Abstract
Knocking out the Lynx1 gene restores plasticity in the visual cortex of adult mice.
The juvenile brain exhibits a high capacity for plasticity and repair that is severely restricted in adulthood. In young mammals, for instance, classic experiments have shown that closing one eye for several days (monocular deprivation) leads visual cortex neurons to shift their responses toward sensory inputs originating from the other, nondeprived eye (1). In adults, however, such plasticity in ocular dominance, while not eliminated, is strongly restricted. This knowledge gap has medical implications, because the restoration of juvenile plasticity in injured or dysfunctional adults has the potential to allow recovery of neurological performance. On page 1238 of this issue, Morishita et al. (2) identify one brake on visual cortex plasticity in adults: Lynx1, a protein that inhibits nicotinic acetylcholine receptors (nAChRs). By eliminating the gene that expresses Lynx1 in mice, the researchers were able to create adult animals that exhibited visual cortex plasticity similar to that exhibited by juveniles.
Nicotinic receptors are present throughout the nervous system. They form “channels” that enable small ions to pass through neuronal membranes, and have “gates” that are opened by acetylcholine, a common neurotransmitter. Lynx1 is similar to snake venom proteins that inhibit nicotinic receptors, and deleting the Lynx1 gene is known to increase cholinergic neurotransmission (the activity of acetylcholine) (3). Morishita et al. noted that, in juvenile mice, Lynx1 expression increases as the critical period for visual cortex plasticity closes. To better understand the role of Lynx1, they created knockout mice that lacked the Lynx1 gene. Then, they used electrophysiological methods to measure the effect of monocular deprivation on neocortical ocular dominance (the eye preference of single cortical neurons) in both the knockout mice and wild-type mice that still expressed Lynx1. After 4 days of monocular deprivation, 60-day-old adult knockout mice exhibited plasticity that matched that of 30-day-old juvenile wild-type mice; in contrast, adult wild-type mice did not show such plasticity. Subsequent experiments with drugs that blocked nicotinic receptors produced results that were consistent with the hypothesis that Lynx1 acts by inhibiting nAChRs.
Previous studies have shown that blocking cholinergic signaling in the juvenile visual cortex during the critical period inhibits ocular dominance plasticity (4). In adults, enhanced cholinergic activity can promote activity-dependent plasticity in both auditory (5) and motor (6) regions of the brain. Morishita et al., however, provide the first demonstration that acetylcholine signaling serves as a brake on adult visual cortex plasticity.
Other pathways have been implicated in preventing adult plasticity. Myelin proteins can act via the receptors NgR1 and/or PirB to inhibit the growth of neurites (7–9). Both NgR1 and PirB limit adult visual cortex plasticity to an extent similar to that shown for Lynx1 (10, 11). In addition, the perineuronal nets that surround inhibitory interneurons are rich in chondroitin sulfate proteoglycans (CSPGs). The development of these nets parallels both Lynx1 expression and the development of intracortical myelin sheaths, and CSPGs function as an additional brake on plasticity (12). Specifically, digesting CSPGs reestablishes ocular dominance plasticity in the adult brain (12). However, Lynx1 appears to be unique in its regulation of neurotransmission, in contrast to the anatomical role proposed for myelin and CSPGs.
Although inhibition of nicotinic signaling appears to be the primary mechanism by which Lynx1 regulates cortical plasticity, the specific cellular targets are not yet defined. Nicotinic receptors are expressed on axonal terminals and postsynaptic membranes of both excitatory and inhibitory cells. Their activation likely alters the balance of synaptic excitation and inhibition, thereby changing the complex patterns of neuronal activity evoked by sensory inputs. Many nAChRs exhibit permeability to calcium ions, enabling them to contribute to calcium-dependent signaling pathways that may regulate synaptic plasticity. Lynx1 expression is also observed in subsets of inhibitory neurons (2), although it is not clear if this site is relevant for control of plasticity.
The mechanism of ocular dominance plasticity also is unclear. Given the persistent nature of cortical plasticity and the anatomical actions of NgR1, PirB, and CSPGs, Lynx1 and nAChR activation might modulate some aspect of intracerebral synaptic connectivity. Future studies will be required to elucidate whether this modulation involves changes in axonal branching, the formation or elimination of synaptic contacts, or simple changes in the efficacy of existing synapses. It also remains unclear whether nAChR function, myelin, and CSPGs act independently or cooperatively in influencing plasticity.
There is strong reason to believe that increasing adult brain plasticity can support neurologic recovery in a range of conditions (13). Morishita et al. examined how mice that experienced 2 weeks of juvenile monocular deprivation recovered from amblyopia (loss of visual acuity due to disuse). In adult mice lacking Lynx1, simply reopening the closed eye caused electrophysiological signals to return to patterns indicating normal acuity. They obtained a similar degree of recovery from amblyopia in wild-type mice by administering an acetylcholinesterase inhibitor that increased acetylcholine transmission. Similar recovery through plasticity may underlie the beneficial effects of digesting CSPG or blocking NgR after spinal cord injury or stroke (14–16), and it will be of great interest to assess whether Lynx1 deletion or facilitation of nAChR activity enhances recovery from such neurological damage.
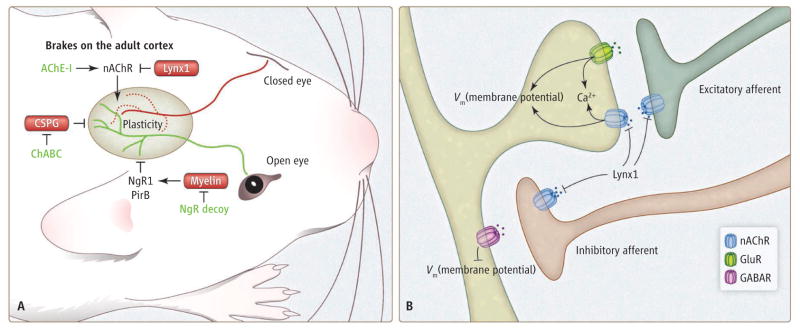
Figure 1. Easing the brakes on plasticity.
(A) In juvenile wild-type mice, closing one eye for several days causes the loss of projections on the inactive pathway from the retina to the visual cortex (red lines for this polysynaptic pathway) and gains on the active pathway (green). In the adult, several pathways function as brakes on this plasticity in ocular dominance. Lynx1 inhibits nAChRs. Myelin inhibitors or CSPG-rich perineuronal nets also prevent rearrangement (11, 12). Specific interventions can remove each of these brakes, including acetylcholinesterase inhibitors (AChE-I), NgR decoy protein, and chondroitinase (ChABC). (B) Nicotinic receptors are found on both pre- and postsynaptic elements of excitatory and inhibitory cortical neurons. Regulation of nAChRs by Lynx1 likely influences the balance of synaptic excitation and inhibition, and calcium influx through nAChRs may contribute to biochemical signaling pathways.
Acknowledgments
S.M.S. is supported by grants from the NIH and the Falk Medical Research Trust. S.M.S is a cofounder of Axerion Therapeutics, seeking to commercialize PrP- and NgR-based therapies.
Contributor Information
Michael J. Higley, Email: michael.higley@yale.edu.
Stephen M. Strittmatter, Email: stephen.strittmatter@yale.edu.
References and Notes
- 1.Hensch TK. Annu Rev Neurosci. 2004;27:549. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 2.Morishita H, et al. Science. 2010;330:1238. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miwa JM, et al. Neuron. 1999;23:105. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- 4.Bear MF, Singer W. Nature. 1986;320:172. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 5.Froemke RC, et al. Nature. 2007;450:425. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 6.Ramanathan D, et al. J Neurosci. 2009;29:5992. doi: 10.1523/JNEUROSCI.0230-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atwal JK, et al. Science. 2008;322:967. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 8.Fournier AE, et al. Nature. 2001;409:341. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 9.Cafferty WB, Strittmatter SM. J Neurosci. 2006;26:12242. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syken J, et al. Science. 2006;313:1795. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 11.McGee AW, et al. Science. 2005;309:2222. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzorusso T, et al. Science. 2002;298:1248. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 13.Cafferty WB, et al. Trends Neurosci. 2008;31:215. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradbury EJ, et al. Nature. 2002;416:636. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, et al. Ann Neurol. 2006;60:540. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JK, Kim JE, Sivula M, Strittmatter SM. J Neurosci. 2004;24:6209. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]