Abstract
Oxidative stress plays a significant role in allergic airway inflammation. Supplementation with alpha-tocopherol (alone or combined with ascorbate/vitamin C) has been assessed as an intervention for allergic airway diseases with conflicting results. Enhancing levels of airway antioxidants with oral supplements has been suggested as an intervention to protect individuals from the effect of inhaled oxidants, although it is unclear whether supplementation changes tocopherol or vitamin C levels in both serum and airway fluids. Our objective was to obtain pilot safety and dosing data from 14 allergic asthmatic volunteers examining the effect of daily combination oral therapy with 500 mg alpha-tocopherol (αT) and 2 g vitamin C for 12 wk. We examined serum and airway fluid and cellular levels of alpha- and gamma-tocopherol (γT) and vitamin C to plan for future studies of these agents in asthma and allergic rhinitis. Six volunteers completed 12 wk of active treatment with αT and vitamin C and 8 completed placebo. Blood and sputum samples were obtained at baseline and at 6 wk and 12 wk of therapy and were analyzed for αT, γT, and vitamin C levels in the serum, sputum supernatant, and sputum cells. Combination treatment increased serum vitamin C and significantly decreased sputum αT and serum γT levels. No changes were found in sputum supernatant or sputum cell vitamin C or serum αT levels in the active treatment group. In conclusion, supplementation with αT and high-dose vitamin C does not augment vitamin C levels in the respiratory-tract lining fluid.
Due to its role in oxygen exchange and its exposure to the external environment, the lung is particularly susceptible to oxidant stress. There is a growing body of evidence that redox homeostasis is an essential element in airway inflammation that is central to the pathophysiology of asthma, environmental lung disease, and smoking-related disorders (Cross et al., 2002). In studies focused on asthma, nutritional deficiencies in antioxidants like vitamin E, vitamin C, and selenium have been linked to asthma prevalence (Gilliland et al., 2003), including large epidemiologic studies associating lower serum levels of vitamin C with increased risk of wheezing (Schwartz & Weiss, 1990), While much remains to be learned about dietary factors and asthma pathogenesis, several studies do suggest that enhancing antioxidant and anti-inflammatory defenses is a logical target for development of complementary and alternative medicine (CAM) therapies for use by persons with allergic diseases.
The data on antioxidant supplementation in asthma is mixed. Several investigators have reported that vitamin C supplementation decreases bronchial reactivity to methacholine and histamine (Zuskin et al., 1973; Mohsenin et al., 1983) and exercise-induced bronchospasm (Schachter & Schlesinger, 1982). A double-blinded, placebo-controlled trial with Nigerian asthmatics taking 1 g/day of vitamin C showed decreased rate and severity of asthma exacerbations. (Anah et al., 1980) However, more recent studies have found no clinical benefit on asthma with regular dietary supplementation with vitamin C (Fogarty et al., 2003). Studies evaluating the effect of α-tocopherol (vitamin E) supplementation have also had mixed results, with one study finding no clinical benefit in asthma patients after 6 wk of supplementation (Pearson et al., 2004), and another study finding no benefit on the incidence of pulmonary symptoms in COPD patients after 3 yr of supplementation (Rautalahti et al., 1997).
In addition to its action as a direct radical scavenger, vitamin C protects lipids from oxidative stress and can transform the oxidized product of α-tocopherol, α-tocopheroxyl, back to its original reduced form in vitro. These observations have led to investigation of combination therapy using vitamin C and α-tocopherol in a number of disease states, including environmental lung disease. In a series of reports on the use of combination antioxidant interventions, several groups have found that such combination therapy attenuated loss of lung function following natural or experimental ozone exposure compared to placebo controls (Romieu et al., 2002) (Trenga, Koenig et al., 2001). Of a cohort of normal volunteers who undertook an antioxidant-depleted diet prior to ozone challenge, those who received supplementation with vitamin E and vitamin C for 1 wk displayed a trend toward reduced decreases in forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) after ozone challenge compared to placebo controls (Samet et al., 2001).
Our group has also reported that levels of vitamin C in respiratory-tract lining fluids of asthmatics sampled by induced sputum are diminished relative to normal volunteers (Kongerud, et al., 2003). This observation is consistent with the hypothesis that asthmatics have increased susceptibility to the effects of ozone, and the idea that antioxidant supplementation would protect asthmatics from the effect of ozone challenge on decreased lung function. However, it is unclear how effective supplementation is in increasing antioxidant levels in persons who consume a vitamin-sufficient diet. In anticipation of undertaking a study of the effect of high-dose vitamin C and α-tocopherol on the effect of ozone in asthmatics, we conducted a randomized, double-blinded, two-group, placebo-controlled Phase I pilot dosing study to determine whether supplementation with 500 mg α-tocopherol and 2 g vitamin C on a daily basis for 12 wk increases serum, repiratory-tract linining fluid, and sputum-cell vitamin C, α-tocopherol, and γ-tocopherol levels in a cohort of allergic asthmatics sampled by induced sputum (IS).
MATERIALS AND METHODS
Subjects
Adult subjects between the ages of 18 and 50 yr were recruited through use of advertisements placed on Clintrials.gov and Center for Environmental Medicine, Asthma and Lung Biology (CEMALB) web sites. Potential subjects were also identified from the CEMALB database of subjects who have expressed interest in participating in studies. In total, 15 allergic, asthmatic volunteers were recruited and randomized to one of the following treatment groups: placebo tocopherol and placebo vitamin C, or 500 mg tocopherol and 2 g vitamin C.
Inclusion into the study required oxygen saturation of >94% at baseline, and blood pressure between 90 and 150 mm systolic and between 60 and 100 mm diastolic. Atopy was demonstrated by positive immediate skin test response to one of the following allergen mixes: two species of house dust mite (Dermatophagoides farinae and Dermatophagoides Pteryonnisuius), cockroach, tree mix, grass mix, weed mix, mold mix 1, mold mix 2, rat, mouse, guinea pig, rabbit, cat, or dog.
Moderate to severe persistent asthma was defined according to NHLBI definitions including history one of the following:
Episodic wheezing, chest tightness, or shortness of breath consistent with asthma symptoms at least 1 time per week that affects activity.
Asthma symptoms occurring at night or during sleep at least once per week.
Measured FEV1 or FVC <80% of predicted.
Physician-diagnosed moderate or severe persistent asthma that was currently controlled with maintenance medication that includes inhaled corticosteroids with or without the addition of long-acting beta agonists.
Exclusion criteria included pregnancy or currently nursing a baby; inability to abstain from nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, antihistamines, or anticoagulants for the length of the study; a current diagnosis of anemia or abnormal blood counts or clotting times at screening; and known vagal response to venipuncture. Volunteers were also excluded if they had any chronic medical condition considered by the Principal Investigator (PI) as a contraindication to receiving the alpha-tocopherol and ascorbate supplements, including significant cardiovascular disease, diabetes, chronic renal disease, chronic thyroid disease, kidney disease, or coagulation defects. Volunteers were also asked to refrain from taking vitamin supplements or other dietary supplements for the length of the study
This study was reviewed and approved by the Committee for the Protection of the Rights of Human subjects of the University of North Carolina (UNC). All study subjects signed a consent form prior to any study procedures. Participants then underwent an assessment of general health including a health history questionnaire, physical exam, measurement of baseline circulating antioxidant levels, measurement of baseline safety laboratory tests, lung function assessment, and symptom scoring prior to receiving the supplements.
Study Design
This was a randomized, double-blinded, placebo-controlled study of 500 mg α-tocopherol and 2000 mg vitamin C, with each supplement orally administered daily for 12 wk in asthmatic volunteers. The two supplements were kindly provided as a gift from Yasoo Health, Inc. (Johnson City, TN), and were then distributed by the UNC investigational drug pharmacy. Subjects were instructed to take each supplement at the same time each day. Primary endpoints included serum levels of γT, αT, and vitamin C, and the levels of these in sputum supernatant and sputum cells. The study was powered on the assumption that dosing of asthmatics with high levels of antioxidants would normalize the lower levels of vitamin C present in the airways of asthmatics, as noted by Kongerud et al. (2003). We estimated a sample size of 20 per group for a 12-wk dosing study, based on determinations of effect size and variability from previous vitamin C studies (Kongerud et al., 2003). After having completed dosing in 14 asthmatic volunteers, interim analysis was undertaken to reconfirm original power estimates to establish the effectiveness of changing antioxidant levels in the serum, respiratory-tract lining fluid (RTLF), and sputum cells after 6 and 12 wk of supplementation. Secondary endpoints included changes in sputum cellularity, symptom scores derived from a multidomain symptom questionnaire (see later description), and changes in lung function measures.
Blood samples were collected at baseline and at 6 wk and 12 wk after the initial dose of supplementation and immediately placed on ice. These blood samples were analyzed for serum levels of α-tocopherol, γ-tocopherol, and vitamin C. Sputum was collected at baseline, at 6 wk, and at 12 wk. Sputum was assessed for levels of α-tocopherol, γ-tocopherol, and vitamin C in the sputum supernatant and sputum cells. Furthermore, the types of inflammatory cells composing the sputum were compared before and after supplementation. Lung function and symptom score measures were made on a weekly basis. Individual subject participation lasted approximately 14 wk and required 14 visits to the study facility. Participants were compensated for their time and expenses.
Symptom Score Questionnaire
All subjects filled out a symptom questionnaire before randomization and on a weekly basis. The questionnaire had the participant score any symptoms they were experiencing on a scale of 0 (none) to 3 (severe), and were given definitions of mild, moderate, and severe. There were 12 symptom choices, including malaise, fatigue, shortness of breath, cough, myalgia, chills, fever, headache, nausea, vomiting, indigestion/stomachache, and unusual bleeding/bruising. The minimum possible score on the questionnaire was 0, and maximum possible score was 36.
Allergy Testing
Skin allergy testing was performed before randomization. Skin testing was performed using skin test reagents from Greer Laboratories, and included extracts for dust mites (D. pteronyssinus and D. farinae), tree mix, weed mix, grass mix, mold, cat, dog, rat, mouse, rabbit, guinea pig, and cockroach. Antigen solution was placed on the anterior forearms of test subject using a specialized 8-pronged applicator and was left on the skin for 15 min before the test was read. Allergic individuals developed indurations corresponding to individual allergens, and the diameters in millimeters of the resulting indurations were measured and traced. A positive allergic response is quantified as a resultant wheal diameter that is equal to or larger than the positive control wheal (histamine). A negative control is also included in the battery to aid in reading the test.
Induction of Sputum and Differential Cell Counts
Sputum induction and processing were performed according to previously published methods with some modifications (Alexis et al., 2001). In brief, subjects inhaled increasing concentrations (3%, 4%, and 5%) of hypertonic saline for 7 min each, for a total of 21 min. Manual selection of plug material was weighed and treated with 0.1% dithiothreitol (DTT, in Dulbecco’s phosphate-buffered saline [DPBS]) that contained 25 μM desferoxamine (DFA) for cell dispersion. DFA was added as a precaution to prevent redox recycling. Differential cell counts were analyzed from Romanowski (Diff-Quik)-stained slides, based on 400 cells, and expressed as a percentage of total nonsquamous nucleated cells. Acceptable slides had a minimum of 50% viability (trypan blue staining) and less than 20% squamous epithelial cell content.
Sputum and Serum Processing for Antioxidant Analysis
Sputum
Following DTT dispersion, filtration, and sample centrifugation, the cell pellet was recovered and then equally distributed into two tubes, one marked for ascorbate analysis, the other for tocopherol analysis. For the ascorbate analysis tube, 500 μl of 10% metaphosphoric acid (MPA) containing 25 μM desferoxamine (DFA) in DPBS was added directly to the cell pellet followed by storage at −80°C. The tocopherol tube was only centrifuged, the supernatant was discarded, and the pellet was stored at −80°C. The sputum supernatant fraction was also equally distributed into two tubes, one for vitamin C and one for tocopherol analysis. Seventy percent MPA was added to the vitamin C tube (10% MPA and 25 μM DFA final concentrations) followed by centrifugation for 20 min, and the supernatant was stored at −80°C. The tocopherol tube was only centrifuged and the supernatant was stored at −80°C.
Serum
Blood was collected in serum separator tubes and allowed to settle in the tubes for 30 min. The tubes were then centrifuged at 1500 × g for 15 min at 4°C. Serum was equally distributed into two tubes (600 μl each). The tocopherol tube was immediately stored at −80°C. The vitamin C tube was treated with 10% MPA containing 25 μM DFA (100 μl 10% MPA + 175 μM DFA), then stored at −80°C.
Analysis of Tocopherols
Quantitation of Tocopherols
αT and γT were measured by a high-performance liquid chromatography (HPLC) assay with electrochemical detection (Christen et al., 2002). Briefly, tocopherols were extracted from serum, sputum cells, and sputum supernatant using a mixture of methanol/hexane (2:5, v/v). All extractions were carried out in the presence of 0.8 mM butylated hydroxytoluene. After centrifugation at 1000 × g for 10 min at 4°C, the top hexane layer was collected and evaporated under N2, and the dried residue was redissolved in ethanol. Tocopherols were separated on a 150 × 4.6 mm, 5-μm Supelcosil LC-18-DB column, and eluted with 95:5 (v/v) methanol/water with final 25 mM lithium acetate (pH 4.75) at a flow rate of 1.2 ml/min. Tocopherols were monitored by coulometric detection (model Coulochem II, ESA, Inc., Chelmsford, MA) at 300 (upstream) and 500 mV (downstream electrode) using a model 5011 analytical cell.
Analysis of Vitamin C
MPA-treated samples were centrifuged (27,000 × g, 20 min at 4°C). Serum, sputum supernatants, and sputum cells were assayed for vitamin C using amperometric detection using an LC-4B detector (Bioanalyticial Systems, West Lafayette, IN) (Kutnink, Hawkes et al., 1987). Each sample was analyzed for about 9.0 min. A glassy carbon electrode was used and the potential was 0.5 V. Standards were run from 39 ng/ml to 10,000 ng/ml. The lower end of the level of detectability is between 18 and 39 ng/ml; samples above 5500 ng/ml are diluted and rerun. The detector sensitivity was set at 100 nA/V. The flow was 1.5 ml/min. The mobile phase was aqueous and consisted of 0.04 M sodium acetate, 0.54 mM ethylenediamine tetraacetic acid (EDTA), 2.5 mM dodecyltriethyl ammonium phosphate, and 6% methanol, with the pH adjusted to 4.75.
Statistical Analysis
To account for differences in baseline antioxidant levels in the experimental groups, we first calculated the difference between the 6-wk (Δ6,0) or 12-wk (Δ12,0) measurements and the baseline measurements for each individual where Δ12,0 = Y12 − Y0 and Δ6,0 = Y6 − Y0, respectively. We then tested the differences between the active and placebo groups with a two-sample t-test when the equality of variances was satisfied; when the equality of variances was not satisfied, a Satterthwaite t-test was adopted. Differences were considered statistically significant if p < .05. To improve the statistical power, we also employed a multivariate regression analysis to take advantage of the repeated observations per individual (at baseline, wk 6, and wk 12).
For the analysis of inflammatory cells in the sputum, the total number of cells isolated from each individual was added over the 12-wk period of observation. The total number of cells from those individuals in the active group versus the number for placebo group were compared using a two-sample t-test. The same procedure was applied to the measurements of FEV1. For the symptom score measurements, we grouped each individual’s symptom scores into two categories: no symptoms (scores 0–3) and mild–moderate symptoms (score >4). We then applied a logistic regression to compare the total symptom scores of those subjects in the active versus placebo groups, adjusting for other covariates that include subject’s age, race, and gender. All analyses were conducted using SAS software (Cary, NC).
RESULTS
Study Participants Characteristics
Fourteen of 15 volunteers completed this randomized double-blinded placebo-controlled Phase I study in which volunteers consumed 2 capsules per day for 12 wk of 500 mg α-tocopherol and 2 g vitamin C, or placebo α-tocopherol and placebo vitamin C. One of the subjects randomized to the treatment group dropped out at wk 6 due to the development of cystitis. This was reported as an adverse event. Upon further review of the subject’s history, she was found to have a history of mild, intermittent right lower quadrant discomfort. This is the same symptom that was reported during the study period. She reported that her personal physician suspected that she had cystitis, and was therefore excluded from the study due to the possibility that vitamin C therapy may aggravate the cystitis.
There were some notable differences in the characteristics of the active and placebo groups at baseline (Table 1): On average, the subjects in the active treatment group were older and had a lower baseline FEV1. The active group was also composed of more subjects using inhaled corticosteroids for asthma therapy.
TABLE 1.
Demographic data of subjects completing the phase I pilot study
| Parameter | Placebo (n = 8) | Active (n = 6) |
|---|---|---|
| Age (mean) | 25.3 | 32.5 |
| Gender (% male) | 12.5% | 33.3% |
| Race (% white) | 87.5% | 66.7% |
| FEV1 (% predicted) | 106% | 97.7% |
| Medications | ||
| Percent on inhaled corticosteroids | 25% | 66.6% |
| Percent on leukotriene receptor antagonists | 25% | 0% |
Vitamin C
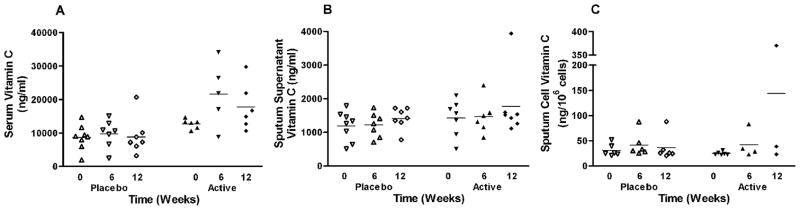
Figure 1 depicts the serum and sputum supernatant vitamin C levels in the placebo and active groups. In the active group, the serum vitamin C levels increased after starting supplementation with a mean level of 21,660 ng/ml at 6 wk and a mean level of 17,760 ng/ml at 12 wk. The serum vitamin C levels at 6 and 12 wk showed a large degree of variation within the active group (Figure 1a). A simple comparison of active and placebo groups, without adjusting for baseline differences, demonstrated a statistically significant difference in serum vitamin C levels between the active and placebo groups following both 6 and 12 wk (p = .046 and p = .018, respectively). A more rigorous assessment of the differences in the 12-wk or 6-wk to baseline serum vitamin C levels was made for the active and placebo groups: This difference reached statistical significance for wk 6 (p = .043) and almost reached statistical significance for wk 12 (p = .069). Despite the observation that high-dose supplementation was associated with increased serum levels of vitamin C, these levels did not translate to increased levels in the sputum supernatant. The difference in wk 12 and baseline sputum supernatant vitamin C levels was not statistically different between the active and placebo groups (p = .604) (Figure 1b). Vitamin C did not appear to be sequestered into sputum cells either: The difference in wk 12 and baseline sputum cell vitamin C levels was not statistically different between the active and placebo groups (p = .429), although this result should be interpreted with caution due to the small number of samples analyzed at wk 12 in the active group.
FIG. 1.
Vitamin C levels recovered in the a. serum, b. sputum supernatant, c. sputum cells of allergic asthmatic subjects. Supplementation augmented serum levels of vitamin C of subjects in the active treatment group (p = 0.069), yet there was no increase in sputum supernatant or sputum cell vitamin C levels. Horizontal bar represents the mean.
Alpha-Tocopherol
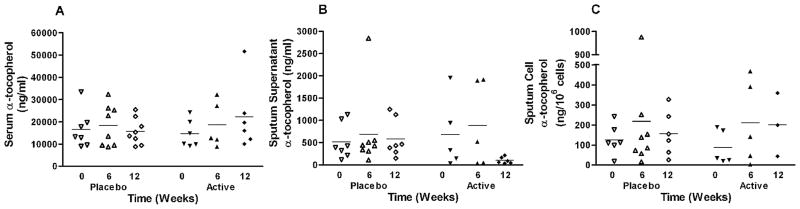
Figure 2 depicts the serum, sputum supernatant, and sputum-cell levels of alpha-tocopherol. Compared to baseline, the serum αT levels increased after 12 wk of supplementation in the active group, but the rise in the active group was not statistically significant when compared to the placebo group (p = .18) (Figure 2a). Unexpectedly, α-tocopherol supplementation decreased levels in the sputum supernatant in the active group (Figure 2b). A two-sample t-test did not detect a statistically significant difference (p = .105) but the repeated measure approach showed that the reduction in levels in the active group almost reached statistical significance compared to the placebo group (p = .077). The differences in α-tocopherol levels in sputum cells between the active and placebo groups did not reach statistical significance (p = .814).
FIG. 2.
Alpha tocopherol levels recovered in the a. serum, b. sputum supernatant, c. sputum cells of allergic asthmatic subjects. Despite supplementation, subjects in the active treatment group experienced no change in serum α-tocopherol levels, and a decline in sputum supernatant α-tocopherol levels (p = 0.01). There were no changes in sputum cell α-tocopherol levels. Horizontal bar represents the mean.
Gamma-Tocopherol
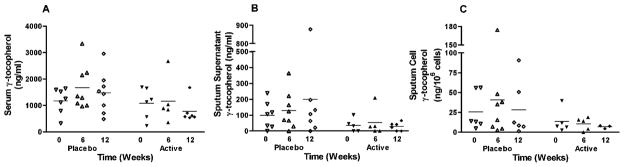
As previous studies have shown interactions between α-and γ-tocopherol levels in the serum (Handelman et al., 1985; Clement & Bourre, 1997), we also evaluated the levels of γ-tocopherol in the serum, sputum supernatant, and sputum cells. We found that γT levels in the serum significantly dropped in the active group during the 12-wk period of α-tocopherol supplementation (p = .035) (Figure 3a). However, this drop in serum γT levels did not effect changes in γT levels in the sputum supernatant of subjects in the active group (p = .22). Supplementation with α-tocopherol had no effect on sputum cell γT levels in the active group (p = .979).
FIG. 3.
Gamma tocopherol levels recovered in the a. serum, b. sputum supernatant, c. sputum cells of allergic asthmatic subjects. A reduction in serum levels of y-tocopherol was noted in those subjects who received active supplementation with α-tocopherol and vitamin C (p = 0.035). There were no changes in sputum cell y-tocopherol levels. Horizontal bar represents the mean.
Sample Size Estimates for the Supplemented Antioxidants
Based on the variability we found in our samples, we calculated the sample sizes required to observe a statistically significant difference between active versus placebo antioxidant supplementation in various physiologic relevant compartments, using the observed serum and sputum supernatant antioxidant levels in this study. These estimates are calculated based on a two-sided test with equal variances for the α-tocopherol data, assuming alpha = .05 and power = 80% and a 1:1 ratio for sample sizes. Due to the different observed variances in vitamin C levels in the active and the placebo groups, unequal variances were assumed, and we used a two-sided Sattherwaite t-test, again assuming alpha = .05 and power = 80% and a 1:1 ratio for sample sizes.
Our initial hypothesis was that supplementation with αT and vitamin C would result in changes in airway αT and vitamin C levels. Based on the effect sizes seen in this study, we estimate that we would need 15 persons in each arm to demonstrate a statistically significant change in serum vitamin C (total n = 30). However, to detect a significant difference in RTLF vitamin C for our treatment regimen would require 133 volunteers in both the placebo and treatments groups (total n = 266). If we base a sample size estimate on serum alpha-tocopherol, we would require 30 volunteers in each group. If we base a sample size estimate on changes in RTLF α-tocopherol, we would require 19 volunteers in each group to detect a significant change in α-tocopherol.
Sputum Differential Cell Counts
There was no significant difference in the cumulative total cell count from induced sputum between the active and placebo groups through wk 12 (p = .36). We also evaluated the types of inflammatory cells isolated from the sputum. The cumulative numbers of neutrophils, over the 12-wk period since randomization, were significantly higher in the active group than in the placebo group (p = .034). There were no significant differences in the cumulative numbers of eosinophils (p = .45) or macrophages (p = .08) between the active or placebo groups over the 12-wk period of observation. Table 2 shows total cell counts from induced sputum at wk 0, wk 6, and wk 12 isolated from subjects in the active and placebo groups and differential cell counts.
TABLE 2.
Cell counts from induced sputum
| Treatment | Week 0
|
Week 6
|
Week 12
|
|||
|---|---|---|---|---|---|---|
| Placebo | Active | Placebo | Active | Placebo | Active | |
| Total cell count (± 1 SD) | 3.26 × 106 (± 2.4 × 106) | 3.64 × 106 (± 4.4 × 106) | 2.97 × 106 (± 1.9 × 106) | 1.87 × 106 (± 2.2 × 106) | 1.89 × 106 (± 1.6 × 106) | 3.63 × 106 (± 3.8 × 106) |
| Percent neutrophils (± 1 SD) | 27.9 (± 15.3) | 34.6 (± 17.5) | 21.1 (± 9.9) | 33.8 (± 20.8) | 21.5 (± 13.3) | 36.6 (± 19.9) |
| Percent eosinophils (± 1 SD) | 2.9 (± 2.9) | 1.3 (± 1.5) | 4.1 (± 4.4) | 4.4 (± 10.1) | 2.7 (± 2.7) | 4.5 (± 9.6) |
| Percent macrophages (± 1 SD) | 63.4 (± 19.2) | 57.7 (± 14.8) | 71.3 (± 13.7) | 60.5 (± 21.6) | 69.5 (± 15.6) | 56.4 (± 21.6) |
Measures of Lung Function
The FEV1 was determined for each volunteer at baseline and every other week during the study. There was no significant difference in the cumulative percent predicted FEV1 between the active and placebo groups through wk 12 (p = .42). Symptom scores were also compared between the active and placebo groups, and no statistically significant difference was noted between the two groups (p = .93).
DISCUSSION
The rationale for using combination treatment with α-tocopherol and vitamin C to prevent inflammation and injury of tissues due to oxidative stress is largely based on in vitro observations that vitamin C can participate in reactions that recycle α-tocopherol after it is oxidized in cellular redox reactions (Kelly et al., 1995). This may be particularly relevant in preventing airway inflammation and injury due to ozone exposure. Different research teams have reported that α-tocopherol and vitamin C combination therapy was effective in mitigating the effect of ozone-induced lung function decrements in asthmatics (Samet et al., 2001; Trenga et al., 2001; Romieu et al., 2002; Romieu et al., 2004), or in normal volunteers after they had undertaken an antioxidant-depleted diet to mimic a state of poor antioxidant nutritional status (Samet, Hatch et al., 2001). Our group has also found that vitamin C levels in airway fluids of asthmatics were significantly decreased compared to those of normal volunteers (Kongerud et al., 2003). Taken together, these observations led to the hypothesis that combination treatment with α-tocopherol and vitamin C would enhance airway vitamin C levels in asthmatics, and would protect them from the adverse effects of ozone exposure.
Before committing to a study examining the effect of combination treatment on response of asthmatics to ozone, we felt it was important to confirm that α-tocopherol and vitamin C modify airway antioxidant levels. Thus, the primary aim of this dosing study was to determine whether oral supplementation with 2 g vitamin C and 500 mg α-tocopherol daily for 12 wk would increase levels of vitamin C (which we used as a sentinel antioxidant) in RTLF of asthmatics to levels observed in normal volunteers. We also examined effects of such supplementation on serum levels of α-tocopherol and vitamin C as well as γ-tocopherol, a vitamin E isoform with antioxidant and radical-scavenging capabilities.
In designing our study, we used a very generous dose of vitamin C, coupled with a dose of α-tocopherol employed in other studies in which a protective effect of this intervention had been reported. Although it is known that vitamin C metabolism is tightly regulated, we chose this high dose of vitamin C to maximize the likelihood that we would achieve changes in RTLF vitamin C levels. Data from this pilot study of 14 volunteers indicate that the combination supplementation regimen does not change vitamin C levels in the RTLF despite a significant increase in serum vitamin C levels of asthmatic subjects. These observations led us to the conclusion that protective effects reported by oral supplementation with α-tocopherol and vitamin C may not be primarily due to changes in airway fluid concentrations of these molecules. Supporting this conclusion is a report that levels of airway antioxidants (including tocopherols and vitamin C) do not predict physiologic or inflammatory responses to ozone challenge (Mudway et al., 2001).
There may be other specific risk factors or populations in which vitamin supplementation may modify airway levels of antioxidants. Our volunteers were persons with moderate to severe asthma, most of whom were using inhaled corticosteroids, but were otherwise generally well nourished and in good health. While persons with asthma per se may not benefit from antioxidant supplementation, those with nutritional deficiencies may derive benefit from antioxidant replacement therapy, given the effect of supplementation in nutritionally impaired volunteers. Genetic factors may also play an important role in determining effectiveness of antioxidant supplements.
Perhaps the best example of this is a series of studies of children with asthma in Mexico City conducted by Romieu and colleagues. They assessed the ability of dietary supplementation of 50 mg/day of vitamin E and 250 mg/day of vitamin C to protect child asthmatics from ozone-induced exacerbation of disease (Romieu et al., 2004). Overall, there was a significant protective effect on forced midexpiratory flow rate (FEF25-75) observed in the entire group of asthmatics, but this effect was much more apparent in those children with the glutathione-S-transferase M1 (GSTM1) null genotype. There are a number of other studies that reveal that defects in GSTM1, an antioxidant response protein, and other antioxidant enzymes regulated by the transcription factor NRF2 are associated with increased risk for pollutant-induced respiratory disease (Cho et al., 2006; Kensler et al., 2007). It may be that focused attention on the pharmacokinetics and efficacy of antioxidant supplements in persons with genetic risk for oxidant injury may demonstrate beneficial effects not observed in a general population.
The dose of antioxidants used may also impact physiologic responses to ozone challenge or routine environmental challenges. We did not find any differences in FEV1 or symptom scores between the active and placebo groups. In another study of normal volunteers who did not undergo presupplement dietary antioxidant depletion, supplementation with 100 mg/day α-tocopherol and 500 mg/day vitamin C did not prevent acute responses to ozone (Mudway et al., 2006). The failure to see protection could be due to possible pro-oxidant effects of vitamin C seen at oral supplementation doses of 400–500 mg (Cooke et al., 1998, 2003), as the protective studies performed by Romieu, Samet, and colleagues used doses of 250 mg/day. Failure to see protective effects could also be due to higher consumption of α-tocopherol by high levels of vitamin C. Serum tocopherol levels have been noted to increase simultaneously with vitamin C supplementation in combination therapy studies using 250 mg/day vitamin C (Samet et al., 2001; Romieu et al., 2002). It is unclear whether a potentially pro-oxidant environment created by higher doses of vitamin C could deplete α-tocopherol levels in various physiologic compartments, accounting for the failure to alter various parameters of lung function at baseline, or to prevent ozone-induced acute responses.
As an additional exploratory endpoint, we also examined changes in airway sputum cellularity across the 12-wk dosing period, primarily to determine whether there had been any decrease in airway eosinophils, an important inflammatory cell in asthma. While we observed no change in sputum eosinophils, more neutrophils were recovered in sputum of those in the active group. This observation is consistent with findings by Samet et al., who observed that α-tocopherol and vitamin C supplementation in persons who were rendered antioxidant depleted by use of a specifically deficient diet had increased sputum neutrophils (Samet et al., 2001). High vitamin C intake also appears to influence the response of neutrophils to oxidative stress induced by exercise, increasing neutrophil activation, as reflected by a significant decrease in superoxide dismutase activity in neutrophils recovered from well-supplemented athletes after exercise. It has also been noted that expression of the CD11b/CD18 adhesion molecule complex is upregulated in athletes supplemented with vitamin C (Tauler et al., 2003). As these observations indicate that vitamin C supplementation may impact neutrophil activity, changes of antioxidant content of cellular and tissue compartments may be more important venues to study than changes of scavenger molecules on mucosal surfaces. The failure to see significant changes in sputum-cell antioxidant concentrations in our study may be primarily due to the limited samples available for these assays, as the study was not powered on this endpoint.
In conclusion, our pilot data indicate that oral supplementation with α-tocopherol and vitamin C does increase serum vitamin C, but has no effect on RTLF vitamin C levels. Supplementation had no effect on serum α-tocopherol and was associated with decreased α-tocopherol levels on the airway surface. Although it is attractive to hypothesize that providing oral supplements would augment levels of airway surface antioxidants and thus protect individuals from the effect of inhaled oxidants, this view is overly simplistic. Asthmatics may have defective antioxidant transport mechanisms to the extracellular compartment, and/or have a highly oxidizing airway environment that consumes more antioxidants than normals. Asthmatics may benefit from topical administration of vitamin C therapy instead of oral supplementation. Finally, intracellular antioxidant content may be even more important as this likely protect cells from exogenous oxidants and minimizes inflammatory processes that are mediated in part by generation of oxidants in situ directly by inflammatory cells. Thus, future dosing studies of α-tocopherol and vitamin C will need to focus on assessing intracellular as well as serum and airway fluid compartments to select appropriate doses for future efficacy studies of antioxidants in asthma.
Acknowledgments
This publication was made possible by grant P01AT002620 from the National Center for Complementary and Alternative Medicine (NC-CAM) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM. The alpha-tocopherol preparation used in this study was supplied as a gift by Yasoo Health, Inc.
Contributor Information
Michelle Hernandez, The Center for Environmental Medicine, Asthma and Lung Biology, University of North Carolina School of Medicine, Chapel Hill, North Carolina.
Haibo Zhou, The Center for Environmental Medicine, Asthma and Lung Biology, University of North Carolina School of Medicine, Chapel Hill, North Carolina. Department of Biostatistics, School of Public Health, University of North Carolina, Chapel Hill, North Carolina.
Bingqing Zhou, The Center for Environmental Medicine, Asthma and Lung Biology, University of North Carolina School of Medicine, Chapel Hill, North Carolina. Department of Biostatistics, School of Public Health, University of North Carolina, Chapel Hill, North Carolina.
Carole Robinette, The Center for Environmental Medicine, Asthma and Lung Biology, University of North Carolina School of Medicine, Chapel Hill, North Carolina.
Kay Crissman, U. S. Environmental Protection Agency Experimental Toxicology Division, Research Triangle Park, North Carolina, USA.
Gary Hatch, U. S. Environmental Protection Agency Experimental Toxicology Division, Research Triangle Park, North Carolina, USA.
Neil E Alexis, The Center for Environmental Medicine, Asthma and Lung Biology, University of North Carolina School of Medicine, Chapel Hill, North Carolina.
David Peden, The Center for Environmental Medicine, Asthma and Lung Biology, University of North Carolina School of Medicine, Chapel Hill, North Carolina.
References
- Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: Confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1964–1970. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- Anah CO, Jarike LN, Baig HA. High dose ascorbic acid in Nigerian asthmatics. Trop Geogr Med. 1980;32(2):132–137. [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8(1–2):76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- Christen S, Jiang W, Shigenaga MK, Ames BN. Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection. J Lipid Res. 2002;43(11):1978–1985. doi: 10.1194/jlr.d200023-jlr200. [DOI] [PubMed] [Google Scholar]
- Clement M, Bourre JM. Graded dietary levels of RRR-gamma-tocopherol induce a marked increase in the concentrations of alpha- and gamma-tocopherol in nervous tissues, heart, liver and muscle of vitamin-E-deficient rats. Biochim Biophys Acta. 1997;1334(2–3):173–181. doi: 10.1016/s0304-4165(96)00090-6. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Podmore ID, Herbert KE, Mistry N, Mistry P, Hickenbotham A, Hussieni A, Griffiths HR, Lunec J. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998;439(3):363–367. doi: 10.1016/s0014-5793(98)01403-3. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Mistry N, Ahmad J, Waller H, Langford L, Bevan RJ, Evans MD, Jones GD, Herbert KE, Griffiths HR, Lunec J. Deoxycytidine glyoxal: Lesion induction and evidence of repair following vitamin C supplementation in vivo. Free Radical Biol Med. 2003;34(2):218–225. doi: 10.1016/s0891-5849(02)01240-6. [DOI] [PubMed] [Google Scholar]
- Cross CE, Valacchi G, Schock B, Wilson M, Weber S, Eiserich J, van der Vliet A. Environmental oxidant pollutant effects on biologic systems: A focus on micronutrient antioxidant–oxidant interactions. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S44–S50. doi: 10.1164/rccm.2206015. [DOI] [PubMed] [Google Scholar]
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, Britton J. Oral magnesium and vitamin C supplements in asthma: A parallel group randomized placebo-controlled trial. Clin Exp Allergy. 2003;33(10):1355–1359. doi: 10.1046/j.1365-2222.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Berhane KT, Li YF, Gauderman WJ, McConnell R, Peters J. Children’s lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol. 2003;158(6):576–584. doi: 10.1093/aje/kwg181. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J Nutr. 1985;115(6):807–813. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Mudway I, Krishna MT, Holgate ST. The free radical basis of air pollution: Focus on ozone. Respir Med. 1995;89(10):647–656. doi: 10.1016/0954-6111(95)90131-0. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kongerud J, Crissman K, Hatch G, Alexis N. Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhal Toxicol. 2003;15(2):101–109. doi: 10.1080/08958370304477. [DOI] [PubMed] [Google Scholar]
- Kutnink MA, Hawkes WC, Schaus EE, Omaye ST. An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal Biochem. 1987;166(2):424–430. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- Mohsenin V, Dubois AB, Douglas JS. Effect of ascorbic acid on response to methacholine challenge in asthmatic subjects. Am Rev Respir Dis. 1983;127(2):143–147. doi: 10.1164/arrd.1983.127.2.143. [DOI] [PubMed] [Google Scholar]
- Mudway IS, Stenfors N, Blomberg A, Helleday R, Dunster C, Marklund SL, Frew AJ, Sandstrom R, Kelly FJ. Differences in basal airway antioxidant concentrations are not predictive of individual responsiveness to ozone: A comparison of healthy and mild asthmatic subjects. Free Radical Biol Med. 2001;31(8):962–974. doi: 10.1016/s0891-5849(01)00671-2. [DOI] [PubMed] [Google Scholar]
- Mudway IS, Behndig AF, Helleday R, Pourazar J, Frew AJ, Kelly FJ, Blomberg A. Vitamin supplementation does not protect against symptoms in ozone-responsive subjects. Free Radical Biol Med. 2006;40(10):1702–1712. doi: 10.1016/j.freeradbiomed.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: A parallel group randomised placebo controlled trial. Thorax. 2004;59(8):652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautalahti M, Virtamo J, Haukka J, Heinonen OP, Sundvall J, Albanes D, Huttunen JK. The effect of alpha-tocopherol and beta-carotene supplementation on COPD symptoms. Am J Respir Crit Care Med. 1997;156(5):1447–1452. doi: 10.1164/ajrccm.156.5.96-11048. [DOI] [PubMed] [Google Scholar]
- Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Tellez-Rojo MM, Moreno Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166(5):703–709. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, Hernandez-Avila M, London SJ. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59(1):8–10. [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Hatch GE, Horstman D, Steck-Scott S, Arab L, Bromberg PA, Levine M, McDonell WF, Devlin RB. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Respir Crit Care Med. 2001;164(5):819–825. doi: 10.1164/ajrccm.164.5.2008003. [DOI] [PubMed] [Google Scholar]
- Schachter EN, Schlesinger A. The attenuation of exercise-induced bronchospasm by ascorbic acid. Ann Allergy. 1982;49(3):146–151. [PubMed] [Google Scholar]
- Schwartz J, Weiss ST. Dietary factors and their relation to respiratory symptoms. The Second National Health and Nutrition Examination Survey. Am J Epidemiol. 1990;132(1):67–76. doi: 10.1093/oxfordjournals.aje.a115644. [DOI] [PubMed] [Google Scholar]
- Tauler P, Aguilo A, Gimeno I, Noguera A, Agusti A, Tur JA, Pons A. Differential response of lymphocytes and neutrophils to high intensity physical activity and to vitamin C diet supplementation. Free Radical Res. 2003;37(9):931–938. doi: 10.1080/1071576031000150454. [DOI] [PubMed] [Google Scholar]
- Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health. 2001;56(3):242–249. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- Zuskin E, Lewis AJ, Bouhuys A. Inhibition of histamine-induced airway constriction by ascorbic acid. J Allergy Clin Immunol. 1973;51(4):218–226. doi: 10.1016/0091-6749(73)90141-3. [DOI] [PubMed] [Google Scholar]