Abstract
RNA interference (RNAi) is a post-transcriptional pathway in which double-stranded RNA (dsRNA) triggers the degradation of complementary mRNA in the cytoplasm of eukaryotic cells. In plants and in some animals, including Caenorhabditis elegans, initiation of RNAi in one cell can lead to sequence-specific RNA silencing in another cell, a phenomenon referred to as non-cell-autonomous RNAi. Until recently, this phenomenon had not been observed in mammalian cells. Here, we review emerging data demonstrating that non-cell-autonomous RNAi occurs in cultured mammalian cells. We discuss possible mechanisms for the transfer of RNAi between mammalian cells and highlight the implications of this phenomenon for the development of in vivo cell-based RNAi delivery.
Keywords: RNAi, siRNA, miRNA, non-cell-autonomous RNAi, systemic RNAi, RNAi delivery, cell-based delivery
INTRODUCTION
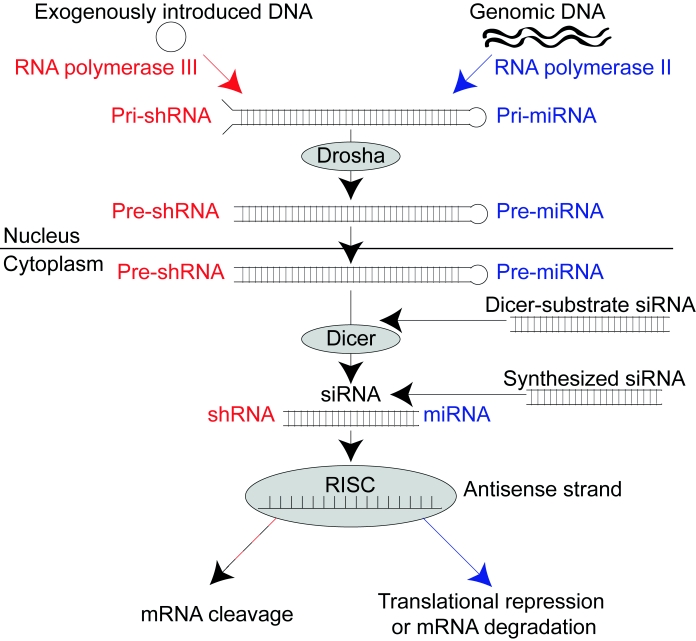
RNA interference (RNAi) is a post-transcriptional pathway in which double-stranded RNA (dsRNA) triggers the degradation of complementary mRNA in the cytoplasm of eukaryotic cells (Fire et al, 1998). In this pathway, the ribonuclease III enzyme Dicer cleaves long dsRNA (Bernstein et al, 2001) into ∼21–25-nucleotide (nt) dsRNA duplexes (Hammond et al, 2000; Zamore et al, 2000) referred to as short hairpin RNAs (shRNAs), microRNAs (miRNAs) or short interfering RNAs (siRNAs). (See Figure 1 for more detail regarding when each term is used). The resulting dsRNA duplex is incorporated into the RNA-induced silencing complex (RISC) (Hammond et al, 2000), where helicase enzymes unwind the duplex and the catalytic protein Argonaute 2 (Ago2) cleaves the sense strand of RNA (Matranga et al, 2005). Guided by the antisense strand of RNA (Martinez et al, 2002), the activated RISC targets complementary mRNAs in the cytoplasm for translational repression, mRNA degradation, or cleavage by Ago2 (Liu et al, 2004; Rand et al, 2004; reviewed in Fabian et al, 2010).
Figure 1.
The RNAi pathway. Dicer cleaves long dsRNA in the form of pre-shRNA, pre-miRNA, or Dicer-substrate siRNA into ∼21–25-nt dsRNA duplexes (Hammond et al, 2000; Zamore et al, 2000), referred to as shRNAs, miRNAs, or siRNAs, respectively, to initiate RNAi. Most shRNAs and siRNAs have exact complementarity with their target mRNAs and result in mRNA cleavage by Ago2. Most miRNAs bind to the 3' untranslated region of their target mRNAs with imperfect complementarity, resulting in translational repression (reviewed in He and Hannon, 2004) or deadenylation and target mRNA degradation (reviewed in Fabian et al, 2010). Alternatively, ∼21–25nt siRNAs can be directly transfected into the cytoplasm of cells to initiate RNAi. Note that the term siRNA can also be used more generally to describe any of the forms of ∼21–25nt dsRNA duplexes that initiate RNAi.
In plants and in some animals, including Caenorhabditis elegans, RNAi can spread intercellularly (reviewed in Mlotshwa et al, 2002; Voinnet, 2005; Jose and Hunter, 2007; Dinger et al, 2008; Kalantidis et al, 2008; Chitwood and Timmermans, 2010), a phenomenon referred to as non-cell-autonomous RNAi. In plants, RNAi spreads locally from cell-to-cell through plasmodesmata (Himber et al, 2003), which connect the cytosols of adjacent plant cells. RNAi also spreads systemically through the phloem system of the plant (Voinnet et al, 1998; Himber et al, 2003). In C. elegans, injection of dsRNA into the pseudocoelomic body cavity or gonad triggers RNAi in somatic tissues (Fire et al, 1998). Additionally, feeding C. elegans with dsRNA or dsRNA-expressing bacteria induces systemic RNAi (Timmons and Fire, 1998).
Despite numerous reports of non-cell-autonomous RNAi in plants and in some animals, until recently, this phenomenon had not been observed in mammalian cells. Here, we review emerging data demonstrating the transfer of RNAi between cultured mammalian cells by both cell contact-independent and cell contact-dependent transfer mechanisms. The existence of non-cell-autonomous RNAi in mammalian cells has important implications for the development of in vivo cell-based RNAi delivery.
MECHANISMS OF CELL CONTACT INDEPENDENT NON-CELL-AUTONOMOUS RNAi
Microvesicles
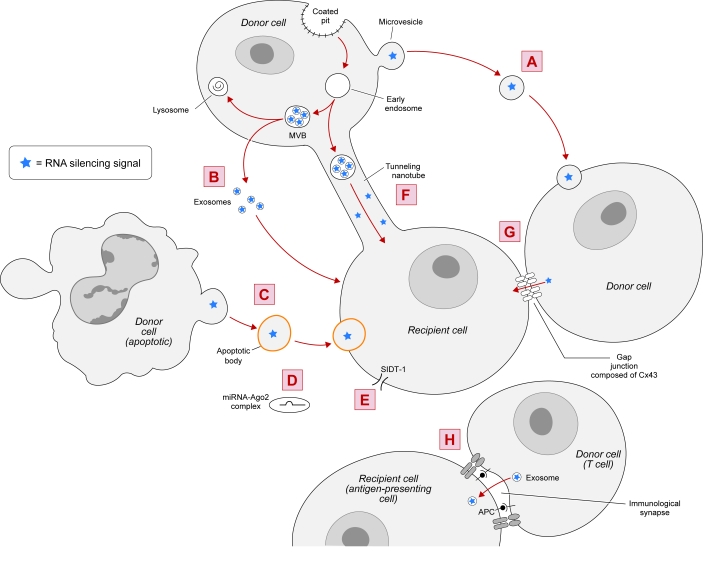
Microvesicles (MVs) are plasma membrane fragments varying in size (0.1–1μm in diameter), shape, and composition that are shed from a variety of healthy or damaged cells during plasma membrane blebbing. MVs are thought to mediate intercellular communication by delivering proteins, RNAs, and other cellular cargos from one cell to another (reviewed in Ratajczak et al, 2006b; Cocucci et al, 2009). In agreement, Ratajczak et al (2006a) showed that MVs derived from embryonic stem cells (ESCMVs) delivered embryonic stem cell-derived mRNA into hematopoietic progenitor cells (HPCs), resulting in target protein production and reprogramming of the HPCs. Furthermore, MVs derived from a variety of cell types contain miRNAs (Hunter et al, 2008; Yuan et al, 2009; Collino et al, 2010), suggesting that MVs may shuttle miRNAs between cells. Interestingly, Collino et al (2010) detected certain miRNAs in MVs, but not in their parental cells, suggesting that such miRNAs are selectively packaged in MVs for non-cell-autonomous RNAi.
In further supporting the hypothesis that MVs shuttle miRNAs between cells, Yuan et al (2009) showed that ESCMVs fused with co-incubated mouse embryonic fibroblasts to deliver miRNAs. Additionally, Collino et al (2010) demonstrated that miRNA-containing MVs released from donor human bone marrow-derived mesenchymal stem cells (MSCs) delivered miRNAs into co-incubated recipient cells, resulting in target-specific reduction in protein levels. These data demonstrate that certain cell types release MVs that can shuttle functional miRNAs between cells (Figure 2A).
Figure 2.
Possible mechanisms of non-cell-autonomous RNAi in cultured mammalian cells. Non-cell-autonomous RNAi can occur by cell contact-independent mechanisms, such as the shuttling of miRNA-containing MVs (A), exosomes (B), or apoptotic bodies (C) between cells. Additionally, miRNA-Ago2 complexes may induce RNAi in recipient cells (D). siRNAs and miRNAs may also utilize SIDT-1, when present at high expression levels, for entry into cells and to generate RNAi (E). Non-cell-autonomous RNAi can also occur by cell contact-dependent mechanisms, relying on TNTs (F), gap junctions (G), or ISs (H).
Exosomes
Also involved in intercellular communication, exosomes are small, 30–100nm in diameter, membrane-bound intraluminal vesicles (ILVs) that are secreted by most cells. Formed during the endolysosomal pathway by the invagination of the limiting endosomal membrane in multivesicular bodies (MVBs), exosomes are released into the extracellular space when MVBs fuse with the plasma membrane. Exosomes contain cytosolic and plasma membrane proteins and express cell recognition molecules that allow for selective cellular uptake (reviewed in Théry et al, 2002; Simons and Raposo, 2009; Mincheva-Nilsson and Baranov, 2010). Similarly, secretory exosomes containing RNAs and proteins may be a major mode of communication within the nervous system (reviewed in Smalheiser, 2007). Exosomes secreted from a variety of cell types contain miRNAs (Valadi et al, 2007; Skog et al, 2008; Taylor and Gercel-Taylor, 2008; Luo et al, 2009; Kosaka et al, 2010; Michael et al, 2010; Ohshima et al, 2010; Pegtel et al, 2010; Zomer et al, 2010; DeIuliis et al, 2011; Levänen et al, 2011; Mittelbrunn et al, 2011), leading to the name "exosomal shuttle RNA" (Lotvall and Valadi, 2007; Valadi et al, 2007). In fact, certain miRNAs exist in higher levels in exosomes than in their parental cells (Valadi et al, 2007; Ohshima et al, 2010; DeIuliis et al, 2011; Mittelbrunn et al, 2011), suggesting that such miRNAs are selectively packaged into MVBs and secreted as exosomes for non-cell-autonomous RNAi.
Pegtel et al (2010) has provided further evidence to support the hypothesis that exosomes shuttle miRNAs between cells by demonstrating that Epstein-Barr virus (EBV)-infected B cells secrete exosomes containing EBV-encoded miRNAs, which knocked down EBV target genes in co-incubated monocyte-derived dendritic cells. Similarly, Kosaka et al (2010) showed that cultured HEK293 human embryonic kidney cells and COS-7 African green monkey kidney fibroblast-like cells secrete miRNA-containing exosomes, which were internalized in recipient cells leading to sequence-specific RNA silencing. These data demonstrate that certain cell types release exosomes that can shuttle functional miRNAs between cells (Figure 2B).
Apoptotic bodies, miRNA-Ago2 complexes and SIDT-1
In addition to MVs and exosomes, apoptotic bodies – a form of MVs released during programmed cell death – may transport miRNAs between cells (Figure 2C). Zernecke et al (2009) demonstrated that apoptotic bodies isolated from cultured endothelial cells could deliver miRNA-126 into recipient vascular cells, inducing the production of chemokine CXC motif ligand 12 (CXCL12). Alternatively, miRNAs were shown to exist extracellularly in human plasma as vesicle-free Ago2 complexes (Arroyo et al, 2011). Because Ago2 is the catalytic component of RISC (Liu et al, 2004; Rand et al, 2004), these miRNA-Ago2 complexes could be responsible for inducing RNAi in recipient cells (Figure 2D).
SID-1 (systemic RNAi-defective-1) is a protein channel that is necessary for the import of dsRNA into most cells in C. elegans (Winston et al, 2002; Feinberg and Hunter, 2003). Overexpression of the mammalian homologue of SID-1 (i.e., SIDT-1) was shown to increase internalization of siRNA and RNAi in mammalian cells soaked in siRNA-containing medium (Duxbury et al, 2005; Tsang et al, 2007) (Figure 2E). However, at endogenous levels, SIDT-1 expression appeared to be insufficient for siRNA uptake into mammalian cells (Tsang et al, 2007).
MECHANSIMS OF CELL CONTACT-DEPENDENT NON-CELL-AUTONOMOUS RNAi
Tunneling nanotubes
Tunneling nanotubes (TNTs) were first identified in cultured rat pheochromocytoma PC12 cells as intercellular structures with diameters of 50–200nm and lengths of up to several cell diameters (Rustom et al, 2004). They were shown to hover between cells, transferring membrane components and organelles between the cytosols of adjoining cells (Rustom et al, 2004). TNTs with a variety of diameters, lengths, and compositions have since been identified in vitro in numerous cell types (reviewed in Gerdes and Carvalho, 2008; Gurke et al, 2008) and in vivo between bone marrow-derived MHC class II-positive cells in the corneal stroma of mice (Chinnery et al, 2008).
Two types of TNTs have been identified in cultured human macrophages: TNTs less than 0.7μm in diameter that contain F-actin, and TNTs greater than 0.7μm in diameter that contain F-actin and microtubules (Önfelt et al, 2006). Bidirectional transfer of mitochondria and intracellular vesicles (including late endosomes and lysosomes) can occur in the latter variety of TNTs (Önfelt et al, 2006), suggesting that TNTs could mediate the transfer of miRNA-containing endosomal vesicles between cells (Figure 2F). In agreement, Belting and Wittrup (2008) reviewed TNTs as a potential mechanism for the intercellular transfer of genetic material, due in part to their ability to transfer endosomal vesicles between cells. Additionally, TNTs are remarkably similar to plasmodesmata (reviewed in Rustom, 2009), which are involved in the transfer of siRNAs between plant cells. In fact, donor human MSCs were shown to infuse siRNAs into co-cultured recipient neural progenitor cells (Mitchell et al, 2011; Olson et al, 2011) through TNTs and other mechanisms (Nolta JA, personal communication).
Gap junctions
Gap junctions are intercellular channels that span the plasma membranes of adjoining cells, connecting the cytosols and allowing for cell-to-cell communication (reviewed in Bruzzone et al, 1996). In a gap junction, each of two adjacent cells contains a connexon, which is a hemichannel composed of six connexin (Cx) proteins. Gap junctions form from two identical connexons (homotypic) or two different connexons (heterotypic). Most tissues express more than one type of connexin and connexins often have distinct tissue and cellular distributions. Gap junction intercellular communication (GJIC) allows water-soluble molecules (e.g., ions, second messengers, and small metabolites) to diffuse between cells (reviewed in Bruzzone et al, 1996). Emerging evidence suggests that GJIC also mediates the transfer of RNAi between cells.
Valiunas et al (2005) demonstrated that there was transfer of RNAi targeting DNA polymerase β from donor normal rat kidney (NRK) cells stably expressing shRNA against DNA polymerase β to co-cultured recipient NRK cells (Valiunas et al, 2005). NRK cells express C×43 (Hand et al, 2002); however, repeated experiments using donor and recipient cells that expressed C×26 and C×32 but not C×43, or that were connexin-deficient, did not show knockdown of DNA polymerase β in recipient cells (Valiunas et al, 2005).
In similar co-culture experiments, Wolvetang et al (2007) demonstrated a gap junction (C×43 and/or C×45)-mediated transfer of RNAi targeting green fluorescent protein (GFP) from donor human embryonic stem cells (hESCs) stably expressing shRNA against GFP to co-cultured recipient hESCs stably expressing GFP. Likewise, Kizana et al (2009) observed the transfer of RNAi targeting enhanced GFP (eGFP) from donor neonatal rat ventricular myocytes (NRVMs) stably expressing shRNA against eGFP to co-cultured recipient NRVMs stably expressing eGFP. NRVMs express C×43 (Kizana et al, 2007); however, there was no change in eGFP levels when the NRVMs expressed a C×43 dominant-negative mutant, suggesting that the reduction in eGFP was dependent on C×43 gap junctions (Kizana et al, 2009). Lastly, Lim et al (2011) reported that miRNAs against CXCL12 were transferred from donor bone marrow stromal cells to co-cultured recipient breast cancer cells through gap junctions composed of C×43, leading to reduced expression of CXCL12 in the recipient cells. Together, these studies demonstrate that RNAi transfers between cells through gap junctions composed of certain connexins, including C×43 (Figure 2G).
Immunological synapses
An immunological synapse (IS) is a junction that forms at the interface of a T cell and an antigen-presenting cell (APC), allowing for cell-to-cell interactions to modulate the immune response (reviewed in Rodríguez-Fernández et al, 2010). Mittelbrunn et al (2011) demonstrated that miRNA-containing exosomes transferred from T cells to APCs upon antigen-induced IS formation, resulting in sequence-specific RNA silencing in the recipient APCs. The T cell MVBs polarized towards the IS, which enhanced the secretion of miRNA-containing exosomes (Mittelbrunn et al, 2011). These data suggest that RNAi may transfer between immune cells through ISs (Figure 2H).
CONSIDERATIONS IN STUDIES OF NON-CELL-AUTONOMOUS RNAi IN MAMMALIAN CELLS
The method of introducing the RNAi agent into the donor cells
Different methods of introducing the RNAi agent into the donor cells may impact the transfer of RNAi between cells. For example, a common method of transfecting cells with siRNA involves complexing the siRNA with a cationic lipid carrier to create siRNA-lipoplexes. Recent data suggest that a majority of siRNA-lipoplexes introduced into cells persist in endolysosomes (Lu et al, 2009). Because endolysosomal trafficking may be involved in intercellular transfer of RNAi (reviewed in Gibbings and Voinnet, 2010), siRNA-lipoplexes within endolysomes may be more likely to transfer between cells than siRNA introduced into cells by a different method.
The method of introducing the RNAi agent into the donor cells may also impact the potency of RNA silencing in the recipient cells. Assuming that the donor cells remain viable at the target site, donor cells stably expressing shRNA or miRNA may induce more potent RNA silencing in the recipient cells than donor cells transiently expressing siRNA.
Donor to recipient cell ratio
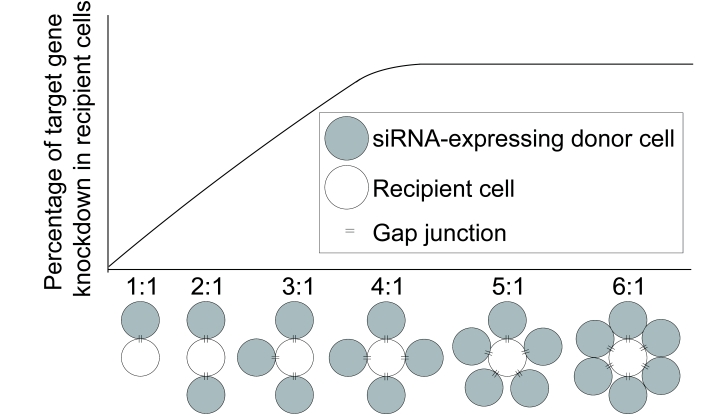
Increasing the ratio of siRNA-expressing donor cells to recipient cells in co-culture increased target gene knockdown in recipient cells (Valiunas et al, 2005; Wolvetang et al, 2007), suggesting that a higher ratio of donor to recipient cells results in more RNA silencing signals entering the recipient cells. By increasing the ratio of donor to recipient cells, we expect to eventually reach a maximum percentage of target gene knockdown in recipient cells (Figure 3). This maximum would depend on a variety of factors including the rate of target mRNA turnover in recipient cells, the number of RNA silencing signals transferred by each donor cell, and the potency of each RNA silencing signal. Additionally, in cell contact-dependent RNAi transfer, limited points of contact between the donor and recipient cells may increase the time of co-culture required to reach the maximum target gene knockdown in recipient cells or may reduce the maximum altogether.
Figure 3.
The predicted transfer of RNAi when the ratio of siRNA-expressing donor cells to recipient cells in co-culture is increased. Valiunas et al (2005) observed a greater percentage of target gene knockdown in recipient cells when the ratio of donor to recipient cells increased from 1:1 to 2:1. Similarly, Wolvetang et al (2007) observed a progressive increase in target gene knockdown in recipient cells when the ratio of donor to recipient cells increased from 1:1 to 2:1 to 3:1. By increasing the ratio of donor to recipient cells, we expect to eventually reach a maximum percentage of target gene knockdown in recipient cells. In cell contact-dependent transfer of RNAi, such as by GJIC, limited points of contact between the donor and recipient cells may reduce the maximum target gene knockdown in recipient cells.
RNAi in donor cells following intercellular transfer of RNAi to recipient cells
While the transfer of RNA silencing signals from donor to recipient cells may lessen the potency of RNAi in the donor cells, we predict that the transfer would not eliminate RNAi in the donor cells. Each activated RISC undergoes multiple rounds of RNA silencing (Hutvágner and Zamore, 2002), suggesting that potent RNA silencing could occur in the donor cells despite the transfer of some RNA silencing signals to the recipient cells. Additionally, an RNA-dependent RNA polymerase (RdRP) has recently been identified in mammalian cells (Maida et al, 2009).
RdRPs are primed by existing siRNAs to produce more dsRNAs, thereby amplifying RNA silencing (Sijen et al, 2001; reviewed in Nishikura, 2001). Thus, the transfer of RNA silencing signals from donor to recipient cells would not necessarily eliminate RNAi in the donor cells. Unfortunately, limited understanding of the molecular nature of the transferred RNA silencing signal makes it difficult to predict how RdRPs or the multi-turnover nature of RISC impacts the transfer of RNAi between mammalian cells.
MECHANISTIC INSIGHTS INTO NON-CELL-AUTONOMOUS RNAi IN MAMMALIAN CELLS
Molecular nature of the transferred RNA silencing signal
In plants, the RNA silencing signal that spreads short distances (10–15 cells) from cell to cell through plasmodesmata is a 21-nt siRNA duplex (Himber et al, 2003; Dunoyer et al, 2005; Dunoyer et al, 2010; reviewed in Chitwood and Timmermans, 2010). However, the molecular nature of the RNA silencing signal that spreads systemically in plants and in C. elegans remains unclear (reviewed in Mlotshwa et al, 2002), as does the molecular nature of the transferred RNA silencing signal in mammalian cells.
It is unlikely that long dsRNA is the RNA silencing signal that transfers between mammalian cells, as dsRNAs longer than 30nt activate the interferon system (Minks et al, 1979; Manche et al, 1992). In agreement, Valiunas et al (2005), Wolvetang et al (2007), and Kizana et al (2009) hypothesized that Dicer-processed shRNA was the RNA silencing signal transferred between mammalian cells through gap junctions. Valiunas et al (2005) demonstrated that oligonucleotides (morphilinos) simulating siRNAs, with molecular weights of ∼2–4kDa, minor diameters of 1.0–1.1nm, and lengths of 7.6nm could diffuse between cells through C×43 gap junctions. The oligonucleotide permeation through the C×43 gap junctions decreased as the length of the oligonucleotides increased, which was expected as the minor diameter of the oligonucleotides was close to the pore diameter of the gap junctions (∼1.0–1.5nm) (Valiunas et al, 2005). A hybridized, double-stranded 12-mer oligonucleotide had significantly decreased permeation through the C×43 gap junctions when compared with a single-stranded 12-mer oligonucleotide, suggesting that single-stranded siRNAs are more likely to transfer through C×43 gap junctions than hybridized siRNAs (Valiunas et al, 2005). Additionally, Kizana et al (2009) determined that, compared with shRNA-expressing donor cells, co-cultured recipient cells contained 25% of the copy number of the antisense strand of shRNA, suggesting that the antisense strand of shRNA, or possibly the shRNA duplex, transfers from donor to recipient cells through C×43 gap junctions.
Rechavi et al (2009) demonstrated the transfer of 22-nt Cy3-labeled dsRNAs, or possibly metabolic products of those dsRNAs, from donor B cells to recipient T cells. However, 22-nt FITC-conjugated locked nucleic acids did not transfer between cells, suggesting that structural specificity is involved in intercellular RNA transfer (Rechavi et al, 2009). Additionally, Rechavi et al (2009) did not detect the movement of the RISC component Ago2 between donor and recipient cells, suggesting that small RNAs transfer between cells independently of Ago2. Based on these studies, we hypothesize that the antisense strand of Dicer-processed siRNA may be the RNA silencing signal that transfers between cells in non-cell-autonomous RNAi; however, we recognize that there may be different RNA silencing signals depending on the mechanism of non-cell-autonomous RNAi.
Endolysosmal trafficking and intercellular transfer of RNAi
There is increasing data linking RNAi to endolysosomal trafficking (reviewed in Siomi and Siomi, 2009; Gibbings and Voinnet, 2010). In the endolysosomal pathway, the endosomal sorting complex required for transport (ESCRT) is required for the invagination of the limiting endosomal membrane in MVBs to form ILVs (reviewed in Babst, 2005). ESCRT is also required for the sorting of endosomal cargo proteins into ILVs. MVBs can fuse with the lysosome for degradation or they can fuse with the plasma membrane to secrete their ILVs, which are then referred to as exosomes.
Gibbings et al (2009) demonstrated that RISC proteins GW182 and Ago2 were present with miRNAs in endosomes/MVBs from cultured monocytes, suggesting that miRNAs and RISC congregate on endosomes and/or MVBs. Furthermore, Lee et al (2009) discovered that in cultured HeLa cervical cancer cells, blocking the maturation of MVBs into lysosomes with Hermansky-Pudlak Syndrome 4 mutants stimulated RNAi. These findings, together with data showing that exosomes contain miRNAs (Valadi et al, 2007; Skog et al, 2008; Taylor and Gercel-Taylor, 2008; Luo et al, 2009; Kosaka et al, 2010; Michael et al, 2010; Ohshima et al, 2010; Pegtel et al, 2010; Zomer et al, 2010; DeIuliis et al, 2011; Levänen et al, 2011; Mittelbrunn et al, 2011), suggest that molecular pathways control the packaging of miRNA and RISC components into endosomes/MVBs and mediate intercellular transfer of RNAi (reviewed in Siomi and Siomi, 2009; Gibbings and Voinnet, 2010). However, the details of such pathways remain unclear. Although the ESCRT machinery appears to be involved in MVB formation (reviewed in Babst, 2005), evidence suggests that sphingomyelinase 2, which regulates ceramide biosynthesis, but not the ESCRT machinery, is involved in the secretion of miRNA-containing exosomes (Kosaka et al, 2010; Mittelbrunn et al, 2011).
IMPLICATIONS FOR IN VIVO CELL-BASED RNAi DELIVERY
Despite the enormous potential of RNAi for disease therapy, current in vivo RNAi delivery strategies (reviewed in Whitehead et al, 2009; Lares et al, 2010), such as those using liposomes, cationic polymers, or viral vectors, have been hindered by a variety of challenges (reviewed in Trehan et al, 2010), including immune system activation and inefficient cell targeting. Cell-based RNAi delivery, in which donor cells act as RNAi delivery vehicles, can potentially overcome these challenges (reviewed in Brink et al, 2010; Brink et al, 2011). Autologous or immunoprivileged allogeneic donor cells may avoid host immune system activation, and certain cell types inherently migrate to tumors or wounds. Furthermore, in areas where traditional RNAi delivery vehicles have limited diffusion, the spread of RNAi may be more efficient by transferring the RNA silencing signal from cell to cell.
MSCs are excellent candidates for in vivo cell-based RNAi delivery because they exhibit innate tumor- and wound-homing abilities, are considered immunoprivileged, and can be isolated in large numbers from bone marrow or adipose tissue (reviewed in Brink et al, 2010; Dwyer et al, 2010, Hu et al, 2010). Furthermore, MSCs express C×43 (Valiunas et al, 2004), allowing for the transfer of RNAi from MSCs to C×43-expressing recipient cells by GJIC. MSCs can also form TNTs (Plotnikov et al, 2010), and release miRNA-containing MVs (Collino et al, 2010) and exosomes (DeIuliis et al, 2011), illustrating their potential to participate in non-cell-autonomous RNAi by multiple mechanisms.
CONCLUSIONS
Emerging evidence demonstrates that non-cell-autonomous RNAi occurs in cultured mammalian cells. This phenomenon can occur by cell contact-independent mechanisms (Figure 2A-D), such as the shuttling of miRNA-containing MVs, exosomes, or apoptotic bodies between cells. Non-cell-autonomous RNAi can also occur by cell contact-dependent mechanisms, relying on TNTs, gap junctions, or ISs (Figure 2F-H). Non-cell-autonomous RNAi in mammalian cells may allow for the development of in vivo cell-based RNAi delivery, which has the potential to overcome major challenges in RNAi delivery and allow for effective RNAi therapies.
Acknowledgments
We would like to acknowledge Sally Griffith-Oh at the University of Wisconsin-Madison for help in creating Figure 2. This work was supported with start-up funds from the University of Wisconsin-Madison School of Pharmacy and through a Department of Defense (DoD) National Defense Science and Engineering Graduate (NDSEG) Fellowship to HC Cohen.
LIST OF ABBREVIATIONS
- Ago2
Argonaute 2
- APC
antigen-presenting cell
- Cx
connexin
- CXCL12
chemokine CXC motif ligand 12
- EBV
Epstein-Barr virus
- eGFP
enhanced GFP
- ESCMVs
embryonic stem cell-derived MVs HPCs; hematopoietic progenitor cells
- ESCRT
endosomal sorting complex required for transport
- GFP
green fluorescent protein
- GJIC
gap junction intercellular communication, NRK; normal rat kidney
- hESCs
human embryonic stem cells
- ILVs
intraluminal vesicles
- IS
immunological synapse
- MSCs
mesenchymal stem cells
- MVBs
multivesicular bodies
- MVs
microvesicles
- NRVMs
neonatal rat ventricular myocytes
- RdRP
RNA-dependent RNA polymerase
- RISC
RNA-induced silencing complex
- SID-1
systemic RNAi-defective-1
- TNTs
tunneling nanotubes
STATEMENT OF COMPETING INTERESTS
None declared
REFERENCES
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183:1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Brink PR, Robinson RB, Rosen MR, Cohen IS. In vivo cellular delivery of siRNA. IDrugs. 2010;13:383–387. [PubMed] [Google Scholar]
- Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS. Can gap junctions deliver? BBA – Biomembranes. 2011. in press. [DOI] [PubMed]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DH, Timmermans MCP. Small RNAs are on the move. Nature. 2010;467:415–419. doi: 10.1038/nature09351. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeIuliis G, Di Giuseppe M, Phinney D, Sala E, Kaminski N, Ortiz LA. Differential expression of microRNA in mesenchymal stem cell derived exosomes. Am J Respir Crit Care Med. 2011;183:A3769. [Google Scholar]
- Dinger ME, Mercer TR, Mattick JS. RNAs as extracellular signaling molecules. J Mol Endocrinol. 2008;40:151–159. doi: 10.1677/JME-07-0160. [DOI] [PubMed] [Google Scholar]
- Dunoyer P, Himber C, Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat Genet. 2005;37:1356–1360. doi: 10.1038/ng1675. [DOI] [PubMed] [Google Scholar]
- Dunoyer P, Schott G, Himber C, et al. Small RNA duplexes function as mobile silencing signals between plant cells. Science. 2010;328:912–916. doi: 10.1126/science.1185880. [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Ashley SW, Whang EE. RNA interference: a mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun. 2005;331:459–463. doi: 10.1016/j.bbrc.2005.03.199. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Khan S, Barry FP, O'Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther. 2010;1:25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;301:1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Gerdes HH, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- Gibbings D, Voinnet O. Control of RNA silencing and localization by endolysosomes. Trends Cell Biol. 2010;20:491–501. doi: 10.1016/j.tcb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539–550. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Hand GM, Muller DJ, Nicholson BJ, Engel A, Sosinsky GE. Isolation and characterization of gap junctions from tissue culture cells. J Mol Biol. 2002;315:587–600. doi: 10.1006/jmbi.2001.5262. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Himber C, Dunoyer P, Moissiard G, Ritzenthaler C, Voinnet O. Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J. 2003;22:4523–4533. doi: 10.1093/emboj/cdg431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Fu Y, Tabata Y, Gao J. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J Control Release. 2010;147:154–162. doi: 10.1016/j.jconrel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvágner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- Jose AM, Hunter CP. Transport of sequence-specific RNA interference information between cells. Annu Rev Genet. 2007;41:305–330. doi: 10.1146/annurev.genet.41.110306.130216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantidis K, Schumacher HT, Alexiadis T, Helm JM. RNA silencing movement in plants. Biol Cell. 2008;100:13–26. doi: 10.1042/BC20070079. [DOI] [PubMed] [Google Scholar]
- Kizana E, Chang CY, Cingolani E, et al. Gene transfer of connexin43 mutants attenuates coupling in cardiomyocytes. Circ Res. 2007;100:1597–1604. doi: 10.1161/CIRCRESAHA.106.144956. [DOI] [PubMed] [Google Scholar]
- Kizana E, Cingolani E, Marban E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 2009;16:1163–1168. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lares MR, Rossi JJ, Ouellet DL. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 2010;28:570–579. doi: 10.1016/j.tibtech.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Pressman S, Andress AP, et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009;11:1150–1156. doi: 10.1038/ncb1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levänen B, Torregrosa-Paredes P, Barbeau R, et al. Differences in exosomal microRNAs in bronchoalveolar lavage fluid from asthmatics and healthy individuals. Am J Respir Crit Care Med. 2011;183:A1006. [Google Scholar]
- Lim PK, Bliss SA, Patel SA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharm. 2009;6:763–771. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SS, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- Maida Y, Yasukawa M, Furuuchi M, et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009;461:230–235. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manche L, Green SR, Schmedt C, Mathews MB. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol Cell Biol. 1992;12:5238–5248. doi: 10.1128/mcb.12.11.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Matranga C, Tomari Y, Shin C, et al. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Michael A, Bajracharya SD, Yuen PST, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Minks MA, West DK, Benvin S, Baglioni C. Structural requirements of double-stranded RNA for the activation of 2′, 5′-oligo (A) polymerase and protein kinase of interferon-treated HeLa cells. J Biol Chem. 1979;254:10180–10183. [PubMed] [Google Scholar]
- Mitchell GA, Olson SD, Pollock KM, et al. Mesenchymal stem cells as a delivery vehicle for intercellular delivery of RNAi to treat Huntington's disease. AAN, IN10-1.010. 2011 [Google Scholar]
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlotshwa S, Voinnet O, Mette MF, et al. RNA silencing and the mobile silencing signal. Plant Cell. 2002;14(Suppl):S289–S301. doi: 10.1105/tpc.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. A short primer on RNAi: RNA-directed RNA polymerase acts as a key catalyst. Cell. 2001;107:415–418. doi: 10.1016/s0092-8674(01)00581-5. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Inoue K, Fujiwara A, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SD, Pollock K, McNerny G, Chuang F, Huser T, Nolta JA. Visualization of siRNA complexed to RISC machinery: demonstrating intercellular siRNA transfer by directly imaging activity. AAN, IN4-1.014. 2011 [Google Scholar]
- Önfelt B, Nedvetzki S, Benninger RK, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010;316:2447–2455. doi: 10.1016/j.yexcr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Rand TA, Ginalski K, Grishin NV, Wang X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc Natl Acad Sci U S A. 2004;101:14385–14389. doi: 10.1073/pnas.0405913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006a;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006b;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- Rechavi O, Erlich Y, Amram H, et al. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 2009;23:1971–1979. doi: 10.1101/gad.1789609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Fernández JL, Riol-Blanco L, Delgado-Martín C. What is an immunological synapse? Microb Infect. 2010;12:438–445. doi: 10.1016/j.micinf.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Rustom A. Hen or Egg? Some thoughts on tunneling nanotubes. Ann N Y Acad Sci. 2009;1178:129–136. doi: 10.1111/j.1749-6632.2009.04997.x. [DOI] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Siomi H, Siomi MC. RISC hitches onto endosome trafficking. Nat Cell Biol. 2009;11:1049–1051. doi: 10.1038/ncb0909-1049. [DOI] [PubMed] [Google Scholar]
- Skog J, Würdinger T, Van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser N. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct. 2007;2:35. doi: 10.1186/1745-6150-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Trehan S, Sharma G, Misra A. siRNA: sojourn from discovery to delivery challenges and clinics. Syst Rev Pharm. 2010;1:1–16. [Google Scholar]
- Tsang SY, Moore JC, Huizen RV, Chan CWY, Li RA. Ectopic expression of systemic RNA interference defective protein in embryonic stem cells. Biochem Biophys Res Commun. 2007;357:480–486. doi: 10.1016/j.bbrc.2007.03.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Doronin S, Valiuniene L, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555:617–626. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Polosina YY, Miller H, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Vain P, Angell S, Baulcombe DC. Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell. 1998;95:177–187. doi: 10.1016/s0092-8674(00)81749-3. [DOI] [PubMed] [Google Scholar]
- Voinnet O. Non-cell autonomous RNA silencing. FEBS Lett. 2005;579:5858–5871. doi: 10.1016/j.febslet.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolvetang EJ, Pera MF, Zuckerman KS. Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun. 2007;363:610–615. doi: 10.1016/j.bbrc.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Yuan A, Farber EL, Rapoport AL, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: fit to deliver small RNA. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]