Abstract
Signal transducer and activator of transcription 3 (Stat3) has emerged as a critical regulator for tumor-associated inflammation. Activation of Stat3 negatively regulates the Th1-type immune response and promotes expansion of myeloid-derived suppressor cells (MDSCs) and regulatory T-cell functions in the tumor microenvironment. Mounting evidence suggests that Stat3 and related pathways may serve as a target for changing the tumor immunologic microenvironment to benefit cancer immunotherapies. Many recent studies support the use of certain tyrosine kinase inhibitors, through inhibition of Stat3, in decreasing immunosuppression in the tumor microenvironment. Other potential therapeutic avenues include the use of targeted delivery of Stat3 siRNA into immune cells. Here, we describe the role of Stat3 in regulating the immunologic properties of tumors as a background for Stat3-based therapeutic interventions.
1 Introduction
The ability of tumors to evade immune surveillance plays a central role in tumor progression (Dunn et al. 2002; Yu et al. 2007). Studies performed in our laboratory, supported by work at other institutions, have suggested an important role of signal transducer and activator of transcription 3 (Stat3), an important oncogenic transcriptional factor, in mediating tumor-induced immune suppression at various levels (Yu et al. 2007, 2009). In the setting of malignancy, Stat3 is activated by many cytokine signaling pathways, which is highlighted by interleukin-6 (IL-6). As a point of convergence for numerous oncogenic signaling pathways, Stat3 is also persistently activated by abnormal signaling of various growth factor receptors, including epidermal growth factor receptor (EGFR) and vascular growth factor receptor (VEGFR), along with oncoproteins such as Src and BCR-ABL. Activated Stat3 not only downregulates Th1 cytokines and other mediators critical for potent anti-tumor immune responses, but also activates many genes involved in immune suppression. Many Stat3 driven tumor-derived factors, including IL-6, IL-10, and VEGF, ensure persistent Stat3 activation in the tumor microenvironment through a crosstalk between tumor cells and tumor-associated immune cells, thereby creating “feed-forward loop” (Kortylewski et al. 2005; Wang et al. 2004; Yu et al. 2007, 2009). Activated Stat3 in tumor-associated immune cells further promotes expression of growth factors and angiogenic factors (Kujawski et al. 2008). As such, Stat3 limits the antitumor effects from host immune system and accelerates tumor growth and metastasis (Kortylewski et al. 2005; Wang et al. 2004; Yu et al. 2007, 2009).
Inhibiting Stat3 using various means induces robust anti-tumor innate and adaptive immune responses in the tumor microenvironment (Kortylewski et al. 2005; Wang et al. 2004; Yu et al. 2007, 2009). Considering the critical role of Stat3 in both tumor cells as well as in tumor-associated immune cells in inducing immune suppression, a more detailed understanding of the mechanism underlying Stat3-mediated immune suppression may lead to advances in cancer therapy. In this review, we will summarize recent findings related to the role of Stat3 in tumor-induced immune suppression and discuss different therapeutic approaches involving abrogation of Stat3 signaling and enhancement of immunotherapy.
2 Stat3-Mediated Immune Suppression
2.1 Inhibition of the Th1 Immune Response
The first study demonstrating Stat3 as a negative regulator of Th1-type immune responses reported that ablation of Stat3 in neutrophils and macrophages increased production of Th1 cytokines, such as IFNγ, TNFα, and IL-1, after LPS stimulation (Takeda et al. 1999). A role of Stat3 in inhibiting immunostimulatory Th1 cytokines and other mediators in tumors was subsequently shown (Nabarro et al. 2005; Sumimoto et al. 2006; Wang et al. 2004). Because of Stat3 is a critical oncogenic molecule, a direct link between oncogenesis and tumor immune evasion was thus substantiated. Further studies revealed that Stat3 activation in immune cells is in part mediated by tumor-derived factors, such as VEGF, IL-10, and IL-6 (Sumimoto et al. 2006; Wang et al. 2004). Conversely, Stat3 ablation in immune cells leads to induction of Th1 mediators involved in both innate and T-cell-mediated adaptive immunity. In turn, this causes increased anti-tumor activity of immune cells that impedes tumor progression (Kortylewski et al. 2005) (Fig. 1).
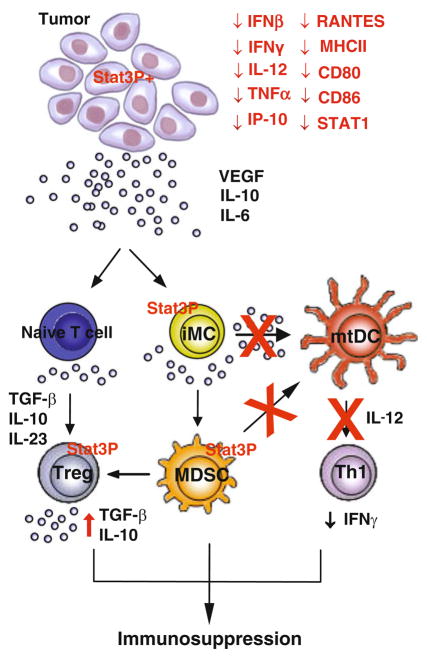
Fig. 1.
Multifaceted role of Stat3 in anti-tumor immunity. Stat3 is persistently activated in tumors and the tumor microenvironment, inducing production of many tumor-derived factors such as VEGF, IL-10, and IL-6. Increased Stat3 activity in tumor-associated immune cells promotes immunosuppressive environment, by mediating the generation of immune suppressor cells, including MDSC and T regs. The expression of MDSC and Treg effector molecules, such as TGF-β, IL-10, and IL-23, is in part mediated by Stat3. Activated Stat3 in tumor-associated immune cells also inhibits DC maturation as well as the production of Th1-type cytokines such as IL-12 and IFN-γ. As such, Stat3 activity in tumor impairs both adaptive and innate immune responses against tumor
Many of the Th1 mediators produced in tumors upon Stat3 ablation are typical targets of other immune regulators, such as NF-κB and/or Stat1, whose role is pivotal in Th1-mediated immune responses (Yu et al. 2009). Deletion of Stat3 facilitates activation of NF-κB (Welte et al. 2003) and Stat1 (Takeda et al. 1999), leading to increased production of Th1 type immune mediators required for anti-tumor immunity (Kortylewski et al. 2009c; Yu et al. 2009). Although various mechanisms have been suggested, how Stat3 antagonizes NF-κB and Stat1 remains to be further defined. It has been implicated from several studies that Stat3 negatively regulates IκB kinase β (IKKβ), which is required for phosphorylation of IκBα and its subsequent degradation (Lee et al. 2009; Welte et al. 2003). This may render NF-κB in a suppressed state, keeping it from activating downstream genes involved in Th1 type immune responses. However, a synergistic interaction between Stat3 and NF-κB is also documented during tumor progression through autocrine/parcrine signaling of IL-6 and IL-10 (Bollrath et al. 2009; Grivennikov et al. 2009; Lam et al. 2008; Lee et al. 2009). Distinct from the interaction between Stat3 and NF-κB, Stat3 and Stat1 often oppose each other in cancer models – if Stat3 is highly activated, Stat1 is downregulated. In the setting of melanoma, it has been noted that increased expression of activated Stat1 is an important predictor of therapeutic responsiveness to interferon-α (IFNα) (Lesinski et al. 2007; Wang et al. 2007; Zimmerer et al. 2007) and correlates with longer overall survival (Wang et al. 2007). These studies suggest that the balance between Stat1 and Stat3 may determine the therapeutic outcome of cancer immunotherapy and targeting Stat3 may shift cellular balance more favorable toward host. It has recently been noted that single nucleotide polymorphism associated with Stat3 expression may be a significant predictor of IFNα response. In a study of 174 patients with chronic myelogenous leukemia (CML), the single nucleotide polymorphism (SNP) rs6503691 was tightly correlated with the level of STAT3 mRNA, and could further reliably distinguish responders and non-responders to IFN-α (Kreil et al. 2010). In a separate assessment of 75 patients with metastatic renal cell carcinoma (mRCC) treated with IFN-α, it was documented that the rs4796793 polymorphism in the 5′ region of Stat3 was a significant predictor of clinical response (odds ratio [OR] = 2.73, 95% CI 1.38–5.78) (Ito et al. 2007). The enhanced growth inhibitory effects of IFNα upon Stat3 suppression in renal cell carcinoma also supports the notion that Stat3 inhibition is a useful tool to boost the efficacy of IFNα therapy in patients with renal cell carcinoma.
2.2 Relevant Immunologic Signaling Pathways
Tumors produce various factors that in turn activate Stat3 by forming feed-forward loops with signaling pathways (Yu et al. 2007, 2009). Persistent Stat3 activation can be propagated from tumor cells to diverse immune cells through factors such as IL-6, IL-10, and VEGF (Kortylewski et al. 2005; Kujawski et al. 2008; Lee et al. 2009; Sumimoto et al. 2006; Wang et al. 2004; Yu et al. 2007, 2009). These factors impede appropriate immune cell functioning in both innate and adaptive immunity. Specifically, release of IL-10, VEGF, and IL-6 prevents immature dendritic cells (DCs) from maturing into antigen-presenting cells (Gabrilovich 2004; Gabrilovich et al. 1996; Ohm et al. 2003; Park et al. 2004). Expression levels of MHC class II, co-stimulatory molecule CD86 and IL-12, all of which are required for proper DC-mediated immune function, are decreased by IL-10-induced Stat3 activation, leading to the generation of tolerogenic DCs (Li et al. 2006; Liang et al. 2008). Moreover, the constitutive activation of Stat3 by IL-10, VEGF, and IL-6 impedes functional maturation of tumor-associated DCs (Bharadwaj et al. 2007; Gabrilovich 2004; Nabarro et al. 2005; Yang et al. 2009), leading to increased tumor growth and metastasis (Kortylewski et al. 2005; Wang et al. 2004). Demonstrating cross-talk amongst these pathways, IL-6 is shown to upregulate production of IL-10 in both colon cancer cell lines and T cells through Stat3 activation (Herbeuval et al. 2004; Stumhofer et al. 2007).
Interplays between tumor cells and immune cells are mainly regulated by cytokines, which can stimulate either tumorigenic or anti-tumorigenic effects. For example, IL-23 was first identified as a proinflammatory cytokine, sharing a common p40 subunit with IL-12 (Oppmann et al. 2000). IL-12 has a critical role in regulating Th1 cells that are essential for tumor suppression. Unlike IL-12, IL-23 does not promote IFN-γ-producing Th1 cells, but is one of the essential factors required for the expansion of a pathogenic memory T cell population, which is characterized by the production of IL-17, IL-6, and tumor necrosis factor (TNF) (Langrish et al. 2005). The production of IL-17 and IL-6 is mediated through Stat3 (Chen et al. 2006; Cho et al. 2006). In addition, IL-23 receptor is shown to be engaged with the Jak2/Stat3 pathway (Parham et al. 2002) and is required for the terminal differentiation of Th17 cell into effector cells in a Stat3-dependent fashion (McGeachy et al. 2009). Impaired Th17 function causes immune deficiencies such as hyper-IgE syndrome (HIES), which harbors dominant-negative Stat3 mutation (Ma et al. 2008; Milner et al. 2008; Minegishi 2009; Minegishi et al. 2007).
In contrast to its role in promoting inflammatory responses, IL-23 has been implicated in tumor-mediated immunosuppression (Kortylewski et al. 2009c; Langowski et al. 2006). While suppressing NF-κB activation is required for IL-12 mediated anti-tumor immune responses, Stat3 markedly upregulates transcriptional activity of IL-23p19 subunit in tumor-associated macrophages (Kortylewski et al. 2009c), thereby promoting IL-23 mediated pro-tumorigenic immune responses. Given that Stat3 inhibits IL-12 expression (Hoentjen et al. 2005; Wang et al. 2004) and that enhanced IL-12 production upon IL-23 blockade intensifies Th1 type immune responses (Uemura et al. 2009), targeting Stat3 may be a relevant approach to shift IL-12/IL-23 balance towards Th1-mediated anti-tumor immune responses. It is noteworthy that IL-17 expression is concomitantly increased in tumors driven by IL-23 (Langrish et al. 2005). IL-17 also enhances tumor angiogenesis and growth (Numasaki et al. 2003) through Stat3 activation in various tumors (Charles et al. 2009; He et al. 2010; Wang et al. 2009). Since both cytokines share regulatory network through Stat3 (Kortylewski et al. 2009c; Wang et al. 2009; Yu et al. 2009), it is possible that IL-23-mediated Th17 response to tumors promote tumor progression. This notion is supported by in vivo studies assessing the link between enterotoxigenic Bacteroides fragilies (ETBF), a common gastrointestinal pathogen linked to colon carcinogenesis. These studies suggested that Stat3 is required for ETBF-mediated IL-17 production. Dual blockade of the receptors for IL-17 and IL-23 resulted in decreased formation of colonic tumors (Wu et al. 2009). Nevertheless, IL-17 may also play an antitumor role (Kryczek et al. 2009), and further studies are required to clarify why IL-17 can both promote and inhibit tumor development.
2.3 Role in Myeloid Derived Suppressor Cells
Tumor myeloid-derived suppressor cells (MDSCs) inhibit CD4+ and CD8+ T cell activation as well as innate immune responses (Gabrilovich and Nagaraj 2009; Sinha et al. 2007). In addition to its role in regulating immunosuppressive cytokines, Stat3 also promotes expansion of MDSCs (Yu et al. 2007, 2009). Several factors regulate tumor MDSC accumulation. These include IL-1β, IL-6 (Bunt et al. 2007), VEGF (Melani et al. 2003), COX2 (Xiang et al. 2009) and GM-CSF (Serafini et al. 2004), all of which trigger signaling pathways activating Stat3 (Yu et al. 2007, 2009). Exposure of myeloid cells to tumor cell conditioned medium upregulates Stat3 activity and triggers MDSC expansion (Nefedova et al. 2004). Moreover, Stat3 is persistently elevated in MDSCs from tumor-bearing mice (Nefedova et al. 2005), indicating that Stat3 activation in MDSCs may result from tumor-derived factors. Conversely, ablation of the Stat3 gene using conditional knockout mice or Stat3 blockade by tyrosine kinase inhibitor significantly reduces the number of tumor-associated MDSCs and consequently elicits robust anti-tumor immune responses (Kortylewski et al. 2005; Xin et al. 2009).
A recent study suggests an integral role of S100A9 in MDSC accumulation in tumors (Cheng et al. 2008). MDSC accumulation appears to result from impaired DC differentiation, caused by overexpressed S100A9 protein. Stat3 serves as transcriptional activator of S100A9, inducing its expression by directly binding to its promoter region (Cheng et al. 2008). S100A9 expression is reduced in myeloid cells isolated from mice with Stat3 deletion in hematopoietic cells compared to wild-type counterpart, further confirming the critical role of Stat3 in regulating S100A9 expression. Mice lacking Stat3-inducible S100A9 mount potent anti-tumor immune responses, leading to the rejection of implanted tumors. A separate series of experiments suggests that mice with a dominant-negative Stat3 mutation have markedly reduced S100A9 expression (Li et al. 2004).
One of the main characteristics of tumor MDSCs is high production of reactive oxygen species (ROS), which is essential for the suppressive function of cells (Gabrilovich and Nagaraj 2009). The increased ROS production by MDSCs is mediated by up-regulated activity of NADPH oxidase (NOX2). Owing to the fact that S100A9 upregulates ROS production by NADPH oxidase (Cheng et al. 2008), it is plausible to speculate that Stat3 may involve the suppressive function of MDSCs. Indeed, MDSCs from tumor-bearing mice had significantly higher expression of NOX2 subunits, primarily p47(phox) and gp91 (phox), as compared to immature myeloid cells from tumor-free mice. Furthermore, Stat3 directly controls transcriptional activity of p47(phox) subunit of NOX2 (Corzo et al. 2009). Treatment of MDSCs with a Stat3 inhibitor dramatically reduces the level of ROS in these cells accompanied by reduction in NOX2 expression (Corzo et al. 2009). In the absence of NOX2 activity, MDSCs lost the ability to suppress T cell responses and quickly differentiated into mature DCs (Corzo et al. 2009). Therefore, Stat3 plays a diverse role in MDSC-mediated immune suppression. Constitutive activation of Stat3 results in expansion of MDSCs that contain a high level of NOX2 components. This drives MDSCs in tumor-bearing mice to release ROS, leading to immunosuppressive activity of these cells.
2.4 Role in Regulatory T-Cells
Regulatory T-cells (Tregs) are critical in the induction of T-cell tolerance to tumor antigens by suppressing immune responses mediated by CD8+ T cells (Curiel et al. 2004; Liyanage et al. 2002; Viguier et al. 2004; Yang et al. 2006). Tregs release several immunosuppressive mediators including TGF-β and IL-10, both of which are activated and upregulated by Stat3 in tumors (Dercamp et al. 2005; Yu et al. 2007). Tumors with Stat3 ablation in hematopoietic cells markedly decrease the number of infiltrating CD4+CD25+Foxp3+ Tregs when compared to tumors with intact Stat3 activity (Kortylewski et al. 2005). This is further associated with a proliferation of CD8+ T cells, leading to potent anti-tumor immune responses (Kortylewski et al. 2005). Moreover, recent findings demonstrate that tumor-associated Tregs maintain constitutive Stat3 activity through IL-23 receptor expression (Kortylewski et al. 2009c). The contribution of constitutive Stat3 activation may be enhanced in Tregs by tumor-derived factors such as IL-23. How constitutive Stat3 activity in tumors contribute to Treg expansion is further illustrated in several studies. Constitutive activation of tumor Stat3 by oncogenes, such as nucleophosmin/anaplastic lymphoma kinase (NPM/ALK), promotes Treg expansion and expression of the Treg specific transcription factor Foxp3 as well (Kasprzycka et al. 2006). Stat3 binds to the promoter of Foxp3, although to a lesser extent compared to Stat5 (Yao et al. 2007; Zorn et al. 2006), and physically interacts with Foxp3 protein (Chaudhry et al. 2009). Conversely, inhibition of Stat3 using either siRNA or upstream tyrosine kinase inhibitor abrogates Foxp3 expression and suppressive function of Tregs. Thus, Stat3 is important for the functional maintenance of Tregs (Kong et al. 2009; Larmonier et al. 2008; Pallandre et al. 2007). Of interest, co-culturing MDSCs with T cells induces the Foxp3+ Treg phenotype in both mouse and human tumor models, leading to tumor-induced T cell tolerance (Hoechst et al. 2008; Serafini et al. 2008).
3 Therapeutic Relevance
As a point of convergence for numerous oncogenic signaling pathways, Stat3 is continuously activated in various human cancers. Numerous genetic studies validate Stat3 as one of the most promising target for cancer immunotherapy. Moreover, new approaches directly targeting Stat3, either alone or in conjunction with other therapeutic modalities, elicit robust anti-tumor immune responses that are highly efficacious in the treatment of cancer (Table 1).
Table 1.
Therapeutic strategies under investigation with the intent of abrogating Stat3-mediated signaling
| Approaches | Mechanism | References |
|---|---|---|
| Stat3 ablation | Preclinical in vivo studies suggest that ablation of Stat3 in tumor cells or tumor-associated immune cells decreases tumor progression | Kortylewski et al. (2005), Wang et al. (2004), Yu and Jove (2004), and Yu et al. (2007, 2009) |
| JAK inhibitors | Use of these agents (i.e., AG490, WP1066, AZD1480) decreases Stat3 activation and augments the tumor-associated immune response in preclinical models of both hematologic and solid tumors | Burdelya et al. (2002), Fujita et al. (2008), Hussain et al. (2007), Kong et al. (2008), Kong et al. (2009), and Nefedova et al. (2005) |
| Other tyrosine kinase inhibitors | Inhibitors of fusion proteins (i.e., products of NPM/ALK or BCR-ABL) Both directly and indirectly inhibit activation of Stat3 Agents such as sunitinib decrease recruitment of Tregs and MDSC to sites of tumor in a Stat3-dependent fashion | Kasprzycka et al. (2006), Larmonier et al. (2008), Ozao-Choy et al. (2009), and Xin et al. (2009) |
| CpG-siRNA | Stat3 siRNA linked to the Toll-like receptor agonist 9 (TLR9), CpG, both silences genes in TLR9(+) myeloid cells and decreases the Stat3-mediated immune response; in preclinical models, a marked antitumor effect is observed | Kortylewski et al. (2009a, b) |
3.1 Genetic Evidence and Potential Toxicity
Mouse studies using knockout mice demonstrate that ablation of Stat3 in either tumor cells or tumor-associated immune cells prevent tumor progression (Bollrath et al. 2009; Chiarle et al. 2005; Kortylewski et al. 2005). Potent anti-tumor immune responses can also be achieved from reconstitution of mice with Stat3-deficient immune cells (Kortylewski et al. 2005). Although long-term ablation of Stat3 in hematopoietic cells can lead to severe inflammatory disease (Welte et al. 2003), there appears to be a therapeutic window for inducing antitumor immune responses (Kortylewski et al. 2009a,b,c). Recent genetic studies in patients afflicted by HIES reveal that dominant-negative mutations in STAT3 are strongly associated with the disease (Minegishi et al. 2007). All mutations are located in the Stat3 DNA-binding domain and as a result, signaling responses to cytokines, including IL-6 and IL-23, are defective (Milner et al. 2008; Minegishi 2009; Minegishi et al. 2007). These studies in individuals with HIES suggest that short term STAT3 blockade may not lead to severe side effects and that STAT3 may be exploited as a molecular target for therapeutic development.
3.2 JAK Inhibitors
Aberrant Stat3 activity in cancer, to a large degree, is the result of overactivation of upstream tyrosine kinases. Owing to the fact that Jak tyrosine kinase is an important activator of Stat3 both in tumor and immune cells in the tumor microenvironment, much effort has been devoted to studying Jak kinase inhibitors in various tumor models. The prototype Jak inhibitor, AG490, prevents Stat3 phosphorylation and activation of its downstream pro-survival genes (Rahaman et al. 2002). The opportunity to use AG490 was shown to enhance immunotherapy by several studies. For examples, in vivo administration of AG490 in conjunction to IL-12 results in better anti-tumor effects than either one alone (Burdelya et al. 2002). A structurally related compound, WP1066, also disrupts Jak/Stat3 activation and reduces the malignant tumor growth (Ferrajoli et al. 2007; Iwamaru et al. 2007). WP1066 has the capacity to penetrate blood–brain barrier and has demonstrated activity in preclinical glioma models (Hussain et al. 2007). Consistent with the role of Stat3 in inducing and maintaining tumor-associated Tregs is the observation that tumors treated with WP1066 show a marked reduction in number of Tregs. This, in turn, results in reversal of immune tolerance elicited by Tregs (Kong et al. 2009). Tumor growth in mice with subcutaneously established syngeneic melanoma was markedly inhibited by WP1066 (Kong et al. 2008).
Another Jak2/Stat3 inhibitor shown to induce anti-tumor immune responses is JSI-124, a member of curcubitacin compounds (Blaskovich et al. 2003). Treatment of tumors with JSI-124 limits the number of tumor-infiltrating MDSCs, inhibits DC differentiation, and thereby inhibits tumor growth (Fujita et al. 2008; Nefedova et al. 2005). Improved anti-tumor immune responses achieved by JSI-124 are associated with prolonged survival in murine glioma models. Tumor response appears to be dependent upon host immunity (Fujita et al. 2008). Importantly, combined use of JSI-124 with DC vaccines for the treatment of mouse sarcoma induces IFNγ production by CD8+ T cells and synergistic eradication of tumors (Nefedova et al. 2005).
As with the previously noted compounds, the novel JAK2 inhibitor AZD1480 also caused growth arrest in solid tumor cell lines with cytokine-induced Stat3 activation (Hedvat et al. 2009). In these studies, JAK2 inhibition resulted in decreased nuclear translocation of Stat3 and proliferation. Studies are underway to evaluate this compound in modulating the tumor immunologic environment. The agent is currently undergoing clinical evaluation in the setting of myelofibrosis (NCT00910728), and further studies in solid tumors are highly anticipated. Collectively, these studies indicate that targeting of Stat3 using Jak2 inhibitors have the potential to revert tumor mediated-immune suppression and generate anti-tumor immune responses.
3.3 Other Oncogenic Kinase Inhibitors
Numerous oncoproteins (including NPM/ALK, Src and BCR-ABL) possess intrinsic kinase activity and may regulate Stat3 activity. For example, chromosomal translocations that juxtapose NPM and ALK lead to ALK overexpression and concomitant Stat3 activation in anaplastic large cell lymphoma (ALCL) (Chiarle et al. 2005). Persistent Stat3 activation by NPM/ALK facilitates induction of Treg-like phenotypes in ALCLs by promoting secretion of IL-10 and TGF-β as well as expression of Foxp3 (Kasprzycka et al. 2006). Moreover, Stat3 activation by NPM/ALK negatively modulates immune responses by activating gene transcription of immunosuppressive cell surface protein CD274 (B7-H1) in T cell lymphoma, where Stat3 directly binds to the promoter region of CD274 (Marzec et al. 2008). Given that antibody-mediated blockade of CD274 in conjunction with T cell depletion therapy leads to complete tumor regression (Webster et al. 2007), targeting NPM/ALK-mediated STAT3 activity may offer therapeutic advantages for the treatment of T cell lymphoma. Two small molecular inhibitors, WHI-131 and 154, effectively inhibit Stat3 phosphorylation by blocking enzymatic activity of NPM/ALK (Marzec et al. 2005). More detailed investigation is required to identify whether desirable anti-tumor immune responses are elicited by these compounds.
Targeting BCR-ABL also reverses Stat3-mediated immune suppression in tumors. The most widely studied BCR-ABL kinase inhibitors, imatinib mesylate is applied as standard therapy for the treatment of Philadelphia chromosome-positive CML and gastrointestinal stromal tumor (GIST), where it has demonstrated significant clinical activity (Blanke et al. 2008; Druker et al. 2001). Intriguing findings related to the immune responses associated with imatinib treatment have been reported. However, treatment with imatinib induces both suppressive as well as stimulating effects on CD4+ and CD8+ T cells or DCs, suggesting the exact nature of imatinib effect on immune cells remains to be further explored. Nonetheless, used at clinically achievable concentrations, imatinib reduces suppressive activity of Tregs as well as Foxp3 expression in Tregs through inhibition of Stat3 (Larmonier et al. 2008). Also, the quantity of tumor-infiltrating Tregs is diminished with imatinib therapy (Larmonier et al. 2008). Similar to Jak inhibitors, treatment of tumor with imatinib significantly enhances the efficacy of DC vaccine against lymphoma, lessening tumor metastasis in conjunction with effective IFNγ production by splenocytes (Larmonier et al. 2008). All of these studies indicate that there is a significant opportunity to advance immunotherapy using imatinib. Although imatinib does have substantial activity in CML, a proportion of patients do become resistance to this agent. Presumably, the bone marrow (which harbors multiple soluble factors that activate Stat3) is an ideal environment for the development of resistance CML clones (Bewry et al. 2008). While Stat3 activity may be downregulated to some extent by imatinib-mediated Abl inhibition, other kinases, such as Jak kinase, may continue to drive Stat3 activation (Sen et al. 2009). Using direct Stat3 inhibitors in conjunction with imatinib may prevent Stat3 reactivation by pleiotropic Stat3 activators present in the tumor microenvironment.
3.4 RTK Inhibitors
In addition to cytokines and oncoproteins, Stat3 is constitutively activated in cancers by many growth factors including EGF, PDGF, and VEGF. Receptors for these growth factors are transmembrane receptor tyrosine kinases (RTKs), which trigger downstream signaling through a number of distinct cascades. Targeting RTKs can be a clinically effective strategy across a wide variety of malignancies, including lung cancer, hepatocellular carcinoma (HCC) and mRCC (Llovet et al. 2008; Motzer et al. 2009; Shepherd et al. 2005). However, a multitude of escape mechanisms exist to circumvent RTK inhibitors. Given that Stat3 represents a point of convergence for numerous growth factor signaling pathways in tumors, targeting Stat3 activation may be a useful strategy.
Sunitinib is a multi-targeted growth factor inhibitor that is widely used in the treatment of mRCC (Motzer et al. 2009). The inhibitory effect of sunitinib on a variety of kinases impairs Stat3 activation in tumors, thereby inducing tumor cell apoptosis (Xin et al. 2009; Yang et al. 2010). Treatment with sunitinib intensifies the anti-tumor immune response by limiting the number of tumor-associated MDSCs and Tregs in mouse tumor models (Xin et al. 2009). mRCC patients show elevated levels of CD33+HLA-DR− and CD15+CD14− MDSCs in peripheral blood (Ko et al. 2009). While treatment with sunitinib results in inhibition of Treg mediators such as IL-10, TGF-β, and Foxp3, it also elevates Th1 cytokine and IFNγ in murine models (Ozao-Choy et al. 2009). Moreover, treatment of tumors with sunitinib increases efficacy of IL-12 based immune activation therapy (Ozao-Choy et al. 2009). Therefore, sunitinib-based therapy has great potential to modulate anti-tumor immunity as an adjunct for the treatment of certain human cancers including RCC.
It is important to note that the effects of sunitinib may not extend across this class of agents. As one example, the small molecule RTK inhibitor sorafenib is also widely used in the treatment of mRCC (Escudier et al. 2007). In contrast to sunitinib, recent studies suggest that sorafenib inhibits the function of DCs and decreases induction of antigen-specific T-cells (Hipp et al. 2008). Thus, the immunologic phenomena triggered by RTK inhibitors should be separately considered in tailoring clinical strategies.
3.5 siRNA
Combined use of kinase-targeted Stat3 inhibitors with other immunotherapeutic approaches such as tumor vaccines may augment efficacy of cancer immunotherapy. For example, combination of DC-based vaccine together with RTK inhibitors leads to a greater therapeutic efficacy in preclinical models, suggesting various levels of Stat3 inhibition facilitate immune cell mediated anti-tumor effect (Larmonier et al. 2008; Nefedova et al. 2005). Alternatively, these inhibitors can synergize with different immune modulators that elicit innate immunity, such as the Toll-like receptor agonist CpG. Given that Stat3 downregulates CpG-mediated innate immune responses, ablation of Stat3 has been shown to enhance and prolong a potent anti-tumor immune responses elicited by CpG in a murine melanoma xenograft model (Kortylewski et al. 2009a). Furthermore, conjugation of CpG to siRNA targeting Stat3 activates various populations of immune cells including DCs and macrophages and ultimately induces robust anti-tumor immune responses (Kortylewski et al. 2009b). Therefore, CpG-coupled siRNA can maximize therapeutic efficacy by inducing anti-tumor responses through CpG while knocking down Stat3.
As previously noted, a major hurdle in the clinical use of RTK inhibitors is the development of resistance mechanisms. This concept is supported by a recent study demonstrating that long-term sunitinib treatment increases tumor cell invasiveness and metastasis (Paez-Ribes et al. 2009). Accelerated tumor progression upon prolonged sunitinib treatment is in part mediated by intense hypoxia during metastatic processes (Paez-Ribes et al. 2009). The role of Stat3 in regulating the expression of hypoxia-inducible factor-1α (HIF-1α), which is a critical regulator of hypoxic response in tumors, has been previously shown (Niu et al. 2008). Directly targeting Stat3 using gene-specific approaches, such as CpG-Stat3 siRNA, may thus overcome undesirable effects of sunitinib by reducing tumor hypoxia. Using CpG-Stat3 siRNA and sunitinib in combination therefore may have clinical merit.
4 Concluding Remarks
Stat3 is persistently activated in diverse cancers, promoting tumor cell survival, proliferation, angiogenesis/metastasis, and immune escape. Targeting Stat3 has the potential to not only directly inhibit tumor growth but also alter the tumor immunologic environment in favor of immunotherapy. Stat3 therefore represents a promising target for cancer therapy. With the emergence of Stat3 inhibitors, both indirect and direct, we are entering a new era of cancer immunotherapy.
Contributor Information
Heehyoung Lee, Beckman Research Institute, City of Hope Comprehensive Cancer Center, 1500 East Duarte Road, Duarte, CA 91010, USA.
Sumanta Kumar Pal, Division of Genitourinary Malignancies, Department of Medical Oncology and Experimental Therapeutics, City of Hope Comprehensive Cancer Center, 1500 East Duarte Road, Duarte, CA 91010, USA.
Karen Reckamp, Division of Thoracic Malignancies, Department of Medical Oncology and Experimental Therapeutics, City of Hope Comprehensive Cancer Center, 1500 East Duarte Road, Duarte, CA 91010, USA.
Robert A. Figlin, Department of Medical Oncology and Experimental Therapeutics, City of Hope Comprehensive Cancer Center, 1500 East Duarte Road, Duarte, CA 91010, USA
Hua Yu, Email: hyu@coh.org, Cancer Immunotherapeutics and Tumor Immunology, City of Hope Comprehensive Cancer Center, 1500 East Duarte Road, Duarte, CA 91010, USA.
References
- Bewry NN, Nair RR, Emmons MF, Boulware D, Pinilla-Ibarz J, Hazlehurst LA. Stat3 contributes to resistance toward BCR-ABL inhibitors in a bone marrow microenvironment model of drug resistance. Mol Cancer Ther. 2008;7:3169–3175. doi: 10.1158/1535-7163.MCT-08-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007;67:5479–5488. doi: 10.1158/0008-5472.CAN-06-3963. [DOI] [PubMed] [Google Scholar]
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VHC, Baker LH, Maki RG, et al. Phase III Randomized, Intergroup Trial Assessing Imatinib Mesylate At Two Dose Levels in Patients With Unresectable or Metastatic Gastrointestinal Stromal Tumors Expressing the Kit Receptor Tyrosine Kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdelya L, Catlett-Falcone R, Levitzki A, Cheng F, Mora LB, Sotomayor E, Coppola D, Sun J, Sebti S, Dalton WS, et al. Combination therapy with AG-490 and interleukin 12 achieves greater antitumor effects than either agent alone. Mol Cancer Ther. 2002;1:893–899. [PubMed] [Google Scholar]
- Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ Regulatory T Cells Control TH17 Responses in a Stat3-Dependent Manner. Science. 2009;326 (5955):986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, Karras JG, Levy DE, Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, et al. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Dercamp C, Chemin K, Caux C, Trinchieri G, Vicari AP. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 2005;65:8479–8486. doi: 10.1158/0008-5472.CAN-05-1319. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in the Blast Crisis of Chronic Myeloid Leukemia and Acute Lymphoblastic Leukemia with the Philadelphia Chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W, et al. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 2007;67:11291–11299. doi: 10.1158/0008-5472.CAN-07-0593. [DOI] [PubMed] [Google Scholar]
- Fujita M, Zhu X, Sasaki K, Ueda R, Low KL, Pollack IF, Okada H. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180:2089–2098. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 Promotes Tumor Development through the Induction of Tumor Promoting Microenvironments at Tumor Sites and Myeloid-Derived Suppressor Cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et al. The JAK2 Inhibitor AZD1480 Potently Blocks Stat3 Signaling and Oncogenesis in Solid Tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol. 2004;172:4630–4636. doi: 10.4049/jimmunol.172.7.4630. [DOI] [PubMed] [Google Scholar]
- Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–696. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, Priebe W, Heimberger AB. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–9636. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- Ito N, Eto M, Nakamura E, Takahashi A, Tsukamoto T, Toma H, Nakazawa H, Hirao Y, Uemura H, Kagawa S, et al. STAT3 Polymorphism Predicts Interferon-Alfa Response in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2007;25:2785–2791. doi: 10.1200/JCO.2006.09.8897. [DOI] [PubMed] [Google Scholar]
- Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–2444. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- Kasprzycka M, Marzec M, Liu X, Zhang Q, Wasik MA. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc Natl Acad Sci USA. 2006;103:9964–9969. doi: 10.1073/pnas.0603507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, Fuller GN, Fokt I, Grimm EA, Schmittling RJ, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–5768. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LY, Wei J, Sharma AK, Barr J, Abou-Ghazal MK, Fokt I, Weinberg J, Rao G, Grimm E, Priebe W, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58:1023–1032. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Herrmann A, Yang C, Wang L, Liu Y, Salcedo R, Yu H. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res. 2009a;69:2497–2505. doi: 10.1158/0008-5472.CAN-08-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009b;27(10):925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009c;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil S, Waghorn K, Ernst T, Chase A, White H, Hehlmann R, Reiter A, Hochhaus A, Cross NC. A polymorphism associated with STAT3 expression and response of chronic myeloid leukemia to interferon alpha. Haematologica. 2010;95:148–152. doi: 10.3324/haematol.2009.011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LT, Wright G, Davis RE, Lenz G, Farinha P, Dang L, Chan JW, Rosenwald A, Gascoyne RD, Staudt LM. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111:3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larmonier N, Janikashvili N, LaCasse CJ, Larmonier CB, Cantrell J, Situ E, Lundeen T, Bonnotte B, Katsanis E. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J Immunol. 2008;181:6955–6963. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesinski GB, Trefry J, Brasdovich M, Kondadasula SV, Sackey K, Zimmerer JM, Chaudhury AR, Yu L, Zhang X, Crespin TR, et al. Melanoma cells exhibit variable signal transducer and activator of transcription 1 phosphorylation and a reduced response to IFN-alpha compared with immune effector cells. Clin Cancer Res. 2007;13:5010–5019. doi: 10.1158/1078-0432.CCR-06-3092. [DOI] [PubMed] [Google Scholar]
- Li C, Zhang F, Lin M, Liu J. Induction of S100A9 Gene Expression by Cytokine Oncostatin M in Breast Cancer Cells Through the STAT3 Signaling Cascade. Breast Cancer Research and Treatment. 2004;87:123–134. doi: 10.1023/B:BREA.0000041594.36418.f6. [DOI] [PubMed] [Google Scholar]
- Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol. 2006;177:1679–1688. doi: 10.4049/jimmunol.177.3.1679. [DOI] [PubMed] [Google Scholar]
- Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Natl Acad Sci USA. 2008;105:8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Kasprzycka M, Ptasznik A, Wlodarski P, Zhang Q, Odum N, Wasik MA. Inhibition of ALK enzymatic activity in T-cell lymphoma cells induces apoptosis and suppresses proliferation and STAT3 phosphorylation independently of Jak3. Lab Invest. 2005;85:1544–1554. doi: 10.1038/labinvest.3700348. [DOI] [PubMed] [Google Scholar]
- Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y. Hyper-IgE syndrome. Curr Opin Immunol. 2009;21:487–492. doi: 10.1016/j.coi.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabarro S, Himoudi N, Papanastasiou A, Gilmour K, Gibson S, Sebire N, Thrasher A, Blundell MP, Hubank M, Canderan G, et al. Coordinated oncogenic transformation and inhibition of host immune responses by the PAX3-FKHR fusion oncoprotein. J Exp Med. 2005;202:1399–1410. doi: 10.1084/jem.20050730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00910728. [Accessed 17 Feb 2010];A PhaseI/II, Open label multi-centre study to assess the safety, tolerability, pharmacokinetics and preliminary efficacy of the JAK2 inhibitor AZD1480 administered orally to patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF) 2010 http://www.clinicaltrials.gov.
- Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–9535. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Briggs J, Deng J, Ma Y, Lee H, Kortylewski M, Kujawski M, Kay H, Cress WD, Jove R, et al. Signal transducer and activator of transcription 3 is required for hypoxia-inducible factor-1alpha RNA expression in both tumor cells and tumor-associated myeloid cells. Mol Cancer Res. 2008;6:1099–1105. doi: 10.1158/1541-7786.MCR-07-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallandre JR, Brillard E, Crehange G, Radlovic A, Remy-Martin JP, Saas P, Rohrlich PS, Pivot X, Ling X, Tiberghien P, et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J Immunol. 2007;179:7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009;69:1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, et al. Erlotinib in Previously Treated Non-Small-Cell Lung Cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Liu TY, Narita Y, Suzuki M, Nakatsuka R, Araki T, Matsumoto M, Iwai LK, Hirosawa N, Matsuoka Y, et al. Cytokine-dependent modification of IL-12p70 and IL-23 balance in dendritic cells by ligand activation of Valpha24 invariant NKT cells. J Immunol. 2009;183:201–208. doi: 10.4049/jimmunol.0900873. [DOI] [PubMed] [Google Scholar]
- Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Wang W, Edington HD, Rao UN, Jukic DM, Land SR, Ferrone S, Kirkwood JM. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin Cancer Res. 2007;13:1523–1531. doi: 10.1158/1078-0432.CCR-06-1387. [DOI] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster WS, Thompson RH, Harris KJ, Frigola X, Kuntz S, Inman BA, Dong H. Targeting molecular and cellular inhibitory mechanisms for improvement of antitumor memory responses reactivated by tumor cell vaccine. J Immunol. 2007;179:2860–2869. doi: 10.4049/jimmunol.179.5.2860. [DOI] [PubMed] [Google Scholar]
- Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Rhee K-J, Albesiano E, Rabizadeh S, Wu X, Yen H-R, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DH, Park JS, Jin CJ, Kang HK, Nam JH, Rhee JH, Kim YK, Chung SY, Choi SJ, Kim HJ, et al. The dysfunction and abnormal signaling pathway of dendritic cells loaded by tumor antigen can be overcome by neutralizing VEGF in multiple myeloma. Leuk Res. 2009;33:665–670. doi: 10.1016/j.leukres.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res. 2010;8:35–45. doi: 10.1158/1541-7786.MCR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer–new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerer JM, Lesinski GB, Kondadasula SV, Karpa VI, Lehman A, Raychaudhury A, Becknell B, Carson WE., 3rd IFN-alpha-induced signal transduction, gene expression, and antitumor activity of immune effector cells are negatively regulated by suppressor of cytokine signaling proteins. J Immunol. 2007;178:4832–4845. doi: 10.4049/jimmunol.178.8.4832. [DOI] [PubMed] [Google Scholar]
- Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]