Abstract
In this review article we address the radiation oncology process improvements in clinical trials and review how these changes improve the quality for the next generation of trials. In recent years we have progressed from a time of limited data acquisition to the present in which we have real time influence of clinical trials quality. This enables immediate availability of the important elements including staging, eligibility, response and outcome for all trial investigators. Modern informatics platforms are well designed for future adaptive clinical trials. We review what will be needed in the informatics architecture of current and future clinical trials.
Introduction
Oncology clinical trials, including those sponsored by the National Cancer Institute (NCI), have become a cornerstone to improvements in patient care and clinical outcome. Clinical trials have touched on every disease site with radiation oncology playing an important role in nearly all areas of epithelial and liquid oncology. Radiation oncology has been either the primary focus of clinical trials or has served as a valuable co-partner with surgical, imaging, and medical endpoints.
Through the NCI, cooperative groups and radiation therapy quality assurance centers have been established, restructured and re-organized. The Radiation Therapy Oncology Group (RTOG) was established as a cooperative group in the late 1960’s. RTOG established case sampling initial radiation therapy review in the late 1970’s.1 The Radiological Physics Center 1 (RPC) has been funded by the NCI continuously since 1968 to provide quality auditing of dosimetry practices at institutions participating in NCI cooperative clinical trials. The RPC was formed through the American Association of Physicists in Medicine (AAPM) and radiation oncologists through the Committee on Radiation Therapy Studies.2 The RPC provides thermoluminescent dosimetry (TLD) services with phantoms to validate dose per machine and physical evidence that dose can be accurately delivered with sophisticated radiotherapy techniques including IMRT and radiosurgery.2,3,4
The Quality Assurance Review Center (QARC) began as a part of the Radiation Oncology Committee for the original Cancer and Leukemia Group B (CALGB) in 1976. At that time, radiation guidelines and protocol compliance were non-uniform and data, including films and radiation treatment plans, were not routinely collected for review. Within a short period, a data collection process was developed by QARC, and study investigators began interventional and retrospective reviews for all subjects registered on clinical trials with radiotherapy review.5,6,7,8 QARC was later independently funded through the NCI Cancer Therapy Evaluation Program (CTEP) in a manner identical to the cooperative groups.
In the 1980’s, three-dimensional (3D) treatment planning matured as computed tomography (CT) became universally available. The use of CT and 3D imaging to define radiation therapy volumes introduced new QA issues. Benchmarks (treatment planning exercises used to assess equipment, staff and capabilities.) were established to provide QA centers with baseline knowledge of the participating RT centers. Target volume and critical organ definition, dose prescription and delivery were issues of the 3D treatment planning era. Protocol guidelines adapted to these changes. As this process matured, the targeting and language for treatment became image driven rather than based on the traditional anatomical guidelines of the previous generation of studies. ICRU 50 and 62 further refined targeting to include areas of clinical concern, internal motion, and patient set up reproducibility. This led to new strategies for both target definition and dose coverage uniformity to a volume.
As radiation therapy technologies and image validation of treatment matured, the use of expanded volumes and intended target volume coverage have further changed to demonstrate the precision of image validation. The radiotherapy data and review required for the advanced radiotherapy protocol guidelines have evolved as the technology has progressed. In 1999, the Advanced Technologies Consortium (ATC) was created to support the development and conduct of advanced-technology clinical trials. The ATC is a partnership of four QA offices including: the Quality Assurance Review Center (QARC) in Lincoln, RI; the RPC in Houston, TX; the Image-Guided Therapy QA Center (ITC) in St. Louis; and the Radiation Therapy Oncology Group (RTOG) dosimetry group in Philadelphia. The ATC was organized so that the centers concerned with these issues could develop uniform standards in a collaborative manner.
The QA centers ensure that institutions participating in clinical trials deliver prescribed radiation doses that are clinically comparable and consistent. They have helped improve compliance with protocols, reduce minor and major deviations, detect systematic errors in clinical practice, as well as identify misunderstanding, misinterpretation, and equipment failure modes. These endeavors have indirectly improved the quality of patient care at participating institutions by training and educating personnel in the safe implementation of new radiotherapy methodologies and techniques in radiotherapy.
There are many challenges in the development and management of clinical protocols to reflect the expanding use of radiation therapy technology and the variety of technology applications in clinical use. Technology and the application of treatment strategies within institutions must be credentialed for use in clinical trials.9,10,11 This insures uniform dose calibration and treatment execution. The process evaluates and validates treatment planning and radiation dose computation capability within each institution. For specific areas within radiation therapy and for potentially varied radiation therapy treatment techniques, credentialing is expanded to include other technologies and treatment techniques including intensity modulation, image guidance, and pediatric applications. As volumes have become better defined through imaging, radiation oncology is re-visiting altered treatment fractionation programs and compressed treatment schedules. Image guidance and motion management have now become important issues in the radiation delivery management of several disease sites for patients treated with altered fractionation treatment programs. As technology has matured, quality assurance has adapted to validate the expanded role of technology in clinical trials. This is balanced by the need to complete the trial, not limit study accrual, and define what technologies are deemed reasonable for specific clinical trial execution. Quality assurance may be different if one is asking a specific radiation therapy treatment question or if radiation therapy is serving as a co-partner in a clinical trial evaluating systemic therapy. We have learned that quality assurance is important whether or not radiation therapy is the primary study endpoint.
Having established the role of quality assurance, we will now explore lessons learned from the quality assurance process and what we can do to improve protocols and adjust quality assurance strategies to reflect changes in treatment standards and execution. Protocols are written and developed to ask a specific study question and may ask a specific question for radiation therapy. We have witnessed an extraordinary change in treatment technology with multiple treatment strategies developed for small field therapy, image validation, and target motion management. Each protocol experience teaches us how investigators use technology in the execution of patient care at their institutional sites. We can build from this experience for the next generation of clinical trials. One goal of quality assurance is to find common ground between investigators and technology.12 The quality assurance program must find the common ground between establishing a uniform study population for review while not limiting accrual to study. Lessons learned from the quality assurance of clinical trials better define protocol parameters which lead to improvements in providing uniformly treated study populations that answer trial objectives.13,14,15
In this paper, we summarize many of the critical QA elements necessary to perform clinical trials: credentialing, protocol development, data acquisition, case review, data management, informatics, and remote review of objects, and then describe several of the problems/issues which these have demonstrated.
Clinical Trial QA Methods
Credentialing
Credentialing is the vehicle used to evaluate treatment planning and execution for each institution planning on participating in clinical trials. Credentialing can include evaluation of dose and treatment planning as well as evaluation of advanced technology. Credentialing was originally designed to make certain dose was uniform between treatment units and that planning could be performed per study guidelines. In early iterations of clinical trials, this was important because there was clear ambiguity in computational algorithms and disparity in using these algorithms among institutions. Accordingly, deviations were largely computational in nature as volume analysis was largely based on anatomical guidelines written into the study. QARC developed a process in the early iterations of clinical trials to review all data within the first three days of treatment in order to insure compliance to study objectives. The timing required for this process was due to carrier delivery of paper and image copy. As imaging became the vehicle used for target volume definition in clinical trials, disparities between site and central imaging reviews often led to target volume deviations on study. Credentialing has now matured to benchmark all aspects of clinical trials that may create ambiguity in study interpretation.
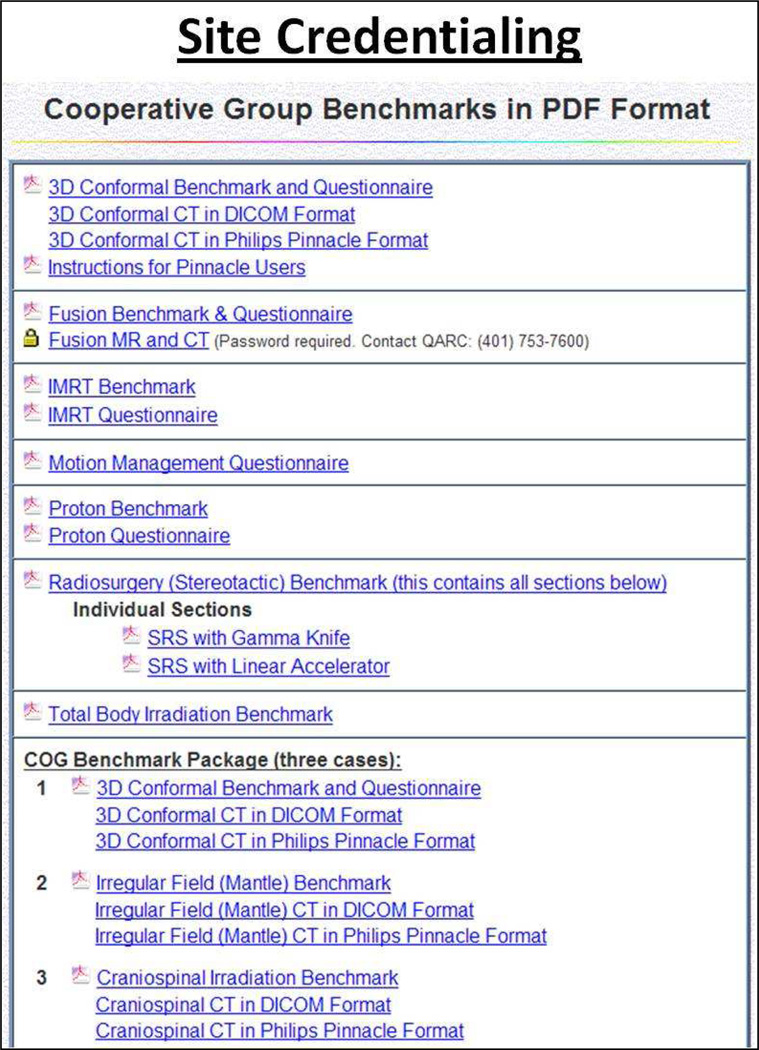
While the RPC validates machine dose and accurate dose delivery with sophisticated radiotherapy techniques, QARC provides complementary credentialing strategies for advanced technology radiation therapy including MR/CT fusion as well as benchmarks to provide uniformity in target volume definition for both adult and pediatric protocols. Credentialing establishes that institutions have the computational and informatics infrastructure to participate in clinical trials.4 When institutions commission new planning systems, benchmarks are repeated to make certain study compliance objectives continue to be met. From 2001–2009, the RPC reviewed 752 anthropomorphic phantom irradiations from 472 institutions.2 QARC has over 4000 approved benchmarks on file. Figure 1 illustrates the current QARC benchmark portfolio.
Figure 1.
The QARC Benchmark Portfolio
Credentialing does not always insure compliance to study objectives. Even with established benchmarks, an institution may perform differently on site in the execution of a clinical trial. These issues may be more apparent as we enter the next generation of clinical trials with full international participation. In one head and neck clinical trial with international participation monitored at QARC, sites that had passed credentialing processes had to cease participation as they could not complete treatment in the protocol specified timeframe. This was largely due to cultural habit and how patients were managed when treated to a sensitive mucosal surface. As part of the QA process QARC acquires the complete clinical record and is able to identify the deviation early in clinical trial execution minimizing on- study deviations.
It became important in clinical trials fully dependent on imaging for target volume definition (i.e. involved field therapy for Hodgkins lymphoma) to adjust the quality assurance strategy to perform pre-treatment review of objects. Deviation rates were as high as 30% on study when data was reviewed in retrospect.16 Pre-treatment review of objects became a very successful strategy for decreasing treatment deviations on study. Accordingly, credentialing has further matured to address issues such as drawing of both tumor and normal tissue objects including image fusion/integration for target definition. As needs mature for modern clinical trials, process improvements in credentialing are required to reflect the changing environment and needs. In the next generation of clinical trials, radiation oncology will need to integrate with our diagnostic radiology colleagues for credentialing in image acquisition and interpretation. Targets for primary and supplemental therapy will be defined by imaging metrics. Metabolic imaging indices and benchmarks will need to be performed for interpretation of these metrics for studies such as positron imaging and contrast associated magnetic resonance imaging.
Protocol Development
It is important for clinical trial QA to involve the QA centers at the time of development and design of the trial concept sheet. QARC radiation therapy and diagnostic radiology templates are used by investigators to place the protocol into language supported by Cancer Therapy Evaluation Program (CTEP). This facilitates the development and subsequent approval of the protocol. Timely completion of guidelines is now an essential component to protocol success. Working with these templates permits facile development of data acquisition and data management strategies and enables uniformity of treatment execution. Every protocol provides an opportunity to improve the templates as problems and pitfalls from previous studies can be corrected and adjusted to meet the needs of the current study with potentially improved strategies.8
Data Acquisition
Protocols need clear and concise definitions of the required data and when/how it is to be transmitted to the QA center. Digital data submissions in clinical trials have grown considerably in the last decade. As trials have matured over the past decade, digital media via multiple formats has become the preferred method to transmit diagnostic imaging and radiation therapy treatment objects. In order to meet accrual standards, QARC accepts data via multiple media formats. Digital transmission can be performed via secure file transfer protocol (FTP) sites as more than 390 institutions have established accounts at QARC. Imaging can be received through multiple mechanisms including direct transfer through the QARC- developed and supported software known as Dicommunicator. This program has the advantages of de-identification, study management and electronic data submission for the research personnel at the participating sites. Many institutions prefer computer disc (CD) as a vehicle for data transmission as it does not require site information technology (IT) interactions. Imaging received on CD is de-identified at the QA center. As we move into global international participation in clinical trials, the QA centers must be prepared to accept protocol objects via multiple methods as efforts towards more uniform informatics transmission platforms evolve.
Individual Case Review (ICR) - Data Integrity QA
Protocol case digital data submitted to a QA Center undergoes what is now referred to as a “Digital Data Integrity QA (DDIQA)” review.17,18 Submitted data are checked for completeness and consistency. Ensuring completeness of protocol required elements, assessing data format and potential format of data, possible data corruption, uniformity in Organ at Risk (OAR)/ Tumor Volume (TV) contour names, and recalculation of dose volume histogram (DVH) have been shown to be important elements of the overall protocol QA review process. Experience has shown that submitted DVHs lack consistency due to algorithmic differences among treatment planning systems (TPSs)19. Thus, re-calculation of DVHs is necessary for consistent correlation of dosimetry with outcomes.
Individual Case Review (ICR) - Protocol Compliance QA
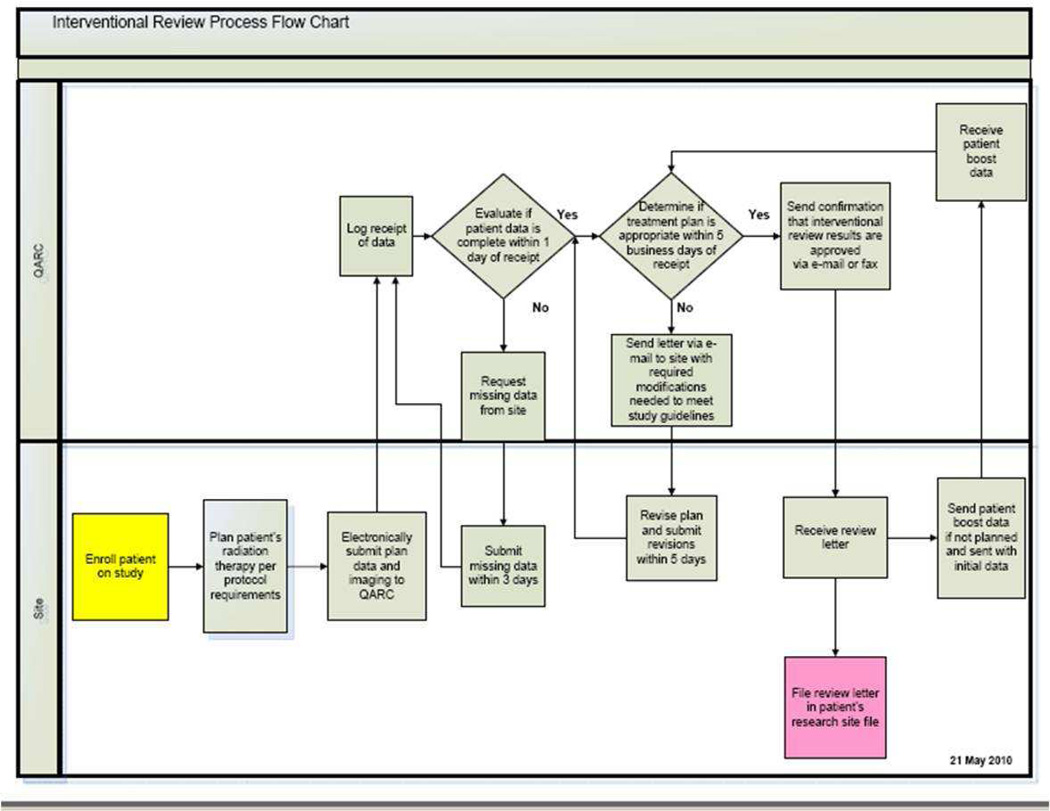
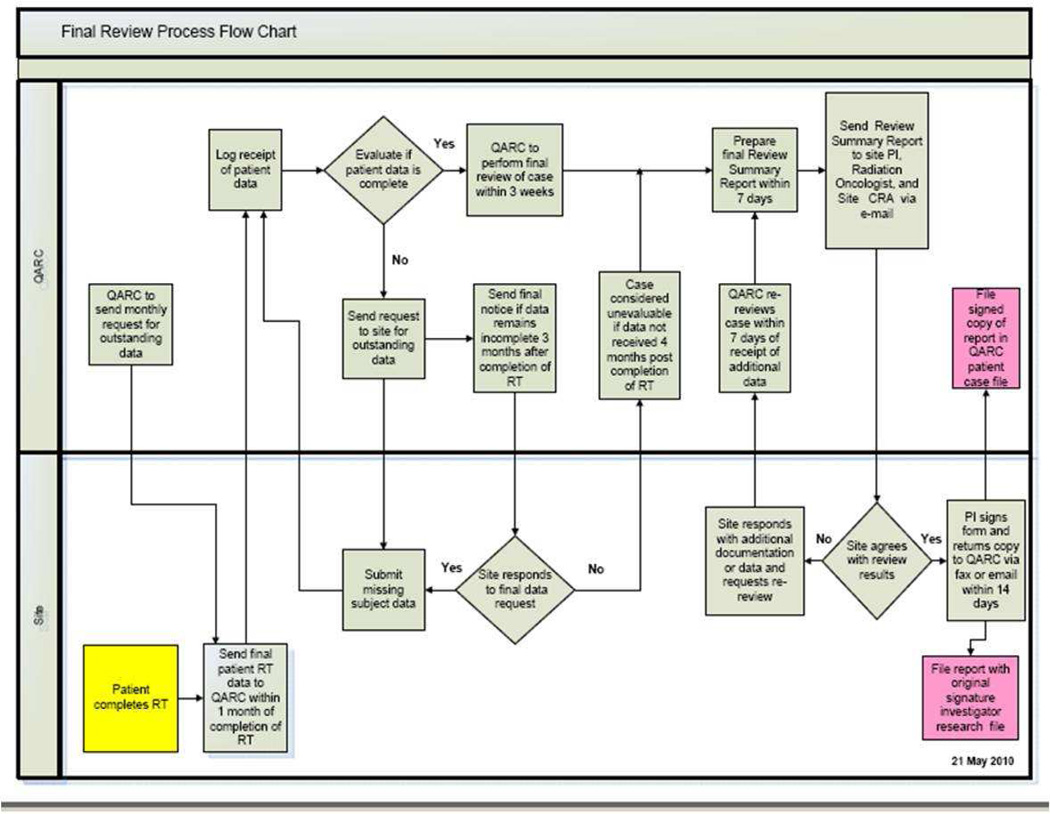
Once the digital data has been processed, the case undergoes what is called “protocol compliance QA (PCQA)” review. The PCQA is a review of the target volume, OAR contours and dose/dose heterogeneity compliance. The current process used for RTOG ATC supported protocols is that contours are reviewed by the study chairs and dosimetry compliance is reviewed by either RTOG Headquarters (HQ) dosimetry staff, or by RPC dosimetry staff (for brachytherapy cases). For QARC-monitored protocols, study chairs or QARC clinical staff reviews the volumes for interventional cases. Retrospective volume case review is performed by the study chairs. QARC dosimetry staff provides integrated dosimetry review for both the interventional and final case reviews. Figures 2 and 3 illustrate the QARC interventional and final review process.
Figure 2.
QARC Interventional Review Process
Figure 3.
QARC Final Review Process
Data Management
Data management is the integration and presentation display of data once it has been acquired by the QA center. Funded clinical research associates (CRA) are crucial to the success of data management. These highly skilled individuals are fluent in the study strategy and objectives, identify and acquire protocol required data and participate in the daily process of integrating the acquired data and information into the study database in an established uniform format. As protocols develop, interactions between study sponsor, cooperative group, and QA centers identify informatics processes that will be used for the trial execution. Delineation of responsibility among the partners is defined for the confidentiality of subject data as well as the data acquisition, management and storage. Often responsibilities are divided among involved parties. For example, patient care information and clinical outcome data are acquired by the study sponsor/cooperative group while the QA center is responsible for image and radiation therapy object acquisition. The imaging and radiation therapy QA objects are acquired and formatted by the QA center and defined elements are integrated into the study sponsor/cooperative group database in a uniform format. These interactions are essential to the execution of the trial. Modern protocols demand real time assessment of imaging response and approval of intended radiation therapy treatment fields therefore exchange of information is done in real time. (Figure 2) In the COG AHOD0031 Hodgkins lymphoma intermediate risk trial, central review assessment of imaging objects for clinical response to chemotherapy was performed at QARC and entered into the COG website in real time. The assessment of response was the gateway for both secondary and tertiary randomizations imbedded in the trial.20,21 Processes required for the successful execution of the modern clinical trial must be built for adaptive strategies.
Informatics
The informatics platform is essential for clinical trial operation. It becomes the center for trial operations and the primary vehicle for data exchange and real time/retrospective review. Databases need to be secure and include query functions. These elements become crucial as we move towards adaptive clinical trial design. The importance of being able to query, both during and at study completion, cannot be overstated. Protocols of the future will be living documents with mechanisms imbedded to add/subtract therapies as interim assessment of results become more commonplace and meaningful.
Systems fully compliant with 21 CFR Part 11 are critical to clinical trial function. This law defines the standards for which electronic records and signatures can be used. It is essential for this full compliance when clinical trials require real time data review with corresponding therapy adjustment and submission of response validation to the FDA.
Remote Review of Objects
In the early generation of clinical trials management, review of data by study investigators and sponsors was largely retrospective and performed well after completion of the clinical trial. In the modern trial, informatics processes must be agile and available for both on site and remote investigator review throughout the world. To achieve this objective QARC developed access to the QARC database through virtual private networking (VPN) accounts to a terminal server. Using an internet browser enables the remote investigator to log in and view the custom designed interface which lists their cases ready for review. Clicking on the case number opens the record. Clicking on the links to the subject's imaging studies and RT treatment plans, objects are reviewed. The functionality is designed to support exactly what is required for a remote review.22 The areas of the database that the remote reviewer can access are limited to the specific tasks required for them to complete. Only subject records specifically assigned to the reviewer are viewable. The fields necessary for the remote reviewer's evaluation are editable, images can be annotated and saved, and all other fields and imaging are “read-only” and cannot be edited. Remote reviewer activity within the database is audited to maintain 21 CFR Part 11 compliance.
Enabling investigators to review their assignments at a time of their convenienceis essential to modern clinical trial function. The goal moving forward will be to insure appropriate radiotherapy planning and delivery as well as staging, eligibility, response, and validation of disease progression/failure on all protocol subjects. A retrospective review of failure images in a medulloblastoma clinical trial revealed that 8 of 60 failure images were consistent with treatment effect, not failure, on central review.23[JT1] Moving these processes into real time function will be essential to the future success of adaptive clinical trial design.
Results and Discussion
The Importance of Quality in Clinical Trials: Real Time Review of Hodgkins Lymphoma
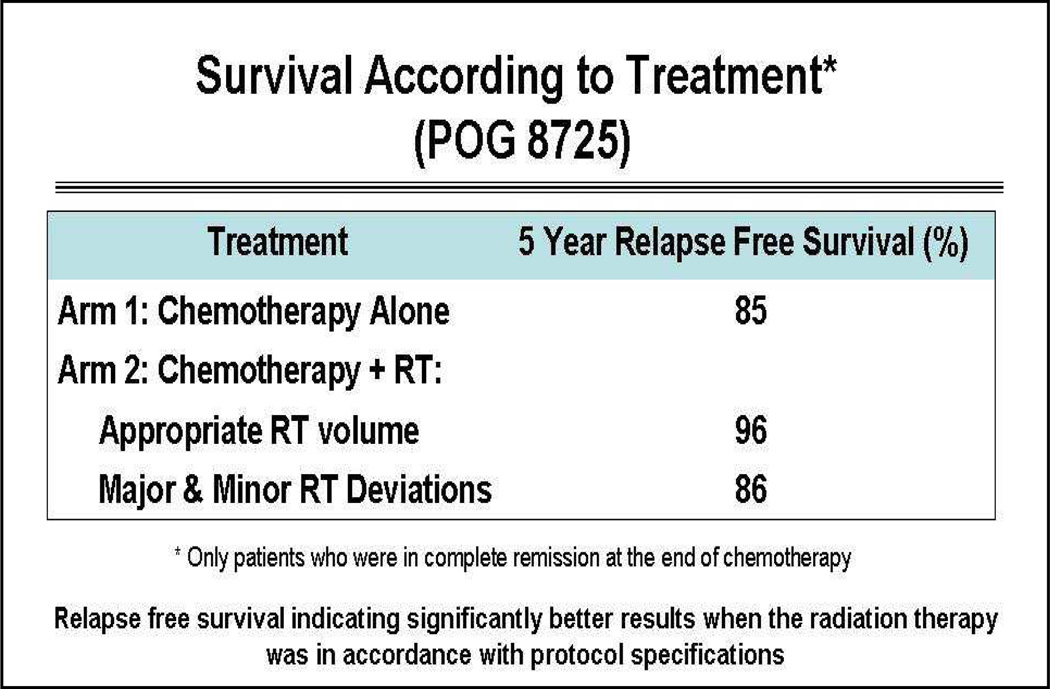
Hodgkins lymphoma (HL) is a model demonstrating process improvements of quality assurance in clinical trials for radiation therapy. Pediatric Oncology Group (POG) clinical trial 8725 was a trial designed to test the importance of consolidation radiation management in intermediate and high-risk patients with HL. The protocol randomized patients for radiation therapy to all sites of original disease defined on imaging after completing 8 cycles of alternating chemotherapy. The final data published in the Journal of Clinical Oncology in 1999 revealed no difference in survival between those who underwent radiation therapy or those who received chemotherapy only.24 A retrospective analysis at QARC evaluated the differences in patients who had treatment delivered per protocol or had treatment delivered in a non-study compliant manner. The study required that all sites of original disease be included in the radiation therapy treatment fields. Investigators off study may prefer to exclude areas of involvement such as pulmonary/hepatic parenchyma, pericardial effusions, axilla in female patients, and other sites of involvement due to preconceived concerns about both acute and late normal tissue toxicity. In this cohort of intermediate and advanced patients, patient survival was affected in a significant manner when radiation therapy was not delivered in a study compliant manner. Of more importance, patient survival and disease free status were statistically improved (Figure 4)8 when radiotherapy given in accordance with protocol specifications. In this study most deviations from protocol compliance were related to excluding areas of original involvement from the intended treatment target. Because there was a perceived disparity in image interpretation, involved disease sites at diagnosis and the subsequent design of the radiation therapy treatment fields, a decision was made by the POG for pre-treatment approval at QARC of the intended radiation therapy treatment targets. In the next iteration of clinical trials in HL (P9425 and P9426), radiation therapy treatment objects were reviewed with imaging pre-treatment in order to insure protocol compliance. The deviation rate was under 10% (improved from 30%) which was considered good from a historical perspective. The compliance rate for pre-therapy data submission for this effort was 90%, again considered excellent.25
Figure 4.
Relapse-free survival indicating significantly better results
Protocol P9426 was a response adaptive clinical trial for pediatric early stage HL. Chemotherapy was abbreviated to 2 cycles if the study subjects had a rapid early response to chemotherapy based on review of images. The protocol was completed in the era prior to the routine use of positron emission tomography (PET). Also analyzed was the assessment of the difference between central and site review of image response. The concordance rate was only 50% for response status; creating a clear need for image reviews to be done on a real time basis to assure consistent interpretation of response. Preliminary data suggests that in this favorable group of patients 2 cycles of chemotherapy with 2100 cGy RT to involved fields had an identical clinical outstanding outcome as those who received 4 cycles of chemotherapy and radiation treatment (paper submitted); therefore the need for uniform interpretation of response is clear.
Based on data from this and other clinical trials, a response adaptive treatment strategy with attenuated chemotherapy for patients deemed both rapid early responders to 2 cycles of chemotherapy and complete responders to 4 cycles of chemotherapy was implemented in the current generation of clinical trials for HL patients with intermediate risk features. AHOD0031 became the first study to employ real time review of anatomic and metabolic images for central review evaluation of response coupled with pre-treatment review of radiation therapy treatment objects. The study accrued 1733 patients with greater than 90% compliance to submission of objects both for imaging and radiation therapy treatment review. If there was a difference between site and central review, the issue was resolved using web conferencing in real time. This provided the Study Chairs the opportunity to review the data in real time and adjudicate issues such as staging, relapse, response, and other matters associated with the trial conduct in an upfront manner, thereby significantly decreasing imaging and radiation therapy deviations and limiting the number of ineligible patients. This important step forward has altered study design and the quality assurance strategy for many studies. Real time review of objects has now become the routine, providing investigators the opportunity to remain in close observation to the conduct of their studies.
The tools for this review have also improved. For example, the initial real time remote review process required QARC staff to be on site to facilitate the review. QARC has developed a terminal server mechanism that permits study investigators to log into the server via a web based mechanism and review and annotate their images. The investigators have full utility of the QARC database system and can log on at any time at their convenience. The annotations and measurements are saved and stored for future review. This maintains 21 CFR Part 11 compliance, which is important for retrospective review of data if needed by the FDA. Many studies now employ real time review of objects performed both on and off site through this mechanism.
Opportunities Lost
Multi-institutional clinical trials involving radiotherapy encompass a range of trial designs and objectives, from investigating the safety or efficacy of new radiotherapy technologies or fractionation schemes to examining more conventional radiotherapy approaches as an adjuvant to novel chemotherapy or biologic agents to studying symptom management interventions. While current QA processes in the United States tend to be “one size fits all,” there may be opportunities to tailor clinical trial QA to the objectives of the trial under study.
Not all potential study issues can be anticipated and opportunities may be lost in not acquiring data sets on study patients. From 1988–2000 the CALGB, together with the intergroup mechanism, developed a sequential series of outstanding clinical trials evaluating adriamycin based chemotherapy in breast cancer. These studies established the role of dose dense chemotherapy and also validated the use of taxol in breast cancer patient care. It was determined in the study, 9344, not to acquire data concerning radiation therapy if treated, nor to inquire as to whether or not the patient received radiation therapy. This was due to the perception that local care did not affect survival and that there was no synergism between local and systemic care with respect to patient survival.
As these studies matured, closed, and re-opened in a sequential manner, data became independently available concerning the survival advantage to node positive breast cancer patients treated with radiation therapy.26 This created limitations in interpreting the studies as no data was captured whether the patients on study received radiation therapy nor to what volume/dose if they were treated with radiation. Dr. Carolyn Sartor made a strong effort to capture this information in retrospect with the support of QARC. Information was captured on the patients treated through the CALGB mechanism. There was a clear trend for patients who received taxol to also be irradiated thus making the overall study data more difficult to interpret.27 Today, as a result of several clinical trials, we are beginning to question the extent of surgery in the axilla. This may alter the role of radiation therapy in breast cancer care and may change the manner in which we approach the axilla from a dose/volume perspective. If we had captured volumetric data on the thousands of node positive patients on trial including images of local regional relapse, we might be much further along and in a better position to define and assign dose/volume constraints to the volume defined axilla in clinical trials. Therefore this lost opportunity will place limitations on our knowledge base for the future. We do not always have to score data as part of a clinical trial. This is well documented in registration trials. If quality assurance centers can build an archive with clinical trial information, this might prove to be an invaluable resource.
A New Paradigm Based on Real Time Review
The success of AHOD0031 has led to a new paradigm for radiation therapy in the treatment of HL. In the current advanced stage clinical trial, AHOD0831, the patients have stage 3B and 4B disease. They receive chemotherapy adjusted to response seen on PET followed by radiation therapy delivered to sites of bulk disease and regions that did not achieve CR status (> 2 cm) to chemotherapy. This strategy moves radiation therapy into a different position with this unique patient cohort as not all areas of original disease are intentionally treated as part of consolidation management. Therefore pre-treatment review of radiation therapy treatment objects is directly involved. The conduct and execution of this trial has been built on the success of the real time review of objects in AHOD0031.
Standardized descriptions of radiotherapy volume and dose definitions and standardized reporting of data and QA results enable the creation, application and update of consensus standards as revealed in clinical trial evidence.28 Adaptive treatments based on centrally confirmed response would not be an option without the standard QA processes for central review, data acquisition, collection, review and reporting.21,29,30
The clinical trials experience for HL over the past 25 years reflects the change in the structure of clinical trials with movement towards real time review of objects in order to insure compliance to study objectives. We have been able to use real time review of imaging and radiation therapy treatment objects to insure appropriate staging and patient eligibility. Real time review ensures uniform interpretation of objects when evaluating response. Clinical trials are now increasingly complex, with multiple endpoints imbedded into study objectives. Endpoints can include imaging, radiation therapy, genomics/proteomics, and other trial objectives beyond traditional patient outcome with chemotherapy. Modern investigators now require patient objects be available on a real time basis for both secondary randomization events and adaptive protocol design. Thus in thirty years of HL clinical trials, we can see how the quality assurance process has evolved from retrospective review of objects to real time integration of a broad informatics portfolio for review of imaging and radiation therapy treatment objects. Integrating pathology objects is the next step forward.
Opportunity for Process Improvements
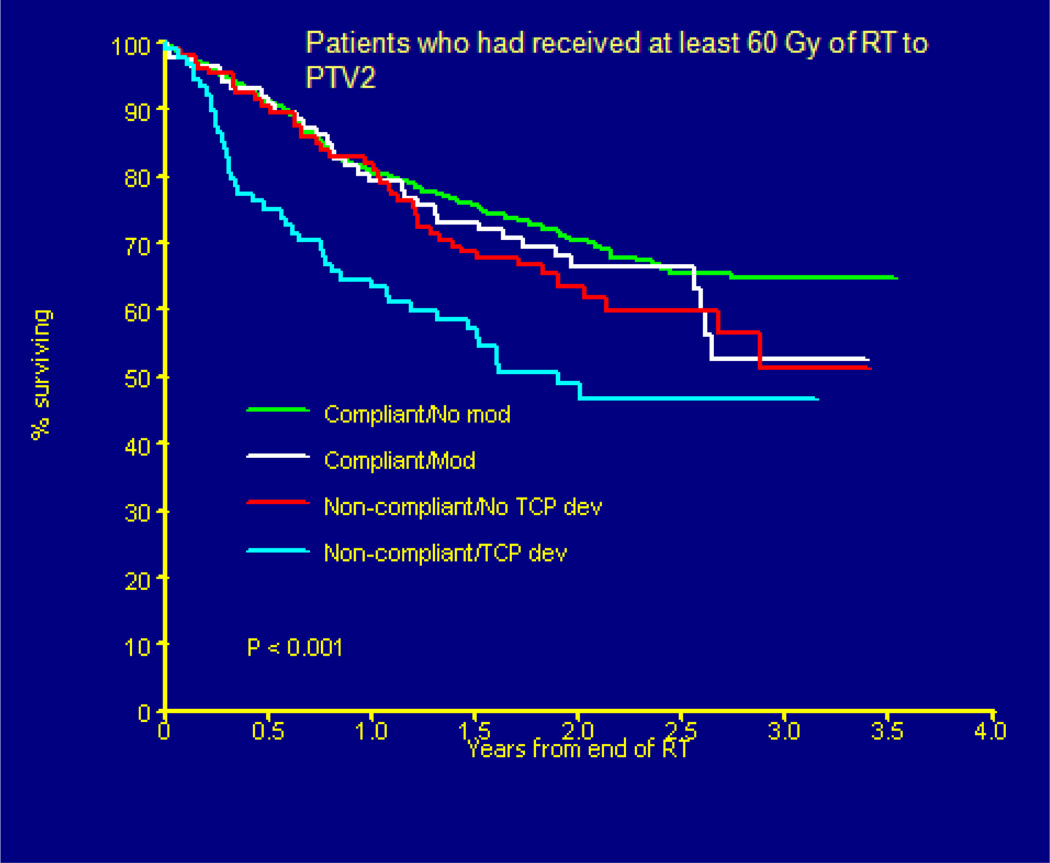
The need for real time review of imaging and radiation therapy treatment objects on an international scale became visible in the Head START clinical trial. This head/neck phase 3 clinical trial evaluated the hypoxic cell sensitizer, Tirapazamine, for locally advanced head/neck cancer. Because the clinical trial was international, a decision was made to review objects within the first three days of treatment in order to provide time for sites to compile data without delaying patient initiation of treatment. The images and plans were reviewed at QARC with evaluation sent to investigators. As can be seen in Figure 5, patient survival was directly correlated to the quality of the treatment plan with a near thirty percent decrease in patient survival if the plan did not meet study objectives on review. Most study deviations were related to incomplete coverage of what was thought to be tumor targets on review. If a plan was revised by site investigators and was thought to be compliant at the time of the final review there was a clear improvement in patient survival, but not as good as if the plan was approved up front. The clinical trial management committee asked QARC to review deviations and score them as to whether or not the clinical intent and execution met a reasonable clinical standard for patient care.
Figure 5.
(Peters L, O’Sullivan B)Head START Trial Results (Overall Survival) by Protocol Deviation Status
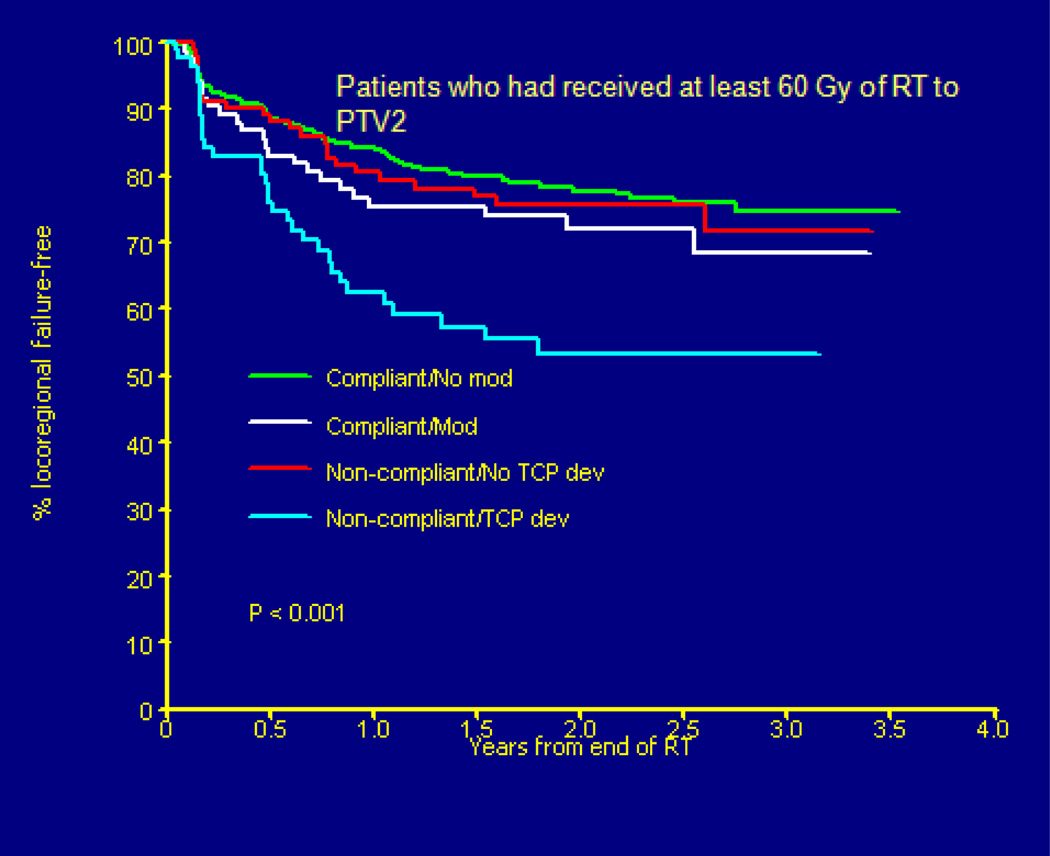
For example, in this group were patients whose objects were drawn too close to the skin which meant that those patients did not meet dose-volume constraints for DVH analysis and were scored as deviations. On secondary review these patients were deemed as being treated in a manner consistent with standard clinical practice. Deviations often remained in patients with photon-electron matches over asymmetric lymph node regions as coverage at depth was not always uniform and largely cold. These patients had a similar survival to those patients who had their plans adjusted for study compliance, again improved but not as good as patients with plans initially compliant to study objectives. The data for local control (Figure 6) is similar to patient survival.
Figure 6.
(Peters L, O’Sullivan B) Head START Trial Results (Failure-Free Survival) by Protocol Deviation Status
This study took place at a time (2002–2005) when radiation oncologists were beginning to use image driven objects in the execution of treatment plans for head and neck cancer. IMRT was not permitted on this study. This may emphasize one key element to quality assurance. As we go through changes in radiation therapy, including changes in both technology and target definition, quality assurance in clinical trials needs to anticipate how sites will adjust to changes in technique including target definition and provide as much support as possible to facilitate the change and insure compliance to study objectives.31 This can include real time review of objects, use of web-based media for providing examples of target definition, and media workshops. The primary objective of quality assurance needs to be limitation of study deviations in order to provide a uniform study population for protocol analysis. With the use of digital media, these objectives can be met in real time.
Future objectives
Clinical translational investigators will soon need to have many tools at their fingertips in order to perform modern research. Digital pathology objects including Digital Imaging and Communications in Medicine (DICOM) compatible genomic/proteomic micro arrays will be linked in a single database with images, RT objects, and patient outcome data. Investigators will review and integrate response data with the pathology objects for protocol analysis. Protocols will be designed to acknowledge genomic/proteomic data including protocols involving radiation therapy. For example, ECOG has a current protocol intentionally decreasing radiation dose to head and neck cancer patients who are positive to HPV and demonstrate a complete response to induction chemotherapy. This protocol requires both validation of pathology data and real time review of imaging data to validate response prior to initiating radiation therapy. International participation will further emphasize the need for integrated informatics formats and data harmonization. Current protocols in high grade glioma require analysis of molecular expression products integrated with advanced technology metabolic imaging to assess response to treatment and disease progression as traditional imaging cannot fully validate early progression or response. Protocols in lung and colo-rectal cancer will integrate selected targeted therapy with RT based on mutation analysis. The current list is extensive and will require an integrated database to facilitate protocol success. Radiation therapy clinical trials will be able to take advantage of this collective knowledge to further promote our discipline in the next generation of clinical trials.
Acknowledgement
Thank you to Maryann Bishop-Jodoin for the work provided on this paper.
Supported in part by NIH/NCI CA 29511 (Quality Assurance Review Center)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. FitzGerald does not have any financial disclosures or other conflicts of interest.
References
- 1.Purdy JA, Harms WB, Michalski J, Bosch WR. Initial experience with quality assurance of multi-institutional 3D radiotherapy clinical trials. A brief report. Strahlenther Onkol. 1998;174:40–42. [PubMed] [Google Scholar]
- 2.Ibbott GS. QA in radiation therapy: The RPC perspective; J Phys Conf Ser; 2010. 012001. [Google Scholar]
- 3.Ibbott GS, Followill DS, Molineu HA, Lowenstein JR, Alvarez PE, Roll JE. Challenges in credentialing institutions and participants in advanced technology multi-institutional clinical trials. Int J Radiat Oncol Biol Phys. 2008;71:S71–S75. doi: 10.1016/j.ijrobp.2007.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olch AJ, Kline RW, Ibbott GS, Anderson JR, Deye J, FitzGerald TJ, Followill D, Gillin MT, Huq MS, Palta JR, Purdy JA, Urie MM. Quality assurance for clinical trials: A primer for physicists. Medical Physics Publishing, Madison. 2004 AAPM Report N0. 86. [Google Scholar]
- 5.Reinstein LE, Peachey S, Laurie F, Glicksman AS. Impact of a dosimetry review program on radiotherapy in group trials. Int J Radiat Oncol Biol Phys. 1985;11:1179–1184. doi: 10.1016/0360-3016(85)90067-7. [DOI] [PubMed] [Google Scholar]
- 6.Glicksman AS, Reinstein LE, Laurie F. Quality assurance of radiotherapy in clinical trials. Cancer Treat Rep. 1985;69:1199–1205. [PubMed] [Google Scholar]
- 7.Bogart JA, Seagren SL, Glicksman A. Radiation oncology research in the cancer and leukemia group B. Clin Cancer Res. 2006;12:3628s–3634s. doi: 10.1158/1078-0432.CCR-06-9011. [DOI] [PubMed] [Google Scholar]
- 8.FitzGerald TJ, Urie M, Ulin K, Laurie F, Yorty J, Hanusik R, Kessel S, Jodoin MB, Osagie G, Cicchetti MG, Pieters R, McCarten K, Rosen N. Processes for quality improvements in radiation oncology clinical trials. Int J Radiat Oncol Biol Phys. 2008;71:S76–S79. doi: 10.1016/j.ijrobp.2007.07.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulin K, Urie MM, Cherlow JM. Results of a multi-institutional benchmark test for cranial CT/MR image registration. Int J Radiat Oncol Biol Phys. 2010;77:1584–1589. doi: 10.1016/j.ijrobp.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palta JR, Deye JA, Ibbott GS, Purdy JA, Urie MM. Credentialing of institutions for IMRT in clinical trials. Int J Radiat Oncol Biol Phys. 2004;59:1257–1259. doi: 10.1016/j.ijrobp.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Ibbott GS, Hanson WF, O'Meara E, Kuske RR, Arthur D, Rabinovitch R, White J, Wilenzick RM, Harris I, Tailor RC. Dose specification and quality assurance of radiation therapy oncology group protocol 95-17; a cooperative group study of iridium-192 breast implants as sole therapy. Int J Radiat Oncol Biol Phys. 2007;69:1572–1578. doi: 10.1016/j.ijrobp.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onar A, Ramamurthy U, Wallace D, Boyett JM. An operational perspective of challenging statistical dogma while establishing a modern, secure distributed data management and imaging transport system: the Pediatric Brain Tumor Consortium phase I experience. Clin Transl Sci. 2009;2:143–149. doi: 10.1111/j.1752-8062.2009.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Movsas B, Vikram B, Hauer-Jensen M, Moulder JE, Basch E, Brown SL, Kachnic LA, Dicker AP, Coleman CN, Okunieff P. Decreasing the adverse effects of cancer therapy: National Cancer Institute guidance for the clinical development of radiation injury mitigators. Clin Cancer Res. 2011;17:222–228. doi: 10.1158/1078-0432.CCR-10-1402. [DOI] [PubMed] [Google Scholar]
- 14.Pappo AS, Krailo M, Chen Z, Rodriguez-Galindo C, Reaman G. Infrequent tumor initiative of the Children's Oncology Group: initial lessons learned and their impact on future plans. J Clin Oncol. 2010;28:5011–5016. doi: 10.1200/JCO.2010.31.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Million L, Anderson J, Breneman J, Hawkins DS, Laurie F, Michalski J, Rodeberg D, Wharam M, Wolden S, Donaldson SS Soft Tissue Sarcoma Committee of theChildren's Oncology Group. Influence of noncompliance with radiation therapy protocol guidelines and operative bed recurrences for children with rhabdomyosarcoma and microscopic residual disease: a report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys. 2011;80:333–338. doi: 10.1016/j.ijrobp.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendenhall NP, Meyer J, Williams J, Tebbi C, Kessel S, Laurie F, et al. The impact of central quality assurance review prior to radiation therapy on protocol compliance: POG 9426, a trial in pediatric Hodgkin’s disease. Blood. 2005;106:753. [Google Scholar]
- 17.Purdy JA, Bosch WR, Straube WL, et al. A review of the activities of the ITC in support of RTOG advanced technology clinical trials. Int J Radiat Oncol Biol Phys. 2006;66:S134. [Google Scholar]
- 18.Straube W, Bosch W, Matthews J, Haynes R, Purdy J. Digital data integrity QA for multi-institutional clinical trials. Med Phys. 2006;33:2087. [Google Scholar]
- 19.Straube W, Matthews J, Bosch W, Purdy JA. DVH analysis: Consequences for quality assurance of multi-institutional clinical trials. Med Phys. 2005;32:2021. [Google Scholar]
- 20.McCarten KM, Rosen N, Friedman D, Schwartz CL, Voss S, Bishop-Jodoin M, Kessel S, Johnson C, Laurie F, FitzGerald TJ. Feasibility of real time diagnostic imaging central review in Multicenter Cancer Trials. American Roentgen Ray Society (ARRS) Annual Meeting; Boston, MA. 2009. [Google Scholar]
- 21.Wolden S, Constine L, Schwartz CL, Friedman D, McCarten K, Laurie F, Kessel S, Johnson C, Bishop-Jodoin M, FitzGerald TJ. PROS Congress. Canada: Montreal; 2009. Real time diagnostic imaging central review and radiotherapy interventional review in multicenter cancer trials. [Google Scholar]
- 22.Hanusik RE, Laurie F, FitzGerald TJ, Kelly S, Bishop-Jodoin M. Extending the availability of a large multi-institutional oncology imaging archive to remote reviewers. Society for Imaging Informatics in Medicine (SIIM) Annual Meeting; Minneapolis, MN. 2010. [Google Scholar]
- 23.Gajjar A, Vezina G, Langston J, Muraszko K, Dias M, Kessel S, Sposto R, Packer R. Results of central imaging review for patients enrolled on average risk Medulloblastoma (MB) Protocol A9961. International Symposium on Pediatric Neuro-Oncology; Boston, MA. 2004. [Accessed Aug 1, 2011]. http://home.comcast.net/~turne038/abstracts.htm. [Google Scholar]
- 24.Weiner MA, Leventhal B, Brecher ML, Marcus RB, Cantor A, Gieser PW, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol. 1997;15:2769–2777. doi: 10.1200/JCO.1997.15.8.2769. [DOI] [PubMed] [Google Scholar]
- 25.Mendenhall NP, Meyer J, Williams J, Tebbi C, Kessel S, Laurie F, et al. The impact of central quality assurance review prior to radiation therapy on protocol compliance: POG 9426, a trial in pediatric Hodgkin’s disease. Blood. 2005;106:753. [Google Scholar]
- 26.Marks LB, Cirrincione C, Fitzgerald TJ, Laurie F, Glicksman AS, Vredenburgh J, Prosnitz LR, Shpall EJ, Crump M, Richardson PG, Schuster MW, Ma J, Peterson BL, Norton L, Seagren S, Craig Henderson I, Hurd DD, Peters WP for the Cancer and Leukemia Group B, Southwest Oncology Group, National Cancer Institute of Canada Clinical Trials Group. Impact of high-dose chemotherapy on the ability to deliver subsequent local-regional radiotherapy for Breast Cancer: Analysis of Cancer and Leukemia Group B Protocol 9082. Int J Radiat Oncol Biol Phys. 2010;76:1305–1313. doi: 10.1016/j.ijrobp.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartor CI, Peterson BL, Woolf S, FitzGerald TJ, Laurie F, Turrisi AJ, Bogart J, Henderson IC, Norton Effect of addition of adjuvant Paclitaxel on radiotherapy delivery and loco regional control of node-positive breast cancer: Cancer and Leukemia Group B 9344. J Clin Oncol. 2005;23:30–40. doi: 10.1200/JCO.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 28.Bekelman JE, Yahalom J. Quality of radiotherapy reporting in randomized controlled trials of Hodgkin's lymphoma and non-Hodgkin's lymphoma: a systematic review. Int J Radiat Oncol Biol Phys. 2009;73:492–498. doi: 10.1016/j.ijrobp.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 29.McCarten KM, Rosen N, Friedman D, Schwartz CL, Voss S, Bishop-Jodoin M, Kessel S, Johnson C, Laurie F, FitzGerald TJ. Feasibility of real time diagnostic imaging central review in multicenter cancer trials. American Roentgen Ray Society (ARRS) Annual Meeting; Boston, MA. 2009. [Google Scholar]
- 30.Fitzgerald TJ, Bishop-Jodoin M, Cicchetti MG, Hanusik R, Kessel S, Laurie F, McCarten KM, Moni J, Pieters RS, Rosen N, Ulin K, Urie M, Chauvenet AR, Constine LS, Deye J, Vikram B, Friedman D, Marcus RB, Jr, Mendenhall NP, Williams JL, Purdy J, Saltz J, Schwartz CL, White KS, Wolden S. Quality of radiotherapy reporting in randomized controlled trials of Hodgkin's lymphoma and non-Hodgkin's lymphoma: in regard to Bekelman and Yahalom (Int J Radiat Oncol Biol Phys 73:492–498, 2009) Int J Radiat Oncol Biol Phys. 2010;77:315–316. doi: 10.1016/j.ijrobp.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters L, O’Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, Bourhis J, Yuen K, Fisher R, Rischin D. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer- results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 32.Arndt CA, Donaldson SS, Anderson JR, Andrassy RJ, Laurie F, Link MP, Raney RB, Maurer HM, Crist WM. What constitutes optimal therapy for patients with rhabdomyosarcoma of the female genital tract? Cancer. 2001;91:2454–2468. [PubMed] [Google Scholar]
- 33.Bekelman JE, Yahalom J. Quality of radiotherapy reporting in randomized controlled trials of Hodgkin's lymphoma and non-Hodgkin's lymphoma: a systematic review. Int J Radiat Oncol Biol Phys. 2009;73(2):492–498. doi: 10.1016/j.ijrobp.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 34.Bishop-Jodoin M, FitzGerald TJ, Urie M, Ulin K, Cicchetti MG, Pieters R, Kessel S, Yorty J, Hanusik R, Laurie F. The QARC quality assurance program- Improving standard of care in the management of cancer: Past, present and future. Int J Rad Oncol Bio Phys. 2008;72:S426. [Google Scholar]
- 35.Blackstock A, Socinski MA, Fitzgerald TJ, Gu L, Rosenman J, Wang X, Bogart JA, Vokes EE, Green MR. Initial pulmonary toxicity evaluation of chemo radiotherapy (CRT) utilizing 74 Gy 3-dimensional (3-D) thoracic radiation in Stage III Non-Small Cell lung cancer (NSCLC): A Cancer and Leukemia Group B (CALGB) Randomized Phase II Trial. Int J Rad Oncol Biol Phys. 2005;63:S43. [Google Scholar]
- 36.Bogart JA, Watson D, McClay EF, Evans L, Herndon JE, Laurie F, Seagren SL, Fitzgerald TJ, Vokes E, Green MR. Interruptions of once-daily thoracic radiotherapy do not correlate with outcomes in limited stage small cell lung cancer: Analysis of CALGB phase III trial 9235. Lung Cancer. 2008;62:929–928. doi: 10.1016/j.lungcan.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch W, Matthews J, Ulin K, Urie M, Yorty J, Straube W, FitzGerald T, Purdy J. Implementation of ATC Method 1 for clinical trials data review at the Quality Assurance Review Center. Med Phys. 2006;33:2109. [Google Scholar]
- 38.Cicchetti MG, Bogart J, Lyss A, Turrisi A, Green M, Herndon J, Bushe S, Briggs K, Koehler I, Bertsch K, Laurie F, FitzGerald TJ. Spinal cord dose in limited stage small cell lung cancer: a preliminary quality assurance review committee (QARC) report on CALGB 39808, using target thoracic radiotherapy (TRT) doses of 60 – 70 Gy. Int J Rad Onc Bio Phys. 2001;51:356. [Google Scholar]
- 39.Constine LS, Marcus R, Chauvenet A, London W, Villaluna D, Kessel S, Fitzgerald TJ, Kamps W, Steven L, Schwartz C. Patterns of failure after response-based, dose-dense therapy for intermediate/high risk pediatric Hodgkin’s disease (POG 9425). Proceedings of the American Society for Therapeutic Radiology and Oncology 47th Annual Meeting, Denver, CO. Int J Radiat Oncol Biol Phys. 2005;63:S21–S22. [Google Scholar]
- 40.DiPetrillo TA, Heron DE, Fernando HC, Landreneau RJ, Logan D, Briggs K, Fitzgerald TJ, Thomas CR, Putnam JB, Meyers BF, et al. Sublobar resection and Iodine-125 brachy-therapy for stage I NSCLC: Initial quality assurance review of ACOSOG Z-4032. American Society for Radiation Oncology (ASTRO) Annual Meeting; Nov 2009; Chicago, IL. [Google Scholar]
- 41.Donahue BR, Marymont MH, Kessel S, Iandoli MK, Fitzgerald TJ, Holmes E, Zhou T, Gajjar A, Packer RJ. Secondary radiation therapy quality analysis CCG/POG intergroup 9961: Implications for cranio spinal irradiation (CSI) Canada: Paediatric Radiation Oncology Society (PROS) Congress, Montreal; 2009. Jul, [Google Scholar]
- 42.Donaldson SS, Asmar L, Breneman J, Fryer C, Glicksman AS, Laurie F, Wharam M, Gehan EA. Hyper fractionated radiation in children with rhabdomyosarcoma--results of an Intergroup Rhabdomyosarcoma Pilot Study. Int J Radiat Oncol Biol Phys. 1995;32:903–991. doi: 10.1016/0360-3016(95)00151-n. [DOI] [PubMed] [Google Scholar]
- 43.Donaldson SS, Meza J, Breneman JC, Crist WM, Laurie F, Qualman SJ, Wharam M Children's Oncology Group Soft Tissue Sarcoma Committee (formerly Intergroup Rhabdomyosarcoma Group) representing the Children's Oncology Group and the Quality Assurance Review Center. Results from the IRS-IV randomized trial of hyper fractionated radiotherapy in children with rhabdomyosarcoma--a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 44.Donaldson SS, Torrey M, Link MP, Glicksman A, Gilula L, Laurie F, Manning J, Neff J, Reinus W, Thompson E, Shuster JJ. A multidisciplinary study investigating radiotherapy in Ewing's sarcoma: end results of POG #8346. Pediatric Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:125–135. doi: 10.1016/s0360-3016(98)00191-6. [DOI] [PubMed] [Google Scholar]
- 45.FitzGerald TJ. Current limitations & identification of key clinical needs: Quality Control for National Trials. Rochester, NY: SBRT; 2008. Jun, [Google Scholar]
- 46.FitzGerald TJ. Cancer Survivor Research and Education (CURED) Adverse Late Effects in Radiation Treatment (ALERT) Rochester, NY: 2008. May, Data management for late effects of cancer treatment. [Google Scholar]
- 47.FitzGerald TJ. Setting Endpoints Using Imaging Biomarkers in Clinical Trials. Imaging and Clinical Trials Conference; Cambridge Healthtech Institute; May 2008; Cambridge, MA. [Google Scholar]
- 48.FitzGerald TJ. QA in Clinical Trials. Bethesda, MD: EORTC-NCI; 2008. Apr, [Google Scholar]
- 49.FitzGerald TJ. Fundamentals & Practical Aspects of Imaging in Clinical Trials. San Francisco, CA: Institute for International Research; 2007. Sep, Setting Endpoints and Biomarkers in Imaging Testing. [Google Scholar]
- 50.FitzGerald TJ, Bishop-Jodoin M, Ulin K, Urie M, Kessel S, Morano K, Hanusik R, Laurie F. Establishing an integrated database for distributed review of imaging, radiation therapy, and pathology objects. Fourth International Conference on Translational Research and Pre-Clinical Strategies in Radiation Oncology; Mar 2009; Geneva, Switzerland. [Google Scholar]
- 51.Fitzgerald TJ, Williams J, Laurie F, Kessel S, Feree C, Mendenhall NP. 2221 Problems in radiation therapy protocol compliance in Hodgkin’s disease: Preliminary analysis of POG study 9426. 41st Annual Meeting of the American Society for Therapeutic Radiology and Oncology, San Antonio, TX. Int J Radiat Oncol Bio Phys. 1999;45(3) S1:392. 1999. [Google Scholar]
- 52.Glicksman AS, Wasserman TH, Bjarngard B, Laurie F. The structure for a radiation oncology protocol. The Committee of Radiation Oncology Group Chairmen. Int J Radiat Oncol Biol Phys. 1992;23:1079–1082. doi: 10.1016/0360-3016(92)90916-6. [DOI] [PubMed] [Google Scholar]
- 53.Halperin EC, Laurie F, Fitzgerald TJ. An evaluation of the relationship between the quality of prophylactic cranial radiotherapy in childhood acute leukemia and institutional experience: a Quality Assurance Review Center-Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 2002;53:1001–1004. doi: 10.1016/s0360-3016(02)02833-x. [DOI] [PubMed] [Google Scholar]
- 54.Providence, RI: Quality Assurance Review Center; [Accessed August 1, 2011]. IMRT Questionnaire and Benchmark. Available at: http://www.qarc.org/benchmarks/IMRTbenchmark.pdf. [Google Scholar]
- 55.Ishikura S. Quality assurance of radiotherapy in cancer treatment: toward improvement of patient safety and quality of care. Jpn J Clin Oncol. 2008;38:723–729. doi: 10.1093/jjco/hyn112. [DOI] [PubMed] [Google Scholar]
- 56.Laprise NK, Hanusik R, Fitzgerald TJ, Rosen N, White KS. Developing a multi-institutional PACS archive and designing processes to manage the shift from a film to a digital-based archive. J Digit Imaging. 2009;22:15–24. doi: 10.1007/s10278-007-9080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin C, Donaldson SS, Meza JL, Anderson JR, Lyden ER, Brown CK, Morano K, Laurie F, Arndt CA, Enke CA, Breneman JC. Effect of radiotherapy techniques (IMRTvs. 3D-CRT) on outcome in patients with intermediate-Risk Rhabdomyosarcoma enrolled in COG D9803-A report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys. 2011 Apr 4; doi: 10.1016/j.ijrobp.2011.01.036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michalski JM, Meza J, Breneman JC, Wolden SL, Laurie F, Jodoin M, Raney B, Wharam MD, Donaldson SS. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys. 2004;59:1027–1038. doi: 10.1016/j.ijrobp.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 59.Miralbell R, Fitzgerald TJ, Laurie F, Kessel S, Glicksman A, Friedman HS, Urie M, Kepner JL, Zhou T, Chen Z, Barnes P, Kun L, Tarbell NJ. Radiotherapy in Pediatric Medulloblastoma: Quality Assessment of Pediatric Oncology Group Trial 9031. Int J Rad Oncol Biol Phys. 2006;64:1325–1330. doi: 10.1016/j.ijrobp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Naranjo A, Parisi MT, Shulkin BL, London WB, Matthay KK, Kreissman SG, Yanik GA. Comparison of 123I-meta iodobenzyl guanidine (MIBG) and 131I-MIBG semi-quantitative scores in predicting survival in patients with stage 4neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;56:1041–1045. doi: 10.1002/pbc.22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pieters RS, Laurie F, Wagner H, Wolden S, Constine LS, Cicchetti MG, Kessel S, Friedman D, Schwartz C, Fitzgerald TJ. Management of adolescent patients with Hodgkin’s disease in cooperative group clinical trials. Proceedings of the American Society for Therapeutic Radiology and Oncology 49th Annual Meeting, Los Angeles, CA; Int J Radiat Oncol Biol Phys; 2007. p. S578. [Google Scholar]
- 62.Raney RB, Stoner JA, Walterhouse DO, Andrassy RJ, Donaldson SS, Laurie F, Meyer WH, Qualman SJ, Crist WM Intergroup Rhabdomyosarcoma Study-IV, 1991–1997. Results of treatment of fifty-six patients with localized retroperitoneal and pelvic rhabdomyosarcoma: a report from The Intergroup Rhabdomyosarcoma Study-IV, 1991–1997. Pediatr Blood Cancer. 2004;42:618–625. doi: 10.1002/pbc.20012. [DOI] [PubMed] [Google Scholar]
- 63.Reinstein LE, Glicksman AS, Laurie F. Technical structure of a radiotherapy protocol. Eur J Cancer Clin Oncol. 1987;23:877–881. doi: 10.1016/0277-5379(87)90296-3. [DOI] [PubMed] [Google Scholar]
- 64.Sartor CI, Peterson BL, Woolf S, FitzGerald TJ, Laurie F, Turrisi AJ, Bogart J, Henderson IC, Norton L. Effect of addition of adjuvant Paclitaxel on radiotherapy delivery and loco regional control of node-positive breast cancer: Cancer and Leukemia Group B 9344. J Clin Oncol. 2005;23:30–40. doi: 10.1200/JCO.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 65.Siegel E, Jaffe CC, Purdy J, FitzGerald TJ, Devlin P. Enterprise use cases: Moving beyond demonstration projects to provide real world community Impact. caBIG Annual Meeting; Jul 2009; Washington, DC. [Google Scholar]
- 66.Shankar LK, Van den Abbeele A, Yap J, Benjamin R, Scheutze S, Fitzgerald TJ. Considerations for the use of imaging tools for phase II treatment trials in oncology. Clin Cancer Res. 2009;15:1891–1897. doi: 10.1158/1078-0432.CCR-08-2030. [DOI] [PubMed] [Google Scholar]
- 67.Trimble EL, Abrams JS, Meyer RM, Calvo F, Cazap E, Deye J, Eisenhauer E, Fitzgerald TJ, Lacombe D, Parmar M, Seibel N, Shankar L, Swart AM, Therasse P, Vikram B, von Frenckell R, Friedlander M, Fujiwara K, Kaplan RS, Meunier F. Improving cancer outcomes through international collaboration in academic cancer treatment trials. J Clin Oncol. 2009;27:5109–5114. doi: 10.1200/JCO.2009.22.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urie M, FitzGerald TJ, Followill D, Laurie F, Marcus R, Michalski J. Current calibration, treatment, and treatment planning techniques among institutions participating in the Children's Oncology Group. Int J Radiat Oncol Biol Phys. 2003;55:245–260. doi: 10.1016/s0360-3016(02)03827-0. [DOI] [PubMed] [Google Scholar]
- 69.Urie M, FitzGerald TJ, Hanusik R, Jodoin M, Kessel S, Laurie F, Ulin K. QARC QA of imaging and radiation therapy improves protocol compliance in advanced technology cancer clinical trials. Imaging for Treatment Assessment in Radiation Therapy (ITART) Meeting, National Harbor; Maryland. Jun 2010. [Google Scholar]
- 70.Vezina G. Personal communication
- 71.Wallner PE, Lustig RA, Pajak TF, Robinson G, Davis LW, Perez CA, Seydel HG, Marcial VA, Laramore GE. Impact of initial quality control review on study outcome in lung and head/neck cancer studies--review of the Radiation Therapy Oncology Group experience. Int J Radiat Oncol Biol Phys. 1989. pp. 893–900. [DOI] [PubMed]
- 72.Williamson JF, Dunscombe PB, Sharpe MB, Thomadsen BR, Purdy JA, Deye JA. Quality assurance needs for modern image-based radiotherapy: recommendations from 2007 inter organizational symposium on "Quality Assurance of Radiotherapy: Challenges of Advanced Technology". Int J Radiat Oncol Biol Phys. 2008;71:S2–S12. doi: 10.1016/j.ijrobp.2007.08.080. [DOI] [PubMed] [Google Scholar]
- 73.Yock TI, Krailo M, Fryer CJ, Donaldson SS, Miser JS, Chen Z, Bernstein M, Laurie F, Gebhardt MC, Grier HE, Tarbell NJ Children's Oncology Group. Local control in pelvic Ewing sarcoma: analysis from INT-0091--a report from the Children's Oncology Group. J Clin Oncol. 2006;24:3838–3843. doi: 10.1200/JCO.2006.05.9188. [DOI] [PubMed] [Google Scholar]