Introduction
The structural basis of the architecture for the cell membrane is a lipid bilayer of about 4 nm thick, made up of two monolayers of lipids1,2. According to the classical Singer-Nicholson model, membrane-embedded proteins perform their functions while floating unencumbered in a sea of lipids3. According to the model the lipids play a passive role as a solvent for membrane proteins and no special consideration is given to the particular environment in which membrane proteins function. However, it has been recognized that many membrane functions (e.g. fusion, signaling, and permeability) are strictly dependent on the particular nano-environment in which these processes take place4,5. Development of emerging techniques to study membrane phenomena at the nanoscale has been instrumental in furthering our understanding of these membrane functions6,7,8. The current view is that membranes are patchy with nanoscale segregated regions of structure and function (nanodomains) and that lipid regions vary in thickness and composition9,10. Monolayers, multilayers and liposomes have frequently been used as simple model membranes in attempts to gain insight into more complex natural structures and nano-domain formation9,11. In order to probe the domain structure and motional dynamics of biological membranes and their model systems, photosensitive moieties have been incorporated into lipid structures12,13,14,15. Photo-polymerizable diacetylenic lipids have been extensively studied in lipid model membranes in the context of membrane structure and domain formation16,17,18,19. Since these photo-polymerizable lipids combine the plasticity of lipids with the robustness of polymers, they have received much attention in the biotechnology arena20,21. The lipid-based scaffolds, once polymerized, form extremely stable structures which may be used in surface coating for biocompatible materials, supporting matrices for bio-sensing molecules, and carrier vehicles for drugs21. The aim of this review is to summarize the biomedical applications of polymerizable lipids (primarily phospholipids) in the context of various nano-platforms that are currently available and being developed. The first part of this review will deal with the stable nano-platforms, which have been used in a variety of theranostics applications. In the second part we will describe a way to trigger nano-platforms that contain photo-polymerizable lipids in a stable lipid matrix for on demand drug delivery applications.
Principles of Polymerization
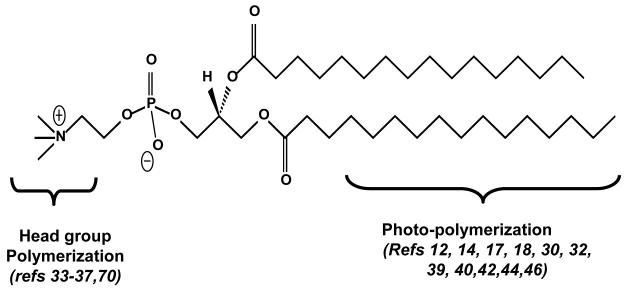
The concept of using phospholipid polymers as tools in the medical field originated in early 1980s22. Biomedical applications of the lipid polymers include biosensors23,24, micropatterned membrane biomemetics25, rechargeable batteries26, imaging agents27 and drug delivery carriers28,29,30,31. The basic design of a photopolymerizable lipid relies on two important parameters, (a) self-assembly properties of the lipids (or related molecules), and (b) strategic chemical synthesis schemes for the introduction of photoactivable bonds in these molecules. Phospholipids such as phosphatidylcholine (PC, Figure 1) can be considered as a prototype molecule to direct the design of polymerizable lipid molecules for multi-faceted applications. The PC molecule can be divided into three major parts, head group, glycerol backbone and fatty acyl chains; each of these regions has been modified either by the introduction of additional groups or modification of existing chemical bonds such as polymerizable moieties to produce light sensitive nanoassemblies of lipids.
Figure 1. Sites for Chemical modifications in phospholipids (photoreactive lipids).
Two major parts of phospholipids that can be chemically modified to generate photosensitive molecules. The lipid parts: head group, and fatty acyl chains the are described with their proposed modifications. The references correspond to the currently available designer lipids respectively. The modifications in glycerol backbone are typically introduced to modulate responses to enzymes such as phospholipases.
In this communication, we will only focus our discussion on the light-activable lipid molecules (including phospholipids and non-phospholipids) that utilize the principle of photopolymerization (photo-crosslinking); and will later summarize their biological applications. A general overview of the drug delivery applications of light-sensitive lipid-based nanoparticles has recently been published20.
The photoreactive chemical bonds in a photopolymerizable molecule are primed to undergo photo-crosslinking (polymerization) upon activation with a light source; the modifications are expected to introduce minimum perturbations in overall self-assembly features of the nano-system being investigated (such as monolayers, bilayers and/or lipid vesicles). Typically, light-triggered photo-crosslinking reactions result in irreversible polymerization due to inter or intra-molecular chemical reactions between the photoactive groups; however, a few examples exist where these reactions have been shown as reversible phenomena. Various polymeric lipids that have been designed to date utilizing distinct polymerization principles are described below:
3a. Reversible Polymerization
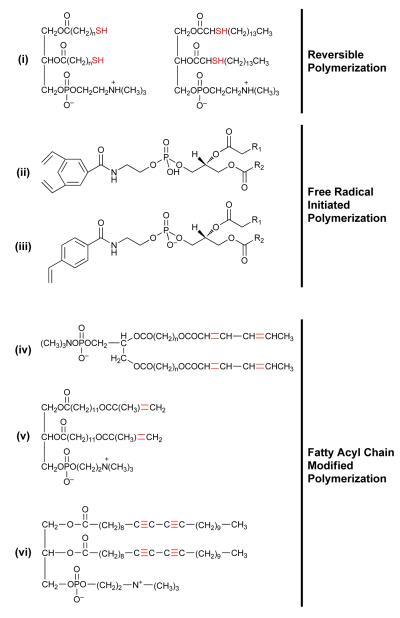
During the early 1980’s, Singh, Regen and colleagues described the synthesis and characterization of a thiol-bearing phospholipid, with an aim to generate vesicles that can undergo reverse polymerization32. The structure of a class of one such lipid (1,2-bis(11-mercaptoundecanoyl)-sn-glycero-3-phosphocholine) is shown in Figure 2(i). The principle of the reversible polymerization of this lipid entails “switched on/switched off” mechanism by oxidation/reduction respectively. Polymerization (via the S-S bond formation) could either be achieved by direct UV (254 nm) treatment or oxidation in the presence of hydrogen peroxide. Although an interesting platform, biological applications of this approach have not been documented yet. Moreover, the light source and the effective concentration of the oxidizing-reducing agents that will be compatible with biological systems may pose limitations for this approach.
Figure 2. Polymeric Lipids.
The chemical structures of various photoactivable phsopholipids are shown. i, lipids beating SH groups for reversible polymerization, ii&iii, Head-group polymerizable lipids to generate stable nanocapsules (ii, DVBA and ii, styryl modifications), iv-vi, fatty acyl modified lipids (iv, bis-Sorb PC, v, di-polymerizable lipid(DPL) and vi, diacetylenic lipid (DC8,9PC).
3b. Free Radical-initiated Photopolymerization
The examples of free radiation-initiated polymerization reaction include the head-group polymerizable phospholipids. Figure 2(ii, iii) shows the chemical structures of two head-group modified phospholipids containing divinylbenzoyl33 (Fig. 2ii) and styryl34 (Fig. 2iii) functionalities. These molecules were synthesized using either saturated or unsaturated fatty acyl chains in the hydrophobic part, with an aim to stabilize liposome bilayer membranes to improve their drug delivery potential in vivo. The light-induced photo-crosslinking in these liposomes is typically achieved under relatively mild conditions in the presence of a water soluble free radical initiator. The choice of monomer functionalities and the flexibility to place these monomers in the liposome membrane prior to cross-linking offers attractive possibilities with potential applications for plasma stable vesicles for theranostics (drug delivery and/or imaging) applications. It is critical that the photoreactive monomers should maintain the integrity of the vesicles and their contents (such as pharmaceutical agents) during the photopolymerization step. Introduction of a photoreactive moiety in the head group of the phospholipid (see Figure 2) appears to be an appropriate choice since these modifications result in polymerization while sustaining the original lipid assemblies. Light treatment of the liposomes (prepared from these phospholipids) has been demonstrated to photo-crosslink without compromising the activity of entrapped enzymes35. Although biophysical studies demonstrate that these head-group polymerizable lipids are potential candidates for generating stable liposomes33,34, further studies are needed to evaluate the merit of these lipids for sustained drug delivery and as theranostic tools70 . In the phospholipid realm, there are only a few examples of head-group photopolymerizable molecules (Figure 2). During the last decade, Jung, German and colleagues have also explored similar design of molecules (non-phospholipids) for in situ polymerization in the vesicle bilayers; the biological applications of these molecules however, have not been explored yet36,37,38.
3c. Fatty acyl chain-modified photopolymerizable lipids and phototriggering
As discussed above, fatty acyl chains (tail region) of the lipids play an important role in self-assembly of the lipidic nanoparticles; modifications in the tail regions are projected to influence stability, structure and physical properties of the polymerizable nanoassemblies. About three decades ago, a number of studies were reported to this end 29,30,31,39. In contrast to relatively few reports concerning chemical modifications in the head group region of the lipid molecules33 (see above), introduction of various photoactivable groups in tail regions of the lipids have extensively been studied. These functionalities include the diacetylenes, methacryloyl, and sulfhydryls modifications19,40,41,42. The objective to introduce light-sensitive fatty acyl modification in the phospholipid structures was multi-fold including generation of stable vesicles, on-demand drug delivery and also as tools to understand the membrane structure and function. In general the light-induced changes in these molecules typically involve direct chemical or photon-catalyzed reactions that lead to polymerization reaction in an organized pattern. Figure 2(iv-vi) shows a partial list of structures of various tail-region modified photopolymerizable lipids (iv, bis-Sorb PC42,43, v, methacryloyl PC (a dipolymerizable lipid)30,44, and vi, DC8,9PC 45).
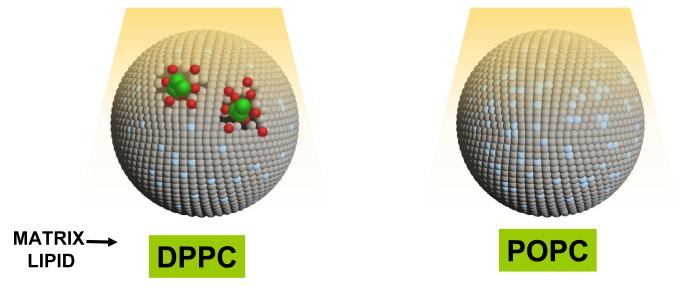
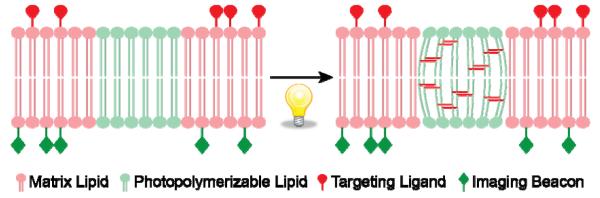
For photoreactive lipids to undergo photo-crosslinking within the liposome bilayer (photopolymerization), appropriate molecular packing of these molecules is an essential component. Apparently, segregation of polymerizable lipids within the lipid bilayer will favor inter-molecular cross-linking of the lipids. This phenomenon is shown in a cartoon form in Figure 3 (adapted from12,20). The next section describes the properties and theranostics potential of two most-studied photopolymerizable phospholipids containing either bis-Sorbyl and diacetylenic groups.
Figure 3. A cartoon depicting effect of bulk (matrix) lipids on self-assembly of a polymerizable lipid, DC8,9PC in the lipid bilayers.
Grey, matrix lipid (Left panel DPPC (Tm, 41°C), Right panel POPC(Tm, −2°C)). Blue, light-activated DC8,9PC (Tm, 44°C). DC8,9PC clustering in DPPC results in light-induced activation of molecules (shown in blue) that leads to DC8,9PC polymerization. This results in release of drugs (green) or imaging molecules (red). Right panel, DC8,9PC is not clustered in POPC molecules; light treatment results in activation of DC8,9PC but no polymerization and hence no release of contents.
Bis-SorbPC
Initial studies conducted by O’Brien and colleagues examined liposomes containing bis-sorbyl phosphatidylcholine (bis-SorbPC, Figure 2iv) as photo-triggerable drug delivery platforms43,46,47. Polymerization of bis-Sorb PC occurs via an oxygen radical reaction and is initiated by a photosensitizing lipophilic dye (such as 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)) by the visible light (550 nm). Oxygen radicals generated by the photo-activation of DiI trigger polymerization of bis-sorbPC leading to required defects in the lipid bilayer (see Figure 3).
DC8,9PC
The diene-containing lipid molecules have attracted considerable attention in the development of biosensors, and as imaging/diagnostics tools. The chemistry of diacetylenes provides opportunities for unique molecular assemblies and light-triggered alterations. A recent review by Cashion and colleagues21 describes in details the fundamental chemical reactions that give rise to defined polymeric lipids and their applications in Biomimetics design. Here we will limit our focus on studies that use diene polymers for their biological applications. The photopolymerizable phospholipid, (1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine (DC8,9PC, Figure 2vi) can be considered as the best-studied example in this class of polymerizable molecules. Our laboratory is investigating DC8,9PC for on-demand drug delivery application (see below). The biophysical traits necessary for UV-triggered polymerization as well as chemical modifications in monomeric DC8,9PC and/or resulting polymers have been reported earlier45,48,49. DC8,9PC is only found in lower organisms50, exhibits unique bilayer packing properties and undergoes UV (254 nm)-induced photopolymerization in a synchronized fashion16,45,51. UV-treatment has profound effects on the plasticity and chromogenic properties of the resulting DC8,9PC polymers. These unique features of DC8,9PC have attracted investigators to explore this lipid for various biological, biomedical and diagnostic applications. We believe that this molecule may prove to be a viable candidate for future theranostic applications. Currently available reports are discussed below (section 4).
4. Biological Applications of Polymeric Lipids
Although several polymeric lipids are currently available, among these DC8,9PC has been examined for multi-faceted applications in the realm of biology including biometics, as sensors for pathogen detection52,53, drug delivery platforms12,30,46,54,55, nano-imaging agents27 and as components for DNA delivery29,56 and vaccine applications57. In this article, we have restricted our discussion to experimental systems where at least cell culture data are available in the literature (see below).
4a. Diagnostic Tools for Pathogen Detection
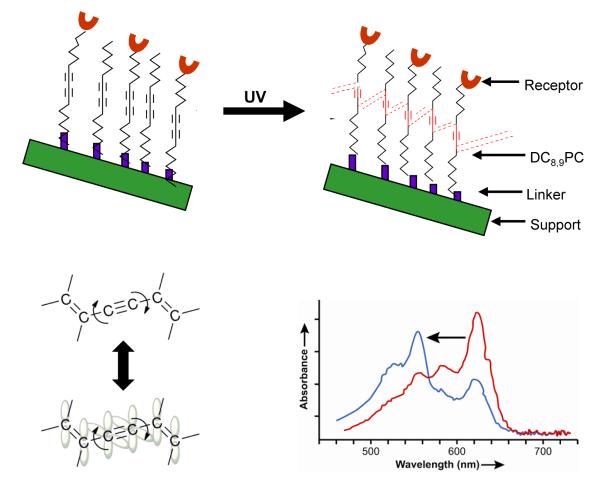
Biosensing devices are considered useful systems for the detection of pathogens such as bacteria and viruses58. The principle relies on changes in optical or electrical properties of these sensors upon pathogen-specific interactions on ligand-coated surfaces. Since light-induced chemical modifications in polymerizable lipid molecules typically result in measurable changes in their chromogenicity and overall lipid organization, these molecules have been tested as response-sensitive components of the detection units. Nagy, Bednarski and colleagues were the first to develop a direct colorimetric detection method based on changes in chromogenic properties of DC8,9PC59,60,61 upon interactions with pathogens. Since influenza virus invades cells via binding to sialic acid residues present on the cellular proteins and/or lipids62,63,64, sialic acid containing lipids were used in this biosensor design65,66. DC8,9PC along with other non-polymerizable lipids and a sialic acid containing lipid was coated on glass slides and then polymerized by exposure to UV (254 nm) yielding a colorimetric reaction. Upon interaction with influenza virus, the polymer linkages presumably undergo a conformational modification resulting in a shift in the chromogenic properties suitable for optical detection. The basic design of this optical sensor and the phenomenon of chromogenic alterations are shown in Figure 4.
Figure 4. DC8,9PC-Supported Monolayers for Pathogen Detection.
This cartoon shows principle and assembly of cross-linked DC8,9PC on planar surfaces for pathogen detection (top panel). Interaction with pathogens results in changes in chromogenic properties of photo-crosslinked DC8,9PC measured by colorimetric methods (bottom, right). Bottom (left), A schematic presentation of π-conjugated diacetylenes in planar configuration and reorganization of inter and intra-molecular rearrangements of bonds in the polymers. This figure is adapted (in part) from reference 59.
Similarly, DC8,9PC was also used as component on the optical biosensor to detect the Escherichia coli enterotoxin and botulinum neurotoxin61 based on selective and sensitive binding to sialic acids. Another application was recently developed by the same group to detect shiga like-toxin producing E.Coli using the glycodiacetylenic lipids53. These colorimetric detection methods may prove to be useful and find practical applications due to the rapid, selective and sensitive design of these units. However, to our knowledge, these biosensors are not currently available in the market.
4b. Nanoimaging Tools
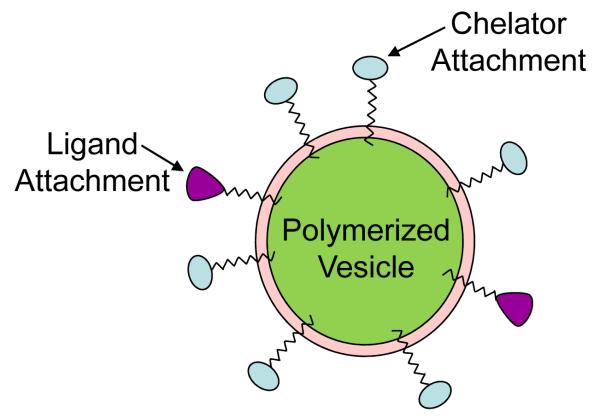
Molecular imaging is a power tool towards diagnosis of cancer and related diseases. Image-contrast agents are widely used for the detection of disease-specific biomarkers (caused by upregulation of specific genes etc) and efforts have led to the development and availability of useful probes (PET/SPECT). However, similar to the targeted delivery issues for drugs/pharmaceuticals, it is critical that imaging probes reach desired areas in the body within defined space and time. In this context, the enhanced bioavailability of nanocapsules carrying the image-contrast agents can be achieved by stabilizing these nanocarriers. Choice of polymeric lipids as the components (to provide mechanical stability to nanocapsules) can be considered a promising strategy, as light-triggered stabilization is expected to cause no or minimum perturbations in the structure and physical properties of the nanocarriers. Principles of such constructs rely on the inclusion of a polymer (such a DC8,9PC) in the nano-assembly containing an imaging agent (such as Gd3+) such that light-triggered photo-crosslinking of the polymer promotes stability to assembled complex. Li and colleagues were the first to develop functionalized polymerized vesicles for vascular-targeted molecular imaging27. The basic assembly of the functionalized polymerized vesicles is shown in Figure 5 (adapted from Li et al., 2002). These vesicles serve as a good example of theranostics as these integrate both an imaging (Gd3+) and a ligand for vascular targeting.
Figure 5. Functionalized Polymerized Vesicles for Vascular Targeted Molecular Imaging Nano-imaging tools.
Core of the vesicle contains a polymerizable lipid, and functionalized lipids for chelator attachment for imaging (blue) as well as for ligand attachment for targeting (purple). This cartoon is adapted from reference 27.
Interestingly, animal studies using these polymeric vesicles revealed promising results based on vascular targeting of receptors such as integrins and ICAM. Nevertheless, clinical translation of this promising theranostic platform for humans remains to be seen. Recently, Kumar and colleagues64 have presented an alternate strategy to generate polymerized liposomes bearing adequate functional groups for ligand attachment; this chemistry involves a click reaction (copper-catalyzed azide-alkyne cycloaddition reaction). Nevertheless, biological testing of this system is subject to future developments.
4c. In vivo Studies
Once the potential of polymeric vesicles as tools in biology was realized, it became important to examine the bioavailability and toxicity of the polymerized lipids. In mid-1980’s Regen, Juliano and colleagues studied the interactions of polymerized liposomes with cultured cells as well their in vivo behavior30,31. Later, Li and colleagues also examined polymeric vesicles as nano-imaging tools and reported biodistribution of Gd3+-loaded polymerized vesicles in animals27 (described above). Regen as well as Juliano’s group used the synthesized phospholipids (dilipoyl lipids and dipolymerizable lipids, Figure 2v) to prepare polymerized vesicles; it may be noted that these formulations did not include pegylated lipids that confer stealth properties. The biodistribution studies in animals revealed that although polymerized vesicles (with similar size distribution) were cleared from the circulation more rapidly, these exhibited more bio-stability67. The bio-distribution analysis in these experiments was based on a radioactive lipid marker C14-cholesteryl oleate. Therefore, the structural integrity of these vesicles in vivo may be questionable; a revisit of these experiments with inclusion of pegylated lipids and entrapped molecules (such as drugs/pharmaceuticals) in polymerized vesicles will shed light on future biological applications.
4d. Drug Delivery Applications
4di. Stable nanocapsules
Polymerization of the preformed vesicles loaded with the drugs and/or imaging agents’ is an attractive technological tool to develop theranostic technologies for future medical applications. Improved stability of the nanocapsules is the primary advantage of this approach. Polymeric lipids bearing either the photo-reactive head group33 or the diacetylenic groups in the fatty acyl tail regions68 have been examined for their potential toxic effects in cell culture systems. In both systems, light treatments had minimal effect of the physico-chemical properties of the vesicles33,34,35,68. We have reported that delivery of Piroxicam, an anti-inflammatory agent, to cultured cells by head-group polymerized lipid vesicles was superior to delivery of Piroxicam by non-polymerized vesicles or free drug alone70; future in vivo studies are needed to assess the practical applications of this system. Recently, Temprana et al. studied the effect of light treatment on membrane interfacial properties of diacetylenic vesicles68,69. These authors showed that polymerization has substantial effect on the stability of the vesicles as the vesicles were reported to be stable up to 30 days at 4 °C. Over all membrane properties were changed as assessed by differential interaction with proteins. Although cell culture experiments indicated that polymerized vesicles did not exhibit cytotoxicity, a detailed examination of the physical and biochemical properties of polymerized vesicles are warranted for their future theranostic applications.
4dii: Localized Drug delivery
Recently, we have examined in situ light-triggered drug release properties of DC8,9PC liposomes12,54. According to our hypothesis, DC8,9PC forms aggregates (self-assembles) in the bilayer of phospholipids containing saturated acyl chains, and this packing is prone to create phase boundary defects in lipid model membranes (see Figure 3). In support of this hypothesis, we demonstrated that UV (254 nm)-triggered calcein release occurs from liposomes containing a mixture of saturated phospholipids and DC8,9PC. Here, the UV-triggered mechanism of calcein release was due to the DC8,9PC photopolymerization.
We are currently developing DC8,9PC formulations for their on-demand drug delivery applications. We have demonstrated that visible light (514 nm) treatment of liposomes loaded with a light-sensitive aqueous compounds also results in release of contents54. Interestingly, in contrast to UV-triggered photopolymerization, visible-light triggered solute release does not occur in concert with the photopolymerization reaction. Visible light triggered release of contents appears to involve reactive oxygen species. The proposed mechanism is based on our observations that the release occurs in a wave-length specific manner and scavengers of oxygen radicals block this release and the (unpublished data). The exact nature of modifications in the triple bonds by the reactive oxygen radicals are unknown at present and are subject to future investigations. The DC8,9PC formulations appear promising candidates for future drug delivery because visible light-triggered release of doxorubicin (an anticancer drug) from these liposomes improved cytotoxicity in our cell culture experiments44. We are hopeful that our formulations can be considered as the next-generation of light-sensitive liposomes for on-demand drug delivery applications.
Although a number of light-triggerable formulations have been examined to date, none of the formulations developed so far have been successful for in vivo applications. Lack of success of light-triggered drug delivery is primarily due to two main limitations, First, lack of adequate photon energy produced by the radiation source(s), and second, limitations of the available light sources suitable for deep tissue penetration. These topics were covered in details in our recent review article20. We believe that the theranostic approach that will combine development of innovative strategies based on suitable photoreactive lipids combined with an appropriate imaging agent (such as metal ions) as “the helper” components will enable in vivo success in this area. One should keep in mind that the light source(s) used should have minimal effects on the biology of normal cells and tissues. The knowledge about the visible and/or infrared light sources currently in use for PDT in patients will certainly be valuable to further develop polymeric vesicles as theranostic tools.
BIOGRAPHICAL INFORMATION
Anu Puri received her Ph.D. degree in chemistry from the Central Drug Research Institute, Lucknow, India studying the chemical synthesis of modified phospholipids and possible use of their liposomes in drug delivery. She came to the United States in 1985 as post-doctoral fellow at the Hormel Institute, University of Minnesota. In 1986, she joined Dr Blumenthal as visiting fellow in the Laboratory of Mathematical Biology, NCI, NIH. Currently she holds the Research Biologist position at the CCR Nanobiology Program, NCI-Frederick, NIH. Her research revolves around several themes including (a) Cell Biology of Viral Entry, (b) Development of Lipid-Based Nanoparticles for Targeted Delivery of Cancer Therapeutics (c) Development of nano-scale diagnostic tools for detection of pathogens and cancer biomarkers, and (d) Mechanisms of opportunistic infections in AIDS and related diseases.
Robert Blumenthal obtained his M.Sc. at the University of Leiden, The Netherlands, and his Ph.D. in physical chemistry at the Weizmann Institute, Israel studying mechanisms of active transport across membranes. Following postdoctoral work at the Institute Pasteur and at Columbia University studying molecular mechanisms of membrane excitability in neurons, he came to the NIH and was ultimately recruited by the NCI. In 1978 he was tenured and in 1980 he became chief of the Section on Membrane Structure and Function. In 2005 he was appointed director of the newly established Center for Cancer Research Nanobiology Program. Dr. Blumenthal has worked in a wide range of areas in membrane biophysics, which includes membrane fusion, membrane transport, membrane domains, membrane channels, cell surface receptors, immune cytotoxic mechanisms, and use of liposomes for delivery of drugs and genes into cells. Dr. Blumenthal’s current interest is in viral entry, pathogenesis and vaccines; multifunctional nanoparticles for triggered and targeted delivery of therapeutics; and photo-induced chemical reactions in membranes.
CONSPECTUS.
Polymeric lipids are of considerable interest in the emerging field of theranostics since they combine the flexibility of nanoassemblies and structural modifications with the stability of polymers. A variety of polymerizable lipids have been used for biological applications from membrane models to imaging platforms, drug delivery systems, vaccines carriers, biosensors or as coating materials. Lipid polymerization leads to a covalent bond between lipid moieties thus improving the non-covalent interactions that keeps lipid lamellar phase architecture maintained. This property has an important impact on the stability of the polymerized system. Moreover, triggerable theranostics can be designed by combining appropriate non-polymerizable lipids with polymerizable lipids.
Polymeric lipids bear promise as nano-tools in the field of medical imaging, targeting, and on-demand drug delivery. Although the field of polymeric nanocapsules (including liposomes) is currently at its developmental stage, intensive efforts are being devoted to further clinical applications to diagnosis and treatment. In this respect polymeric lipids bear an advantage over non-polymerizable molecules as these have the propensity to provide stability to nano-assemblies. In addition, being lipidic in nature, long-term toxicity issues can be predicted to be minimal. It can be envisioned that nano-imaging platforms coupled with localized drug delivery technology will have significant impact on cancer therapy and other related diseases. The wealth of clinical knowledge available on the photochemistry of imaging agents and/or drugs as well as light-induced modifications in the context of patient’s treatments will prove to be an asset to develop polymeric theranostic lipid-based nanoparticles.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- (1).Gabrielli G. Monolayers and Planar Or Curved Bilayers. Advances in Colloid and Interface Science. 1991;34:31–72. doi: 10.1016/0001-8686(91)80046-m. [DOI] [PubMed] [Google Scholar]
- (2).Bonosi F, Gabrielli G. Dodac in Bidimensional States - Monolayers, Langmuir-Blodgett-Films and Vesicles. Colloids and Surfaces. 1991;52:277–285. [Google Scholar]
- (3).Singer SJ, Nicolson GL. The Fluid Mosaic Model of the Structure of Cell Membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- (4).Simons K, Ikonen E. Functional Rafts in Cell Membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- (5).Simons K, Vaz WLC. Model Systems, Lipid Rafts, and Cell Membranes. Annual Review of Biophysics and Biomolecular Structure. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- (6).Stockl MT, Herrmann A. Detection of Lipid Domains in Model and Cell Membranes by Fluorescence Lifetime Imaging Microscopy. Biochim. Biophys. Acta. 2010;1798:1444–1456. doi: 10.1016/j.bbamem.2009.12.015. [DOI] [PubMed] [Google Scholar]
- (7).Garcia-Saez AJ, Schwille P. Stability of Lipid Domains. FEBS Lett. 2010;584:1653–1658. doi: 10.1016/j.febslet.2009.12.036. [DOI] [PubMed] [Google Scholar]
- (8).Elson EL, Fried E, Dolbow JE, Genin GM. Phase Separation in Biological Membranes: Integration of Theory and Experiment. Annu. Rev. Biophys. 2010;39:207–226. doi: 10.1146/annurev.biophys.093008.131238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lasic D. Liposomes. American Scientist. 1992;80:20–31. [Google Scholar]
- (10).Dobereiner HG, Dubin-Thaler B, Giannone G, Xenias HS, Sheetz MP. Dynamic Phase Transitions in Cell Spreading. Phys. Rev. Lett. 2004;93:108105. doi: 10.1103/PhysRevLett.93.108105. [DOI] [PubMed] [Google Scholar]
- (11).Lasic D. Liposomes - An Industrial View. Chemistry & Industry. 1996:210–214. [Google Scholar]
- (12).Yavlovich A, Singh A, Tarasov S, Capala J, Blumenthal R, Puri A. Design of Liposomes Containing Photopolymerizable Phospholipids for Triggered Release of Contents. Journal of Thermal Analysis and Calorimetry. 2009;98:97–104. doi: 10.1007/s10973-009-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shum P, Kim JM, Thompson DH. Phototriggering of Liposomal Drug Delivery Systems. Adv. Drug Deliv. Rev. 2001;53:273–284. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- (14).Lasic DD, Bolotin E, Brey RN. Polymerized Liposomes: From Biophysics to Applications. Part I. Chimica Oggi-Chemistry Today. 2000;18:48–51. [Google Scholar]
- (15).Lasic DD, Bolotin E, Brey RN. Polymerized Liposomes: From Biophysics to Applications. Part II. Chimica Oggi-Chemistry Today. 2001;19:45–48. [Google Scholar]
- (16).Singh A, Markowitz MA. The Stabilization of Tubules Formed From Heterobifunctional Phospholipids. New Journal of Chemistry. 1994;18:377–385. [Google Scholar]
- (17).Rhodes DG, Singh A. Structure of Polymerizable Lipid Bilayers IV. Mixtures of Long Chain Diacetylenic and Short Chain Saturated Phosphatidylcholines and Analogous Asymmetric Isomers. Chem. Phys. Lipids. 1991;59:215–224. doi: 10.1016/0009-3084(91)90021-3. [DOI] [PubMed] [Google Scholar]
- (18).Pons M, Villaverde C, Chapman D. A C-13-Nmr Study of 10,12-Tricosadiynoic Acid and the Corresponding Phospholipid and Phospholipid Polymer. Biochimica et Biophysica Acta. 1983;730:306–312. [Google Scholar]
- (19).Johnston DS, Sanghera S, Pons M, Chapman D. Phospholipid Polymers--Synthesis and Spectral Characteristics. Biochim. Biophys. Acta. 1980;602:57–69. doi: 10.1016/0005-2736(80)90289-8. [DOI] [PubMed] [Google Scholar]
- (20).Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A. Light-Sensitive Lipid-Based Nanoparticles for Drug Delivery: Design Principles and Future Considerations for Biological Applications. Mol. Membr. Biol. 2010;27:364–381. doi: 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cashion MP, Long TE. Biomimetic Design and Performance of Polymerizable Lipids. Accounts of Chemical Research. 2009;42:1016–1025. doi: 10.1021/ar800191s. [DOI] [PubMed] [Google Scholar]
- (22).Albrecht O, Johnston DS, Villaverde C, Chapman D. Stable Biomembrane Surfaces Formed by Phospholipid Polymers. Biochim. Biophys. Acta. 1982;687:165–169. doi: 10.1016/0005-2736(82)90542-9. [DOI] [PubMed] [Google Scholar]
- (23).Song J, Cheng Q, Zhu SM, Stevens RC. “Smart” Materials for Biosensing Devices: Cell-Mimicking Supramolecular Assemblies and Colorimetric Detection of Pathogenic Agents. Biomedical Microdevices. 2002;4:213–221. [Google Scholar]
- (24).Cheng Q, Song J, Stevens RC. Polydiacetylenic Lipid Assemblies: “Smart” Materials for Colorimetric Biosensing and Structural Transformation in Charge-Induced Chromatic Transition. Abstracts of Papers of the American Chemical Society. 2002;223:D44. [Google Scholar]
- (25).Morigaki K, Baumgart T, Jonas U, Offenhausser A, Knoll W. Photopolymerization of Diacetylene Lipid Bilayers and Its Application to the Construction of Micropatterned Biomimetic Membranes. Langmuir. 2002;18:4082–4089. [Google Scholar]
- (26).Stanish I, Lowy DA, Hung CW, Singh A. Vesicle-Based Rechargeable Batteries. Advanced Materials. 2005;17:1194. + [Google Scholar]
- (27).Li KC, Bednarski MD. Vascular-Targeted Molecular Imaging Using Functionalized Polymerized Vesicles. J. Magn Reson. Imaging. 2002;16:388–393. doi: 10.1002/jmri.10174. [DOI] [PubMed] [Google Scholar]
- (28).Zhou Y. Lipid Nanotubes: Formation, Templating Nanostructures and Drug Nanocarriers. Critical Reviews in Solid State and Materials Sciences. 2008;33:183–196. [Google Scholar]
- (29).Zarif L. Elongated Supramolecular Assemblies in Drug Delivery. J. Control Release. 2002;81:7–23. doi: 10.1016/s0168-3659(02)00010-x. [DOI] [PubMed] [Google Scholar]
- (30).Juliano RL, Hsu MJ, Peterson D, Regen SL, Singh A. Interactions of Conventional or Photopolymerized Liposomes With Platelets in Vitro. Exp. Cell Res. 1983;146:422–427. doi: 10.1016/0014-4827(83)90144-1. [DOI] [PubMed] [Google Scholar]
- (31).Bonte F, Hsu MJ, Papp A, Wu K, Regen SL, Juliano RL. Interactions of Polymerizable Phosphatidylcholine Vesicles With Blood Components: Relevance to Biocompatibility. Biochim. Biophys. Acta. 1987;900:1–9. doi: 10.1016/0005-2736(87)90271-9. [DOI] [PubMed] [Google Scholar]
- (32).Regen SL, Yamaguchi K, Samuel NKP, Singh M. Polymerized Depolymerized Vesicles - A Reversible Phosphatidylcholine-Based Membrane. Journal of the American Chemical Society. 1983;105:6354–6355. [Google Scholar]
- (33).Lawson GE, Lee Y, Singh A. Formation of Stable Nanocapsules From Polymerizable Phospholipids. Langmuir. 2003;19:6401–6407. [Google Scholar]
- (34).Lawson GW, Breen JJ, Marquez M, Singh A, Smith BD. Polymerization of Vesicles Composed of N-(4-Vinylbenzoyl)Phosphatidylethanolamine. Langmuir. 2003;19:3557–3560. [Google Scholar]
- (35).Lawson GE, Lee YW, Raushel FM, Singh A. Phospholipid-Based Catalytic Nanocapsules. Advanced Functional Materials. 2005;15:267–272. [Google Scholar]
- (36).Jung M, Hubert DHW, van Veldhoven E, Frederik P, van Herk AM, German AL. Vesicle-Polymer Hybrid Architectures: A Full Account of the Parachute Architecture. Langmuir. 2000;16:3165–3174. [Google Scholar]
- (37).Jung M, den Ouden I, Montoya-Goni A, Hubert DHW, Frederik PM, van Herk AM, German AL. Polymerization in Polymerizable Vesicle Bilayer Membranes. Langmuir. 2000;16:4185–4195. [Google Scholar]
- (38).Jung M, Hubert DHW, van Veldhoven E, Frederik PM, Blandamer MJ, Briggs B, Visser AJWG, van Herk AM, German AL. Interaction of Styrene With DODAB Bilayer Vesicles. Influence on Vesicle Morphology and Bilayer Properties. Langmuir. 2000;16:968–979. [Google Scholar]
- (39).Freeman FJ, Hayward JA, Chapman D. Permeability Studies on Liposomes Formed From Polymerizable Diacetylenic Phospholipids and Their Potential Applications As Drug Delivery Systems. Biochim. Biophys. Acta. 1987;924:341–351. doi: 10.1016/0304-4165(87)90032-8. [DOI] [PubMed] [Google Scholar]
- (40).Stanish I, Singh A. Highly Stable Vesicles Composed of a New Chain-Terminus Acetylenic Photopolymeric Phospholipid. Chem. Phys. Lipids. 2001;112:99–108. doi: 10.1016/s0009-3084(01)00173-6. [DOI] [PubMed] [Google Scholar]
- (41).Singh A. An Efficient Synthesis of Phosphatidylcholines. J. Lipid Res. 1990;31:1522–1525. [PubMed] [Google Scholar]
- (42).Clapp PJ, Armitage BA, Obrien DF. Two-Dimensional Polymerization of Lipid Bilayers: Visible-Light-Sensitized Photoinitiation. Macromolecules. 1997;30:32–41. [Google Scholar]
- (43).Lamparski H, Liman U, Barry JA, Frankel DA, Ramaswami V, Brown MF, Obrien DF. Photoinduced Destabilization of Liposomes. Biochemistry. 1992;31:685–694. doi: 10.1021/bi00118a008. [DOI] [PubMed] [Google Scholar]
- (44).Bae SK, Kim SH, Kim JD, Koo KI, Ryeom TK, Ryeom K, Fu XL, Chang YH. Simplified Syntheses of Polymerizable Bis-Substituted Phosphatidylcholines With Various Chain Lengths. Tetrahedron Letters. 2000;41:8495–8498. [Google Scholar]
- (45).Singh A, Wong EM, Schnur JM. Toward the Rational Control of Nanoscale Structures Using Chiral Self-Assembly: Diacetylenic Phosphocholines. Langmuir. 2003;19:1888–1898. [Google Scholar]
- (46).Mueller A, Bondurant B, O’Brien DF. Visible-Light-Stimulated Destabilization of PEG-Liposomes. Macromolecules. 2000;33:4799–4804. [Google Scholar]
- (47).Bondurant B, O’Brien DF. Photoinduced Destabilization of Sterically Stabilized Liposomes. Journal of the American Chemical Society. 1998;120:13541–13542. [Google Scholar]
- (48).Singh A, Marchywka S, Gaber BP. Polymerization Properties of Aqueous Dispersions of Diacetylenic and Short Chain Phospholipid Mixtures. Abstracts of Papers of the American Chemical Society. 1989;198:203. MSE. [Google Scholar]
- (49).Regen SL, Singh A, Oehme G, Singh M. Polymerized Phosphatidyl Choline Vesicles - Stabilized and Controllable Time-Release Carriers. Biochemical and Biophysical Research Communications. 1981;101:131–136. doi: 10.1016/s0006-291x(81)80020-4. [DOI] [PubMed] [Google Scholar]
- (50).Leaver J, Alonso A, Durrani AA, Chapman D. The Biosynthetic Incorporation of Diacetylenic Fatty Acids into the Biomembranes of Acholeplasma Laidlawii A Cells and Polymerisation of the Biomembranes by Irradiation With Ultraviolet Light. Biochim. Biophys. Acta. 1983;727:327–335. doi: 10.1016/0005-2736(83)90418-2. [DOI] [PubMed] [Google Scholar]
- (51).Leaver J, Alonso A, Durrani AA, Chapman D. The Physical-Properties and Photo-Polymerization of Diacetylene-Containing Phospholipid Liposomes. Biochimica et Biophysica Acta. 1983;732:210–218. [Google Scholar]
- (52).Nagy JO, Wang P, Gilbert JH, Schaefer ME, Hill TG, Callstrom MR, Bednarski MD. Carbohydrate Materials Bearing Neuraminidase-Resistant C-Glycosides of Sialic Acid Strongly Inhibit the in Vitro Infectivity of Influenza Virus. J. Med. Chem. 1992;35:4501–4502. doi: 10.1021/jm00101a031. [DOI] [PubMed] [Google Scholar]
- (53).Nagy JO, Zhang Y, Yi W, Liu X, Motari E, Song JC, Lejeune JT, Wang PG. Glycopolydiacetylene Nanoparticles As a Chromatic Biosensor to Detect Shiga-Like Toxin Producing Escherichia Coli O157:H7. Bioorg. Med. Chem. Lett. 2008;18:700–703. doi: 10.1016/j.bmcl.2007.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Yavlovich A, Singh A, Blumenthal R, Puri A. A Novel Class of Photo-Triggerable Liposomes Containing DPPC:DC(8,9)PC As Vehicles for Delivery of Doxorubcin to Cells. Biochim. Biophys. Acta. 2011;1808:117–126. doi: 10.1016/j.bbamem.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Miller CR, Clapp PJ, O’Brien DF. Visible Light-Induced Destabilization of Endocytosed Liposomes. FEBS Lett. 2000;467:52–56. doi: 10.1016/s0014-5793(00)01122-4. [DOI] [PubMed] [Google Scholar]
- (56).Chiaramoni NS, Speroni L, Taira MC, Alonso S. V Liposome/DNA Systems: Correlation Between Association, Hydrophobicity and Cell Viability. Biotechnol. Lett. 2007;29:1637–1644. doi: 10.1007/s10529-007-9454-y. [DOI] [PubMed] [Google Scholar]
- (57).Alonso-Romanowski S, Chiaramoni NS, Lioy VS, Gargini RA, Viera LI, Taira MC. Characterization of Diacetylenic Liposomes As Carriers for Oral Vaccines. Chem. Phys. Lipids. 2003;122:191–203. doi: 10.1016/s0009-3084(02)00190-1. [DOI] [PubMed] [Google Scholar]
- (58).Lazcka O, Del Campo FJ, Munoz FX. Pathogen Detection: a Perspective of Traditional Methods and Biosensors. Biosens. Bioelectron. 2007;22:1205–1217. doi: 10.1016/j.bios.2006.06.036. [DOI] [PubMed] [Google Scholar]
- (59).Charych DH, Nagy JO, Spevak W, Bednarski MD. Direct Colorimetric Detection of a Receptor-Ligand Interaction by a Polymerized Bilayer Assembly. Science. 1993;261:585–588. doi: 10.1126/science.8342021. [DOI] [PubMed] [Google Scholar]
- (60).Charych D, Nagy JO. Artificial Cell Membranes for Diagnostics and Therapeutics. Chemtech. 1996;26:24–28. [Google Scholar]
- (61).Charych D, Cheng Q, Reichert A, Kuziemko G, Stroh M, Nagy JO, Spevak W, Stevens RC. A ‘Litmus Test’ for Molecular Recognition Using Artificial Membranes. Chemistry & Biology. 1996;3:113–120. doi: 10.1016/s1074-5521(96)90287-2. [DOI] [PubMed] [Google Scholar]
- (62).Skehel JJ, Wiley DC. Influenza Viruses and Cell Membranes. Am. J. Respir. Crit Care Med. 1995;152:S13–S15. doi: 10.1164/ajrccm/152.4_Pt_2.S13. [DOI] [PubMed] [Google Scholar]
- (63).Skehel JJ, Wiley DC. Receptor Binding and Membrane Fusion in Virus Entry: the Influenza Hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- (64).Rogers GN, Paulson JC. Receptor Determinants of Human and Animal Influenza Virus Isolates: Differences in Receptor Specificity of the H3 Hemagglutinin Based on Species of Origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- (65).Reichert A, Ahn DJ, Nagy J, Charych D. Recognition and Detection at Tailored Polydiacetylene Molecular Assemblies. Abstracts of Papers of the American Chemical Society. 1995;209:163. COLL. [Google Scholar]
- (66).Nagy JO, Spevak W, Charych DH, Schaefer ME, Gilbert JH, Bednarski MD. Polymerized Liposomes Containing C-Glycosides of Sialic-Acid Are Potent Inhibitors of Influenza-Virus Hemagglutination and Invitro Infectivity. Journal of Cellular Biochemistry. 1993:382. [Google Scholar]
- (67).Krause HJ, Juliano RL, Regen S. In Vivo Behavior of Polymerized Lipid Vesicles. J. Pharm. Sci. 1987;76:1–5. doi: 10.1002/jps.2600760102. [DOI] [PubMed] [Google Scholar]
- (68).Temprana CF, Amor MS, Femia AL, Gasparri J, Taira MC, del Valle AS. Ultraviolet Irradiation of Diacetylenic Liposomes As a Strategy to Improve Size Stability and to Alter Protein Binding Without Cytotoxicity Enhancement. J. Liposome Res. 2011;21:141–150. doi: 10.3109/08982104.2010.492477. [DOI] [PubMed] [Google Scholar]
- (69).Temprana CF, Duarte EL, Taira MC, Lamy MT, del Valle AS. Structural Characterization of Photopolymerizable Binary Liposomes Containing Diacetylenic and Saturated Phospholipids. Langmuir. 2010;26:10084–10092. doi: 10.1021/la100214v. [DOI] [PubMed] [Google Scholar]
- (70).Singh A, Lawson G, Shivakrupa R, Johnson B, Blumenthal R, Puri A. Piroxicam Entrapped In Head-Group Polymerized Liposomes Inhibits Proliferation of IC2 Mast Cells In Vitro (Materials Research Symposium on Engineered Nanoscale Materials for the Diagnosis and Treatment of Diseases) pp. 1019–FF02-07. [Meeting Proceeding]