Abstract
Intrauterine growth restricted (IUGR) newborns have increased risk of adult metabolic syndrome, including fatty liver. However, it is unclear whether the fatty liver development is “programmed” or secondary to the accompanying obesity. In this study, we examined hepatic lipid accumulation and lipid-regulatory factors (sterol regulatory element-binding protein-1c and fatty acid synthase) in IUGR and Control fetal (embryonic day 20; e20) and newborn (postnatal day 1; p1) rat pups. Notably, despite of in utero undernutrition state, IUGR fetuses demonstrated ‘fatty liver’ with upregulation of these lipogenic indices at as early as e20. Both IUGR and Control newborns exhibited the same extent of massive increase in hepatic lipid content whereas IUGR newborns continued to exhibit upregulated lipogenic indices. The persistent upregulation of the lipogenic indices in fetal and newborn IUGR suggests that fatty liver is gestationally programmed. Our study suggested that IUGR offspring were born with an altered metabolic life strategy of increased fuel/lipid storage which could be a distinct metabolic pathway of the thrifty phenotype.
Keywords: Programming, fatty liver, intrauterine growth retardation, sterol regulatory element-binding protein 1, fatty acid synthase
Introduction
Epidemiologic studies and animal models have irrevocably demonstrated that intrauterine growth-restricted (IUGR) newborns have increased risk of adult metabolic syndrome, as characterized by obesity, type 2 diabetes, hypertension, cardiovascular diseases and fatty liver (Barker et al. 1993; Martin-Gronert & Ozanne 2007). Select animal studies have shown direct associations between permanent structural and functional changes in tissues/organ systems during fetal development, and risk for adult diseases. For example, studies have shown increased adipocyte cell size as a predecessor of obesity, reduced nephron number as a predecessor of hypertension and decreased pancreatic β cell mass preceding insulinopenia (Desai et al. 2005a; Abdel-Hakeem et al. 2008; Desai et al. 2008; Schwitzgebel et al. 2009).
More recent animal studies of IUGR newborns have indicated increased hepatic lipid accumulation and increased susceptibility for development of “fatty liver” in adults (Thompson et al. 2007; Magee et al. 2008). The causes of fatty liver were multi-factorial and their clinical and pathological presentation varies raging from a simple fatty liver (steatosis) to steatosis with a necroinflammatory component (steatohepatitis) associated with cirrhosis (Matteoni et al. 1999; Larter et al. 2010). The underlying mechanisms contributing to lipid dysregulation and accumulation in the liver include increased de novo lipogenesis, decreased fatty acid oxidation, increased fatty acid transport, decreased fatty acid release, and/or hepatic insulin resistance (Donnelly et al. 2005). Hepatic de novo lipogenesis is one of the critical contributory factors for fatty liver as evident by studies of genetically engineered mouse models (Postic & Girard 2008). In the liver, de novo lipogenesis is regulated by lipogenic transcription factor, sterol regulatory element binding protein-1c (SREBP1c) which in turn, induces the key lipogenic enzyme, fatty acid synthase (Shimano et al. 1999; Horton et al. 2003).
Using a rat model of maternal food-restriction, we have previously demonstrated that IUGR newborns, when nursed by ad libitum fed dams and subsequently fed an ad libitum standard rat chow diet, exhibit adult obesity, lipid abnormalities and notably, fatty liver. We have specifically shown increased hepatic triglyceride content together with upregulated SREBP1 and fatty acid synthase (Magee et al. 2008), suggesting that increased hepatic lipid synthesis likely contributes to the development of fatty liver in the adult IUGR offspring. Nonetheless, it is still unclear whether the fatty liver seen in IUGR adult offspring is a programmed effect occurring during the early developmental period or secondary to obesity-mediated phenomena. In view of this, we studied hepatic lipid metabolism in IUGR fetuses and newborns. We determined lipid content of fetal (embryonic day 20; e20) and newborn (postnatal day 1; p1) livers, as well as the protein expression of SREBP1c and fatty acid synthase. Furthermore, as fatty acid uptake and oxidation by hepatocytes also maintain lipid homeostasis (Qiu et al. 1998; Stefan et al. 2005; Reddy & Rao 2006), we determined the protein expression of hepatic lipase (hepatic lipid uptake enzyme) and mRNA expression of CPT1 (rate-limiting enzyme for fatty acid oxidation).
The present study demonstrated that at e20, IUGR fetuses had ‘fatty liver’ with upregulation of lipogenic indices. Both IUGR and Control newborns exhibited the same extent of massive increase in hepatic lipid content whereas IUGR newborns continued to exhibit upregulated lipogenic indices. The persistent upregulation of lipogenic indices in fetal and newborn IUGR suggests that fatty liver is gestationally programmed.
Materials and methods
Animals
Studies were approved by the Animal Research Committee of the Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles and were in accordance with the American Association for Accreditation of Laboratory Care and National Institutes of Health guidelines. A food-restricted rat model of dams that we have previously described was used (Desai et al. 2005b). Briefly, first-time pregnant Sprague Dawley rats (Charles River Laboratories, Hollister, CA) were housed in a facility with constant temperature and humidity and a controlled 12:12-hour light/dark cycle. At 10 days of gestation, rats were provided either an ad libitum diet of standard laboratory chow (Control, Lab Diet 5001, Brentwood, MO; protein, 23%; fat, 4.5%; metabolizable energy, 3030 kcal/kg) or a 50% food restricted diet (IUGR) that was determined by quantification of normal intake in the ad libitum fed rats. The respective diets were given from 10 days of pregnancy to term.
Tissue preparation
The day when a vaginal plug was observed in the morning was defined as embryonic day 0 (e0). At e20, dams were euthanized by an overdose of pentobarbital (200 mg/kg i.p.) and fetuses operatively removed. At e20 and p1 (next day after birth), fetuses/pups were separated by gender, weighed and sacrificed. In both fetuses and newborns, whole livers were collected and weighed. Liver volumetry at p1 was determined by Archimedes’ principle, whereby the volume of sterile 0.9% NaCl displaced by the liver was measured. In all cases, left lobe was collected for fresh frozen section for osmium staining, right lobe was processed for paraffin sections for standard histology, middle lobe was collected for protein expression analysis, and a part of the middle lobe was used for transmission electron microscopy(TEM).
Lipid quantification by osmium staining
Fresh liver was briefly rinsed in 0.9% NaCl, transferred to 20% sucrose/PBS, embedded in OCT compound (Sakura Finetek USA Inc., Torrance, CA) and frozen at -80 °C. 10 μm sections were fixed in 4% paraformaldehyde/PBS for 20 minutes and incubated in 1% osmium tetroxide (OsO4) for 2 hours. Osmium staining has been long established as fat staining method, in which highly volatile osmium tetroxide which dissolves only in fat (triglyceride) oxides lipids while it is reduced into osmium precipitated in forms of black metal droplets. The area of lipid droplets (stained black) and parenchymal area (without black stain) were measured using ImageJ software (NIH), and the ratio (lipid/parenchyma) calculated. For each liver, 3 sections were taken and from each section 10 images were captured to cover the entire section area.
Transmission electron microscopy (TEM)
A mid portion of middle lobe was fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer at 4°C overnight, subsequently post-fixed in 1% osmium tetroxide for 1 hour. After dehydration, specimens were embedded in Epon 812 epoxy resin. The semi-thin (1 μm) sections for light microscopy were stained with toluidine blue. The ultra-thin sections were stained with 3% uranyl acetate and lead nitrate, and examined with a HITACHI H-600 electron microscope at 75 kV. Three male livers per group were studied.
Western blot
Antibodies used were against fatty acid synthase (1:1000, Santa Cruz Biotechnlogy, Santa Cruz, CA), SREBP1c (68 kD) (1:1000, Santa Cruz), and hepatic lipase (1:1000, Santa Cruz). Valosin-containing protein (VCP) was used as a reference protein. Total protein was extracted in radioimmuno precipitation assay (RIPA) buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate) with protease inhibitors (HALT inhibitor cocktail, Pierce, Rockford, IL). Supernatant protein concentration was determined by bicinchoninic acid (BCA) solution (Pierce). Equal amounts of protein (100 μg) were mixed with sodium dodecylsulfate sample buffer, boiled for 3 minutes, and separated on a 12% Bis-Tris gel. The separated proteins were transferred electrophoretically to pure polyvinylidene fluride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA). Nonspecific antibody binding was blocked by incubation for 1 hour at room temperature with 5% nonfat dry milk in Tris buffered saline solution with 0.1% Tween 20 (TBST). The membrane was then incubated with antibody in 5% milk in TBST overnight at 4°C and washed 3 times for 10 minutes each with TBST at room temperature. Anti-rabbit or anti-mouse immunoglobulin G secondary antibody that was labeled with horseradish peroxidase (1:5000; Bio-Rad Laboratories) in TBST was added to the membrane and incubated for 1 hour at room temperature. The membrane was washed 3 times. SuperSignal West Pico Chemiluminescent Substrate (Pierce) was used to detect the targeted protein. The band density was analyzed with the Alpha DigiDoc Gel Documentation & Image Analysis System (Alpha Innotech Corporation, San Leandro, CA). Western blot data were normalized to the reference protein and presented as fold change.
Real-time qPCR
The p1 liver was placed in RNAlater (Ambion, Austin, TX) at 4°C overnight. RNA was extracted by Trizol (Invitrogen, Carlsbad, CA) method with Turbo DNase treatment (Ambion). RNA purity was assessed by Nanodrop (260/280 ratio: 1.94-1.97) and the ratio of 28S/18S bands (2.0) was confirmed by gel electrophoresis. Two (2) μg was used as a template to prepare cDNA using SuperScript III First-Strand Synthesis System (Invitrogen). Primer sequences for CPT1 are: forward CAC GAA GCC CTC AAA CAG ATC; reverse CCA TTC TTG AAC CGG ATG AAC T (NM_031559) (Suchankova et al. 2005). The qPCR was run on ABI 7000 in a total volume of 25 μL with qPCR Master Mix Plus For Sybr Green (Eurogentec, Seraing, Belgium) according to the manufacturer’s instruction. mRNA expression was normalized using reference gene, GAPDH, using the ΔCt method (Livak & Schmittgen 2001; Schmittgen & Livak 2008). mRNA expression of GAPDH was not impacted by maternal food restriction.
Statistical analysis
Differences between Control and IUGR were compared using ANOVA (groups/gender for weights) with Dunnett’s posthoc test or unpaired t-test (groups for lipid content and gene expression). Numbers of offspring studied are presented in each Table or Figure legend. No sex differences were seen in body and liver weights, hence combined data for males and females are presented. In view of this, only male livers at e20 and p1 were studied for western blot. Values are expressed as means ± standard errors (SE). Mann-Whitney U test was used for non-parametric data (osmium staining).
Results
Body and liver weight and volume
At e20, IUGR fetuses were 13% lighter with a 19% reduction in liver weight as compared to Controls (Table 1). However, the relative liver weight was comparable to that of Controls.
Table 1. Body weight, liver weight and volume.
| Body Weight:BW (g) | Liver Weight:LW (mg) | Liver Volume:LV (μl) | LW/BW (mg/g) | LV/BW (μl/g) | |
|---|---|---|---|---|---|
| p1 Control (n=12) | 7.51±0.11 | 318±9 | 324±14 | 42.4±1.2 | 2.16±0.09 |
| p1 IUGR (n=12) | 6.82±0.18** | 260±6*** | 260±12*** | 38.2±0.5** | 1.91±0.06* |
| e20 Control (n=10) | 2.64±0.04 | 218±6 | ND | 82.5±2.6 | ND |
| e20 IUGR (n=13) | 2.29±0.03*** | 175±10** | ND | 75.8±3.8 | ND |
Body weight, and liver weight and volume in IUGR and Control fetuses (e20) and newborns (p1). Values are mean±SE of specified n from 6 litters;
p<.05;
p<.01;
p<.001 vs. Control.
ND; not determined.
At p1, IUGR newborns were 9% lighter with a greater reduction in liver weight and volume (~18-19%) as compared with Control newborns (Table 1). As a result, the relative liver weight/volume was significantly reduced in IUGR as compared to Control newborns.
Hepatocyte morphology
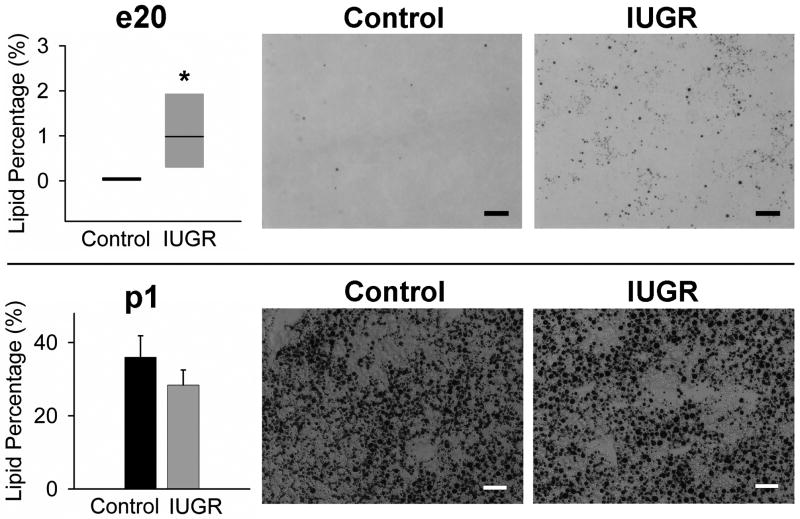
At e20, there were no gross anomalies (e.g., abnormal lobulation) in IUGR liver. The PAS staining exhibited moderate amount of glycogen in both Control and IUGR hepatocytes (data not shown). Osmium staining showed that the fat deposition was significantly more in the IUGR liver than that in Control livers where it was virtually undetectable (Figure 1).
Figure 1. Lipid quantification by osmium staining at e20 and p1.
Osmium stained sections were used to quantify lipid deposition by image analysis. Black dots in the images indicate lipid (triglyceride) droplets stained with osmium. For e20 (upper), n=6 (3 males and 3 females from 3 litters) were studied per group. For p1 (lower), 6 Control and 7 IUGR males from 6/7 litters were studied. *p<.05 vs. Control. Scale bars; 40 μm.
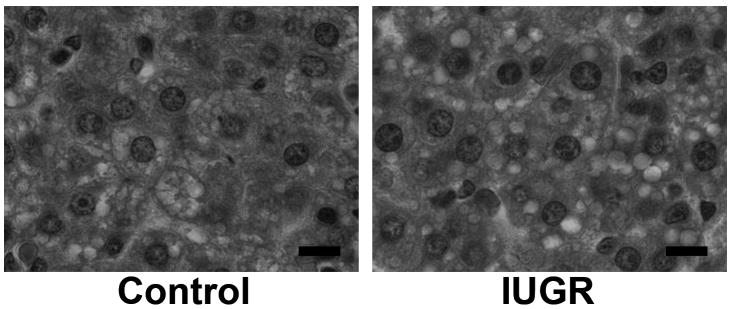
At p1, small amounts of bile were observed in both groups, as previously reported (data not shown) (Wolf-Peeters et al. 1971; Belknap et al. 1981). In both Control and IUGR newborn livers, numbers of small vacuolar structures were observed in the cytoplasmic space, which appeared to be lipid droplets (Figure 2). As confirmation, osmium staining demonstrated massive lipid deposition in both groups, though no significant difference in the lipid deposition between IUGR and Control newborns (Figure 1).
Figure 2. Liver histology at p1.
Hematoxylin & eosin staining was performed. Numerous lipid droplets are observed in both Control and IUGR hepatocytes. Scale bars; 10 μm.
Electron microscopy
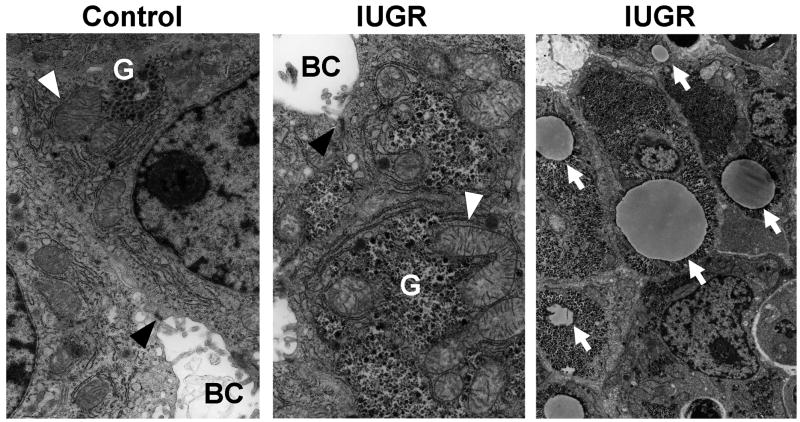
As compromised mitochondrial energy metabolism often leads to microvesicular steatosis in clinical settings, we examined fetal hepatocyte and mitochondrial morphology at e20 by TEM. The TEM revealed no abnormal appearance in mitochondrial shape, size or cristae. Hepatocytes in IUGR and Controls showed intact mitochondrial shape and structure with well-developed bile canaliculi and junctional complexes between neighboring hepatocytes, indicating normal epithelial morphology acquisition (Figure 3). Furthermore, hepatocytes of both IUGR and Control had spacious cytoplasma with abundant glycogen granules and rough endoplasmic reticuli. Importantly, IUGR e20 hepatocytes again exhibited various sizes of fat droplets occupying cytoplasmic space, unlike Control e20 hepatocytes (Figure 3).
Figure 3. Transmission electron microscopic images of livers from e20 Control and IUGR fetuses.
Representative images of liver mitochondria (white arrowheads) from Control (left) and IUGR (middle) fetuses. BC is bile canaliculi and black arrowheads show junctional complexes between two neighboring hepatocyte cell membranes. G indicates glycogen granules. Arrows in right panel indicate fat droplets in the IUGR hepatocytes. The magnifications are 7052x for the left and middle images and 2343x for the right image. n=3 (males) from 3 litters per group were observed.
Lipid metabolism physiology in the liver
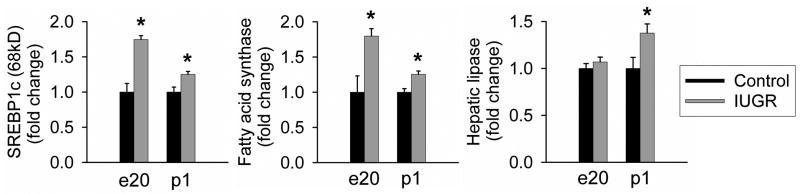
To explore the physiology of lipid metabolism which could explain the fat accumulation in e20 IUGR livers, we measured protein expression of key regulators of lipid metabolism. First, we focused on the lipogenic enzyme, fatty acid synthase and one of its major upstream transcriptional factors (SREBP1c; 68 kD). At both e20 and p1, IUGR newborns showed significant increase in SREBP1c and fatty acid synthase expression as compared to Controls (Figure 4).
Figure 4. Lipogenic indices (SREBP1c and fatty acid synthase) and hepatic lipase.
Hepatic protein expression of SREBP1c (68kD), fatty acid synthase, and hepatic lipase at e20 and p1 from Control and IUGR males. The numbers of animals studied were 6 males from 6 litters per group. Asterisk indicates p<.05 vs. Control.
Secondly, we measured protein expression of hepatic lipase to evaluate lipid uptake into hepatocytes. Although hepatic lipase expression was similar in both groups at e20, it was significantly upregulated in IUGR newborns at p1 as compared to Controls (Figure 4).
Finally, we quantified CPT1 mRNA as a measure of mitochondrial fatty acid oxidation. We performed real-time qPCR since there was no suitable and commercially-available antibody. At p1, IUGR and Controls demonstrated similar CPT1 expression (IUGR was 1.1±0.36 fold of Control), which was consistent with the absence of mitochondrial abnormality by TEM.
Discussion
The principal findings of the present study demonstrates that fatty liver in IUGR offspring is a programmed effect which is evident during fetal and newborn life prior to the onset of adult obesity. We have specifically shown decreased liver weight and increased lipid accumulation with upregulation of lipogenic indices in IUGR fetuses. Furthermore, there was continued reduction in liver weight and increase in lipogenic indices in IUGR newborns compared to Controls.
This is the first study to report that near-term IUGR rat fetuses (e20) exhibit early hepatic fat deposition together with increased expression of lipogenic regulators, (SREBP1c and fatty acid synthase). Hepatic lipid uptake alone was not a contributory factor for the enhanced lipid deposition in IUGR liver since hepatic lipase expression in the IUGR liver was comparable to that of Controls. Pathologically, the fat deposition presented in our study is defined as ‘microvesicular steatosis’ which may be induced by severe impairment of mitochondrial β-oxidation process (e.g., Reye’s syndrome, drug-induced liver disease, and acute fatty liver of pregnancy (Fromenty & Pessayre 1997; Pessayre et al. 1999; Pugliese et al. 2008; Joshi et al. 2010)). However, by our TEM observation, the IUGR hepatocytes showed intact mitochondrial structures. Although we did not directly examine fatty acid oxidation capacity of IUGR hepatocytes, the morphological findings by TEM can rule out severe mitochondrial damage as the etiology for conventional ‘microvesicular steatosis’.
Furthermore, during the newborn period, CPT1 mRNA level was unchanged in IUGR livers. This is inconsistent with results reported from models of uteroplacental insufficiency, in which the mRNA expression of CPT1 was downregulated in IUGR newborns (Lane et al. 2001; Fu et al. 2004). In uteroplacental insufficiency rat model, fetal oxygen supply is compromised (Ogata et al. 1986), which may impact the gene expression of CPT1, a rate limiting enzyme for mitochondrial oxidation of fatty acid. On the other hand, in nutrient restriction models, fetal oxygen status is normal (Williams et al. 2005) suggesting that hypoxia can produce a distinct effect on energy metabolism in the fetal liver. Another possibility for the hepatic lipid deposition is decreased hepatic export of triglyceride to the systemic circulation, which remains to be investigated in future.
An important finding in our study was that p1 IUGR livers showed persistent upregulation of de novo lipogenesis. Despite this, the hepatic fat deposition was comparable in IUGR and Control livers, most likely due to the masking effect of massive lipid accumulation seen in the newborns following birth (discussed below). In our previous study, we demonstrated increased triglyceride content in the liver and increased de novo lipogenesis in IUGR obese adult offspring (Magee et al. 2008). Taken together, de novo lipogenesis in the IUGR liver is already upregulated in the perinatal period, much earlier than the time of obesity onset (Table 2). This persistent upregulation in lipogenesis suggests the fatty liver in IUGR adult offspring is gestationally programmed. In view of the demonstrated epigenetic modifications on energy metabolism-related genes in the liver in several IUGR models (Fu et al. 2004; Burdge et al. 2007; Nijland et al. 2010), it is likely that hepatic genes responsible for lipogenesis are epigenetically modified to be upregulated from fetal through perinatal periods.
Table 2. The changes in hepatic lipid deposition and lipogenic indices in IUGR offspring at three stages of development.
| Hepatic lipid deposition (morphology) | Lipogenic indices (protein expression) | |
|---|---|---|
| IUGR fetus | +* | ↑* |
| IUGR newborn | ++ | ↑* |
| IUGR adult | +*(Magee et al. 2008) | ↑*(Magee et al. 2008) |
+ increased deposition; ++ markedly increased deposition; ↑ upregulation in lipogenic indices (SREBP1c and fatty acid synthase) compared to Controls. Asterisk* denotes p<.05 vs. Control.
Importantly, hepatic de novo fatty acid synthesis is the metabolic pathway that synthesizes fatty acids from excess carbohydrates (Strable & Ntambi 2010). Under normal physiological state in rats, hepatic de novo lipogenesis is lower during fetal than neonatal periods (Eritani et al. 1993). Concomitantly, the ability of gluconeogenesis is very low during fetal life and rapidly develops immediately after birth (Girard 1986). In several experimental models of IUGR, the early onset of gluconeogenesis in the IUGR fetal liver has been observed (Thorn et al. 2009; Nijland et al. 2010). We speculate that this gluconeogenic condition may result in abundant hepatic fuel substrates, favoring the lipogenic environment in the IUGR hepatocyte.
Another possible explanation for the lipid accumulation in the fetal IUGR hepatocyte in our model can be related to ‘lipid burden hypothesis’. This hypothesis has been proposed for a decade as the pathophysiology of type 2 diabetes or metabolic syndrome in adults, in which the extent of lipogenesis is determined by the functional interrelationship (i.e., organ cross-talk) among the three major organs: liver, muscle, and adipose tissue (Nadler & Attie 2001). Early decreased lipogenesis in adipose tissue may adversely impact lipogenesis in other metabolic organs, such as liver and muscle, resulting in triglyceride storage in these two organs (Nelson & Cox 2008). Indeed in our IUGR model, at newborn stage, the total mass of adipose tissue of the IUGR was significantly decreased by 18% (43.7±1.6 vs. 53.2±1.9 mg) (unpublished data) while lipogenesis in the liver was upregulated, evidenced by increased SREBP1c and fatty acid synthase in the newborn period. Taken together, the possible dysfunction of the adipose tissue in our IUGR model may shift lipogenic burden to the liver in the early course of life.
Despite maternal undernutrition and reduced adipose tissue mass in our model, IUGR fetuses have more lipid accumulation in the liver than Control fetuses. One study demonstrated that rabbit fetuses from 48 hour-fasted mothers had increased fat stores both in the liver and adipose tissue (Edson et al. 1975). That study also suggested that increased amounts of maternal free fatty acids crossed the placenta into the fetal circulation and were incorporated into the fetal fat stores. Similarly, prolonged protein restriction in rats elevates maternal free fatty acids (Fernandez-Twinn et al. 2003). In rat fetuses, plasma fatty acids are derived equally from maternal and fetal fatty acid synthesis sources (Hummel et al. 1978). Therefore, in the setting of maternal energy restriction, alterations in maternal source of fatty acid concentration may affect the lipid content in the plasma and liver of the IUGR fetuses.
Regarding the massive ‘physiological steatosis’ of 24 hour-newborn rat in both IUGR and Control groups, to our best knowledge, there has been no report describing the lipid accumulation in the liver in the perinatal period in rats. We firstly speculate that the massive fat deposition, which occupies 20-40% of liver parenchyma, is attributable to rat milk which has the highest lipid content in mammals (69% of the total content) (Ferre et al. 1986). Since the lipid deposition both in IUGR and Control newborn livers was massive, a similar degree of difference to that at e20, i.e. 2%, would be undetectable at p1. The newborn ‘physiological steatosis’ seems to be a distinct phenomenon occurring only in rats which are developmentally immature at birth as compared to other mammals (Gauda 2006). For instance, guinea pig and rabbit fetuses are known to develop physiological fatty liver towards the end of the gestation, which subsides after birth as the liver starts to actively metabolize lipids from the milk (Bohmer et al. 1972; Duee et al. 1985; Girard et al. 1985). In contrast, in Control near-term rats, lipid deposition in the liver is negligible, indicating lack of high-energy fuel storage at term. As a result, the high-fat content in rat milk may serve as a normal compensatory source, and could explain the marked increased lipid accumulation in liver during the newborn period. Another rat-specific development is that rat newborns have far less adipose tissue (less than 1.5%) as compared to humans (15-18%), guinea pig (12%) or rabbit newborns (7%) (Duee et al. 1985; Greenwood & Hirsch 1974). As such, adipose tissue may not be adequate to store lipids from the high-fat milk, with the overall lipid burden being shifted to the liver. Although not yet examined, we postulate that following lactation, hepatic lipid content decreases in both Control and IUGR newborns, though with a continued upregulation of hepatic lipids and lipid storage promoting factors in IUGR offspring. In humans, instead, triglyceride accumulation most rapidly occurs in the adipose tissue in the last trimester (Herrera & Amusquivar 2000). Despite the inter-species difference in functional development of lipid handling, both IUGR humans and rats are predisposed to adult fatty liver, likely a result of programmed upregulation of hepatic lipogenic factors.
In conclusion, our study demonstrates that IUGR fetuses exhibit increased hepatic lipid accumulation with upregulated lipogenic indices. The persistent upregulation of lipogenesis in IUGR newborn livers suggest that molecular mechanism contributing to fatty liver development in IUGR offspring is gestationally programmed.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01DK081756, R01HD054751 and R03HD060241. We acknowledge technical assistance from William Lungo (TEM), Thomas R. Magee and Tatsuya Fukami (qPCR), and Linda Day and Stacy Behare (tissue collection). We thank Professor Hiroki Otani for reviewing our manuscript.
References
- Abdel-Hakeem AK, Henry TQ, Magee TR, et al. Mechanisms of impaired nephrogenesis with fetal growth restriction: altered renal transcription and growth factor expression. Am J Obstet Gynecol. 2008;199:252 e1–7. doi: 10.1016/j.ajog.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Belknap WM, Balistreri WF, Suchy FJ, Miller PC. Physiologic cholestasis II: serum bile acid levels reflect the development of the enterohepatic circulation in rats. Hepatology. 1981;1:613–6. doi: 10.1002/hep.1840010608. [DOI] [PubMed] [Google Scholar]
- Bohmer T, Havel RJ, Long JA. Physiological fatty liver and hyperlipemia in the fetal guinea pig: chemical and ultrastructural characterization. J Lipid Res. 1972;13:371–82. [PubMed] [Google Scholar]
- Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr. 2007;97:435–9. doi: 10.1017/S0007114507352392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Gayle D, Babu J, Ross MG. Permanent reduction in heart and kidney organ growth in offspring of undernourished rat dams. Am J Obstet Gynecol. 2005a;193:1224–32. doi: 10.1016/j.ajog.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005b;288:R91–6. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- Desai M, Guang H, Ferelli M, Kallichanda N, Lane RH. Programmed upregulation of adipogenic transcription factors in intrauterine growth-restricted offspring. Reprod Sci. 2008;15:785–96. doi: 10.1177/1933719108318597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duee PH, Pegorier JP, el Manoubi L, Herbin C, Kohl C, Girard J. Hepatic triglyceride hydrolysis and development of ketogenesis in rabbits. Am J Physiol. 1985;249:E478–84. doi: 10.1152/ajpendo.1985.249.5.E478. [DOI] [PubMed] [Google Scholar]
- Edson JL, Hudson DG, Hull D. Evidence of increased fatty acid transfer across the placenta during a maternal fast in rabbits. Biol Neonate. 1975;27:50–5. doi: 10.1159/000240758. [DOI] [PubMed] [Google Scholar]
- Eritani N, Fukuda H, Matsumura Y. Lipogenic enzyme gene expression in rat liver during development after birth. J Biochem. 1993;113:519–25. doi: 10.1093/oxfordjournals.jbchem.a124076. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE, Ekizoglou S, et al. The maternal endocrine environment in the low-protein model of intra-uterine growth restriction. Br J Nutr. 2003;90:815–22. doi: 10.1079/bjn2003967. [DOI] [PubMed] [Google Scholar]
- Ferre P, Decaux JF, Issad T, Girard J. Changes in energy metabolism during the suckling and weaning period in the newborn. Reprod Nutr Dev. 1986;26:619–31. doi: 10.1051/rnd:19860413. [DOI] [PubMed] [Google Scholar]
- Fromenty B, Pessayre D. Impaired mitochondrial function in microvesicular steatosis. Effects of drugs, ethanol, hormones and cytokines. J Hepatol. 1997;26(Suppl 2):43–53. doi: 10.1016/s0168-8278(97)80496-5. [DOI] [PubMed] [Google Scholar]
- Fu Q, McKnight RA, Yu X, Wang L, Callaway CW, Lane RH. Uteroplacental insufficiency induces site-specific changes in histone H3 covalent modifications and affects DNA-histone H3 positioning in day 0 IUGR rat liver. Physiol Genomics. 2004;20:108–16. doi: 10.1152/physiolgenomics.00175.2004. [DOI] [PubMed] [Google Scholar]
- Gauda EB. Knowledge Gained from Animal Studies of the Fetus and Newborn: Application to the Human Premature Infant. ILAR J. 2006;47:1–4. [Google Scholar]
- Girard J. Gluconeogenesis in late fetal and early neonatal life. Biol Neonate. 1986;50:237–58. doi: 10.1159/000242605. [DOI] [PubMed] [Google Scholar]
- Girard J, Duee PH, Ferre P, Pegorier JP, Escriva F, Decaux JF. Fatty acid oxidation and ketogenesis during development. Reprod Nutr Dev. 1985;25:303–19. doi: 10.1051/rnd:19850221. [DOI] [PubMed] [Google Scholar]
- Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res. 1974;15:474–83. [PubMed] [Google Scholar]
- Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev. 2000;16:202–10. doi: 10.1002/1520-7560(200005/06)16:3<202::aid-dmrr116>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Horton JD, Shah NA, Warrington JA, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027–32. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel L, Zimmermann T, Wagner H. Quantitative evaluation of the fetal fatty acid synthesis in the rat. Acta Biol Med Ger. 1978;37:229–32. [PubMed] [Google Scholar]
- Joshi D, James A, Quaglia A, Westbrook RH, Heneghan MA. Liver disease in pregnancy. Lancet. 2010;375:594–605. doi: 10.1016/S0140-6736(09)61495-1. [DOI] [PubMed] [Google Scholar]
- Lane RH, Kelley DE, Gruetzmacher EM, Devaskar SU. Uteroplacental insufficiency alters hepatic fatty acid-metabolizing enzymes in juvenile and adult rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R183–90. doi: 10.1152/ajpregu.2001.280.1.R183. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Chitturi S, Heydet D, Farrell GC. A fresh look at NASH pathogenesis. Part 1: the metabolic movers. J Gastroenterol Hepatol. 2010;25:672–90. doi: 10.1111/j.1440-1746.2010.06253.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Magee TR, Han G, Cherian B, Khorram O, Ross MG, Desai M. Down-regulation of transcription factor peroxisome proliferator-activated receptor in programmed hepatic lipid dysregulation and inflammation in intrauterine growth-restricted offspring. Am J Obstet Gynecol. 2008;199:271 e1–5. doi: 10.1016/j.ajog.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. J Intern Med. 2007;261:437–52. doi: 10.1111/j.1365-2796.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- Nadler ST, Attie AD. Please pass the chips: genomic insights into obesity and diabetes. J Nutr. 2001;131:2078–81. doi: 10.1093/jn/131.8.2078. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Hormonal regulation and integration of mammalian metabolism. In: Nelson DL, Cox MM, editors. Lehninger Principals of Biochemistry. 5. W. H. Freeman and Company; New York, NY: 2008. pp. 938–939. [Google Scholar]
- Nijland MJ, Mitsuya K, Li C, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588:1349–59. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism. 1986;35:970–7. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Mansouri A, Haouzi D, Fromenty B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol Toxicol. 1999;15:367–73. doi: 10.1023/a:1007649815992. [DOI] [PubMed] [Google Scholar]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–38. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese A, Beltramo T, Torre D. Reye’s and Reye’s-like syndromes. Cell Biochem Funct. 2008;26:741–6. doi: 10.1002/cbf.1465. [DOI] [PubMed] [Google Scholar]
- Qiu S, Bergeron N, Kotite L, Krauss RM, Bensadoun A, Havel RJ. Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice. J Lipid Res. 1998;39:1661–8. [PubMed] [Google Scholar]
- Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852–8. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Somm E, Klee P. Modeling intrauterine growth retardation in rodents: Impact on pancreas development and glucose homeostasis. Molecular and Cellular Endocrinology. 2009;304:78–83. doi: 10.1016/j.mce.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Shimano H, Yahagi N, Amemiya-Kudo M, et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274:35832–9. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- Stefan N, Schafer S, Machicao F, et al. Liver fat and insulin resistance are independently associated with the -514C>T polymorphism of the hepatic lipase gene. J Clin Endocrinol Metab. 2005;90:4238–43. doi: 10.1210/jc.2004-2479. [DOI] [PubMed] [Google Scholar]
- Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun. 2005;326:851–8. doi: 10.1016/j.bbrc.2004.11.114. [DOI] [PubMed] [Google Scholar]
- Thompson NM, Norman AM, Donkin SS, et al. Prenatal and postnatal pathways to obesity: different underlying mechanisms, different metabolic outcomes. Endocrinology. 2007;148:2345–54. doi: 10.1210/en.2006-1641. [DOI] [PubMed] [Google Scholar]
- Thorn SR, Regnault TR, Brown LD, et al. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–30. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R360–7. doi: 10.1152/ajpregu.00178.2004. [DOI] [PubMed] [Google Scholar]
- Wolf-Peeters CD, Vos RD, Desmet V. Histochemical evidence of a cholestatic period in neonatal rats. Pediatr Res. 1971;5:704–710. [Google Scholar]