (Jackson Laboratory).
Imaging Agents
Synthesis of the MPO molecular agent bis-5-hydroxytryptamide-diethylenetriaminepentatacetate-Gd (MPO-Gd; molecular weight = 863 g) has been described in detail previously (20). In brief, in the presence of excess triethylamine, diethylenetriaminepentaacetic acid (DTPA) bis anhydride was reacted with serotonin in dimethylformamide to obtain bis-5-hydroxytryptamide-DTPA. Isolation of MPO-Gd proceeded with recrystallization from methanol and acetone. Complexation of bis-5-hydroxytryptamide-DTPA through the DTPA moiety with gadolinium was performed in the presence of 1% citric acid (wt/wt) and was purified by using high-performance liquid chromatography. Gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Montville, NJ) (molecular weight = 590 g) was used. The r1 (r2 of small chelate gadolinium-based agents are similar to r1) of MPO-Gd is 4.3 L · mmol−1 · sec−1 and 10.5 L · mmol−1 · sec−1 after peroxidase-mediated activation in vitro at 0.47 T (20), with a blood half-life of 5.4 minutes ± 0.9 (standard error of measurement) in mice (23). The values for unactivated MPO-Gd are comparable to published values for gadopentetate dimeglumine (r1 of 4.7 L · mmol−1 · sec−1 at 0.47 T [24] and blood half-life of 6.1 minutes [25]). In the presence of active MPO, MPO-Gd is radicalized and can combine to form oligomers (up to 5 units in length) (21,26) and bind to proteins containing phenolic amino acids such as tyrosine (26). The resultant activated agent, because of its increased molecular size and protein binding, exhibits higher relaxivity and greater MR signal intensity (signal amplification) that persist for an extended time, while unactivated agents do not show increased signal intensity or prolonged enhancement, allowing for identification and confirmation of MPO activity in vivo on delayed images (21,23,26–30). In vivo, the signal from activated MPO-Gd was no longer observed within 6 hours after administration and activation in the mouse brain (23) and within 24 hours after administration and activation in the rabbit aortic wall (29). Cytotoxicity studies have revealed no substantial toxicity for either the unactivated or the activated MPO-Gd agent, and transmetallation studies have shown that the gadolinium is greater than 10-fold more stable in MPO-Gd than in gadopentetate dimeglumine (26). In mice, more than 90% of the administered MPO-Gd agent is eliminated after 6 hours (21).
MR Imaging
Because mice induced with vasculitis might experience substantial mortality beyond 5 weeks after induction (16), we performed our experiments at 5 weeks after disease induction to avoid unnecessary loss of animals. Mice were imaged with a 7.0-T MR imager (PharmaScan; Bruker, Billerica, Mass) by using gas isoflurane anesthesia (Sigma Aldrich, St Louis, Mo). All MR imaging was performed with electrocardiographic and respiratory gating by using a dedicated mouse heart birdcage coil (Rapid Biomedical, Würzburg, Germany). Short-axis T1-weighted gradient-echo fast low-angle shot images (bright blood) were obtained of the aortic root; imaging parameters were as follows: echo time of 2.7 msec, repetition time of 7.0–12.0 msec depending on the heart rate, 60° flip angle (higher flip angle for T1 weighting), 200 × 200-μm in-plane resolution, 1-mm section thickness, eight signals acquired. Mice were initially imaged without contrast material. Then, after intravenous injection of either MPO-Gd (0.3 mmol per kilogram of body weight in 10% dimethyl sulfoxide in phosphate-buffered saline, pH = 7.4) or gadopentetate dimeglumine (0.3 mmol/kg), imaging was performed at 30, 60, and 90 minutes after injection. To prevent changes in the animal position before and after agent administration, a long catheter extending out of the magnet bore was utilized so that the animal did not have to be moved out of the imager for injection. When imaging the same animals on different days, care was taken to reproduce as closely as possible the same anatomic location by matching the aortic root and its relationship to the pulmonary arteries (Fig E1 [online]).
To determine if MPO-Gd can identify the diseased vessel wall, we imaged mice induced with vasculitis through intraperitoneal C albicans extract injection and compared with sham-operated control mice (n = 6) injected with saline. In vasculitis DBA/2 mice (n = 7), MPO-Gd and gadopentetate dimeglumine were administered 1 day apart, with mice randomly assigned to receive either MPO-Gd first or gadopentetate dimeglumine first. For the sham-operated mice, three mice received MPO-Gd and three other mice received gadopentetate dimeglumine.
To understand the role MPO plays in the induction of vasculitis and to assess the specificity of the MPO-Gd imaging agent for MPO activity, we injected the C albicans extract into MPO-deficient mice (n = 8) and in C57BL/6 background wild-type control mice (n = 5). Mice were imaged without contrast material, followed by serial MR imaging at 30, 60, and 90 minutes after intravenous injection of MPO-Gd as above.
Histopathologic Analysis and Immunohistochemistry
To validate imaging findings, mice with prolonged MPO-Gd enhancement confirming high levels of MPO activity and thus inflammation were subjected to histopathologic analysis. Mice were euthanized, and hearts with the aortic roots were harvested. Tissue samples were embedded in optimal-cutting-temperature embedding medium (Sakura Finetek, Torrance, Calif). Serial 6-mm-thick cryosections were prepared for histopathologic analysis. The tissue slices were stained with hematoxylin-eosin for overall morphology, and the adjacent slices were stained for cell markers identifying macrophages (Mac-3; BD Biosciences, San Diego, Calif), neutrophils (NIMP-R14; Santa Cruz Biotechnology, Santa Cruz, Calif), and MPO (Ab-1; NeoMarkers, Fremont, Calif). Biotinylated secondary antibodies (avidin–biotinylated enzyme complex kit, Vector Laboratories, Burlingame, Calif) and 3-amino-9-ethylcarbazole substrate (DakoCytomation, Carpinteria, Calif) were used for colorimetric development. All slices for immunohistochemistry were counterstained with Harris hematoxylin. The resultant slides were interpreted by authors (E.A., pathologist; Y.I., pathologist; J.W.C., radiologist; each with more than 10 years of experience in histopathologic analysis).
Peroxidase Activity Assay
To quantify MPO activity in isolated aortic root specimens, we performed peroxidase activity assays by using the substrate tetramethylbenzidine with an ultraviolet-visible spectrometer (Cary 50 Bio UV-Vis; Varian, Palo Alto, Calif) at 655 nm in CAWS-injected vasculitis mice, sham-operated control mice, and MPO-deficient mice. One hundred microliters of cell supernatants was added to 500 μL of assay solution in a 600-μL cuvette. The units of activity were computed according to the following formula: activity = (OD · Vt · 4)/(E · Δt · Vs), where OD is change in absorbance, Vt is total volume, Vs is sample volume, E (extinction coefficient) is 39 L · mmol−1 · cm−1, and Δt is change in time. The result was normalized to 1 mg of protein.
Statistical Analysis
Image analysis was performed by authors (M.N., cardiologist; J.W.C., radiologist; each with more than 10 years of experience in image analysis) who were blinded to the agent used or the mouse identity by using a Digital Imaging and Communications in Medicine viewer (OsiriX, Geneva, Switzerland; www.osirix-viewer.com). Because the animals were not disturbed during each imaging session, the same regions of interest (ROIs) were copied and used for all the time points for each animal for each imaging session (Fig E2 [online]). Signal from the aortic root and normal skeletal muscle was determined by placing equal ROIs in each anatomic region. For the aortic roots of the CAWS-injected mice, the ROIs were drawn to enclose thickened areas in the wall. For the aortic roots of the control mice, the ROIs were placed to enclose the entire vessel wall. One ROI per time point per animal was measured. Contrast-to-noise ratios (CNRs) were computed for each ROI according to the following formula: CNR = (ROIsite − ROImuscle)/SDnoise, where ROIsite is the region of possible MPO activity, ROImuscle is the ROI of muscle, and SDnoise is the standard deviation of noise. The resultant CNRs were corrected by subtracting out the precontrast CNR. The data for each time point comparing the imaging agents were tested for normality by using the Kolmogorov-Smirnov test (n = 7 for each group), which did not reject normality in the data sets. Therefore, for each time point, we performed the paired t test to test for significance between the paired groups (imaged with MPO-Gd or gadopentetate dimeglumine). For comparison of peroxidase activity, we performed one-way analysis of variance to compare between the vasculitis, sham-operated control, and MPO-deficient groups. A P value less than .05 was considered to indicate a statistically significant difference. The analysis was performed by using software (Prism, version 5.0; GraphPad Software, La Jolla, Calif).
Results
MPO-Gd Enhancement in Mice with and without Vasculitis
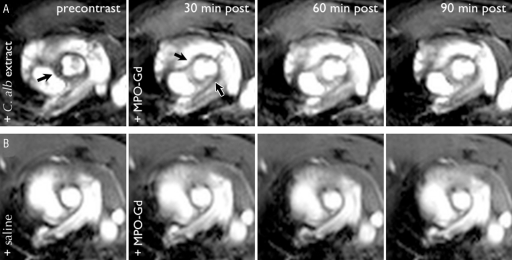
On precontrast images, the aorta in mice with vasculitis showed increased focal thickening of the wall compared with that in the sham-operated control mice without vasculitis (Figs 1, 2). In the control animals, the aortic root was smooth and regular in contour and appeared homogeneous in thickness and signal characteristics (Fig 1, B). After injection of MPO-Gd, we found increased contrast enhancement in the wall of the aortic root. The contrast enhancement increase was only mild in the control animals (mean CNR = 2.9) but marked in the CAWS-injected experimental animals (mean CNR = 28.1) at the 30-minute time point (Fig 1; Fig 2, C). Within all the mice in the CAWS-injected experimental group, there was focal enhancement in the thickened aortic wall (Fig 1, A). The aortic roots of the control mice showed rapid washout of contrast enhancement, and the CNR quickly returned to baseline (actually below baseline, indicating differential washout pharmacokinetics between the vascular wall and muscle) (Fig 1, B; Fig 2, C). In contrast, the mice with vasculitis showed persistent enhancement for greater than 90 minutes. This revealed that there was increased enhancement and altered pharmacokinetics in diseased vessel wall when imaged with the use of MPO-Gd.
Figure 1:
MPO-Gd–enhanced MR images of vasculitis. Serial MR images in, A, DBA/2 mouse induced with vasculitis through intraperitoneal injection of C albicans (C. alb) and in, B, DBA/2 mouse intraperitoneally injected with saline as sham-operated control mouse MR imaging of the aortic root was performed before contrast material administration followed by imaging at 30, 60, and 90 minutes after intravenous MPO-Gd administration. Aortic root enhancement (arrows) in an experimentally induced mouse is increased after contrast material administration. Little enhancement is present in the control mice. Note that because the imaging was performed by using a bright-blood technique, the hyperintense signal seen within the vessel lumen is from the imaging technique, not from the imaging agents.
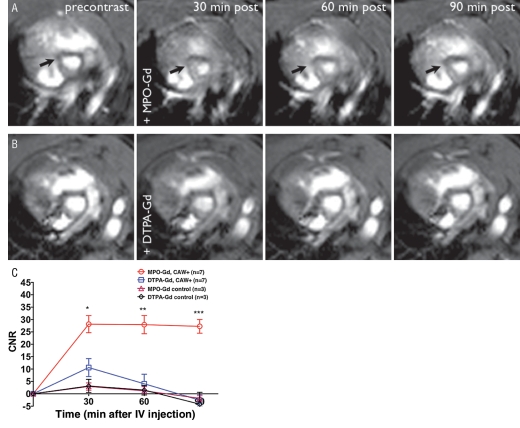
Figure 2:
A, MPO-Gd–enhanced MR images confirm the presence of elevated MPO activity and vasculitis. MPO-Gd identified areas of vasculitis (arrows) at the aortic root that cannot be visualized on precontrast images or on, B, gadopentetate dimeglumine (DTPA-Gd)–enhanced MR images in the same DBA/2 mouse. Persistent prolonged enhancement at 60 and 90 minutes is only seen in the MPO-Gd experimental group and confirms the presence of MPO activation of the agent. C, For CNRs, there was a nearly threefold increase in enhancement within the aortic root of vasculitis mice compared with sham-operated control mice at 30 minutes, and this difference in signal enhancement increased over time (more than sixfold at 60 minutes). At 90 minutes, while CNR on gadopentetate dimeglumine–enhanced images had returned to baseline, MPO-Gd–enhanced images retained 97% of the peak signal enhancement. The enhancement is maximally sustained when using the MPO-Gd agent in vasculitis mice but not when using MPO-Gd or gadopentetate dimeglumine in sham-operated mice or gadopentetate dimeglumine in vasculitis mice. (Figs E1, E2 [online]). ∗ = P < .05, ∗∗ = P < .01, ∗∗∗ = P < .001. CAW = C albicans water-soluble fraction, IV = intravenous.
Comparing MPO-Gd with Gadopentetate Dimeglumine in Vasculitis Mice
We found areas of significantly higher contrast enhancement with MPO-Gd (mean CNR = 28.1) than with gadopentetate dimeglumine (mean CNR = 10.6) (Fig 2) (P = .021) at the 30-minute time point, giving a 2.7-fold difference. The agents also differed in the temporal change in CNR. MPO-Gd–injected mice showed sustained contrast enhancement for up to 90 minutes (Fig 2, C). In contradistinction, CNR in the gadopentetate dimeglumine–injected mice decreased rapidly at 60 minutes before returning to baseline precontrast levels at 90 minutes (Fig 2, C). Thus, at the 60-minute time point, there was a 6.9-fold increase. At the 90-minute time point, while gadopentetate dimeglumine images had returned to precontrast levels, MPO-Gd images still retained 97% of the signal intensity increase (mean CNR of 28.1 at 30 minutes, 27.3 at 90 minutes). In control mice, similar to MPO-Gd, gadopentetate dimeglumine also showed only a small increase in CNR (Fig 2, C). In one mouse, MPO-Gd imaging apparently was able to depict areas of inflammation that could not be detected on the precontrast images or with conventional gadopentetate dimeglumine imaging (Fig 2, Fig E1 [online]).
Immune Cell Migration and MPO Immunoreactivity
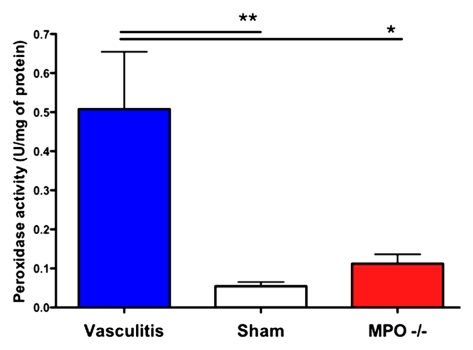
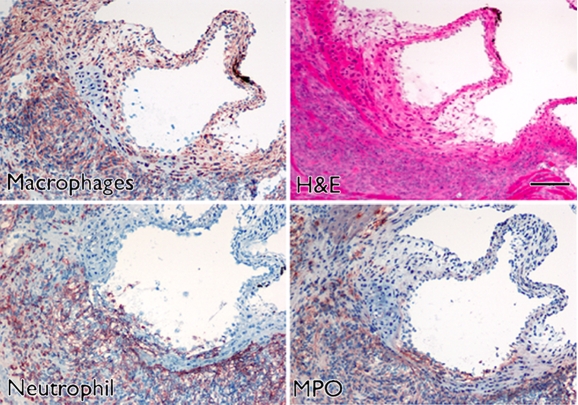
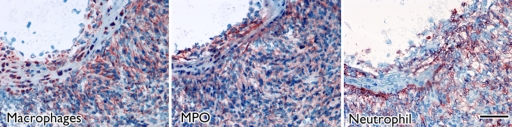
Focal thickening within the aortic root which demonstrated MPO-Gd enhancement on MR images showed diffuse myeloid cell infiltration, including neutrophils and macrophages that had a positive result for MPO (Fig 3), which identified them as the main cellular source of MPO. Further biochemical peroxidase assays confirmed elevated MPO activity in CAWS-injected vasculitis mice (Fig 4c). Extracts of the aortic root from mice induced with vasculitis showed that there was a 10-fold increase in MPO activity compared with that of the sham-operated control mice (P < .05) (Fig 4c).
Figure 3a:
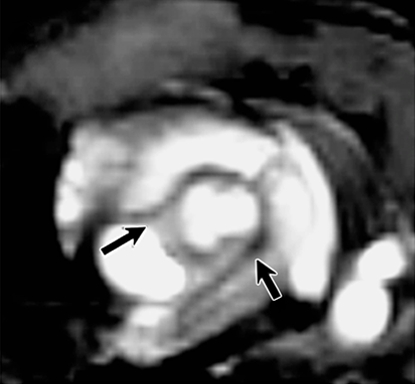
Aortic root enhancement corresponds to the presence of inflammatory cells and MPO immunostaining. (a) MPO-Gd–enhanced in vivo MR image at the level of the thickened aortic root (arrows). (b) Hematoxylin-eosin (H&E) staining was performed at the same anatomic level. Bar = 1 mm. (Original magnification, ×10.) (c) Immunostaining shows presence of migratory inflammatory cells (neutrophils and macrophages) that have a positive result for MPO (red staining) (bar = 250 μm). (Original magnification, ×100.) (d) Magnified views (bar = 60 μm). (Original magnification, ×400.)
Figure 4c:
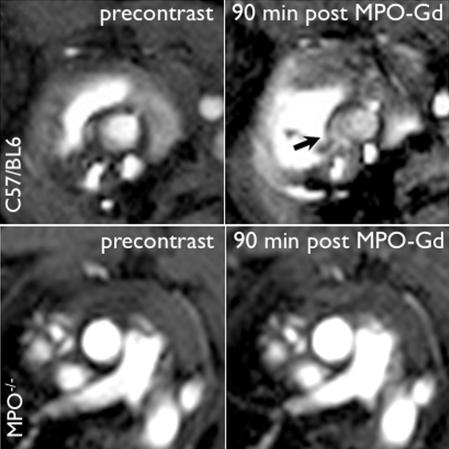
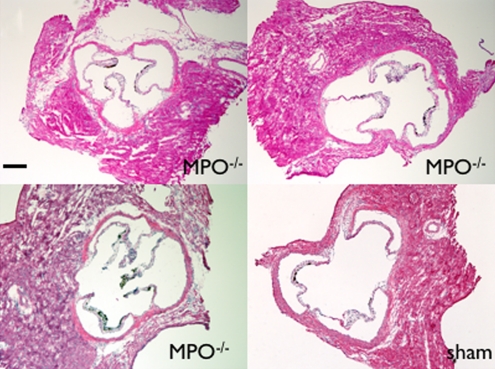
MPO-deficient (MPO−/−) mice cannot be induced with vasculitis. (a) MR images show that MPO-deficient (C57BL/6 background) mice imaged with MPO-Gd do not show wall thickening or prolonged enhancement, whereas control C57BL/6 mice showed wall thickening and prolonged MPO-Gd enhancement (arrow). (b) Graph of CNR image analysis at the 90-minute time point shows no evidence of elevated MPO activity in vivo for the MPO-deficient mice (n = 3) compared with control vasculitis mice (n = 5). Inset = polymerase chain reaction analysis confirmed the genotype of the MPO-deficient mice. Error bar = standard error of measurement. ∗∗∗ = P < .001. (c) Graph of peroxidase activity assays shows elevated peroxidase activity in the vasculitis mice (n = 7) but not in sham-operated (n = 3) or MPO-deficient (n = 3) mice. Error bars = standard error of measurement. ∗ = P < .05, ∗∗ = P < .01. (d) Histopathologic findings (n = 5, three representative specimens are shown) did not show wall thickening or inflammatory cell infiltration in MPO-deficient or sham-operated mice. Bar = 1 mm. (Hematoxylin-eosin stain; original magnification, ×10.)
Figure 3b:
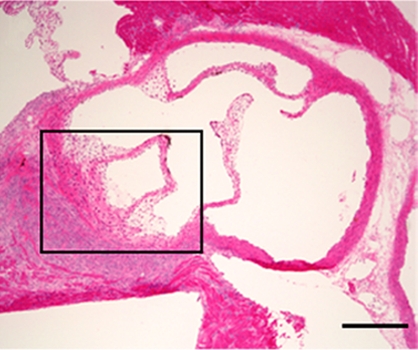
Aortic root enhancement corresponds to the presence of inflammatory cells and MPO immunostaining. (a) MPO-Gd–enhanced in vivo MR image at the level of the thickened aortic root (arrows). (b) Hematoxylin-eosin (H&E) staining was performed at the same anatomic level. Bar = 1 mm. (Original magnification, ×10.) (c) Immunostaining shows presence of migratory inflammatory cells (neutrophils and macrophages) that have a positive result for MPO (red staining) (bar = 250 μm). (Original magnification, ×100.) (d) Magnified views (bar = 60 μm). (Original magnification, ×400.)
Figure 3c:
Aortic root enhancement corresponds to the presence of inflammatory cells and MPO immunostaining. (a) MPO-Gd–enhanced in vivo MR image at the level of the thickened aortic root (arrows). (b) Hematoxylin-eosin (H&E) staining was performed at the same anatomic level. Bar = 1 mm. (Original magnification, ×10.) (c) Immunostaining shows presence of migratory inflammatory cells (neutrophils and macrophages) that have a positive result for MPO (red staining) (bar = 250 μm). (Original magnification, ×100.) (d) Magnified views (bar = 60 μm). (Original magnification, ×400.)
Figure 3d:
Aortic root enhancement corresponds to the presence of inflammatory cells and MPO immunostaining. (a) MPO-Gd–enhanced in vivo MR image at the level of the thickened aortic root (arrows). (b) Hematoxylin-eosin (H&E) staining was performed at the same anatomic level. Bar = 1 mm. (Original magnification, ×10.) (c) Immunostaining shows presence of migratory inflammatory cells (neutrophils and macrophages) that have a positive result for MPO (red staining) (bar = 250 μm). (Original magnification, ×100.) (d) Magnified views (bar = 60 μm). (Original magnification, ×400.)
MPO-deficient Mice
On MPO-Gd images in MPO-deficient mice, there was no evidence of elevated in vivo MPO activity (Fig 4a, 4b) or vascular wall thickening (Fig 4a). Similar findings as for DBA/2 mice were found for the C57BL/6 control mice with increased MPO-Gd enhancement consistent with elevated MPO activity (Fig 4a, 4b). The lack of in vivo MPO activity in the MPO-deficient mice was corroborated by enzymatic assays (Fig 4c) that demonstrated no significant increase in peroxidase activity compared with that in sham-operated mice (P = .56). Histopathologic findings also revealed no evidence of cellular infiltration or wall thickening (Fig 4d).
Figure 4a:
MPO-deficient (MPO−/−) mice cannot be induced with vasculitis. (a) MR images show that MPO-deficient (C57BL/6 background) mice imaged with MPO-Gd do not show wall thickening or prolonged enhancement, whereas control C57BL/6 mice showed wall thickening and prolonged MPO-Gd enhancement (arrow). (b) Graph of CNR image analysis at the 90-minute time point shows no evidence of elevated MPO activity in vivo for the MPO-deficient mice (n = 3) compared with control vasculitis mice (n = 5). Inset = polymerase chain reaction analysis confirmed the genotype of the MPO-deficient mice. Error bar = standard error of measurement. ∗∗∗ = P < .001. (c) Graph of peroxidase activity assays shows elevated peroxidase activity in the vasculitis mice (n = 7) but not in sham-operated (n = 3) or MPO-deficient (n = 3) mice. Error bars = standard error of measurement. ∗ = P < .05, ∗∗ = P < .01. (d) Histopathologic findings (n = 5, three representative specimens are shown) did not show wall thickening or inflammatory cell infiltration in MPO-deficient or sham-operated mice. Bar = 1 mm. (Hematoxylin-eosin stain; original magnification, ×10.)
Figure 4b:
MPO-deficient (MPO−/−) mice cannot be induced with vasculitis. (a) MR images show that MPO-deficient (C57BL/6 background) mice imaged with MPO-Gd do not show wall thickening or prolonged enhancement, whereas control C57BL/6 mice showed wall thickening and prolonged MPO-Gd enhancement (arrow). (b) Graph of CNR image analysis at the 90-minute time point shows no evidence of elevated MPO activity in vivo for the MPO-deficient mice (n = 3) compared with control vasculitis mice (n = 5). Inset = polymerase chain reaction analysis confirmed the genotype of the MPO-deficient mice. Error bar = standard error of measurement. ∗∗∗ = P < .001. (c) Graph of peroxidase activity assays shows elevated peroxidase activity in the vasculitis mice (n = 7) but not in sham-operated (n = 3) or MPO-deficient (n = 3) mice. Error bars = standard error of measurement. ∗ = P < .05, ∗∗ = P < .01. (d) Histopathologic findings (n = 5, three representative specimens are shown) did not show wall thickening or inflammatory cell infiltration in MPO-deficient or sham-operated mice. Bar = 1 mm. (Hematoxylin-eosin stain; original magnification, ×10.)
Figure 4d:
MPO-deficient (MPO−/−) mice cannot be induced with vasculitis. (a) MR images show that MPO-deficient (C57BL/6 background) mice imaged with MPO-Gd do not show wall thickening or prolonged enhancement, whereas control C57BL/6 mice showed wall thickening and prolonged MPO-Gd enhancement (arrow). (b) Graph of CNR image analysis at the 90-minute time point shows no evidence of elevated MPO activity in vivo for the MPO-deficient mice (n = 3) compared with control vasculitis mice (n = 5). Inset = polymerase chain reaction analysis confirmed the genotype of the MPO-deficient mice. Error bar = standard error of measurement. ∗∗∗ = P < .001. (c) Graph of peroxidase activity assays shows elevated peroxidase activity in the vasculitis mice (n = 7) but not in sham-operated (n = 3) or MPO-deficient (n = 3) mice. Error bars = standard error of measurement. ∗ = P < .05, ∗∗ = P < .01. (d) Histopathologic findings (n = 5, three representative specimens are shown) did not show wall thickening or inflammatory cell infiltration in MPO-deficient or sham-operated mice. Bar = 1 mm. (Hematoxylin-eosin stain; original magnification, ×10.)
Discussion
Currently, the imaging-based diagnosis of vasculitis may be difficult because it relies on anatomic changes and lacks a specific method that can confirm inflammation in vivo. This study demonstrated that a molecular imaging approach that targets the inflammatory enzyme MPO can be used to noninvasively identify sites of vascular wall inflammation in a murine model of Kawasaki disease. To validate MPO-Gd imaging results, we compared images obtained by using the MPO-specific agent MPO-Gd (27,28) and conventional gadopentetate dimeglumine in the same mice, as well as images in sham-operated control and MPO-deficient mice imaged with MPO-Gd. The in vivo findings were corroborated with biochemical and histopathologic analyses in tissue specimens. Our results showed that MPO is important in the development of Kawasaki vasculitis and can be a key functional imaging biomarker for assessing vasculitis at high anatomic resolution afforded by MR molecular imaging.
MPO-Gd MR imaging uses an activatable functional molecular marker of inflammation. MPO activates MPO-Gd by oxidizing the agent (21,26). The oxidized form of the agent can combine to form oligomers (21) or bind to certain phenolic amino acids (eg, tyrosine) in proteins (26). The activated form of MPO-Gd thus possesses slower molecular dynamics that result in shortening of the proton T1 and increased T1-weighted MR imaging signal, as well as prolonged enhancement due to retention of the agent at the site of MPO activity (21,26). Vasculitis imaging by using MPO-Gd takes advantage of both increased and prolonged enhancement to improve detection and confirm sites of elevated MPO activity and vascular wall inflammation. Potential applications for MPO-Gd imaging in vasculitides include detection of primary and secondary forms of cerebral vasculitis or glomerular renal disease.
A key aspect of a molecular imaging agent is that of specificity. Specificity of the MPO-Gd agent for MPO has been demonstrated in vitro and in vivo. In vitro, only MPO, but not eosinophil peroxidase, can significantly activate MPO-Gd (26). In vivo, we have performed imaging in MPO-deficient mice in mouse models of myocardial infarction (28), stroke (27), and heart transplantation (30). In these MPO-deficient mice, the signal enhancement did not increase at the sites of inflammation, consistent with lack of MPO-Gd activation and thus specificity for MPO. Interestingly, in heterozygous MPO-deficient mice with intermediate levels of MPO, there was correspondingly intermediate signal enhancement relative to the wild-type mice, further underscoring high specificity of the probe for MPO activity. An analog of MPO-Gd (bis-tyramide-diethylenetriaminepentaacetic acid-Gd) that is activatable by horseradish peroxidase but not by MPO was not able to report regions of MPO activity in tissue embedded with MPO (21) or the aortic vessel wall (29). We have also previously performed MPO MR imaging and MPO immunohistochemical correlation in an atherosclerosis model (29) where we found a high correlation value of 0.91 (P < .0001) between imaging and pathologic findings. A recent article (31) on activatable matrix metalloproteinase imaging agents that were reported to be tumor-specific revealed that there was activation and retention of that agent in the blood prior to reaching the tumor. While MPO-Gd is not organ or disease specific, we did not observe increased retention and activation in the blood in our biodistribution studies (21). In embedded tissue matrix with MPO, we observed a twofold increase in signal enhancement when imaged with MPO-Gd, while in the matrix devoid of MPO, we did not observe an increase in signal when using MPO-Gd. The signal intensity obtained with MPO-Gd in the absence of MPO was similar to the results from using gadopentetate dimeglumine as well as a nonfunctional analog of MPO-Gd in the presence or absence of MPO (21), which supports that there is lack of activation in the blood and no nonspecific distribution of the activated agent. We also did not find increased retention of the agent in the matrix experiments without MPO by using nuclear imaging and biodistribution methods (21).
Several other molecular imaging agents have been developed to image inflammation and have been approved for use clinically. Fluorine 18 fluorodeoxyglucose uptake in positron emission tomographic (PET) imaging is a sensitive functional technique that aims to measure glucose uptake as an indirect marker of inflammation. Use of PET to image large-vessel vasculitis has been reported, and one benefit may lie in the identification of additional regions of disease with the potential for follow-up and tracking of response to treatment (32). However, spatial resolution of PET is about 4 mm and may not be suitable for vasculitides affecting medium and small vessels, although PET/CT may improve the localization of the positron signal. A molecular imaging approach combined with contrast-enhanced MR imaging may provide both high anatomic resolution and pathologic information to improve diagnosis of vasculitis. A class of MR molecular imaging agents in use clinically is the iron oxide nanoparticles, which have been used to image vascular wall inflammation involved in atherosclerosis (33,34). These nanoparticles provide negative contrast as opposed to positive contrast from MPO-Gd and gadopentetate dimeglumine. The nanoparticles can be phagocytosed by inflammatory cells such as macrophages, monocytes, neutrophils, and lymphocytes but also by smooth muscle cells and endothelial cells (35). In addition, not all phagocytic cells are proinflammatory, with some subsets of these cells having been found to have attenuated inflammatory or even anti-inflammatory properties (36–39). Therefore, imaging only phagocytes could cause overestimation of the degree of inflammation. A combination of iron oxide nanoparticle and MPO-Gd imaging may provide information regarding how distinct cell types and subsets participate in inflammation.
Our study had a number of limitations. We used a bright-blood sequence instead of a black-blood sequence, such as inversion-recovery-prepared imaging techniques (29,40), which decreased the dynamic range of the images because the blood was the brightest structure on the images. This made the abnormal vascular wall more difficult to identify as the images saturated quickly at narrower (higher contrast) windows and levels. However, while a black-blood sequence would have allowed easier identification of the abnormal vessel wall, such a sequence would have also limited our ability to identify key vascular structures confidently because most of the structures on the image, prior to agent administration, would all be hypointense. This would have made it difficult to consistently reproduce, as closely as possible, the same anatomic location in the same animal on different days (eg, when comparing MPO-Gd with gadopentetate dimeglumine in the same mice). Even though the use of the bright-blood sequence decreased detection sensitivity, with use of MPO-Gd imaging, we were still able to identify the abnormal areas within the vessel walls. Another limitation was that despite best efforts, it was not possible to always exactly reproduce the same anatomic location in the same mice when they were imaged on different days. We minimized the effect of this by using well-defined anatomic landmarks, especially the pulmonary artery bifurcation, as the internal reference. Minor variations in the vessel locations can also occur during the imaging session from cardiac motion even though the animal has not moved. We also attempted to induce vasculitis in MPO-deficient mice to determine if we can see inflammatory cell infiltration indicating vasculitis without a corresponding positive MPO-Gd enhancement to further confirm MPO-Gd specificity in vivo. Surprisingly, despite successful inductions in DBA/2 and C57BL/6 mice, we were not able to observe any evidence of vascular wall inflammation in the MPO-deficient mice with MPO-Gd imaging, biochemical assays, and histopathologic analysis. Therefore, MPO appears to be essential in the development of vasculitis in this model. A previous study measuring MPO antineutrophil cytoplasmic antibody sera titer in MPO-deficient mice in a similar coronary vasculitis model did not find elevation of MPO antineutrophil cytoplasmic antibody compared with wild-type control mice (41).
Molecular and functional noninvasive assessment of vascular inflammation such as what we have demonstrated by using MPO-Gd in this study has the potential to aid in the clinical and imaging diagnosis of vasculitis when successfully translated. We are working toward translating MPO-Gd for human use. Toward that end, we have recently shown that MPO-Gd is not cytotoxic and that the gadolinium stability is substantially higher in MPO-Gd than in gadopentetate dimeglumine and gadodiamide (26) and have demonstrated that the agent is safe to use and remains highly sensitive and specific in large animals (rabbits) at clinical imaging field strengths (29).
Advances in Knowledge.
Myeloperoxidase (MPO)-targeted MR imaging increased contrast enhancement by more than 2.5-fold compared with gadopentetate dimeglumine in inflamed vascular walls and can be used to noninvasively identify and confirm highly inflamed areas in the vascular wall in vasculitis.
MPO-targeted MR imaging can help identify areas of vasculitis that could be missed at unenhanced and conventional contrast-enhanced MR imaging.
MPO plays an important role in the development of vasculitis in a mouse model of Kawasaki disease.
Disclosures of Potential Conflicts of Interest: H.S.S. No potential conflicts of interest to disclose. M.N. No potential conflicts of interest to disclose. P.R.P. No potential conflicts of interest to disclose. M.O.B. No potential conflicts of interest to disclose. E.R. No potential conflicts of interest to disclose. Y.I. No potential conflicts of interest to disclose. E.A. No potential conflicts of interest to disclose. R.W. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author receives money for board membership to T2 BioSystems (author is founder) and has stock in T2 BioSystems; author is consultant for Lumicell; author is employee of Massachusetts General Hospital; institution has many grants pending; author has many patents. Other relationships: none to disclose. J.W.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author is employee of Massachusetts General Hospital; institution has grants pending from Pfizer; author receives royalties from Elsevier for a book. Other relationships: none to disclose.
Supplementary Material
Received January 5, 2011; revision requested March 23; revision received June 21; accepted July 21; final version accepted August 15. E.R.
Current address: Department of Neuroradiology, Klinikum Rechts der Isar, Technische Universität München, Munich, Germany.
supported by a Marie Curie Fellowship from the European Commission.
Funding: This research was supported by the National Institutes of Health (grants K08 HL081170, R01 NS070835, R01 NS072167, and R01 HL078641).
From the 2008 RSNA Annual Meeting.
Abbreviations:
- CAWS
- Candida albicans water-soluble fraction
- CNR
- contrast-to-noise ratio
- MPO
- myeloperoxidase
- ROI
- region of interest
References
- 1.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009;68(3):310–317 [DOI] [PubMed] [Google Scholar]
- 2.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009;68(3):318–323 [DOI] [PubMed] [Google Scholar]
- 3.Imboden JB, Hellmann DB, Stone JH. Current rheumatology: diagnosis & treatment. New York, NY: Lange Medical Books/McGraw-Hill, Medical Pub. Division, 2004 [Google Scholar]
- 4.Jennette JC, Falk RJ. Nosology of primary vasculitis. Curr Opin Rheumatol 2007;19(1):10–16 [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Kallenberg CG. ANCA-associated vasculitides: advances in pathogenesis and treatment. Nat Rev Rheumatol 2010;6(11):653–664 [DOI] [PubMed] [Google Scholar]
- 6.Kallenberg CG, Heeringa P, Stegeman CA. Mechanisms of disease: pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol 2006;2(12):661–670 [DOI] [PubMed] [Google Scholar]
- 7.Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 2003;349(17):1595–1604 [DOI] [PubMed] [Google Scholar]
- 8.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005;25(6):1102–1111 [DOI] [PubMed] [Google Scholar]
- 9.Rutgers A, Heeringa P, Tervaert JW. The role of myeloperoxidase in the pathogenesis of systemic vasculitis. Clin Exp Rheumatol 2003;21(6 suppl 32):S55–S63 [PubMed] [Google Scholar]
- 10.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002;110(7):955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet 2006;368(9533):404–418 [DOI] [PubMed] [Google Scholar]
- 12.Falcini F. Kawasaki disease. Curr Opin Rheumatol 2006;18(1):33–38 [DOI] [PubMed] [Google Scholar]
- 13.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110(17):2747–2771 [DOI] [PubMed] [Google Scholar]
- 14.Murata H. Experimental candida-induced arteritis in mice: relation to arteritis in the mucocutaneous lymph node syndrome. Microbiol Immunol 1979;23(9):825–831 [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Oharaseki T, Wakayama M, Yokouchi Y, Naoe S, Murata H. Histopathological features of murine systemic vasculitis caused by Candida albicans extract: an animal model of Kawasaki disease. Inflamm Res 2004;53(2):72–77 [DOI] [PubMed] [Google Scholar]
- 16.Nagi-Miura N, Harada T, Shinohara H, et al. Lethal and severe coronary arteritis in DBA/2 mice induced by fungal pathogen, CAWS, Candida albicans water-soluble fraction. Atherosclerosis 2006;186(2):310–320 [DOI] [PubMed] [Google Scholar]
- 17.Geiger J, Ness T, Uhl M, et al. Involvement of the ophthalmic artery in giant cell arteritis visualized by 3T MRI. Rheumatology (Oxford) 2009;48(5):537–541 [DOI] [PubMed] [Google Scholar]
- 18.Küker W, Gaertner S, Nagele T, et al. Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis 2008;26(1):23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markl M, Uhl M, Wieben O, et al. High resolution 3T MRI for the assessment of cervical and superficial cranial arteries in giant cell arteritis. J Magn Reson Imaging 2006;24(2):423–427 [DOI] [PubMed] [Google Scholar]
- 20.Querol M, Chen JW, Weissleder R, Bogdanov A., Jr DTPA-bisamide-based MR sensor agents for peroxidase imaging. Org Lett 2005;7(9):1719–1722 [DOI] [PubMed] [Google Scholar]
- 21.Chen JW, Querol Sans M, Bogdanov A, Jr, Weissleder R. Imaging of myeloperoxidase in mice by using novel amplifiable paramagnetic substrates. Radiology 2006;240(2):473–481 [DOI] [PubMed] [Google Scholar]
- 22.Uchiyama M, Ohno N, Miura NN, et al. Chemical and immunochemical characterization of limulus factor G-activating substance of Candida spp. FEMS Immunol Med Microbiol 1999;24(4):411–420 [DOI] [PubMed] [Google Scholar]
- 23.Chen JW, Breckwoldt MO, Aikawa E, Chiang G, Weissleder R. Myeloperoxidase-targeted imaging of active inflammatory lesions in murine experimental autoimmune encephalomyelitis. Brain 2008;131(Pt 4):1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell DH, Ni Dhubhghaill OM, Pubanz D, et al. Structural and dynamic parameters obtained from 17O NMR, EPR, and NMRD studies of monomeric and dimeric Gd3+ complexes of interest in magnetic resonance imaging: an integrated and theoretically self-consistent approach. J Am Chem Soc 1996;118(39):9333–9346 [Google Scholar]
- 25.Bui T, Stevenson J, Hoekman J, Zhang S, Maravilla K, Ho RJ. Novel Gd nanoparticles enhance vascular contrast for high-resolution magnetic resonance imaging. PLoS ONE 2010;5(9):pii: e13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez E, Nilges M, Weissleder R, Chen JW. Activatable magnetic resonance imaging agents for myeloperoxidase sensing: mechanism of activation, stability, and toxicity. J Am Chem Soc 2010;132(1):168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breckwoldt MO, Chen JW, Stangenberg L, et al. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A 2008;105(47):18584–18589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahrendorf M, Sosnovik D, Chen JW, et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation 2008;117(9):1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronald JA, Chen JW, Chen Y, et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation 2009;120(7):592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swirski FK, Wildgruber M, Ueno T, et al. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest 2010;120(7):2627–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Duijnhoven SM, Robillard MS, Nicolay K, Grüll H. Tumor targeting of MMP-2/9 activatable cell-penetrating imaging probes is caused by tumor-independent activation. J Nucl Med 2011;52(2):279–286 [DOI] [PubMed] [Google Scholar]
- 32.Walter MA. [(18)F]fluorodeoxyglucose PET in large vessel vasculitis. Radiol Clin North Am 2007;45(4):735–744, viii [DOI] [PubMed] [Google Scholar]
- 33.Sigovan M, Boussel L, Sulaiman A, et al. Rapid-clearance iron nanoparticles for inflammation imaging of atherosclerotic plaque: initial experience in animal model. Radiology 2009;252(2):401–409 [DOI] [PubMed] [Google Scholar]
- 34.Hyafil F, Laissy JP, Mazighi M, et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: relationship between signal loss and macrophage infiltration. Arterioscler Thromb Vasc Biol 2006;26(1):176–181 [DOI] [PubMed] [Google Scholar]
- 35.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 2008;117(3):379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol 2002;72(1):101–106 [PubMed] [Google Scholar]
- 37.Sunderkötter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 2004;172(7):4410–4417 [DOI] [PubMed] [Google Scholar]
- 38.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5(12):953–964 [DOI] [PubMed] [Google Scholar]
- 39.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204(12):3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helm PA, Caravan P, French BA, et al. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology 2008;247(3):788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishida-Okawara A, Oharaseki T, Takahashi K, et al. Contribution of myeloperoxidase to coronary artery vasculitis associated with MPO-ANCA production. Inflammation 2001;25(6):381–387 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.