Abstract
Triple negative breast cancer (TNBC) is an aggressive clinical phenotype characterized by lack of expression (or minimal expression) of estrogen receptor (ER) and progesterone receptor (PR) as well as an absence of human epidermal growth factor receptor–2 (HER2) overexpression. It shows substantial overlap with basal-type and BRCA1-related breast cancers, both of which also have aggressive clinical courses. However, this overlap is not complete, and the expression of ER, PR, and HER2 has been noted in basal-like tumors. TNBC also includes the normal-like subtype, and not all patients with TNBC harbor BRCA1 mutations. Because of its expression profile, TNBC is not amenable to treatment with hormone therapy or the anti-HER2 monoclonal antibody trastuzumab, and systemic treatment options are currently limited to cytotoxic chemotherapy. Overall survival, whether in early-stage or advanced disease, is poor compared with that in patients who have other phenotypes. A number of targeted approaches to TNBC are undergoing clinical evaluation, including the use of agents with poly(ADP-ribose) polymerase inhibitory properties such as iniparib (the United States Adopted Name for the investigational agent BSI-201), olaparib (AZD2281), and veliparib (ABT-888), antiangiogenic agents such as bevacizumab and sunitinib, and epidermal growth factor receptor blockers such as cetuximab and erlotinib. Encouraging results with some of these agents have been reported, thereby offering the promise for improved outcomes in patients with TNBC. The clinical characteristics of TNBC and clinical experience to date with novel targeted agents under development for this aggressive phenotype is reviewed.
Keywords: Breast cancer, triple negative, phenotype, basal-like, BRCA1, targeted therapy
Introduction
Breast cancer is the most common malignancy and second leading cause of cancer death among women in the United States, with an estimated 192,370 new cases diagnosed and 40,170 deaths reported in 2009 [1]. Among women aged 20 to 59 years, breast cancer remains the leading cause of cancer death despite a steady decrease in breast cancer mortality since 1990 [1]. Breast cancer comprises multiple diseases harboring different genetic alterations that can be classified into distinct molecular subtypes based on DNA microarray expression profiling [2–4]. The 5 initial subtypes identified are luminal A, luminal B, human epidermal growth factor receptor–2 (HER2)-overexpressing, normal breast tissue–like, and basal-like. More recently, another subtype has been identified: “claudin low,” which appears to be enriched for stem cell markers and cells capable of forming new tumors [5, 6]. These subtypes respond differently to therapy and are associated with differing outcomes, with the shortest survival times seen in patients who have the basal-like and HER2-overexpressing subtypes [3, 4, 7].
Triple negative breast cancer (TNBC) comprises a heterogeneous subgroup of tumors including, but not limited to those classified as basal-like and claudin-low subtypes by expression profiling, and accounts for ~15% of all breast cancers [5–10]. TNBC is characterized by an aggressive clinical course and poor survival and, unlike tumors overexpressing hormone receptors or HER2, is not amenable to hormone therapy or HER2-directed agents such as trastuzumab [8, 9, 11–13]. These factors highlight the need for new treatment options for patients with TNBC. Here we describe the clinical characteristics of TNBC and provide an overview of targeted agents under development for this aggressive phenotype.
Definition of TNBC
As mentioned above, TNBC is characterized by a lack of expression (or minimal expression) of estrogen receptor (ER) and progesterone receptor (PR) as well as an absence of HER2 overexpression. According to guidelines published by the American Society of Clinical Oncology (ASCO), ER/PR negativity by immunohistochemical (IHC) analysis is defined as <1% of tumor cell nuclei immunoreactive for ER or PR [14], although clinical trials of patients with TNBC may allow the enrollment of patients with ≤10% expression. Non-overexpression of HER2 (HER2 negative or equivocal) is defined in the ASCO guidelines as IHC ≤2+ for HER2 or a fluorescence in situ hybridization ratio of <2.2 (HER2 to CEP17) or average HER2 gene copy number of fewer than 6 signals per nucleus without an internal control probe [15].
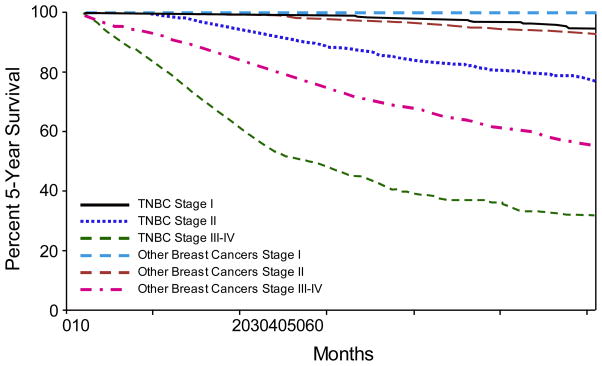
TNBC exhibits a more aggressive clinical course than non-TNBC. This point is illustrated in a population-based study from the California Cancer Registry, which involved 51,074 women with primary breast cancer [8]. TNBCs were present at a more advanced stage and were associated with shorter 5-year survival times than non-TNBC phenotypes (77% vs 93%). Moreover, within each disease stage at diagnosis, TNBC was consistently associated with poorer survival than non-TNBC (Fig. 1) [8]. When compared with other breast cancer subtypes in the pre-trastuzumab era, TNBC was associated with outcomes worse than those for the luminal subtypes and similar to that for the HER2-overexpressing subtype. In a follow-up analysis of the California Cancer Registry, which included 61,309 women, the 5-year survival rates were 96% among women with ER positive, PR positive, HER2 negative breast cancer and 76% among women with TNBC or the HER2-overexpressing subtype [16]. Comparable findings were also reported in other studies [7, 9, 11, 12, 17]. Notably, the poorer outcome in patients with TNBC is observed despite comparable responses to cytotoxic chemotherapy [18, 19].
Fig. 1.
Comparison of overall survival by disease stage for women with triple negative breast cancer (TNBC) and those with other phenotypes
Adapted from Bauer et al [8]
TNBC often presents as advanced-stage disease. In a study of 1134 patients with invasive breast cancer, the age-adjusted odds ratio (OR) for advanced disease stage at diagnosis was significantly higher in patients with TNBC than in patients with luminal type A (OR, 2.2; 95% confidence interval [CI], 1.0 to 4.9) [7]. Studies have also shown that, unlike with most breast cancer subtypes, there is a lack of correlation between tumor size and lymph node positivity among TNBC cases [11, 20, 21]. Most TNBCs are of high histologic grade, with frequencies of grade 3 histology across TNBC cohorts ranging from 66–91% [7, 9, 11, 17, 22]. Grade 1 histology is uncommon, although it has been detected in up to 10% of patients with TNBC [11]. Most TNBCs are ductal carcinomas of no special type, but some cases may have features of metaplastic carcinomas and medullary cancers [9, 23–25]. On microscopic analysis, TNBC is associated with a high proliferative rate, a pushing border of invasiveness often with some degree of a stromal lymphocytic infiltrate at the tumor edge, and central necrotic regions [24]. IHC analyses show that TNBC is associated with a high expression of the proliferation marker Ki-67 [17] as well as several other markers favoring cancer cell growth, including p53 and mutated p53 [3, 9, 20, 26], cyclin E [20, 26], epidermal growth factor receptor (EGFR) [24, 26, 27], vimentin [24, 26], P-cadherin [9], and BRCA1 mutations [4, 12].
Overlap of TNBC with basal-like and BRCA1-related disease
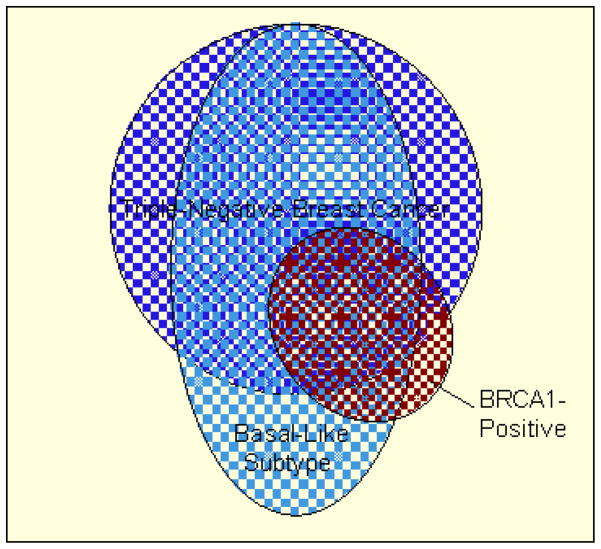
Although TNBC shows considerable overlap with the basal-like subtype and BRCA1-related breast cancer, the overlap is not complete and there are important distinctions between these subtypes (Fig. 2) [28]. The basal-like subtype is best identified by DNA microarray expression profiling, but this methodology is not readily available in clinical practice, and the use of RNA from formalin-fixed paraffin-embedded tissue is not reliable [29]. Thus, IHC profiles have been proposed as a surrogate for the basal-like subtype, the best of which is defined by ER negativity, HER2 negativity, CK5/6 positivity, and/or EGFR positivity, which would suggest complete overlap with TNBC [27]. However, studies using microarray analyses indicate that 15–54% of basal-like breast cancers express at least 1 non-TNBC marker (ie, ER, PR, HER2) [27, 30–33]. Moreover, TNBC includes the normal-like subtype, which lacks the expression of markers for the basal-like phenotype (ie, CK5/6, EGFR) [4, 27]. For example, in a recent single-institution study, 16 (12%) of 132 patients with TNBC had tumors classified as normal-like [34]. Overall, it is estimated that 65–85% of TNBCs are of the basal-like subtype [4, 27, 32, 34].
Fig. 2.
Schematic illustration of overlap among triple negative breast cancer (TNBC), basal-like, and BRCA1-related tumors
Adapted from Pal et al [28]
BRCA1-related tumors account for ~5% of all breast cancers, with higher rates of BRCA1-related breast cancer occurring among patients of Ashkenazi Jewish decent [35]. The BRCA1 protein plays a role in DNA repair and transcriptional regulation; therefore, the disruption of BRCA1 via somatic mutation or epigenetic mechanisms may promote genetic instability and favor tumor growth [36]. BRCA1-related breast cancer and TNBC both have aggressive clinical courses and share pathologic and clinical features and both are frequently of high histologic grade, with pushing borders, and are associated with high Ki-67 expression, p53 mutation, and basal-like expression profiles [26, 37, 38]. While >50% of BRCA1 carriers have TNBC, as indicated by microarray and IHC analyses [4, 38, 39], patients with TNBC do not necessarily harbor BRCA1 mutations. However, it has been shown that TNBCs with wild-type BRCA1 frequently exhibit a downregulation of BRCA1 expression or alterations in BRCA1 function, which may occur through methylation of the BRCA1 promoter or overexpression of proteins (eg, inhibitor of differentiation–4) that normally regulate BRCA1 expression [36, 37, 40–42].
Risk factors for TNBC
Several risk factors for TNBC have been consistently identified in population-based cohorts, and include younger age, African American race, BRCA1 mutation, and a strong family history of breast cancer [8, 12, 13, 21, 43]. In a cohort of 482 patients with early-stage breast cancer, each of these factors, particularly BRCA1 mutations, was shown to be significantly more common among women with TNBC than among women with other phenotypes (25.9% vs 1.7%; P = 0.001) [12]. A multiple logistic regression analysis of demographic factors in the previously mentioned California Cancer Registry cohort found that risk of TNBC increased with younger age (OR, 1.53; 95% CI, 1.37 to 1.70 in a comparison of women aged <40 years with women aged 60 to 69 years) and was higher among African Americans than Caucasians (OR, 1.77; 95% CI, 1.59 to 1.97) [8]. Similarly, the Carolina Breast Cancer Study confirmed the association of younger age and African American race to basal-like breast cancer and also found significant associations for younger age at menarche, higher parity, and shorter duration of breastfeeding [43]. In the Polish Breast Cancer Study, younger age at menarche, higher body mass index among postmenopausal women, and a family history of breast cancer were also associated with the basal-like phenotype [44].
Considerations for screening and diagnosis
Compared with other breast cancer types, TNBC may be less likely to be detected by mammography or alternative screening methods. In a cohort of 1050 women aged ≥50 years, TNBC was significantly more likely to be detected clinically by the patient or physician than radiographically by mammography or ultrasound compared with other phenotypes [11]. Similarly, in the Norwegian Breast Cancer Screening Program, women who developed breast cancer between regular mammography screening intervals, and whose cancer was detected clinically by palpation, were approximately 2 times more likely to have TNBC than were women whose disease was detected at screening [45]. Patients with these so-called interval breast cancers were more likely to be younger and to have dense breasts compared with those who had tumors detected on screening. Moreover, the interval breast cancers were associated with high expressions of Ki-67, p53, and basal-like markers [45]. The higher detection rate of TNBC by clinical examination may reflect a greater difficulty in detection by mammography due to higher breast tissue density, or it may relate to the more rapid growth of this phenotype compared with other subtypes.
Treatment and outcomes
Unlike patients with ER/PR positive or HER2 overexpressing disease, systemic treatment options for patients with TNBC are limited to cytotoxic chemotherapy due to the lack of a molecular target [46]. DNA-damaging agents may be useful based on the association of TNBC with BRCA1-related tumors and their inherent DNA repair dysfunction; however, this suggestion is based on findings from small clinical studies [47]. Neoadjuvant single-agent cisplatin has been reported to produce pathologic complete responses (pCRs) in patients with TNBC [48]. In a study investigating the efficacy and predictors of response to neoadjuvant cisplatin in women with stage II or III TNBC, 6 (22%) of 28 women had a pCR, including both patients with BRCA1 germline mutations, and 18 patients (64%) had a clinical complete or partial response. Factors associated with good cisplatin response include younger age (P = 0.001), low BRCA1 mRNA expression (P = 0.03), BRCA1 promoter methylation (P = 0.04), p53 nonsense or frameshift mutations (P = 0.01), and a gene expression signature of E2F3 activation (P = 0.03) [48]. Higher rates of pCR were noted in a second retrospective study that examined pCR rates achieved with neoadjuvant chemotherapy in102 women with breast cancer who carried the BCRA1 mutation. Of the 12 patients who received single-agent cisplatin, 10 (83%) achieved a pCR [49]. In another study, neoadjuvant platinum-based combination therapy (cisplatin, epirubicin, and 5-fluorouracil) produced pCRs in 15 (88%) of 17 patients with TNBC compared with 39 (51%) of 77 patients who did not have the TNBC phenotype [50]. Other cytotoxic regimens are also active, suggesting that TNBC is chemosensitive. In a prospective cohort treated at the M.D. Anderson Cancer Center, neoadjuvant chemotherapy—mostly anthracycline-based combinations with or without taxanes—produced pCRs in 57 (22%) of 255 patients with TNBC, which was significantly higher than the 11% rate seen among patients with non-TNBC phenotypes (P = 0.034) [51]. High clinical response rates were also seen with anthracycline-based regimens in patients with TNBC in other studies [18, 49].
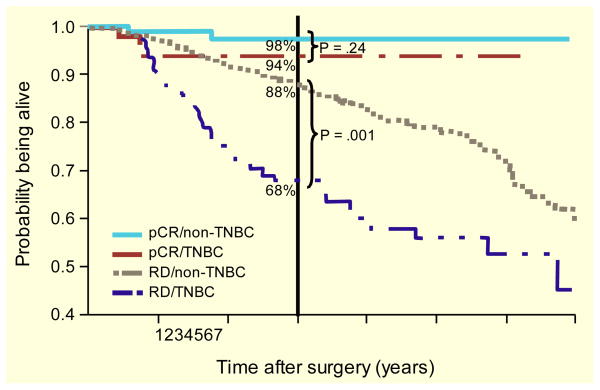
Despite its chemosensitivity, TNBC is still associated with a poor prognosis. In a cohort of 1601 patients with breast cancer, Dent et al showed a rapid increase in the risk of recurrence following diagnosis among patients with TNBC compared with those who had other subtypes, with a peak risk of recurrence at 1 to 3 years [11]. The median time to death among patients with TNBC was also shorter than that with other subtypes (4.2 vs 6.0 years; P < 0.0001). In a retrospective cohort of 1134 patients diagnosed with breast cancer between 1998 and 2005, patients with TNBC had 5-year overall survival (OS) rates of 79.0%, which was comparable to rates in patients with ER/PR negative HER2-overexpressing tumors but poorer than rates in patients with ER/PR positive disease (Table 1) [7]. Similarly, in the M.D. Anderson Cancer Center cohort, the 3-year OS was significantly shorter for patients with TNBC than for patients without TNBC (74% vs 89%; P < 0.0001) [51]. However, differences in recurrence rates and mortality between patients with and without TNBC were evident only during the first 3 years. Moreover, patients with TNBC who had residual disease after neoadjuvant chemotherapy had a particularly poor outcome (Fig. 3) [51].
Table 1.
Five-year Overall and Disease-free Survival by Tumor Subtype and ER/PR and HER2 Status
| Parameter | OS, % (95% CI) | DFS, % (95% CI) |
|---|---|---|
| Subtype | ||
| ER/PR+, HER2+ | 88.7 (79.2–91.4) | 83.2 (74.0–89.6) |
| ER/PR+, HER2 | 90.3 (87.6–92.5) | 86.8 (83.8–89.4) |
| ER/PR−, HER2+ | 78.8 (66.0–87.7) | 66.0 (53.9–76.3) |
|
| ||
| ER/PR−, HER2 | 79.0 (70.8–85.3) | 73.5 (65.0–80.5) |
|
| ||
| ER/PR Status | ||
| ER/PR+ | 90.1 (87.5–92.2) | 86.4 (83.6–88.8) |
| ER/PR− | 79.0 (72.4–84.4) | 70.8 (63.9–76.8) |
| HER2 Status | ||
| Positive | 84.6 (77.3–89.9) | 75.9 (68.6–81.9) |
| Negative | 88.5 (85.9–90.6) | 84.7 (81.9–87.2) |
| Overall | 87.8 (85.4–89.9) | 83.1 (80.5–85.5) |
Note: Highlighting denotes the TNBC category.
Abbreviations: CI, confidence interval; DFS, disease-free survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; OS, overall survival; PR, progesterone receptor.
Reprinted with permission. Source: Onitílo et al [7].
Fig. 3.
Overall survival in women with triple negative breast cancer (TNBC) compared with non-TNBC based on response to neoadjuvant cytotoxic chemotherapy. RD indicates residual disease
Adapted from Liedtke et al [51]
The pattern of disease recurrence may also differ between TNBCs and other subtypes. TNBC is associated with a high risk of distant recurrence compared with other subtypes, with rapid progression from distant recurrence to death [11, 52]. Additionally, patients with TNBC are less likely to experience local recurrence before distal recurrence than are patients with other subtypes [11]. Irrespective of the type of breast cancer, the most common first sites of distant metastasis are bone followed by lung and then brain and liver, with the latter associated with the poorest prognosis [53]. However, for TNBC and basal-like tumors, high rates of visceral metastases (compared with bone metastases) and brain metastases have been reported [10, 52, 54, 55].
In a cohort of 1608 patients with breast cancer, a higher frequency of visceral metastases was reported among patients with TNBC than with other subtypes (84% vs 61%; P = 0.0003) [52]. Moreover, patients with TNBC were 4 times more likely to show visceral metastases within the first 5 years of diagnosis than patients with other subtypes (hazard ratio [HR], 4.0; 95% CI, 2.7 to 5.9; P < 0.0001) [52]. In a cohort of 443 patients with high-grade breast cancer, patients with basal-like tumors were more likely to develop brain metastases but less likely to develop bone or liver metastases compared with patients who had non-basal tumors [54]. In another analysis of 85 intracerebral metastases from breast cancer, the basal-like phenotype was identified in 22 cases (26%)—higher than its overall frequency in primary breast tumors [56].
Chemotherapy is recommended once metastatic disease develops in patients with TNBC [46]. Although a variety of single-agent and combination regimens are available, none is recommended specifically for TNBC. As noted above, several cytotoxic agents, including platinum-based therapies and the anthracyclines, have shown promising activity in small neoadjuvant studies [18, 48–51] and consequently may be rational choices for patients with metastatic disease. However, treatment choices are limited for patients with metastatic disease as many receive adjuvant therapy with anthracyclines, taxanes, or cyclophosphamide for primary tumor treatment. Despite the aggressive nature of TNBC, it is important to recognize that considerable heterogeneity has been observed in the treatment of patients with metastatic disease. This point is illustrated in a cohort of 111 patients with TNBC (median age, 51 years), most of whom presented with multiple sites of metastatic disease [57]. The patients received first-line single-agent (67%) or combination (33%) chemotherapy for a median of 11.9 weeks (range, 0.0 to 73.1 weeks), and 87 patients (78%) then went on to receive second-line systemic therapy for a median of 9 weeks (range, 0.0 to 120.9 weeks). Fifty-five patients (49%) received third-line therapy for a median of 4 weeks (range, 0.0 to 59.0 weeks). Median OS for the entire cohort was 13.3 months (range, 0.8 to 99.8 months). On multivariate analysis, independent factors associated with poorer survival were previous neoadjuvant and/or adjuvant chemotherapy, visceral metastases at first presentation, age <50 years, distant disease-free interval <1 year, and elevated alkaline phosphatase levels [57]. Therefore, most women with recurrent and metastatic TNBC progress quickly on systemic therapy, but some may have a slower progressive course.
Future directions
Numerous targeted agents are under development for TNBC (Table 2). Poly(ADP-ribose) polymerase (PARP) inhibitors target key enzymes involved in repairing single-strand breaks in DNA [47]. These enzymes assume a greater role in DNA repair when the preferred homologous recombination mechanism for repairing double-strand breaks is lost due to BRCA1 dysfunction, and preclinical studies have shown that cancer cells with deficient BRCA1 activity are hypersensitive to PARP inhibition [58]. Based on promising preclinical findings, several agents with PARP inhibitory activity, including iniparib (the United States Adopted Name for the investigational drug BSI-201), olaparib (AZD2281), and veliparib (ABT-888) have begun clinical evaluation for the treatment of patients with TNBC, either as monotherapy or in combination with chemotherapeutic agents. In a phase II trial, iniparib was evaluated in combination with gemcitabine/carboplatin for the treatment of patients with TNBC [59]. The rationale for combining iniparib with chemotherapeutic regimens such as this stems from the hypothesis that agents with PARP inhibitory activity can augment the DNA-damaging effects of platinum-based chemotherapy, which has been demonstrated in preclinical models [28, 60]. This activity may be of particular relevance in TNBC given the reduced expression and/or functionality of BRCA1 frequently observed [36, 37, 40–42]. Indeed, studies have shown that PARP1 is upregulated in TNBC [61]. Results from the phase II trial showed that the addition of iniparib to gemcitabine/carboplatin significantly improved median survival compared with chemotherapy alone [59]. Iniparib was well tolerated and did not potentiate the toxicities associated with chemotherapy. A phase III trial of iniparib plus gemcitabine/carboplatin in patients with metastatic TNBC is currently ongoing, and a phase II trial is under way to evaluate this combination in the neoadjuvant setting. The oral PARP inhibitor olaparib (400 mg BID or 100 mg BID) has been evaluated in a phase II trial involving 54 patients with chemotherapy-refractory advanced breast cancer who carried a BRCA1 or BRCA2 mutation [62]. Of the patients studied, 13 (50%) of 26 assigned to the 400-mg cohort and 16 (64%) of 25 assigned to the 100-mg cohort had TNBC. Eleven (41%) of 27 patients in the 400-mg cohort and 6 (22%) of the 27 patients in the 100-mg cohort had objective responses. Of the patients with TNBC, 7 (54%) of 13 in the 400-mg cohort and 4 (25%) of 16 in the 100-mg cohort had an overall response. None of the patients who had TNBC achieved a pCR. The median progression-free survival was 5.7 months for patients in the 400-mg cohort and 3.8 months for patients in the 100-mg cohort. Olaparib was generally well tolerated, with most common toxicities being fatigue and nausea in both treatment cohorts. One patient in each treatment cohort had grade 4 treatment-related anemia [62]. A second, smaller study of olaparib 400-mg monotherapy involving 15 patients with TNBC reported an ORR of 0% [63]. Clinical trials are also under way to evaluate olaparib plus various cytotoxic chemotherapy agents (paclitaxel, carboplatin, or cisplatin) or cediranib for the treatment of patients with TNBC. In a phase I trial of 19 patients with TNBC, olaparib in combination with paclitaxel showed a manageable toxicity profile; however, there was a high incidence of neutropenia leading to a reduced paclitaxel dose intensity. Nonetheless, responses were observed with this combination [64]. Additionally, the oral PARP inhibitor veliparib is being evaluated in combination with temozolomide in a phase II trial of patients with metastatic breast cancer. Results presented at the 2010 ASCO annual meeting showed that responses were observed with this combination, but were limited to patients with BRCA-associated disease [65]. Although resistance to PARP inhibition has not yet been observed in patients, results from preclinical studies may provide insights into the potential for acquired resistance to this class of drugs. Edwards et al showed that PARP inhibitor–resistant (PIR) clones could be derived from the CAPAN1 pancreatic cancer cell line in vitro [66]. Parental CAPAN1 cells harbor the loss-of-function c.6174delT frameshift mutation in BRCA2, and are therefore exquisitely sensitive to PARP inhibition. Resistance to PARP inhibition in the PIR clones was due to the expression of a new, functional BRCA2 isoform resulting from an intragenic deletion of the c.6174delT mutation and restoration of the open-reading frame. Interestingly, similar mutations restoring BRCA2 function have also been observed in patients with carboplatin-resistant ovarian cancer who harbored the c.6174delT mutation [66].
Table 2.
Ongoing Clinical Trials of Targeted Agents in TNBC
| Agent | Mechanism | Phase | Setting | Other Agents in Combination | NCT Registry Number |
|---|---|---|---|---|---|
| Iniparib (BSI-201) | PARP inhibitory activity | III | Metastatic | Gemcitabine/carboplatin | 00938654 |
| II | Metastatic | Gemcitabine/carboplatin | 00540358, 01045304 | ||
| II | Neoadjuvant | Gemcitabine/carboplatin | 00813956 | ||
| Veliparib (ABT-888) | II | Metastatic | Temozolomide | NCT01009788 | |
| I | Metastatic | Cisplatin/vinorelbine | NCT01104259 | ||
| Olaparib (AZD2281) | II | Metastatic | Paclitaxel | 00707707 | |
| II | Neoadjuvant | None | 0078254 | ||
| PF-01367338 | II | Neoadjuvant | Cisplatin | 01074970 | |
|
| |||||
| Bevacizumab | VEGF monoclonal | III | Adjuvant | None | 00528567 |
| II | Metastatic | Nab-paclitaxel | 00472693 | ||
| II | Metastatic | Paclitaxel/carboplatin | 00691379 | ||
| II | Metastatic | Paclitaxel/capecitabine | 01069796 | ||
| II | Metastatic | Docetaxel/carboplatin | 00608972 | ||
|
| |||||
| Cetuximab | EGFR monoclonal | II | Neoadjuvant | Docetaxel | 00600249 |
| II | Metastatic | Cisplatin | 00463788 | ||
| II | Metastatic | Ixabepilone | 00633464 | ||
| Panitumumab | II | Metastatic | Paclitaxel/carboplatin | 01009983 | |
| II | Metastatic | Gemcitabine/carboplatin | 00894504 | ||
|
| |||||
| Erlotinib | EGFR kinase inhibitor | II | Metastatic | None | 00739063 |
| II | Neoadjuvant | Chemotherapy | 00491816 | ||
|
| |||||
| Dasatinib | Src/Abl kinase inhibitor | II | Metastatic | None | 00371254, 00817531 |
|
| |||||
| Sunitinib | Multikinase inhibitor | II | Metastatic | None | 00246571 |
| II | Neoadjuvant | Paclitaxel/carboplatin | 00887575 | ||
|
| |||||
| Everolimus | mTOR inhibitor | II | Metastatic | None | 00827567 |
| II | Neoadjuvant | Cisplatin/paclitaxel | 00930930 | ||
Abbreviations: EGFR, endothelial growth factor receptor; mTOR, mammalian target of rapamycin; NCT, National Clinical Trials; PARP, poly(ADP-ribose) polymerase; TNBC, triple negative breast cancer; VEGF, vascular endothelial growth factor.
Antiangiogenic agents are another class of inhibitors under evaluation in TNBC. Bevacizumab, a monoclonal antibody directed against vascular endothelial growth factor, prolonged PFS when added to first-line paclitaxel in women with metastatic breast cancer in the phase III ECOG 2100 trial [67]. This PFS benefit with bevacizumab was maintained in the subgroup with ER negative/PR negative disease, which likely represents TNBC since <2% of patients in each arm had HER2 positive disease (HR, 0.53; 95% CI, 0.40 to 0.70). Grade 3/4 toxicities that were more common in the bevacizumab arm included hypertension (14.8% vs 0.0%; P < 0.001), proteinuria (3.6% vs 0.0%; P < 0.001), headache (2.2% vs 0.0%; P = 0.008), and cerebrovascular ischemia (1.9% vs 0.0%; P = 0.02) [67]. Bevacizumab was evaluated in TNBC in the neoadjuvant setting in combination with cisplatin in a single-arm phase II trial [68]. Of 46 evaluable women, 7 (15%) had pCRs (Miller-Payne grade 5) and an additional 10 (22%) had responses of 4 on the Miller-Payne scale. Five patients did not complete neoadjuvant therapy due to toxicity, including 2 with tinnitus/hearing loss, 2 with grade 4 pulmonary embolism, and 1 with grade 4 hypertension.
The antiangiogenic tyrosine kinase inhibitor sunitinib was evaluated in a phase II study of patients previously treated with anthracycline/taxane chemotherapy [69]. Partial responses were achieved in 7 (11) of 64% patients overall, with the response rate being slightly higher in the TNBC subgroup (15%; 3 of 20 patients). The most common grade 3 nonhematologic adverse events included fatigue (14%) and hand-foot syndrome (9%). Grade 4 laboratory abnormalities were reported in 3 patients (2 with hyperuricemia and 1 with elevated alanine aminotransferase and alkaline phosphatase levels).
EGFR may also be a viable target in TNBC given that it is often overexpressed in basal-like breast cancers. The anti-EGFR monoclonal antibody cetuximab was evaluated alone and in combination with carboplatin in a randomized phase II trial involving 102 patients with metastatic basal-like TNBC who had received up to 3 prior chemotherapy regimens but no prior platinum or EGFR-targeting agents [70]. Although cetuximab monotherapy had little activity (partial responses in 2 [6] of 31% patients), cetuximab plus carboplatin produced partial responses in 13 (18) of 71% patients and stable disease lasting ≥6 months in 6 additional patients (9%). Response rates did not differ by line of therapy, with rates of 14%, 31%, and 17% in the first-, second-, and third-line settings, respectively. However, most patients progressed rapidly with a median PFS of 2.0 months. The cetuximab/carboplatin regimen was well tolerated, with grade 3 toxicity consisting of rash, fatigue, nausea, and vomiting, each occurring in 6% of the patients. The EGFR tyrosine kinase inhibitor erlotinib also has shown activity when combined with capecitabine/docetaxel chemotherapy [71]. It remains to be seen whether this agent will be more effective in a selected population of patients with TNBC.
A number of other targeted agents are currently being evaluated in phase II clinical trials in metastatic TNBC—the Src and Abl kinase inhibitor dasatinib, and the mammalian target of rapamycin inhibitor everolimus (Table 2).
Conclusion
TNBC comprises a heterogeneous breast cancer subgroup that overlaps substantially with basal-like tumors. However, there are distinct differences as some patents with basal-like tumors express non-TNBC markers (ie, ER, PR, HER2) and have a normal breast-like phenotype. TNBC also shows overlap with BRCA1-related tumors. A substantial proportion of BRCA1 carriers have TNBC but not all patients with TNBC harbor BRCA1 mutations. Thus, TNBC is a composite of a heterogeneous group of multiple molecular subtypes of breast cancer. In general, OS in patients with TNBC remains poor compared with that in patients who have other breast cancer phenotypes, thus highlighting the need for better treatment options at all stages of disease. Given the lack of hormone receptor and HER2 overexpression, patients with TNBC are not candidates for hormone therapy or trastuzumab-based regimens. However, numerous targeted agents are currently under development for TNBC, which may hold promise for improving treatment outcomes.
Supplementary Material
Acknowledgments
The authors thank Johnathan C. Maher, PhD, of BlueSpark Healthcare Communications for medical and editorial assistance with this manuscript. Financial support for medical and editorial assistance was provided by sanofi-aventis US. The authors were fully responsible for all content and editorial decisions. Dr. Childs is an employee of sanofi-aventis US. Drs Pegram and Pal did not receive financial support or compensation related to the development of the manuscript.
Table of Abbreviations
- ASCO
American Society of Clinical Oncology
- CI
Confidence interval
- EGFR
Epidermal growth factor receptor
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor–2
- HR
Hazard ratio
- IHC
Immunohistochemical
- OR
Odds ratio
- OS
Overall survival
- PARP
Poly(ADP-ribose) polymerase
- pCR
Pathologic complete response
- PIR
PARP inhibitor–resistant
- PR
Progesterone receptor
- TNBC
Triple negative breast cancer
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein LP, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, Liu W, Stivers D, Baggerly K, Carey M, Lluch A, Monteagudo C, He X, Weigman V, Fan C, Palazzo J, Hortobagyi GN, Nolden LK, Wang NJ, Valero V, Gray JW, Perou CM, Mills GB. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. [Accessed July 29, 2010];Genome Biol. 2007 8:R76. doi: 10.1186/gb-2007-8-5-r76. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1929138/?tool=pubmed. [DOI] [PMC free article] [PubMed]
- 7.Onitílo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and HER2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 9.Rakha EA, El-Sayed ME, Green AR, Lee AHS, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda H, Takarabe T, Hasegawa F, Fukutomi T, Hirohashi S. Large, central acellular zones indicating myoepithelial tumor differentiation in high-grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol. 2000;24:197–202. doi: 10.1097/00000478-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 12.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 13.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute‘s Surveillance, Epidemiology, and End Results Database. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 14.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 16.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 17.Hugh J, Hanson J, Cheang MCU, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, Magherini E, Mackey J, Martin M, Vogel C. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 19.Harris LN, Broadwater G, Lin NU, Miron A, Schnitt SJ, Cowan D, Lara J, Bleiweiss I, Berry D, Ellis M, Hayes DF, Winer EP, Dressler L. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342. [Accessed July, 29, 2010];Breast Cancer Res. 2006 8:R66. doi: 10.1186/bcr1622. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1797029/ [DOI] [PMC free article] [PubMed]
- 20.Foulkes WD, Brunet J-B, Stefansson IM, Straume O, Chappuis PO, Begin LR, Hamel N, Goffin JR, Wong N, Trudel M, Kapusta L, Porter P, Akslen LA. The prognostic implication of the basal-like (cyclin Ehigh/p27low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 2004;64:830–835. doi: 10.1158/0008-5472.can-03-2970. [DOI] [PubMed] [Google Scholar]
- 21.Foulkes WD, Stefansson IM, Chappuis PO, Bégin LR, Goffin JR, Wong N, Trudel M, Akslen LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 22.Tischkowitz M, Brunet J-S, Bégin LR, Huntsman DG, Cheang MC, Akslen LA, Nielsen TO, Foulkes WD. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. [Accessed July 29, 2010];BMC Cancer. 2007 7:134. doi: 10.1186/1471-2407-7-134. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1948892/pdf/1471-2407-7-134.pdf. [DOI] [PMC free article] [PubMed]
- 23.Jacquemier J, Padovani L, Rabayrol L, Lakhani SR, Penault-Llorca F, Denoux Y, Fiche M, Figueiro P, Maisongrosse V, Ledoussal V, Martinez Penuela J, Udvarhely N, El Makdissi G, Ginestier C, Geneix J, Charafe-Jauffret E, Xerri L, Eisinger F, Birnbaum D, Sobol H. Typical medullary breast carcinomas have a basal/myoepithelial phenotype. J Pathol. 2005;207:260–268. doi: 10.1002/path.1845. [DOI] [PubMed] [Google Scholar]
- 24.Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 25.Reis-Filho JS, Milanezi F, Steele D, Savage K, Simpson PT, Nesland JM, Pereira EM, Lakhani SR, Schmitt FC. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- 26.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 28.Pal SK, Mortimer J. Triple-negative breast cancer: novel therapies and new directions. Maturitas. 2009;63:269–274. doi: 10.1016/j.maturitas.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Reis-Filho JS, Tutt ANJ. Triple negative tumors: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 30.Calza S, Hall P, Auer G, Bjohle J, Klaar S, Kronenwett U, Liu ET, Miller L, Ploner A, Smeds J, Bergh J, Pawitan Y. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. [Accessed July 29, 2010];Breast Cancer Res. 2006 8:R34. doi: 10.1186/bcr1517. Available at http://www.ncbi.nlm.nih.gov/pubmed?term=%22Breast+Cancer+Res%22%5BJour%5D+AND+2006%5Bpdat%5D+AND+Calza%5Bauthor%5D&cmd=detailssearch. [DOI] [PMC free article] [PubMed]
- 31.Jumppanen M, Gruvberger-Saal S, Kauraniemi P, Tanner M, Bendahl PO, Lundin M, Krogh M, Kataja P, Borg A, Ferno M, Isola J. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. [Accessed July 29, 2010];Breast Cancer Res. 2007 9:R16. doi: 10.1186/bcr1649. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1851391/ [DOI] [PMC free article] [PubMed]
- 32.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5684. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 33.Sotiriou C, Neo S-Y, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nofech-Mozes S, Trudeau M, Kahn HK, Dent R, Rawlinson E, Sun P, Narod SA, Hanna WM. Patterns of recurrence in the basal and non-basal subtypes of triple-negative breast cancers. Breast Cancer Res Treat. 2009;118:131–137. doi: 10.1007/s10549-008-0295-8. [DOI] [PubMed] [Google Scholar]
- 35.Robson M. Are BRCA1- and BRCA2-associated breast cancers different? Prognosis of BRCA1-associated breast cancer. J Clin Oncol. 2000;18:113s–118s. [PubMed] [Google Scholar]
- 36.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness‘ in sporadic cancers. Nature Rev. 2004;4:1–6. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi Y, Murase K, Oh K. Basal-like subtype and BRCA1 dysfunction in breast cancers. Int J Clin Oncol. 2008;13:395–400. doi: 10.1007/s10147-008-0831-x. [DOI] [PubMed] [Google Scholar]
- 38.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 39.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, Hortobagyi GN, Arun BK. Clinical and pathological characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JF, Macmillan D, Blamey RW, Ellis IO. High-throughput protein expression analysis using tissue microarray technology of a well-characterized series identifies biologically distinct classes of breast cancer confirming recent DNA expression analyses. Int J Cancer. 2005;116:340–350. doi: 10.1002/ijc.21004. [DOI] [PubMed] [Google Scholar]
- 41.Beger C, Pierce LN, Krüger M, Marcusson EG, Robbins JM, Welcsh P, Welch PJ, Welte K, King MC, Barber JR, Wong-Staal F. Identification of Id4 as a regulator of BRCA1 expression by using a ribozyme-library-based inverse genomics approach. Proc Natl Acad Sci USA. 2001;98:130–135. doi: 10.1073/pnas.98.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, Tutt AN. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 43.Millikan RC, Newman B, Tse C-K, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, Hewitt SM, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Cartun R, Mandich D, Rymkiewicz G, Ligaj M, Lukaszek S, Kordek R, Garcia-Closas M. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 45.Collett K, Stefansson IM, Eide J, Braaten A, Wang H, Eide GE, Thoresen SO, Foulkes WD, Akslen LA. A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol Biomarkers Prev. 2005;14:1108–1112. doi: 10.1158/1055-9965.EPI-04-0394. [DOI] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™. [Accessed June 8, 2010];Breast cancer. 2010 1.2010 Available at http://www.nccn.org.
- 47.Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8018. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 48.Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Dent R, Lubinski J, Narod S. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 50.Sirohi B, Arnedos M, Popat S, Ashley S, Nerurkar A, Walsh G, Johnston S, Smith IE. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol. 2008;19:1847–1852. doi: 10.1093/annonc/mdn395. [DOI] [PubMed] [Google Scholar]
- 51.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 52.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 53.Patanaphan I, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109–1112. [PubMed] [Google Scholar]
- 54.Fulford LG, Reis-Filho JS, Ryder K, Jones C, Gillett CE, Hanby A, Easton D, Lakhani SR. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. [Accessed July 29, 2010];Breast Cancer Res. 2007 9:R4. doi: 10.1186/bcr1636. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1851397/ [DOI] [PMC free article] [PubMed]
- 55.Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Budd GT, Tubbs RR, Casey G, Weil RJ. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 56.Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, Christgen M, von Wasielewski R, Kreipe HH. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20:864–870. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- 57.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, Fralick M, Kumar R, Clemons M. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 58.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O’Connor MJ, Tutt AN, Zdzienicka MZ, Smith GC, Ashworth A. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 59.O‘Shaughnessy J, Osborne C, Pippen J. Final results of a randomized phase II study demonstrating efficacy and safety of BSI-201, a poly (ADP-ribose) polymerase (PARP) inhibitor, in combination with gemcitabine/carboplatin (G/C) in metastatic triple negative breast cancer (TNBC) (abstract no. 3122). Presented at the 32nd Annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 9–13, 2009; 2009. [Accessed July 28, 2010]. Available at http://www.abstracts2view.com/sabcs09/view.php?nu=SABCS09L_998. [Google Scholar]
- 60.Ossovskaya V, Li L, Broude EV, Lim C, Roninson IB, Bradley C, Sherman B. BSI-201 enhances the activity of multiple classes of cytotoxic agents and irradiation in triple negative breast cancer (abstract no. 5552). Presented at the 2009 American Association for Cancer Research 100th Annual Meeting; Denver, Co. April18–22, 2009; 2009. [Accessed July 28, 2010]. Available at http://www.abstractsonline.com/viewer/viewAbstract.asp?CKey={A98A01B0-1623-4F71-99C7-FCE19F299C1F}&MKey={D007B270-E8F6-492D-803B-7582CE7A0988}&AKey={728BCE9C-121B-46B9-A8EE-DC51FDFC6C15}&SKey={CCA05FCE-642E-4E26-AD12-29C831335BE1} [Google Scholar]
- 61.Ossovskaya V, Alvares C, Kaldjian E, Sherman B. The PARP1 gene is over-expressed in triple negative breast cancer (abstract no. P57) [Accessed July 29, 2010];EJC Supplements. 2007 5(8):31. Available at http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B75GT-4RNTY81-2S&_user=10&_coverDate=11%2F30%2F2007&_alid=1018721483&_rdoc=5&_fmt=high&_orig=search&_cdi=13103&_sort=r&_docanchor=&view=c&_ct=5&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=a0fa3dfd4f586b560109e6ae1bc0f7ff.
- 62.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 63.Gelmon KA, Hirte HW, Robidoux A, Tonkin KS, Tischkowitz M, Swenerton K, Huntsman D, Carmichael J, Macpherson E, Oza AM. Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer (abstract no. 3002). Presented at the Annual Meeting of the American Society of Clinical Oncology; June 4–8, 2010; Chicago, Illinois. [Google Scholar]
- 64.Dent R, Lindeman GJ, Clemons M, Wildiers H, Chan A, McCarthy NJ, Singer CF, Lowe ES, Kemsley K, Carmichael J. Safety and efficacy of the oral PARP inhibitor olaparib (AZD2281) in combination with paclitaxel for the first- or second-line treatment of patients with metastatic triple-negative breast cancer: results from the safety cohort of a phase I/II multicenter trial (abstract no. 1018). Presented at the Annual Meeting of the American Society of Clinical Oncology; June 4–8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 65.Isakoff SJ, Overmoyer B, Tung NM, Gelman RS, Giranda VL, Bernhard KM, Habin KR, Ellisen LW, Winer EP, Goss PE. A phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancer (abstract no. 1019). Presented at the Annual Meeting of the American Society of Clinical Oncology; June 4–8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 66.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1116. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 67.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 68.Ryan PD, Tung NM, Isakoff SJ, Golshan M, Richardson A, Corben AD, Smith BL, Gelman R, Winer EP, Garber JE. Neoadjuvant cisplatin and bevacizumab in triple negative breast cancer (TNBC): safety and efficacy (abstract no. 551) [Accessed July 29, 2010];J Clin Oncol. 2009 27(15) Available at http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=34135.
- 69.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 70.Carey LA, Rugo HS, Marcom PK, Irvin W, Jr, Ferraro M, Burrows E, He X, Perou CM, Winer EP. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer (abstract no. 1009) J Clin Oncol. 2008;26(15):43s. [Google Scholar]
- 71.Twelves C, Trigo JM, Jones R, De Rosa F, Rakhit A, Fettner S, Wright T, Baselga J. Erlotinib in combination with capecitabine and docetaxel in patients with metastatic breast cancer: a dose-escalation study. Eur J Cancer. 2008;44:419–426. doi: 10.1016/j.ejca.2007.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.