Abstract
Background
Sedentary behavior is associated with adiposity and cardiometabolic risk.
Purpose
To determine the associations between sedentary behavior and measures of adiposity-associated inflammation.
Methods
Between 2002 and 2005, a total of 1543 Multi-Ethnic Study of Atherosclerosis participants completed detailed health history questionnaires, underwent physical measurements and had blood assayed for adiponectin, leptin, tumor necrosis factor – alpha (TNF - α) and resistin. Analyses included linear regression completed in 2010. The mean age was 64.3 years and nearly 50% were female. Forty-one percent were non-Hispanic white, 24% Hispanic-American, 20% African-American, and 14% Chinese-American.
Results
In linear regression analyses and with adjustment for age, gender, ethnicity, education, BMI, smoking, alcohol consumption, hypertension, diabetes mellitus, dyslipidemia, hormone therapy and waist circumference, sedentary behavior was associated with higher natural log (“ln”) of leptin and ln TNF - α but a lower ln adiponectin-to-leptin ratio (β = 0.07, β = 0.03 and –0.07, p < 0.05 for all). Compared to the first tertile, and after the same adjustment, the second and third tertiles of sedentary behavior were associated with higher levels of ln leptin (β = 0.11and β = 0.12, respectively; p < 0.05 for both) but lower levels of the adiponectin-to-leptin ratio (β = –0.09 and –0.11, respectively; p < 0.05 for both).
Conclusions
Sedentary behavior is associated with unfavorable levels of adiposity-associated inflammation.
Introduction
It is well established that higher levels of physical activity are associated with lower risk for cardiometabolic diseases (CMD) including the metabolic syndrome, type 2 diabetes (T2D) and cardiovascular disease (CVD).1,2 On the other hand, epidemiologic studies are now beginning to show that prolonged sedentary behavior is associated with an increased risk for these same disorders.3–6 More specifically, large prospective studies have indicated that time spent sitting, and TV viewing in particular, are associated with risk of mortality.7,8 These findings are important because the observed associations are independent of demographic factors (age, gender), negative health behaviors (smoking, alcohol consumption) and positive health behaviors such as moderate-to-vigorous physical activity begging the question of what links sedentary behavior to CMD.
Unlike the evidence for the association between both physical activity and sedentary behavior and risk of CMD, relatively little is known about specific mechanisms linking these behaviors and CMD, as well as pathways involving body composition.9 In this regard, adipokines are signaling proteins (cytokines) produced mainly by adipocytes. While the principal function of adipokines is to regulate glucose metabolism and insulin resistance, as well as fatty acid metabolism in skeletal muscle, these cytokines are also involved in a number of other regulatory and inflammatory processes.10 For example, leptin regulates appetite and influences monocyte activation, phagocytosis, and cytokine production,11 whereas adiponectin reduces the production and activity of TNF-α and interleukin-6 while also limiting induction of the endothelial adhesion molecules ICAM-1 and vascular cell adhesion molecule 1.12,13 Based on these properties, adiponectin has been considered to be anti-inflammatory and anti-atherogenic.
Given the links between sedentary behavior and the accumulation of adipose tissue, and that adipokines are physiologically relevant to CMD, the purpose of this study was to examine, within the context of a multi-ethnic cohort, the associations of sedentary behavior with adiposity-associated measures of inflammation (adiponectin, leptin, TNF-α, resistin) and to determine if the associations are independent of abdominal obesity.
Methods
Subjects
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study of four ethnic groups including African, Chinese and Hispanic Americans, as well as non-Hispanic whites. Details about the study design for the MESA have been published.14 In brief, between July 2000 and August 2002, a total of 6814 men and women aged 45–84 years who were free of clinically apparent cardiovascular disease (CVD) were recruited from six U.S. communities. Individuals with a history of physician-diagnosed heart attack, angina, heart failure, stroke or transient ischemic attack (TIA), or having undergone an invasive procedure for cardiovascular disease (coronary artery bypass graft, angioplasty, valve replacement or pacemaker placement) were excluded from participation. Enrolled participants returned for follow-up clinic visits approximately 2, 4 and 6 years after the baseline clinic visit.
At clinic visits 2 and 3 (from 2002 to 2005), a random subsample of 1970 participants (approximately one half at each visit) enrolled in an ancillary study that is examining the association of body composition and inflammation with both clinical and subclinical CVD. The data obtained on these participants comprise the sample for the current study.
Data Collection
At all clinic visits, standardized questionnaires were used to obtain sociodemographic, ethnicity and health history information. Cigarette smoking was defined as current, former, or never. Height and weight were measured with participants wearing light clothing and no shoes. BMI was calculated by standard formula. Waist and hip circumferences were measured using a standard flexible tape measure.
Physical Activity Assessment
At each clinic visit, MESA participants completed the Typical Week Physical Activity Survey (TWPAS), which was adapted from the Cross-Cultural Activity Participation Study15 and designed to identify the time spent in and frequency of various physical activity during a typical week in the past month. The survey has 28 items in categories of household chores, lawn/yard/garden/farm, care of children/adults, transportation, walking (not at work), dancing and sport activities, conditioning activities, leisure activities, and occupational and volunteer activities. Participants were first asked if they participated in these categories of activity (yes/no), and if yes, they answered the questions regarding the average number of days per week and time per day engaged in these activities. If appropriate, questions also differentiated the intensity of activities as light, moderate and vigorous. The survey also queried for sedentary behavior to include time spent reading, sitting, nonrecreational computer, and watching TV.
Laboratory
At the clinic visits and after a 12-hour fast, venous blood was collected and then shipped to the MESA central laboratory for measurement of total and HDL cholesterol, triglycerides, and glucose levels.16 Fasting blood was also assayed for measures of systemic inflammation (C-reactive protein [CRP], fibrinogen, interleukin-6) and insulin concentration. Dyslipidemia was defined as a total-cholesterol/HDL-cholesterol ratio > 5.0 or if the participant used medication to reduce cholesterol. Diabetes was defined as fasting glucose ≥ 126 mg/dL or use of hypoglycemic medication.
Stored fasting blood samples from visits 2 and 3 were analyzed to provide levels of adiponectin, leptin, TNF - α and resistin. These adipokines were measured using Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Average analytic coefficients of variation across several control samples for these analytes ranged from 6.0% to 13.0%.
Statistical Analysis
Analyses utilized contemporaneous data from clinic visits 2 and 3 and were completed in 2010. Some MESA participants did not complete the physical activity questionnaire. These, along with those missing other covariates resulted in 427 being excluded from the analyses, resulting in a final analytic sample of 1,543 participants. Characteristics of the population were determined with an M and SD for continuous variables, while categoric variables were summarized as a count and percentage of the study population. Skewed variables are presented by median (interquartile ranges). ANCOVA was used to determine the means of adipokines by tertiles of sedentary behavior after adjusting for age, gender, and race. To normalize the distribution, adipokines (denoted by ‘ln’ before the name of the specific marker) were log-transformed and then linear regression analysis was used to determine the association between these variables and both physical activity and sedentary behavior in continuous and categoric (tertiles) forms.
As previous studies have shown the importance of contemporaneous information on adiponectin and leptin17, the adiponectin-to-leptin ratio was computed and this variable was utilized in the analysis. The initial Model 1 was adjusted for age, gender, race/ethnicity and education. Model 2 included Model 1 + BMI, smoking, alcohol consumption, dyslipidemia, hypertension, diabetes mellitus and hormone replacement therapy. Model 3 included Model 2 + waist circumference. Additional adjustments for the waist-to-hip ratio and moderate-to-vigorous physical activity were also conducted. Multiplicative interactions between race and physical activity/sedentary behavior for the different adipokines were assessed. None were significant. A two-tailed p-value < 0.05 was considered significant. All statistical analyses were conducted using STATA (Version 11.0; StataCorp,).
Results
The characteristics of the study cohort are provided in Table 1. The mean age was 64.3 years and nearly 50% were female. Forty-one percent were non-Hispanic white, 24.4% were Hispanic-American, 20% were African-American and just over 14% were Chinese-American. The mean BMI was 28.0; waist circumference and waist-to-hip ratio were 97.5 cm and 0.93, respectively. Over half the sample was current or former smokers while 45% had hypertension, 16.6% had dyslipidemia, 8.5% had diabetes mellitus, and 15.1% had a family history of cardiovascular disease. The median adiponectin, leptin, TNF-α, resistin and adiponectin-to-leptin ratio values were 17.3 μg/ml, 13.0 ng/ml, 4.6 pg/ml, 14.7 ng/ml and 1.37, respectively. The mean level of sedentary behavior was 2205 MET-minutes/week, which was equivalent to 46% of the [waking] time. Sixty-four percent of the population reported no vigorous physical activity.
Table 1.
Characteristics of the study cohort
| Characteristic | Value |
|---|---|
| Age (years)* | 64.3 (9.6) |
| Female† | 50.2 (775) |
| Ethnicity† | - |
| Caucasian | 41.2 (635) |
| Chinese-American | 14.3 (220) |
| African-American | 20.2 (312) |
| Hispanic | 24.4 (376) |
| Smoking† | - |
| Former | 42.1 (650) |
| Current | 11.0 (164) |
| Current Alcohol Use† | 52.4 (808) |
| BMI* | 28.0 (5.1) |
| Hypertension† | 45.1 (696) |
| Dyslipidemia† | 16.6 (256) |
| Diabetes† | 8.5 (131) |
| Family History of CVD† | 15.1 (233) |
| Waist Circumference (cm)* | 97.5 (14.0) |
| Hip Circumference (cm)* | 104.2 (11.0) |
| Waist:Hip Ratio* | 0.93 (0.07) |
| CRP (mg/L)∞ | 1.39 (0.7–3.2) |
| IL-6 (pg/mL)∞ | 1.8 (1.2–2.9) |
| Fibrinogen (mg/dL)∞ | 425 (375–478) |
| Adiponectin (μg/mL)∞ | 17.3 (11.8–26.1) |
| Leptin (ng/mL)∞ | 13.0 (5.5–27.8) |
| TNF-alpha (pg/mL)∞ | 4.6 (3.4–6.3) |
| Resistin (ng/mL)∞ | 14.7 (11.7–18.6) |
| Adiponectin:Leptin Ratio∞ | 1.37 (0.58–3.59) |
M (SD)
% (Freq)
Median (interquartile range)
To aid in determining if there are threshold or nonlinear associations, the mean adipokine levels by tertile of the sedentary activity are provided in Table 2. With adjustment for age, gender, and race, the mean level of adiponectin decreased across increasing tertiles of sedentary behavior while leptin levels increased. Similarly, as sedentary activity increased, the ratio of adiponectin to leptin decreased. The increase in resistin was more modest and there did not appear to be a trend for TNF - α.
Table 2.
Adjusted mean adipokine levels by tertile of sedentary behaviora
| Adipokine | Sedentary Behavior | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| Adiponectin (μg/mL) | 17.9 | 17.2 | 16.9 |
| Leptin (ng/mL) | 9.32 | 12.8 | 13.4 |
| Resistin (ng/mL) | 14.6 | 14.9 | 14.9 |
| TNF-alpha (pg/mL) | 4.48 | 4.79 | 4.60 |
| Adiponectin/Leptin Ratio | 1.91 | 1.35 | 1.26 |
Adjusted for age, gender, and race
Tertile cut-points (metabolic syndrome—min/week × 1000): T1 = <1.6, T2 = 1.6 – 3.1, T3 = >3.1
Tertile cut-points (minutes/week): T1 = <1280, T2 = 1280 – 2480, T3 = >2480
Multivariable linear regression models were constructed to determine the independent associations between sedentary behavior and the adipokines (Table 3). With adjustment for age, gender, ethnicity and education, a 1-SD increment in sedentary behavior was associated with higher ln leptin and ln TNF- α (β = 0.12 [1.13 ng/ml] and 0.04 [1.04 pg/ml], respectively; p < 0.05 for both), as well as the ratio of adiponectin to leptin (β = –0.17, p < 0.01). When the models were additionally adjusted for BMI, smoking, alcohol consumption, dyslipidemia, as well as waist circumference, the associations were attenuated but remained significant (β = 0.07 [1.07 ng/ml], 0.03 [1.03 pg/ml] and –0.08, respectively; p < 0.05 for all). None of these associations was materially altered when waist circumference was replaced by the waist-to-hip ratio or when sedentary behavior was adjusted for moderate-to-vigorous physical activitiy.
Table 3.
Multivariable linear regression models for the association between sedentary behavior and various adipokines
| Model | Ln Adiponectin | Ln Leptin | Ln ALR | Ln TNF - α | Ln Resistin |
|---|---|---|---|---|---|
| 1 | –0.02 (–0.05, 0.00) | 0.15 (0.10, 0.20)** | –0.17 (–0.23, –0.11)** | 0.04 (0.01, 0.06)** | 0.02 (0.00, 0.04) |
| 2 | 0.00 (–0.03, 0.03) | 0.07 (0.04, 0.11)** | –0.08 (–0.12, –0.03)** | 0.03 (0.01, 0.06)** | 0.02 (–0.01, 0.03) |
| 3 | 0.00 (–0.02, 0.02) | 0.07 (0.03, 0.10)** | –0.07 (–0.11, –0.02)** | 0.03 (0.00, 0.06)** | 0.01 (–0.01, 0.03) |
*Per SD Increment of Sedentary Behavior (790 MET-minutes/week)
p< 0.05
Parameter Estimate [in ln(adipokine) units] (95% CI)
Ln = Natural Log, ALR = Adiponectin-to-Leptin Ratio
Model 1 = Adjusted for age, gender, ethnicity, education
Model 2 = Adjusted for variables in model 1 + BMI, smoking, alcohol consumption, hypertension, diabetes mellitus, dyslipidemia, hormone therapy
Model 3 = Adjusted for variables in model 2 + waist circumference
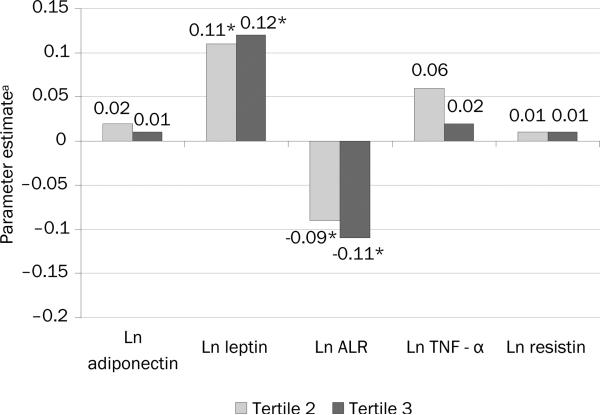
To determine if the associations were stronger by level of sedentary behavior or if there were threshold effects, the regression analysis was performed using tertiles. After adjustment for age, gender, ethnicity, education, BMI, smoking, alcohol consumption, dyslipidemia and waist circumference, and compared to the first tertile, the second and third tertiles of sedentary behavior were associated with significantly higher levels of ln leptin (β = 0.11 (1.12 ng/ml), 95% CI= 0.02, 0.21 and β = 0.12 (1.13 ng/ml), 95% CI=0.02, 0.22, respectively) and a nearly significant lower adiponectin-to-leptin ratios (β = – 0.09, 95% CI= –0.19, 0.02 and β = –0.11, 95% CI= –0.23, 0.01; [Figure 1]).
Figure 1.
Multivariable associations between tertiles of sedentary behavior and levels of adipokines
There were no other associations for sedentary behavior. For the associations provided above, none was not altered when waist circumference was replaced by the waist-to-hip ratio. This was also true when the associations were adjusted for moderate-to-vigorous physical activity, except for the case of the adiponectin to leptin ratio. In this situation, the associations were modestly attenuated and became less significant (β = –0.07, [95% CI= –0.18, 0.04] and β = –0.08, [95% CI= –0.20, 0.03], for the second and third tertiles respectively).
Discussion
In this cross-sectional study of a relatively large multi-ethnic sample from several sites across the U.S., there were associations between sedentary behavior and markers of adiposity-associated inflammation. Specifically, higher levels of sedentary behavior were associated with higher levels of both leptin and TNF - α, as well as lower adiponectin-to-leptin ratios, independent of relevant covariates to include moderate-to-vigorous physical activity. Notably, the magnitudes of the associations did not vary by ethnic group. These results suggest that sedentary behaviors may influence levels of certain markers of adiposity-associated inflammation irrespective of relevant anthropometric measures including those related to central adiposity.
There is a large body of literature indicating that higher levels of sedentary behavior are associated with increased weight and/or fatness.18 For instance, data from the Nurses Health Study indicate that every 2 hours per day of watching TV was associated with a 23% higher risk for becoming obese over 6 years of follow-up.4 Similarly, sedentary behavior is also associated with unfavorable metabolic characteristics. Adult women who spend ≥2hours per day watching TV have a 14% higher risk for developing diabetes.4 Moreover, compared to adults who spent <1 hour watching TV or using a computer, those who spent >4 hours in these kinds of activities had over twice the odds for metabolic syndrome.19
Importantly, the results suggest that the association between sedentary behavior and adiposity-associated inflammation is independent of BMI and central adiposity. In this regard, sedentary behavior has been linked to immune-system dysfunction, chronic systemic inflammation, pulmonary diseases, musculoskeletal disorders and other chronic diseases.19–22 More specifically, there is evidence that although not the predominant source of leptin, this adipokine is also secreted by skeletal muscle.23
Additionally, contracting skeletal muscle elaborates interleukin-6 (IL6); a cytokine with numerous metabolic influences and that has local and distant effects. For instance, with exercise, IL6 causes lipolysis of adipose tissue while also stimulating hepatic glucose production and increasing IL6 sensitivity in skeletal muscle.24 While the increased consumption of fat (lipolysis) may result in a decrease in fat mass and therefore a decrease in leptin, decreases in intramyocellular triglyceride with exercise are associated with improved insulin sensitivity of skeletal muscle and can thereby result in lower levels of leptin.25 Taken together, it is plausible that the changes in leptin levels are due to not only changes in adipose tissue mass, but also metabolism in skeletal muscle.
Much of the research on physical activity and cardiometabolic diseases has focused on moderate-to-vigorous physical activity. There are few data on how adipokines or subsequent inflammatory biomarkers of CMD are related to sedentary behavior independent of moderate-to-vigorous physical activity. Although exercise and other structured moderate-to-vigorous physical activity contributes meaningfully to physical activity thermogenesis, most individuals spend less than 5% of their waking hours engaged in this type of activity when it is measured objectively.26
The vast majority of the between-subject variance in physical activity thermogenesis can be explained by low- and very low–intensity movement such as posture (lying, sitting, and standing), incidental movement (e.g., fidgeting, talking, and typing), and light-intensity ambulation (e.g., walking, doing household chores). As such, the study of sedentary behavior and its links to adiposity, muscle physiology and inflammation is very important to efforts aimed at understanding and preventing the spread of obesity and its consequences.27,28
The strengths of this study include a relatively large sample that is multi-ethnic and free of clinical cardiovascular disease at baseline thereby reducing the likelihood of reverse confounding. Also, the inflammatory markers were performed at a central laboratory with a high level of reproducibility. Limitations of this study include self-reported measures of physical activity and sedentary behavior, as well as the use of anthropometric measures as a proxy for body composition. The latter may contribute to residual confounding in the association between sedentary behavior and the adipokines. Also, the amount of time reported in sedentary behavior is somewhat less than previously reported.29 Importantly, as the MESA was not conducted using a probability-based sampling scheme, the results of this study may not be generalizable to the overall population in the U.S.
Conclusion
In a cohort composed of multiple ethnic groups who were free of clinical CVD at baseline, sedentary behavior was associated with a less favorable profile of inflammatory markers related to adiposity. These results provide additional evidence for the detrimental effects of sedentary behavior with respect to cardiometabolic health and that appear to be independent of other relevant factors.
Acknowledgements
This research was supported by a grant (5R01-HL-088451) and contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-NHLBI.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
References
- 1.Physical activity guidelines advisory committee . Physical activity guidelines for Americans advisory committee report, 2008. DHHS; Washington, D.C.: 2008. [Google Scholar]
- 2.DHHS . Physical activity and health: a report of the Surgeon General. DHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- 3.Sisson SB, Camhi SM, Church TS, et al. Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metab Syndr Relat Disord. 2009 Dec;7(6):529–536. doi: 10.1089/met.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003 Apr 9;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 5.Beunza JJ, Martinez-Gonzalez MA, Ebrahim S, et al. Sedentary behaviors and the risk of incident hypertension: the SUN Cohort. Am J Hypertens. 2007 Nov;20(11):1156–1162. doi: 10.1016/j.amjhyper.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Thorp AA, Healy GN, Owen N, et al. Deleterious associations of sitting time and television viewing time with cardio-metabolic risk biomarkers: AusDiab 2004-2005. Diabetes Care. 2009 Nov 16; doi: 10.2337/dc09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunstan DW, Barr EL, Healy GN, et al. Television Viewing Time and Mortality. The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation. 2010 Jan 11; doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009 May;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 9.Telford RD. Low physical activity and obesity: causes of chronic disease or simply predictors? Med Sci Sports Exerc. 2007 Aug;39(8):1233–1240. doi: 10.1249/mss.0b013e31806215b7. [DOI] [PubMed] [Google Scholar]
- 10.Dyck DJ, Heigenhauser GJF, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiologica. 2006;186(1):5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 11.La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med. 2004 Jan;82(1):4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- 12.Masaki T, Chiba S, Tatsukawa H, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004 Jul;40(1):177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999 Dec 21–28;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002 Nov 1;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999 Jul–Aug;8(6):805–813. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 17.Zaletel J, Barlovic DP, Prezelj J. Adiponectin-leptin ratio: a useful estimate of insulin resistance in patients with Type 2 diabetes. Journal of endocrinological investigation. 2010 Sep;33(8):514–518. doi: 10.1007/BF03346639. [DOI] [PubMed] [Google Scholar]
- 18.Must A, Tybor DJ. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes Relat Metab Disord. 2005;29(S2):S84–S96. doi: 10.1038/sj.ijo.0803064. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES, Kohl HW, Mokdad AH, Ajani UA. Sedentary Behavior, Physical Activity, and the Metabolic Syndrome among U.S. Adults. Obesity. 2005;13(3):608–614. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 20.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman-Goetz L, Pedersen BK. Exercise and the immune system: a model of the stress response? Immunology Today. 1994;15(8):382–387. doi: 10.1016/0167-5699(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 22.Handschin C, Spiegelman BM. The role of exercise and PGC1[alpha] in inflammation and chronic disease. Nature. 2008;454(7203):463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Practice & Research Clinical Endocrinology & Metabolism. 2002;16(4):653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen BK, Edward F. Adolph Distinguished Lecture: Muscle as an endocrine organ: IL-6 and other myokines. Journal of Applied Physiology. 2009 October 1;107(4):1006–1014. doi: 10.1152/japplphysiol.00734.2009. 2009. [DOI] [PubMed] [Google Scholar]
- 25.Meier U, Gressner AM. Endocrine Regulation of Energy Metabolism: Review of Pathobiochemical and Clinical Chemical Aspects of Leptin, Ghrelin, Adiponectin, and Resistin. Clin Chem. 2004 September 1;50(9):1511–1525. doi: 10.1373/clinchem.2004.032482. 2004. [DOI] [PubMed] [Google Scholar]
- 26.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the U.S. measured by accelerometer. Med Sci Sports Exerc. 2008 Jan;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 27.Marshall SJ, Ramirez E. Reducing sedentary behavior: a new paradigm for physical activity promotion. American Journal of Lifestyle Medicine. in press. [Google Scholar]
- 28.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010 Jul;38(3):105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews CE, Chen KY, Freedson PS, et al. Amount of Time Spent in Sedentary Behaviors in the U.S., 2003‚Äì2004. American Journal of Epidemiology. 2008 April 1;167(7):875–881. doi: 10.1093/aje/kwm390. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]