Abstract
Embryonic stem cells have the capacity to differentiate into a wide range of cell types. We previously described that blastocyst injection of wild type (WT) embryonic stem cells (ESCs) into various knockout (KO) mouse models of human disease prevents disease from occurring. In this study we ask if the blastocyst approach can also correct defects in a mouse model of transgenic (Tg) overexpression of a pro-apoptotic factor. We injected ROSA26 (LacZ-marked) WT ESCs into human mammalian sterile 20 like-kinase 1 (Mst1) Tg blastocysts. Mst1 Tg mice overexpress Mst1, a pro-apoptotic factor, in a cardiac-specific manner. As a result, Mst1 Tg mice develop adult dilated cardiomyopathy driven by apoptosis, reduction in cell density and no hypertrophic compensation. Incorporation of WT ESCs generated WT/Mst1 chimeric mice with normal hearts at histological and functional levels. Accordingly, apoptosis and cell density parameters were normalized. The experiments suggest that an adult-onset cardiac myopathy induced by overexpression of the pro-apoptotic Mst1 can be reversed by developmental incorporation of WT ESCs. The findings also suggest that since forced expression of the Mst1 transgene is not abolished in the rescued chimeras, the WT ES-derived cells normalize pathways that lie downstream of Mst1. The results expand the therapeutic capability of the ESCs to mouse models that overproduce detrimental proteins.
Keywords: Mst1, ESCs, Chimera, Cardiomyopathy, Apoptosis
Introduction
ESCs possess the unique capacity to differentiate into a diverse range of cell types that compose the human body. For this reason, ESC research is a vital area of investigation that holds significant promise for the continued development of regenerative cell therapies. An emerging technique is the development of chimeric mice derived from the injection of WT ESCs into mutant blastocysts–preimplantation embryos that would otherwise be prone to disease development. Blastocyst injection results in the generation of chimeras that are a combination of innately derived mutant cells and externally derived WT ESCs. The WT ESCs undergo mutual recognition with the host mutant cells and incorporate into murine development. This approach opens the opportunity to unravel the underlying molecular mechanisms involved in the corrections by the ES-derived cells [1, 2]. To date, blastocyst injection of WT ESCs has been shown to rescue mice with loss-of-function mutations of key genes. The “thin myocardial wall syndrome” is a result of a deletion of members of the Inhibitor of DNA binding (Id) family of nuclear factors, causing embryonic death at midgestation [3, 4]. Blastocyst injection of WT ESCs showed that incorporation of ESCs rescues embryonic lethality in Id1Id3 double KO embryos by correcting the prominent cardiac phenotype [3–5]. Another disease model rescued by blastocyst injection is Duchenne muscular dystrophy (DMD). DMD is caused by a mutation in the dystrophin gene that ablates the production dystrophin [6], a structural protein which is essential for muscle membrane stability [7]. The DMD phenotype is recapitulated in mdx mice [8]. WT ESCs were injected into mdx blastocyts, and the resultant chimeras showed complete morphological and histological recovery of the skeletal muscle [9]. The blastocyst rescue is accomplished by the combination of conventional mechanisms of protein complementation and induction of neomorphic pathways [1–4, 9, 10]. While the former mechanism involves the supply of signals from the ESCs that are lacking in the mutant compartment, the latter mechanism involves the appearance of novel pathways that would not exist in the WT or in the mutant environment if they were not mixed [1, 2].
We wanted to examine the potential of WT ESCs in their capacity to rescue a model of forced overexpression. Mammalian sterile 20-like kinase 1 (Mst1) is a ubiquitiously expressed serine threonine kinase known primarily to activate apoptotic cell death in response to environmental stressors [11]. Mst1 is activated by caspases. This elevated activity can in turn trigger the activation of caspase-3 resulting in an amplification loop for cell death. When active, Mst1 will translocate to the nucleus where it will phosphorylate pro-apoptotic transcription factors and histones [12]. Cardiac-specific Mst1 transgenic overexpression results in dilated cardiac myopathy as a result of excessive cardiomyocyte apoptosis via caspase-3 activation [13]. Because compensatory ventricular hypertrophy is not observed, an extreme circumstance occurs which ultimately results in increased wall stress [13, 14].
In the current study, we employ the blastocyst injection method to ascertain if cardiac-specific pathological overexpression of a protein, in this case Mst1, can be overcome by blastocyst injection of WT ESCs. We report that even in this model of forced overproduction, the ESCs present therapeutic capability.
Materials and Methods
Embryonic Stem Cells
R26 LacZ-marked WT ESCs were developed and provided by Dr. Phillipe Soriano. R26 WT ESCs grow in DMEM with high glucose, 15% FCS, glutamine, nonessential amino acids, β-mercaptoethanol, antibiotics and on SNLa76/7 STO cells, which constitutively express LIF.
Generation of Chimeric Mice
Three week-old WT (B6/C57, Jax Labs) females were superovulated (PMSG, 5000 IU and HCG, 3100 IU, VWR) and mated with Mst1 Tg males generously provided by Dr. Junichi Sadoshima (line 28, high overexpressor [13]). Mst1 mice express transgenic human Mst1 in the adult heart, driven by the α-myosin heavy chain (αMHC) promoter [13]. Blastocysts were collected at 3.5 days after mating and injected with 15 R26 WT ESCs. Injected blastocysts were then transferred into the uteri of pseudopregnant females and allowed to develop to term. The Mst1 transgene of WT/Mst1 chimeras was identified by genomic PCR [13] using DNA from tail tips of 1 week-old pups. The percentage of ESC incorporation was determined by X-gal staining on tail tip cryosections and later confirmed upon sacrifice at 5 months of age through X-gal staining on 10 µm cryosections of tail tips, liver and heart tissue [4, 9]. Immunofluorescence for Mst1 on heart cryosections as described below further confirmed the percentage of ESC incorporation.
Histology, Immunofluorescence, X-gal Staining, Cell Density and Western Blot
Immunofluorescence was performed on 10 µm thick heart cryosections at a 1:50 dilution using mouse anti-human Mst1 primary antibody (BD Transduction Laboratories) and goat anti-mouse Alexa 488 (Invitrogen) secondary antibody. For apoptosis assessment, fluorescent detection of apoptosis was performed on 6 µm thick paraffin sections using Terminal Transferase, recombinant (Roche), Biotin-16-dUTP (Roche), and Streptavidin Alexa 488 (Invitrogen). Sections were pretreated with proteinase K (Qiagen) incubation [13]. X-gal staining was performed on tail tip, heart, lung, and liver cryosections with X-gal (1 mg/mL) in PBS buffer containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 overnight at 37° C followed by subsequent eosin counterstaining[4, 9]. Visualization of fibrosis was performed using a Masson Trichrome Stain Kit (Richard-Allan Scientific). Western Blot was performed for Mst1 (BD Transduction Laboratories) with tubulin control (abcam) using standard methods. Cell density and myocyte cross-sectional area was determined on digitized images of rhodamine-labeled wheat germ agglutinin-stained sections of paraffin-embedded samples [15].
Echocardiography
Echocardiographs were performed on WT, WT/Mst1 and Mst1 mice at 5 months of age to determine left ventricular Stem Cell Rev and Rep (LV) systolic fraction [13]. Mice were anesthetized by intraperitoneal injection 2.5% Avertin 290 mg/kg. Trans-thoracic echocardiography (Sequoia C256; Acuson, Mountain View, CA) was performed using a 13-MHz linear ultrasound transducer. The chest was shaved. Mice were placed on a warm saline bag in a shallow left lateral position and warm coupling gel applied to the chest. Small-needle electrocardiographic leads were attached to each limb. Two-dimensional images and LV M-mode tracing (sweep speed = 100–200 mm/s) were recorded from the parasternal short-axis view at the mid papillary muscle level. M-mode measurements of LV internal diameter (LVID) and wall thicknesses were made from 3 consecutive beats and averaged using the leading edge-to-leading edge convention adopted by the American Society of Echocardiography. End-diastolic measurements were taken at the peak of R wave of EKG. End-systolic measurements were made at the time of the most anterior systolic excursion of the posterior wall. LV ejection fraction (EF) was calculated by the cubed methods as follows: LVEF(%) = 100*[(LVIDd)3 − (LVIDs)3]/(LVIDd)3, LV fractional shortening: (LVFS%) = 100*(LVIDd − LVIDs)/LVIDd, where d indicates diastolic and s indicates systolic. Heart rate was determined from at least three consecutive RR intervals on the LV M-mode tracing. Following echocardiography at P180, mice were sacrificed and hearts harvested for histological analyses as described above.
Microarray Analysis
Total RNA from heart tissue was isolated from 5-month old WT, Mst1 or WT/Mst1 mice (RNeasy, QIAGEN). RNAwas converted to cDNA, cRNA, and hybridized to DNA sequences contained in the MOEA2.0 (14,000 murine well-characterized genes, Affymetrix) chip. Information from at least duplicate samples was compared and filtered by fold change >2 and statistical p-value<0.001.
Data Analysis
Results are presented as mean ± s.e.m. Statistical comparison was performed with nonparametric two-tailed unpaired analysis of variance. A probability value of <0.05 was considered to be statistically significant.
Results
ESCs Do Not Abolish Mst1 Transgenic Overexpression in WT/Mst1 Chimeras
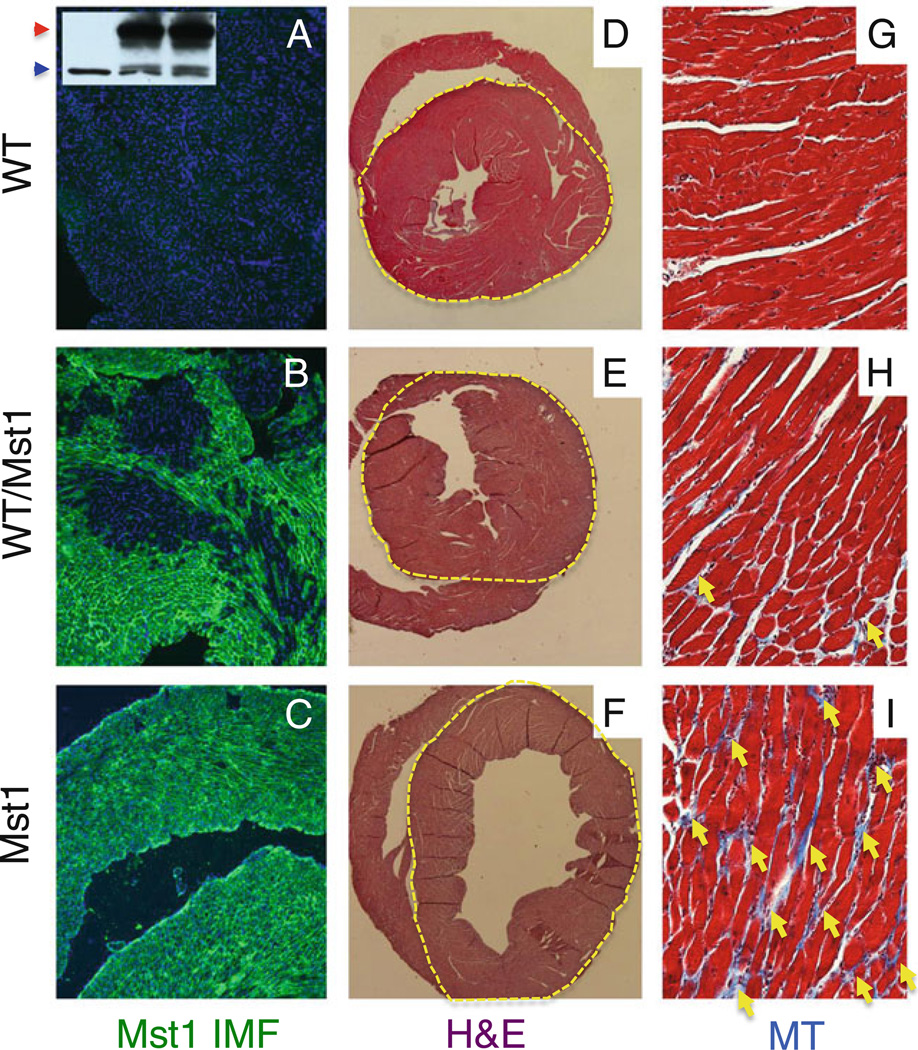
We injected 15 ROSA26 (R26) LacZ-marked WT ESCs into Mst1 transgenic blastocysts that are predisposed to overexpress Mst1 in the heart leading to adult dilated cardiomyopathy. We generated 5 WT/Mst1 chimeric mice with an average of WT mosaicism of 40%, which were compared to 7 WT and 6 Mst1 Tg mice. The degree of ESC incorporation was initially determined by X-gal staining of tail biopsies taken from 1 week-old pups (data not shown), and later confirmed at time of sacrifice (5 months) by Mst1 immunofluorescence on heart sections (Fig. 1a–c). Patchy areas of Mst1 positive cells, derived from the Mst1 transgenic blastocyst, and Mst1 negative cells, derived from the WT ESCs, were observed in the WT/Mst1 chimeric hearts while WT control was negative and Mst1 Tg positive (Fig. 1a–c). Cardiac-specific overexpression of Mst1 was confirmed by western blot analysis (Fig. 1a inset). X-gal staining was observed in all tissues analyzed (liver, muscle, heart, tail) indicating ESC incorporation globally (data not shown).
Fig. 1.
ESCs reverse cardiomyopathy in WT/Mst1 chimeras. a–c Immunofluorescence showing detection of Mst1 in Mst1 transgenic (c) and WT/Mst1 chimeric (b) (note patchy areas of Mst1 staining in the WT/Mst1 chimera), but not in WT (a) heart sections. a inset: WB showing detection of Mst1 (red arrowhead) in Mst1 transgenic (right lane) and WT/Mst1 chimeric (center lane), but not in WT (left lane) hearts (blue arrowhead: tubulin control). d–f H&E staining showing reversion of dilated cardiomyopathy in the WT/Mst1 chimeric hearts (yellow dotted circle demarcates the left ventricle). g–i Masson Trichrome staining showing reduction of fibrosis (yellow arrows) in WT/Mst1 chimeric hearts. DAPI in blue (a–c) demarcates nuclei. Magnification: 100× (a–c); 10× (d–f) and 400× (g–i)
ESCs Partially Reverse Cardiomyopathy in WT/Mst1 Chimeras
Five month-old WT/Mst1 chimeric mice displayed normal hearts (Fig. 1d–i and Table 1). H&E staining of transverse heart sections showed a rescue of cardiac enlargement in WT/Mst1 chimeric mice (Fig. 1d–f). Accordingly, WT/Mst1 chimeras display no congested lungs (Table 1), indicative of no deficit in cardiac function. Masson Trichrome staining for fibrosis showed a reversion in interstitial fibrosis in WT/Mst1 chimeric hearts to levels comparable to WT (Fig. 1g–i). Accordingly, reversion of the expression of collagen markers was observed at gene expression profile levels (Table 2). Remarkably, a significant amelioration of myocardial apoptosis and a concomitant increase in cell density was observed in WT/Mst1 hearts relative to Mst1 hearts (Table 1). Reversion of morphological and molecular parameters in WT/Mst1 was accompanied by reversion of functional deficits, as echo-cardiographic measurements of WT/Mst1 showed ejection fraction readings of 60%, similar to those of WT mice (Table 1).
Table 1.
Apoptosis, cell density and echocardiographic readings are partially reversed in WT/Mst1 chimeras
| WT | WT/Mst1 | Mst1 | |
|---|---|---|---|
| Heart (mg)/body weight (g) a,b | 4.69±0.69 | 4.14±0.60 | 4.95±0.85 |
| Lung (mg)/body weight (g) a,c | 5.21±0.11 | 5.18±0.37 | 6.92±0.34 |
| TUNEL positive myocytes (%)c,d | 0.04±0.01 | 0.11±0.01 | 0.29±0.01 |
| Myocyte density (number/mm2) c,d | 3,982±102 | 3,640±93 | 2,942±24 |
| LVEDD (mm) a,c | 4.14±0.21 | 4.48±0.45 | 4.77±0.45 |
| LVESD (mm) a,c | 2.80±0.14 | 3.30±0.33 | 4.06±0.35 |
| LVEF (%)a,c | 69±3 | 60±7 | 39±5 |
| %FSa,c | 32±3 | 26±4 | 15±3 |
LVEDD, LVESD: Left Ventricular End Diastolic and Systolic Dimension respectively
P>0.05, WT/Mst1 versus WT
P>0.05, WT/Mst1 versus Mst1
P<0.05, WT/Mst1 versus Mst1
P<0.05 WT/Mst1 versus WT
Table 2.
Collagen markers are partially reversed in WT/Mst1 chimeras
| Gene symbol | ID | fold (vs WT) | |
|---|---|---|---|
| Mst1 | WT/Mst1 | ||
| Col8a1 | NM_007739 | +3.90 | +3.35 |
| Col12a1 | NM_007730 | +3.34 | UC |
| Col1a2 | NM_007743 | +2.18 | UC |
| Col14a1 | NM_181277 | +2.01 | UC |
UC: unchanged (cut-off: fold change > 2)
Discussion
We use the chimeric approach to study mechanisms of corrections exerted by neighbor cells [1–4, 9, 10]. In previous studies, WT ES-derived cells were able to prevent disease from occurring in a loss-of-function (KO) mutation [4, 9]. As a result, the tissue (heart, muscle) was corrected as a whole [4, 9]. In this case however, it is remarkable to note that WT ES-derived cells can also correct the overproduction of a pro-apoptotic protein. The reversion is not complete, but as in the case of the KO corrections, the effects are global in terms of important histological, functional and molecular parameters. This indicates that not only the WT ES-derived cells are normal, but also the mutant cells (in this case the Mst1 Tg cells) experience corrections. The reversion is not due to inhibition of the expression of the transgene, as Mst1 Tg expression is observed at high levels in the mutant portion of the chimeric heart. Thus, there must be mechanisms put in place downstream of the Mst1 pathway whereby signals from the WT cells enter the Mst1 apoptotic pathway, circumvent the deleterious role of Mst1 excess and inhibit the cascade of apoptotic events that lead to dilated cardiomyopathy. Because the mutant portion of the Mst1 Tg heart does not lack a key protein as in the case of KO models—rather it produces excessive protein, it would be intriguing to identify the potential WT ES-derived mechanisms (perhaps neomorphic) involved in the neutralization of the pro-apoptotic properties of the Mst1 Tg portion of the chimeric heart. Future experiments aimed at addressing this important question will allow us to identify key cell-to-cell rescue factors that may replace the therapeutic capabilities of the pluripotent stem cells in this model of forced overproduction.
Acknowledgements
We thank E. Stillwell, A. Altaf and F. Khadim for initial experiments. Chimeric mice were generated at the Transgenic and Knockout Mouse Shared Resource of UMDNJ-NJMS and of UMDNJ-RWJMS-CINJ. This work was funded by a Stem Cell Grant (DF) from the New Jersey Commission on Science and Technology.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
Contributor Information
Qingshi Zhao, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA.
Amanda J. Beck, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA
Joseph M. Vitale, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA
Joel S. Schneider, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA
Corey Chang, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA.
Shumin Gao, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA.
Dominic del Re, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA.
Mantu Bhaumik, Department of Pediatrics, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
Ghassan Yehia, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA.
Junichi Sadoshima, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA.
Diego Fraidenraich, Email: fraidedi@umdnj.edu, Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, UMDNJ, Newark, NJ, USA.
References
- 1.Schneider JS, Vitale JM, Terzic A, Fraidenraich D. Blastocyst injection of embryonic stem cells: a simple approach to unveil mechanisms of corrections in mouse models of human disease. Stem Cell Reviews and Reports. 2009;5(4):369–377. doi: 10.1007/s12015-009-9089-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Q, et al. Rescue of developmental defects by blastocyst stem cell injection: towards elucidation of neomorphic corrective pathways. Journal of Cardiovascular Translational Research. 2009;3(1):66. doi: 10.1007/s12265-009-9140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraidenraich D, Benezra R. Embryonic stem cells prevent developmental cardiac defects in mice. Nature Clinical Practice. Cardiovascular Medicine. 2006;3 Suppl 1:S14–S17. doi: 10.1038/ncpcardio0402. [DOI] [PubMed] [Google Scholar]
- 4.Fraidenraich D, et al. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306(5694):247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien KR, Moretti A, Laugwitz KL. Development. ES cells to the rescue. Science. 2004;306(5694):239–240. doi: 10.1126/science.1104769. [DOI] [PubMed] [Google Scholar]
- 6.Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends in Molecular Medicine. 2007;13(12):520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Current Opinion in Genetics & Development. 2002;12(3):349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 8.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stillwell E, et al. Blastocyst injection of wild type embryonic stem cells induces global corrections in mdx mice. PLoS ONE. 2009;4(3):e4759. doi: 10.1371/journal.pone.0004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada S, et al. Stem cell transplant into preimplantation embryo yields myocardial infarction-resistant adult phenotype. Stem Cells. 2009;27(7):1697–1705. doi: 10.1002/stem.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling P, Lu TJ, Yuan CJ, Lai MD. Biosignaling of mammalian Ste20-related kinases. Cellular Signalling. 2008;20(7):1237–1247. doi: 10.1016/j.cellsig.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Radu M, Chernoff J. The DeMSTification of mammalian Ste20 kinases. Current Biology. 2009;19(10):R421–R425. doi: 10.1016/j.cub.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto S, et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. Journal of Clinical Investigation. 2003;111(10):1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odashima M, et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circulation Research. 2007;100(9):1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [DOI] [PubMed] [Google Scholar]
- 15.Peter PS, et al. Inhibition of p38 alpha MAPK rescues cardiomyopathy induced by overexpressed beta 2-adrenergic receptor, but not beta 1-adrenergic receptor. Journal of Clinical Investigation. 2007;117(5):1335–1343. doi: 10.1172/JCI29576. [DOI] [PMC free article] [PubMed] [Google Scholar]