Abstract
Objective
To estimate the effect of porcine subintestinal submucosal graft augmentation on improving anatomic and subjective rectocele repair outcomes compared to native tissue repair.
Methods
We conducted a randomized controlled trial at two sites, including women with at least Stage 2 symptomatic rectocele. Anatomic and subjective outcomes (vaginal bulge and defecatory) were collected 12 months postoperatively, including blinded pelvic organ prolapse quantification (POP-Q) examinations. Anatomic failure was defined as points Ap or Bp ≥ −1 on POPQ. Subjective failure was defined as no improvement or worsening of symptoms. We estimated number needed to treat (NNT) and adjusted odds ratios (AOR). Assuming graft use is associated with 93% anatomic success, 63 women per group would be needed to detect a 20% difference at alpha=0.05 and beta=0.20.
Results
One-hundred sixty women were randomized; 137 had 12-month anatomic data (67 graft; 70 control). There was no difference in anatomic failure (12% versus 9%, P=0.5), vaginal bulge symptom failure (3% versus 7%, P=0.4, NNT 26) or defecatory symptom failure (44% versus 45%, P=0.9, NNT 91) for graft versus control, respectively. Both groups reported improvement in vaginal bulge and defecatory symptoms (P<.05 for all). On multiple logistic regression graft use was not associated with a decreased odds of anatomic failure (AOR 1.36, 95% CI 0.44–4.25), vaginal bulge symptoms (AOR 0.46, 95% CI 0.08–2.68), or defecatory symptoms (AOR 0.98, 95% CI 0.48–2.03).
Conclusions
Although rectocele repair by either approach is associated with improved symptoms, subintestinal submucosal graft augmentation was not superior to native tissue for anatomic or subjective outcomes at 12 months.
INTRODUCTION
Transvaginal graft use has been increasingly used in pelvic organ prolapse repair. Although the scientific literature has also been increasing, there remain few randomized trials comparing the efficacy of graft augmentation compared to native tissue repairs.1 The Food and Drug Administration recently highlighted the lack of evidence supporting the efficacy of transvaginal graft and mesh use for pelvic organ prolapse and their associated complications in a public health notification.2 Randomized trials are urgently needed to provide efficacy and safety data.
Posterior vaginal wall prolapse, or rectocele, is associated with symptoms of vaginal bulging or protrusion, defecatory symptoms, and sexual dysfunction.3, 4,5, 6 The prevalence of rectocele in women ranges from 12.9–18.6% and the average annual incidence is estimated to be 5.7 cases per 100 women-years.7, 8 Consequently, rectocele repair is a common gynecologic procedure and is performed in up to 40–69% of women undergoing surgical correction of prolapse.9, 10 In an effort to improve anatomic and subjective outcomes, graft-augmentation in rectocele repairs is proposed as a superior technique, despite lacking evidence to support this practice.1
Grafts can be biologic (typically non-permanent) or synthetic (can be absorbable or permanent). Porcine sub-intestinal submucosal (SIS) graft (SurgiSIS™, Cook, Biotech) is a freeze-dried, non cross-linked, extracellular matrix graft obtained from the submucosa of porcine small intestine. In theory it functions as a scaffold and host tissue in-growth ultimately replaces the graft prior to its degradation, but the repair is strengthened due to the reinforcing graft. SIS has been used in a variety of hernia repairs in the general surgery literature including inguinal, ventral, and gastroschisis to name a few, with an aggregate long-term failure rate of 6.7%.11 The primary objective of this study was to estimate the effect of SIS graft augmentation on improving anatomic outcomes for symptomatic rectocele repair at 12 months compared to native tissue repair. Our secondary objective was to compare subjective outcomes, including vaginal bulging, defecatory symptoms, and sexual complaints in women randomized to graft versus no graft.
MATERIALS AND METHODS
We performed a randomized, double-blind controlled trial at 2 sites to estimate the effect of SIS graft augmented rectocele repair versus native tissue repair on improving outcomes. Double-blinding in this trial refers to the fact that both the participant and the outcome assessor were masked to the treatment assignment until the 12-month visit.12 No funding or support was provided by the manufacturer of the graft for any portion of this study. We chose SIS due to concerns that a permanent mesh may increase the risk of dyspareunia. The two sites included Women and Infants Hospital in Providence, Rhode Island and Hartford Hospital in Hartford, Connecticut. The study protocol was approved by the institutional review boards of both sites.
Enrollment occurred over a 5-year period beginning in January 2004. An interim analysis was performed from November 2007–January 2008 after approximately half of the targeted sample had been enrolled due to findings from a study by Paraiso et al13 showing that porcine graft was associated with worse outcomes. Our interim analysis revealed no differences in anatomic, subjective, or adverse event outcomes between groups at a P=0.001. The level of significance maintained an overall P-value of 0.05 for our final analysis based on the Haybittle-Peto approach.14 We resumed enrollment and waited for all subjects to complete 12 month follow up before analyzing our final data.
Women with Stage 2 or greater symptomatic rectocele (defined as vaginal bulge and/or defecatory symptoms) electing surgical repair were eligible. Exclusion criteria included age <18 years, women undergoing concomitant sacrocolpopexy or colo-rectal procedures, history of porcine allergy, connective tissue disease, pelvic malignancy, pelvic radiation, and inability to understand English, or unable or unwilling to consent or comply with follow-up. All other vaginal prolapse repairs and anti-incontinence procedures were included. Patients with previous rectocele repair were also included.
All women were evaluated by one of six attending fellowship-trained Urogynecologists at one of the two sites preoperatively. Women scheduled for surgical correction of the rectocele were approached for potential enrollment at their preoperative visit, typically 2–3 weeks prior to surgery, when details of the trial were discussed and informed consent was obtained by the attending physician, a fellow, and/or research staff member. At baseline all women underwent a complete history and physical examination, including the pelvic organ prolapse quantification (POPQ) exam in a 30 degree supine lithotomy position.15 Preoperative multichannel urodynamics were performed as clinically indicated. All women completed a self-administered symptom questionnaire at baseline regarding subjective symptoms of vaginal bulge, defecatory symptoms (including constipation, splinting, and incomplete evacuation), and sexual function. Specific relevant items from the Pelvic Floor Distress Inventory16 were included.
Patients were randomly assigned to SIS graft augmented rectocele repair versus rectocele repair with native tissue using a computer-generated randomization schedule developed by a statistician. Randomization was 1:1 allocation in random blocks ranging from 5–10 assignment blocks and stratified by site. Allocation concealment was ensured using sequentially numbered, opaque, sealed envelopes. Envelopes were opened sequentially in the operating room after anesthesia was administered to the patient. Except for surgeons and those in the operating room, patients, other investigators, office and research staff were kept blind to randomization assignments. Research staff and patients were unblinded at the 12 month visit. The randomization code was broken prior to 12 months only when deemed medically necessary by the subject’s physician. Efforts to maintain blinding included keeping the randomization assignments within locked research files and providing only “blinded charts” to the outcome assessors for study visits which did not include any information on treatment assignment.
All patients received perioperative antibiotic prophylaxis. For both procedures, a posterior vaginal incision was made in the midline and extended to the superior aspect of the rectocele. The vaginal epithelium was dissected away from the underlying recto-vaginal connective tissue laterally to the levator ani muscles. Women randomized to control then underwent either midline plication of the recto-vaginal connective tissue as described by Maher, et al17 or a site specific repair as described by Cundiff, et al18 using No. 2-0 polyglycolic acid sutures (Vicryl, Ethicon, Inc) at the discretion and opinion of the attending Urogynecologist. No levator midline plications were performed.
Women randomized to SIS graft augmentation also underwent either midline plication or site specific repair as described above at the discretion of the attending Urogynecologist. This was followed by augmenting the repair with a 4 × 7 SIS graft. The graft was trimmed to appropriate size, secured over the native tissue repair and sutured laterally to the levator ani fascia using interrupted No. 2-0 polyglycolic acid sutures (Vicryl, Ethicon, Inc) bilaterally. The graft was secured superiorly to the recto-vaginal connective tissue and inferiorly to the perineal body using No. 2-0 polyglycolic acid sutures.
Excess vaginal tissue was trimmed in all women and the posterior vaginal incision was closed using running No. 2-0 polyglycolic acid sutures, but taking care to keep the closure tension free. The deep and superficial transverse perineal muscles and bulbocavernosus muscles were reapproximated using No.0 polyglycolic acid sutures and concomitant perineorrhaphy was performed in all women.
The rectocele repair portion of all surgeries was timed, and estimated blood loss for the rectocele repair portion was also recorded. Any intraoperative and postoperative complications were recorded. Both sites are teaching hospitals with residents and fellows: all procedures were directly overseen by a fellowship-trained attending Urogynecologist.
Postoperatively patients were asked to return for routine visits at 2 weeks, 6 weeks, 6 months and 12 months. All women were placed on stool softeners during the first 4 weeks and laxatives if needed during the first week. Women were discouraged from strenuous activity for 6 weeks. At all visits, women were assessed for wound and graft complications and pain using a 10-point visual analog scale. In addition, at the 6 and 12 month visits patients underwent a standardized history and POPQ exam by a blinded outcome assessor and completed the self-administered symptom questionnaire. At the 12 month visit, the patient was unblinded to her treatment assignment after her exam and questionnaires were complete. Subjects who did not return for 12 month follow-up were contacted and mailed the subjective symptom questionnaire also.
Our primary outcome was anatomic failure of the posterior vaginal wall at 12 months, defined as points Ap or Bp ≥ −1 on POPQ (Stage 2 or greater rectocele). Patients with postoperative data 10 months and beyond were considered as having 12 month data; patients without data beyond 10 months were not included and were considered “lost to follow-up” for this primary analysis. Subjective prolapse symptom failure was defined as no improvement/worsening of bother or de novo vaginal bulge symptoms based on the PFDI item #3 “Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?”. Subjective defecatory symptom failure was defined as no improvement/worsening of bother or de novo defecatory symptoms based on a composite of 3 items: 1) PFDI item #4 “Do you usually have to push on the vagina or around the rectum to have or complete a bowel movement?”; 2) PFDI item #7 “Do you feel you need to strain too hard to have a bowel movement?”; 3) PFDI item #8 “Do you feel you have not completely emptied your bowels at the end of a bowel movement?”. Any woman who did not improve, worsened, or had de novo bowel symptoms was defined as reporting “defecatory symptom failure”, although the outcomes of each individual bowel symptom was also evaluated. Pain with intercourse was assessed with the question “Do you experience pain with intercourse?”
At the time we started our study, there were only case series to base our sample size analysis. Based on a study by Kohli et al, assuming that graft use is associated with a 93% anatomic success rate19, 63 women per group would be needed to detect a 20% difference at alpha=.05 and beta=.20. We aimed to recruit 160 women (80 women per group) to account for drop out. Univariable analyses were performed as appropriate. Student’s T-tests and paired t-tests were used to compare means between and within groups. Chi-square was used to compare proportions, and McNemar’s test was used to compare ordinal data. We used generalized estimating equations to evaluate change over time separately by group, and the overall group difference was tested with adjustment for time to assess any group by time interactions. We calculated risk differences (RD) with 95% confidence intervals (CI), with a negative RD indicating graft use reduced the risk. We estimated number needed to treat (NNT) for outcomes in which there was a reduction in RD in the graft group. We used intention to treat analysis and multiple logistic regression analysis to estimate the effect of SIS graft augmentation on improving anatomic and subjective (vaginal bulge, and defecatory symptoms) outcomes compared to control, adjusting for covariates known to be confounders based on the literature that also statistically changed the effect estimates in the models. Time to anatomic failure was also assessed using Cox proportional hazards regression to determine if graft use was associated with a decreased time to failure compared to control. We performed sensitivity analysis for our primary outcome using propensity scores and then assuming all subjects lost to follow-up were failures to estimate the effect of graft use on outcomes. All statistical analyses were performed using SAS 8.2 (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
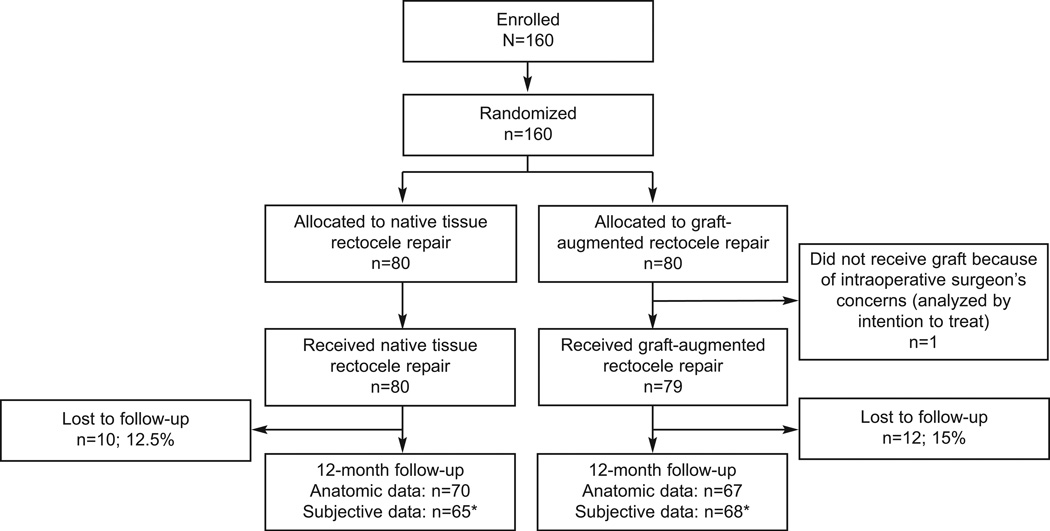
One hundred sixty women were randomized. Twelve month anatomic data were available for 137 women (67 graft and 70 controls) and subjective data was available for 133 women (68 graft and 65 controls). Allocation and follow-up are presented in Figure 1. There were no differences in baseline rectocele stage, median Ap or Bp, or symptoms of vaginal bulge, constipation, splinting, or incomplete evacuation between women who did and did not follow up. There was also no difference in failure rates, follow-up or subjective outcomes between the two sites. One subject randomized to graft did not receive the allocated intervention due to the surgeon’s intraoperative concern that the quality of the rectovaginal connective tissue and vaginal epithelium were extremely poor. This patient received native tissue repair, returned at 22 months for follow-up and was analyzed in the graft group based on intent to treat analysis.
Figure 1.
Flow of study participants throughout protocol. *Subjective questionnaires were mailed to women who did not return for follow-up. Some subjective outcomes missing.
The median follow up time in the graft group was 12.2 months (range 10–43 months) and the control group was 12.5 months (range 10.3–38 months), P=0.7. Baseline demographic and clinical characteristics were not different between groups (Table 1). In addition, there was no difference between groups in women who had previous urogynecologic procedures or rectocele repairs (P>.05). Table 2 includes intraoperative and postoperative details. Concomitant procedures, postoperative complication, and the proportion of women who underwent site-specific versus midline plication rectocele repairs did not differ between groups. Women who were randomized to graft augmentation had longer rectocele repair operative times and higher estimated blood loss compared to controls. There was no difference in postoperative pain at 2 weeks and 6 weeks postoperative. There were no graft erosions or granulomas seen and there were no differences between groups in postoperative wound separation or infection in the posterior vaginal wall. One subject, a 42 year old woman who received graft augmentation and concomitant midurethral sling and anterior repair was unblinded at 4 weeks postoperative due to symptoms of persistent diarrhea and Bell’s Palsy requiring work up with a Neurologist and Rheumatologist. The leading diagnosis was a viral syndrome and her symptoms eventually resolved. She did not return for 12 month follow-up.
Table 1.
Baseline Demographic and Clinical Characteristics by Randomization Group
| Characteristic | Graft (n=80) |
No Graft (n=80) |
P |
|---|---|---|---|
| Age (mean, std) | 54.5 (11.0) | 54.8 (11.2) | 0.9 |
| Race White Nonwhite |
79 (100) 0 |
77 (97.5) 2 (2.5) |
0.5 |
| Comorbidities Diabetes Hypertension Asthma |
6 (7.5) 22 (27.5) 7 (8.9) |
9 (11.4) 27 (34.2) 12 (15.2) |
0.4 0.4 0.2 |
| Prior urogynecologic procedure | 16 (20.0) | 18 (22.8) | 0.7 |
| Preoperative POP-Q Stage Stage II Stage III Stage IV |
56 (70.0) 23 (28.8) 1 (1.3) |
61 (76.3) 18 (22.5) 1 (1.3) |
0.7 |
| Preoperative rectocele POP-Q Stage Stage II Stage III Stage IV |
65 (81.3) 14 (17.5) 1 (1.3) |
66 (82.5) 13 (16.3) 1 (1.3) |
1.0 |
| Preoperative POP-Q rectocele examination: cm (median, range): | |||
| Point AP | 0 (−1.0, 3.0) | 0 (−1.0, 3.0) | 1.0 |
| Point BP | 0 (−1.0, 4.0) | 0 (−1.0, 5.0) | 0.9 |
| Preoperative straining with bowel movements | 48/74 (64.9) | 46/71 (64.8) | 1.0 |
| Preoperative splinting with bowel movements | 38/74 (51.4) | 42/73 (57.5) | 0.5 |
| Preoperative incomplete evacuation with bowel movements | 59/74 (79.7) | 54/71 (76.1) | 0.6 |
| Sexually active | 50/75 (66.7) | 54/75 (72.0) | 0.5 |
POP-Q, pelvic organ prolapsed quantification.
Data presented as n (%) unless otherwise specified.
Numbers may not add to 100% due to missing data.
Table 2.
Intraoperative and Postoperative Details by Randomization Group
| Variable | Graft (n=80) |
No Graft (n=80) |
P |
|---|---|---|---|
| Concomitant procedures Hysterectomy Anterior colporrhaphy Uterosacral suspension Sacrospinous suspension Mersilene sling Midurethral sling None |
10 (12.5) 30 (37.5) 7 (8.8) 3 (3.8) 5 (6.3) 42 (52.5) 7 (8.9) |
8 (10.0) 24 (30.0) 2 (2.5) 6 (7.5) 2 (2.5) 37 (46.3) 4 (5.3) |
0.6 0.3 0.2 0.5 0.4 0.4 0.4 |
| Operative time for rectocele repair (median minutes, range | 60 (15–145) | 45 (15–140) | <0.001 |
| Blood loss for posterior repair (median milliliters, range) | 125 (25–450) | 100 (10–500) | 0.005 |
| Site specific repair | 54 (67.5) | 48 (60.0) | 0.3 |
| Intraoperative complications Rectal injury Bladder injury Transfusion |
1 (1.3) 0 0 |
0 1 (1.3) 0 |
1.0 1.0 -- |
| Postoperative complications Fever Wound separation Wound infection |
1 (1.3) 11 (13.8) 2 (2.5) |
0 5 (6.2) 4 (5.3) |
1.0 0.1 0.7 |
| Pain at 2 weeks (mean score on visual analog scale, SD) | 1.0 (1.6) | 0.8 (1.4) | 0.3 |
| Pain at 6 weeks (mean score, SD) | 0.4 (0.9) | 0.4 (1.2) | 0.9 |
| Vaginal stricture/band | 1/67 (1.4) | 1/70 (1.4) | 1.0 |
SD, standard deviation.
Data presented as n (%) unless otherwise specified.
Numbers may not add to 100% due to missing data.
Two women required a return to the operating room postoperatively. The first was a 64 year old who underwent an uncomplicated rectocele repair without graft. On postoperative day 1, she had poor pain control and perineal ecchymosis. A rectovaginal exam was suspicious for a hematoma and a CT scan confirmed a 6.6×4.0 cm rectovaginal hematoma. She was taken back to the operating room that day, the posterior vaginal incision was opened, and 150 cc of clot was expelled and the vaginal incision was re-approximated again using No. 2-0 polyglycolic acid sutures. Her immediate postoperative course was uncomplicated but she did not return for 6 or 12 month follow-up. The second patient was a 45 year old with history of multiple sclerosis and irritable bowel syndrome randomized to graft placement. She underwent an anterior and posterior repair with graft. Two weeks postoperatively, she was found to have a separation of the posterior vaginal incision without evidence of infection. The patient subsequently underwent exam under anesthesia and re-approximation of the posterior vaginal wall in two layers using No. 2-0 polyglycolic acid sutures. Her subsequent postoperative course was uncomplicated. At 12 month follow-up her rectocele repair was intact (Ap and Bp both -2 on POPQ).
Anatomic and subjective failure outcomes at 12 months are presented in Table 3. At 12 months, 8/67 (12%) in the graft group and 6/70 (8.6%) in the control group experienced anatomic failure, RD=3.4% (95% CI −6.8–13.5%), P=0.5. NNT was not calculated for anatomic failure because graft use did not decrease risk. Of the anatomic failures, 6 subjects had Stage 2 rectocele and 2 subjects had Stage 3 rectocele in the graft group. In the control group, all 6 subjects with anatomic failure had Stage 2 rectocele. The median point Ap was −3.0 for both groups, P=0.7 (graft group range −3.0, 3.0) (control group range −3.0, 1.0) and median point Bp was -3 for both groups, P=0.7 (graft group range −3.0, 3.0) (control group range −3.0, 1.0). There were no differences between groups for subjective failure (defined as no improvement/worsening in bother or de novo symptoms) for vaginal bulge (RD −3.8%, 95% CI −11.6–4%, NNT 26) or any of the three defecatory symptoms (composite defecatory outcome RD −1.1%, 95% CI −18.7–16.6%, NNT 91), P>.05 for all. Although a very small proportion of women experienced vaginal bulge symptom failure, up to 45% of women had some sort of persistent defecatory symptom at 12 months. Regarding dyspareunia, 7/56 (12.5%) in the graft and 4/57 (7%) in the control group reported postoperative dyspareunia (P=0.3).
Table 3.
Anatomic and Subjective Failure Outcomes at 12 Months by Randomization Group
| Outcome | Graft | No Graft | P |
|---|---|---|---|
| Anatomic failure (Ap or Bp≥−1) | 8/67 (11.9) | 6/70 (8.6) | 0.5 |
| Vaginal bulge subjective failure* | 2/64 (3.1) | 4/58 (6.9) | 0.4 |
| Defecatory function subjective failure* | |||
| Straining with bowel movements | 21/64 (32.8) | 18/57 (31.6) | 0.9 |
| Splinting with bowel movements | 6/62 (9.7) | 9/58 (15.5) | 0.3 |
| Sensation of incomplete evacuation | 15/63 (23.8) | 12/57 (21.1) | 0.7 |
| Composite any defecatory symptom failure | 28/64 (43.8) | 26/58 (44.8) | 0.9 |
Data presented as n (%) unless otherwise specified.
Numbers may not add to 100% due to missing data.
Defined as no improvement or worsening in bother or de novo symptoms.
We also analyzed postoperative subjective vaginal bulge and defecatory symptom “resolution”, defined as a dichotomous response of “yes”/”no”, regardless of degree of bother, (see Table 4). Within group and between group comparisons are presented. Both groups demonstrated significant improvements in bother for vaginal bulge and all three defecatory symptoms at both 6 and 12 months (P<.05 within groups for all). These improvements did not change over time and there were no statistically significant group by time interactions detected. At 12 months, 4 subjects in the graft group and 8 subjects in the control group reported vaginal bulge symptoms. Of these subjects, 2 in the graft group and 1 in the control group experienced anatomic rectocele failure. One subject in the graft group and 4 subjects in the control group had stage 2 anterior vaginal wall prolapse but no anatomic rectocele failure. The remaining subjects did not have any significant prolapse to explain their vaginal bulge symptoms.
Table 4.
Resolution of Subjective Symptoms, Within and Between Groups by Randomization Group
| Symptom* | Preoperative | 6 months Postoperative |
12 months Postoperative |
P-value (Within Graft Group)† |
P-value (Within No Graft Group)† |
P-value (Between Groups)‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Graft | No Graft |
Graft | No Graft |
Graft | No Graft |
6 Mos vs Preop |
12 Mos vs Preop |
6 Mos vs Preop |
12 Mos vs Preop |
6 mos | 12 mos | |
| Vaginal bulge | 48/74 (64.9) |
63/73 (86.3) |
5/67 (7.5) |
6/63 (9.5) |
4/68 (5.9) |
8/64 (12.5) |
<0.001 | <0.001 | <0.001 | <0.001 | 0.7 | 0.2 |
| Straining with bowel movements | 48/74 (64.9) |
46/71 (64.8) |
25/67 (37.3) |
30/63 (47.6) |
27/68 (39.7) |
28/64 (43.8) |
<0.001 | 0.005 | 0.003 | 0.001 | 0.2 | 0.6 |
| Splinting with bowel movements | 38/74 (51.4) |
42/73 (57.5) |
9/64 (14.1) |
9/62 (14.5) |
12/66 (18.2) |
16/64 (25.0) |
<0.001 | <0.001 | <0.001 | <0.001 | 0.9 | 0.3 |
| Incomplete evacuation after bowel movements | 59/74 (79.7) | 54/71 (76.1) |
24/67 (35.8) |
26/62 (41.9) |
25/67 (37.3) |
29/64 (45.3) |
<0.001 | <0.001 | <0.001 | 0.002 | 0.5 | 0.4 |
Preop, preoperatively.
Data presented as n (%).
Symptoms defined as affirmative response, regardless of degree of bother.
P-values by McNemar’s test.
P-values by chi-square test.
On multiple logistic regression, after adjusting for preoperative POPQ stage, graft use was not associated with a decreased odds of anatomic failure (AOR 1.36, 95% CI 0.44–4.25). These findings did not change when we assumed all subjects lost to follow up were failures (AOR 1.39, 95% CI 0.66–2.93) or after adjusting for propensity score (AOR 1.58, 95% CI 0.45–5.51). Adjusting for preoperative POPQ stage, graft use was not associated with a decreased odds of vaginal bulge symptom failure (AOR 0.46, 95% CI 0.08–2.68), or defecatory symptom failure (AOR 0.98, 95% CI 0.48–2.03). On survival analysis, graft use was not associated with a decreased time to anatomic failure compared to control (adjusted hazard ratio 1.44, 95% CI 0.51–4.04), adjusting for preoperative POPQ stage. Our results did not change when adjusting for site.
DISCUSSION
In our study, SIS graft augmented rectocele repair was not associated with improved anatomic or subjective outcomes compared to native tissue repair at 12 months. In addition, it was not associated with decreased time to failure. Both methods of repair were associated with improvement in vaginal bulge and defecatory symptoms compared to baseline, although over 40% of women can experience persistent defecatory symptoms.
The primary goal of rectocele repair is aimed at restoring anatomy without causing de novo symptoms. Presumably, restoring anatomy should then lead to subjective symptom improvement (including vaginal bulge sensation and defecatory symptoms) and improved quality of life. A randomized trial by Paraiso et al13 using Fortagen (Organogenesis, Inc, Canton, MA), also a porcine-derived, acellular collagen matrix graft, demonstrated that graft-augmented rectocele repair was associated with worse anatomic outcomes (46% anatomic failure) compared to either midline plication (14% failure) or site-specific native tissue repairs (22% failure) (12). They also found that time to development of rectocele recurrence occurred sooner in the graft group compared to the midline plication group. Aside from this study by Paraiso et al, there are limited randomized trials evaluating the use of either biologic graft or synthetic mesh use in rectocele repair and the remaining literature includes predominantly retrospective comparative studies. One long-term, prospective, uncontrolled study by Altman et al20 evaluating rectocele repair using porcine dermis (Pelvicol, CR Bard, Murray Hill, NJ) found failure rates of 38% at one year and 41% at three year follow-up in a small cohort of 29 patients.
These anatomic failure rates are significantly higher at one year compared to our study. Possible reasons for the discrepant failure rates include the differences in types of grafts used. The SIS is not cross-linked whereas the Fortagen graft is which may increase the host inflammatory response and degradation rate. Thirty percent of women in the graft group had prior urogynecologic surgery in Paraiso’s study, whereas only 20% in our study had prior surgery. In Paraiso’s paper, although the proportion of women with Stage 3 or greater rectocele was not provided, almost 50% of women had Stage 3 or greater overall prolapse. In our study, 30% of women had Stage 3 or greater overall prolapse and 19% had Stage 3 or greater rectocele. Therefore it is possible that our study population was at lower risk for recurrence.
Despite worse anatomic outcomes in the graft group in Paraiso’s study, women in all groups experienced significant improvement in prolapse and bowel symptoms. This is consistent with our study, showing improvement in subjective outcomes in both the graft and control groups. Gustilo-Ashby performed a secondary analysis of Paraiso’s study specifically evaluating defecatory symptoms at 1 year and found that on average, bowel symptoms including straining, splinting, incomplete evacuation, fecal incontinence all improved after rectocele repair; however, up to 35% had persistent or worse symptoms, with the most common symptom being incomplete emptying.21 Similarly, we found that although the majority of women reported improved symptoms in straining, splinting and incomplete evacuation, almost 45% had persistent or worsening of any defecatory symptom with the most common being straining with bowel movements. Evaluating bowel symptoms can be challenging in that definitions can be variable and highly subjective and the literature is conflicting regarding the association with rectocele. Some studies support that rectocele repair results in improvement in bowel symptoms whereas others have shown worsening of symptoms.6, 17, 22 In theory anatomic correction of a rectocele could improve anorectal function by improving rectal caliber. However, by the time most women present for evaluation of prolapse symptoms, many already have both defecatory symptoms and a rectocele thus making any causal assumptions difficult (eg: is the rectocele a cause or a result of the defecatory dysfunction?). It is possible that depending on the underlying cause of the defecatory symptoms, rectocele repair may or may not be more likely to result in improvement. Based on our findings, it seems appropriate to counsel women with defecatory symptoms that their bowel habits may or may not improve after rectocele repair.
Earlier studies showed that rectocele repair was often associated with dyspareunia, especially when levator plication was performed.. In Paraiso’s study, they found that sexual function measured by the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12)23 improved after rectocele repair and that there was no significant change in the rate of dyspareunia at 1 year (range 11–25% dyspareunia rate). A prospective cohort study by Novi et al assessed sexual function at 6 months postoperatively in two separate cohorts: porcine-dermis (Pelvicol CR Bard, Covington, GA, USA) reinforced rectocele repair versus site-specific repair.24 Within groups, this study found improved PISQ-12 scores in the graft group but no significant improvement in the no graft group. Between groups, the graft group had significantly higher improvements in PISQ-12 compared to the no graft group (P=.01). Both groups had a decrease in dyspareunia rates at 6 months (range 8–10% dyspareunia). A systematic review by Abed et al demonstrated dyspareunia rates of 9.6% for biological grafts.25 The postoperative dyspareunia rate in our study was comparable to the existing literature; 12.5% in the graft group and 7% in the control group.
Graft complications were rare in our study, and no women in the graft group experienced graft exposure or required reoperation for graft exposure. This was similar to the study by Paraiso et al.13 In the systematic review by Abed et al, the authors reported a pooled erosion rate for biological grafts of 10.1% and wound granulation of 9.1%.25 They found that most biological graft erosions were managed conservatively and occur within 1 year of surgery.
There are limitations to our study. One challenge frequently encountered in prolapse studies is the inclusion of women undergoing concomitant repairs making it difficult to deconstruct which compartment repair is responsible for vaginal bulge symptoms. Currently, we do not have longer-term data beyond 12 months. Also, our failure rate in the native tissue group was lower than anticipated (9%), making it more difficult to detect differences between groups. Due to the small number of failures, it is also difficult to determine if there is a subgroup of women who would benefit from graft augmentation. Although our recruitment occurred over 5 years, the procedure and graft did not change during this time. We did not administer complete PFDI and PFIQ questionnaires, but used specific items to measure subjective symptoms of interest to minimize respondent burden. Although we did evaluate dyspareunia, we did not fully evaluate sexual function using a validated instrument. Also, we used one type of graft, SIS which is absorbable and these findings may not be applicable to other biologics or permanent meshes. These findings are likely not applicable to the repair of other compartmental defects. Finally, these cases were performed by fellowship trained Urogynecologists and efficacy and safety rates may reflect subspecialty training and/or a referral population.
Despite these limitations, there are few prospective, randomized trials evaluating graft use in posterior vaginal wall prolapse. Our study supports that SIS graft use is not associated with improved anatomic or subjective outcomes. Although the cost to any individual institution may vary, this particular graft can cost $600 or more. We suggest that the associated increases in surgical time, costs of the graft, and the potential risks of implanting a foreign body are not warranted. Innovations that may improve the success rates of surgical therapy for pelvic organ prolapse may be extremely beneficial; however, well designed studies demonstrating efficacy and effectiveness are needed before a procedure is commonly adopted by surgeons.
Acknowledgements
The authors thank Drs. Eric Sokol, B. Star Hampton, Paul Tulikangas, and Renee Ward for their assistance in topic development and data collection.
Funding:
Dr. Sung is supported by grant K23HD060665 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the author and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Presented at the 32nd Annual Scientific Meeting of the American Urogynecologic Society, September 14–17, 2011, Providence, Rhode Island.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131–1142. doi: 10.1097/AOG.0b013e3181898ba9. [DOI] [PubMed] [Google Scholar]
- 2. http://wwwfdagov/MedicalDevices/Safety/AlertsandNotices/ucm262435htm.
- 3.Rogers GR, Villarreal A, Kammerer-Doak D, Qualls C. Sexual function in women with and without urinary incontinence and/or pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:361–365. doi: 10.1007/s001920170012. [DOI] [PubMed] [Google Scholar]
- 4.Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99:281–289. doi: 10.1016/s0029-7844(01)01727-6. [DOI] [PubMed] [Google Scholar]
- 5.Weber AM, Walters MD, Ballard LA, Booher DL, Piedmonte MR. Posterior vaginal prolapse and bowel function. Am J Obstet Gynecol. 1998;179:1446–1449. doi: 10.1016/s0002-9378(98)70008-0. discussion 9–50. [DOI] [PubMed] [Google Scholar]
- 6.Cundiff GW, Fenner D. Evaluation and treatment of women with rectocele: focus on associated defecatory and sexual dysfunction. Obstet Gynecol. 2004;104:1403–1421. doi: 10.1097/01.AOG.0000147598.50638.15. [DOI] [PubMed] [Google Scholar]
- 7.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 8.Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27–32. doi: 10.1016/j.ajog.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 10.Whiteside JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Am J Obstet Gynecol. 2004;191:1533–1538. doi: 10.1016/j.ajog.2004.06.109. [DOI] [PubMed] [Google Scholar]
- 11.Hiles M, Record Ritchie RD, Altizer AM. Are biologic grafts effective for hernia repair?: a systematic review of the literature. Surg Innov. 2009;16:26–37. doi: 10.1177/1553350609331397. [DOI] [PubMed] [Google Scholar]
- 12.Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359:696–700. doi: 10.1016/S0140-6736(02)07816-9. [DOI] [PubMed] [Google Scholar]
- 13.Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762–1771. doi: 10.1016/j.ajog.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Schulz KF, Grimes DA. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet. 2005;365:1657–1661. doi: 10.1016/S0140-6736(05)66516-6. [DOI] [PubMed] [Google Scholar]
- 15.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 16.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193:103–113. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Maher CF, Qatawneh AM, Baessler K, Schluter PJ. Midline rectovaginal fascial plication for repair of rectocele and obstructed defecation. Obstet Gynecol. 2004;104:685–689. doi: 10.1097/01.AOG.0000139833.48063.03. [DOI] [PubMed] [Google Scholar]
- 18.Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451–1456. doi: 10.1016/s0002-9378(98)70009-2. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 19.Kohli N, Miklos JR. Dermal graft-augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:146–149. doi: 10.1007/s00192-002-1013-4. [DOI] [PubMed] [Google Scholar]
- 20.Altman D, Zetterstrom J, Mellgren A, Gustafsson C, Anzen B, Lopez A. A three-year prospective assessment of rectocele repair using porcine xenograft. Obstet Gynecol. 2006;107:59–65. doi: 10.1097/01.AOG.0000192547.58102.ab. [DOI] [PubMed] [Google Scholar]
- 21.Gustilo-Ashby AM, Paraiso MF, Jelovsek JE, Walters MD, Barber MD. Bowel symptoms 1 year after surgery for prolapse: further analysis of a randomized trial of rectocele repair. Am J Obstet Gynecol. 2007;197:76, e1–e5. doi: 10.1016/j.ajog.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 22.Kahn MA, Stanton SL. Posterior colporrhaphy: its effects on bowel and sexual function. Br J Obstet Gynaecol. 1997;104:82–86. doi: 10.1111/j.1471-0528.1997.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 23.Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:164–168. doi: 10.1007/s00192-003-1063-2. discussion 8. [DOI] [PubMed] [Google Scholar]
- 24.Novi JM, Bradley CS, Mahmoud NN, Morgan MA, Arya LA. Sexual function in women after rectocele repair with acellular porcine dermis graft vs site-specific rectovaginal fascia repair. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1163–1169. doi: 10.1007/s00192-006-0295-3. [DOI] [PubMed] [Google Scholar]
- 25.Abed H, Rahn DD, Lowenstein L, Balk EM, Clemons JL, Rogers RG. Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: a systematic review. Int Urogynecol J Pelvic Floor Dysfunct. 22:789–798. doi: 10.1007/s00192-011-1384-5. [DOI] [PubMed] [Google Scholar]