Abstract
Radiation-based therapies aided by nanoparticles have been developed since decades, and can be primarily categorized into two main platforms. First, delivery of payload of photo-reactive drugs (photosensitizers) using the conventional nanoparticles, and second, design and development of photo-triggerable nanoparticles (primarily liposomes) to attain light-assisted on-demand drug delivery. The main focus of this review is to provide an update of the history, current status and future applications of photo-triggerable lipid-based nanoparticles (light-sensitive liposomes). We will begin with a brief overview on the applications of liposomes for delivery of photosensitizers, including the choice of photosensitizers for photodynamic therapy, as well as the currently available light sources (lasers) used for these applications. The main segment of this review will encompass the details on the strategies to develop photo-triggerable designer liposomes for their drug delivery function. The principles underlying the assembly of photoreactive lipids into nanoparticles (liposomes) and photo-triggering mechanisms will be presented. We will also discuss factors that limit the applications of these liposomes for in vivo triggered drug delivery and emerging concepts that may lead to the biologically viable photo-activation strategies. We will conclude with our view point on the future perspectives of light-sensitive liposomes in the clinic.
Keywords: lipid-based nanoparticles, drug delivery, laser, cancer therapy, photodynamic therapy, liposomes, photo-triggering
2. Introduction
Nano-drug delivery systems coupled with site-specific targeting ligands constitute a promising system to boost efficacy and bioavailability of existing drugs and pharmaceuticals [4,5,71,73,75]. Optimal drug delivery systems feature multifunctional nanoparticles with imaging molecules, a pay-load of drugs, targeting ligands, destabilization elements as well as sensors that probe the efficacy of the drug in real time [2,5,6,29,70,78]. Some widely examined nanocarriers aimed at delivering either DNA, pharmaceuticals and/or imaging agents include dendrimers[45,69], nano-gold shells [8,43], nano-emulsions [68], drug-polymer conjugates [40,53], drug-antibody conjugates [33], and quantum dots [19,51,58]. Interestingly, bioactive lipids, such as ceramide, that are involved in cell signaling pathways have also been examined via nanoliposomes delivery systems [65,66]. Each of these nanotechnology platforms entails unique fabrication components that rely on self-assembly of the structural motifs, while accommodating the pharmaceutical agent and the targeting ligand. The latest developments for some of these platforms have been discussed in this issue (?????Nanoemulsions, Ganta, Amiji., Micelles, Sawant, Torchilin, nanoaprticulate systems, R.N. Saha).
In spite of these efforts, only a limited number of the drug-loaded nanoparticles are successful for their clinical applications [9,20]. Therefore drawbacks and limitations associated with currently available nanoparticle formulations require a re-assessment and further investigation [52]. A nanocarrier with low or no toxicity (as well as its byproducts) in vivo is highly desirable (please see comments by Dr. D. Ranamukhaarachchi, FDA, U.S.A.). Therefore, nanoparticles derived from the assembly of biological molecules can be predicted to be successful in clinical applications [20].
3. Liposome Drug Delivery: General considerations
Liposomes are the amongst the longest-studied nanoparticles and these primarily consist of phospholipids (major components of biological membranes) [26,27]. It is notable to mention that Doxil/caylex (a liposome-based formulation of an anticancer drug Doxorubicin, Ortho-Biotech), was the first formulation approved for its application in the clinic and hence may be regarded as an honorary nanoparticle for patient care [9,20]. Historically, the important milestones that led to the research and development of clinically suitable liposome formulations can be summed up in two major technological achievements; (i) inclusion of pegylated lipids in the liposomes to bypass reticulo-endothelial system, resulting in preferential accumulation in the tumors [28,50], and (ii) successful development of a remote drug loading process based on ammonium sulfate gradient method to achieve significantly high quantities of doxorubicin (as well as other weak bases) in the interior of the liposomes[31,32]. We have recently provided a detailed overview of lipid-based nanoparticles elsewhere (Puri et al, 2009). To date, a number of liposome formulations are currently used in the clinic while others await clinical trials. However, two important features, namely targeting potential and on-demand drug release properties (Triggering) of liposomes are the subject of current and future considerations to further improve patient's treatment.
Targeting potential
Although liposomes bearing various targeting ligands (such as antibodies, peptides, affibody etc) appear to be promising candidates, the clinical benefits associated with targeting are under intense debate and are beyond the scope of this article (please refer to contributions by Omolola Eniola-Adefeso, Raymond Schiffelers and Robert Lee in this issue).
Triggering
It can be envisioned that modalities resulting in selective release of drugs from liposomes (once they have reached their target site) will have a significant impact on therapeutic index of the drugs. Toward, this end several strategies have been described in literature that can be broadly classified into internal & external trigger. The examples of internal trigger typically include either exploitation of low pH in the endosome or use of enzymes that are overexpressed in diseased states (the reader is referred to contributions by Yana Reshetnyak and Andresen/Kaasgaard in this issue). Examples of external trigger that are used to destabilize liposomes are heat and light, the two forces of nature. It is noteworthy that thermosensitive liposomes (currently in Phase 3 clinical trials and first described in late 1970's) are thus far the best studied example of triggerable nanoparticles.
Since conventional liposomes (that are not sensitive to light per se) have been used to deliver photoreactive molecules for radiation-based therapies, the information gained from these data presents a foundation for developing light-triggerable liposomes. Therefore, we will first provide an update of conventional liposome formulations used for delivery high concentration of various photosensitizers (photodynamic therapy, PDT). The main part of this review will deal with the design principles of photo-triggerable lipids and molecular mechanisms that result in photo-destabilization of liposome membrane. We will also discuss alternate mechanisms of photo-triggering of liposome entrapped drugs (from conventional liposomes) that are based on unique light absorption properties of metal ions such as gold nanoparticles (AuNP). Lastly, we will present our view on opportunities that may lead to success of photo-triggerable liposomes in the clinic.
4. Liposomes in Photodynamic Therapy
Since 1980's photodynamic therapy (PDT) has served as a promising treatment protocol and involves the use of a photosensitizing agent (PSA) coupled with an appropriate light source. Light-mediated activation of PSA results in the generation of radical oxygen species (ROS) which destroys the target tissues or cells [25,62]. A recent review by Juarranz et al [34] provides a detailed description of the activation mechanisms of PSAs used in PDT. Typically PSAs absorb photons and are excited to a triplet state, which generates ROS (Examples: superoxide anion, hydroxyl radical, hydrogen peroxide) or can transfer energy to a ground-state molecular oxygen, resulting in the production of a highly reactive singlet oxygen. It is the latter reaction that is thought to be the primary agent behind cell death in PDT, although both mechanisms may play a role depending on numerous variables, such as PSA type, localization, and levels of oxygen. In animal models, tumor regression is well-documented using the PDT, which presumably occurs by killing of cells, damage of tumor vasculature, and/or stimulation of immune responses that cause cell death [34] [55].
Although numerous PSA formulations have been tested in laboratory settings, only a few have made it in to the clinic. The majority of PSA fall into one of four categories: porphyrin derivatives, chlorins, phthalocyanines, and porphycenes (Figure 1) [21]. Porphryins have traditionally been the most extensively studied and used. The first clinically available PSA for PDT of multiple cancers was Photofrin, which may be classified as a hematoporphryin derivative. The second type, chlorins, contains compounds derived from chlorophyll and porphryins, including Benzoporphyrin derivative monoacid ring A (BPD-MA) which has been found effective in treating some forms of carcinomas. Second-generation PSA, phthalocyanines feature a diamagnetic metal ion, reduced phototoxic side effects, and have shown high potential in PDT in animal tumor models. The last group, porphycenes, has also proven to be effective in reducing tumor size through their efficient generation of singlet oxygen (Figure 1).
Figure 1. Chemical structures of various photosensitizing chemical agent (PSA).
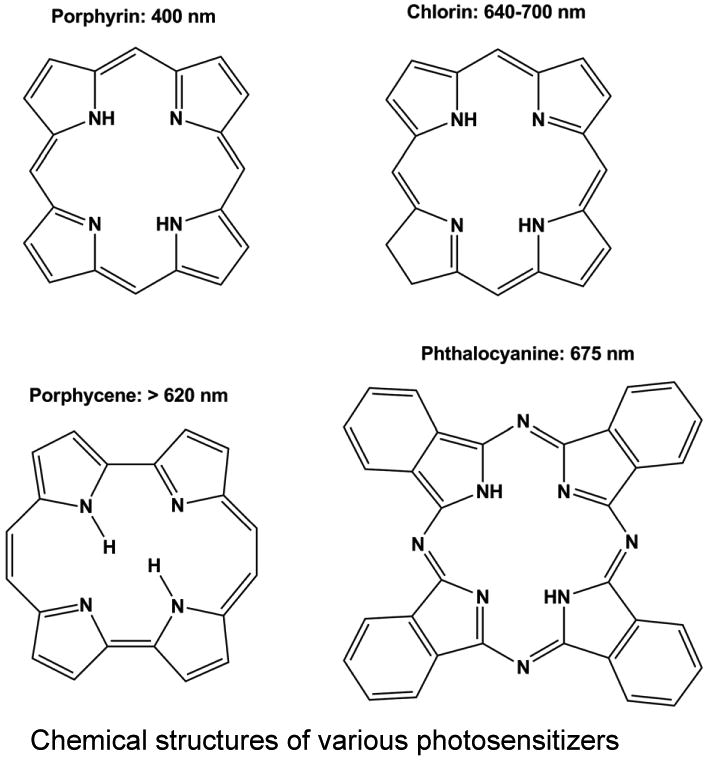
Majority of photosensitizing chemical agents (PSA) may be classified into four main categories according to their chemical structures, including porphyrins, chlorins, porphycenes, and phthalocyanines. Each PSA features a peak absorbance wavelength, typically in the red light region of the spectrum.
Despite advances made by clinically available PSA, there are still several drawbacks limiting their use. For example the hydrophobic nature of most PSA makes them highly susceptible to aggregation in aqueous solutions, and therefore also limits their efficiency and delivery in the body [16,17]. Additionally, an inadequate affinity by most PSA to tumor sites also results in some damage of normal tissue following PDT in patients. To resolve these issues and curb potential side effects, alternative formulations for PSA are required to achieve greater solubility and selective delivery to tumor sites. Liposomes have the ability to encapsulate the mostly hydrophobic PSA molecules and avoid any aggregation issues associated with interactions in the aqueous environment [25]. The inclusion of PEG-lipids within the liposomes also greatly enhances their efficacy and biodistribution through the avoidance of the reticuloendothelial system [3]. Recent advances in the liposomal field with regard to the conjugation of ligands to the nanoparticles' surface (for targeting) may even further enhance the potential of conventional liposomes as PSA delivery vehicles for PDT. Below we review recent studies from the literature and provide specific examples of targeted liposomal formulations for the delivery of PSA.
Recently, Oku and Ishii [48] suggested that actively targeted liposomes for PDT is the most efficient delivery system, since binding and internalization of the nanoparticles will be greater than that of control, or non-targeted PEG-coated liposomes. As the PEG-lipid molecule decreases interactions with cells, they are less likely to stay in close enough proximity to deliver an effective dose of singlet oxygen species generated from the PSA. Since the reactive oxygen species are short-lived, the onset of photodynamic reaction (in close proximity to the cells and/or tissues) at the cancer site is important. This phenomenon is shown in a cartoon form (Figure 2). Using the PSA, benzoporphryin derivative monoacid ring A (BPD-MA), the same group studied tumor regression in a Meth-A (?) sarcoma mouse model with a 689 nm diode-laser system (SP689) as the irradiation source. They modified a PEG-Liposomal BPD-MA formulation to include the APRPG peptide, which is known to target angiogenic endothelial cells, and found that the APRPG-targeted PEG-Liposomal BPD-MA displayed a 5 fold difference in tumor reduction compared to the non-targeted PEG-Liposomal BPD-MA. It was hypothesized that the close interaction of the APRPG-targeted formulation with endothelial cells resulted in greater cytotoxicity as the generated oxygen species need not travel far to induce cell death [48] (Figure 2). Additionally, another recent study investigated the delivery of PSA by targeted liposomes in vitro and in vivo. Derycke et al [22] took advantage of the upregulation of transferrin receptors on tumor cells, and conjugated the transferrin molecule to PEGylated liposomes loaded with the PSA aluminum phthalocyanine tetrasulfonate (AlPcS4) [22]. Utilizing a 1000W halogen lamp system with a filter (center wavelength = 651 nm), researchers measured in vitro photodynamic effects of the transferrin-conjugated AlPcS4 liposomes on a rat bladder carcinoma cell line and tumor model. The targeted formulation displayed a cell killing greater than 3-fold in the log scale compared to the control, non-targeted AlPcS4 liposomes. Moreover, in the tumor model, accumulation of the PSA, AlPcS4, was shown to be significantly enhanced by the conjugation of transferrin to the PEGylated liposomes. Hence, targeted liposomes may be of benefit over non-targeted liposomes.
Figure 2. The effects of targeting on the delivery and cytotoxicity of liposome-encapsulated PSA.
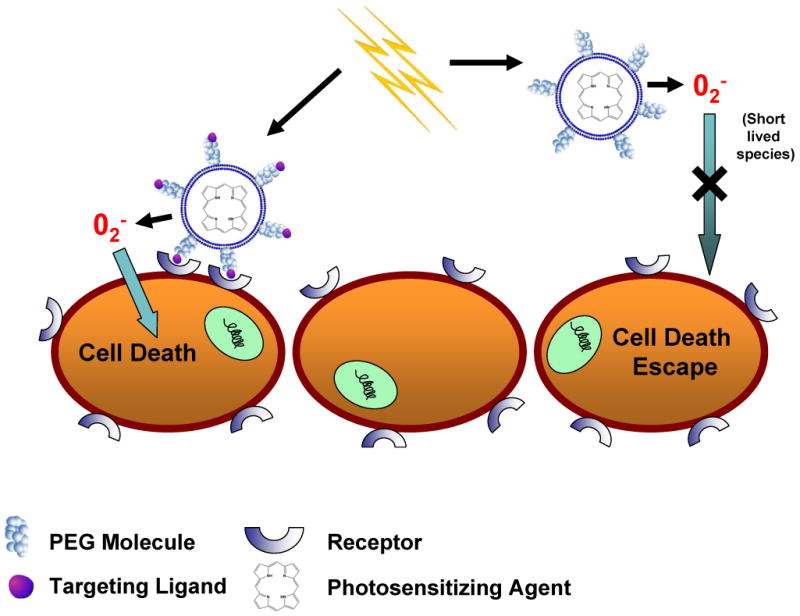
The addition of targeting ligands to liposomes encapsulating PSA improves cytotoxicity compared to non-targeted, PEG-coated liposomes. Exterior PEG-molecules do not allow for close contact between liposomes and cells, whereas targeted formulations induce binding between the two. This close interaction is required for efficient delivery of short-lived radical oxygen species, which are generated from the light-activated encapsulated PSA.
In conclusion, pre-clinical studies using a variety of PSA (ex: Photofrin, Visudyne, aminolevulinic acid) indicate that PDT may be a suitable treatment for a number of diseases, including several cancer types.. However, alternate formulations appear to be required to increase the bio-availability of PSA at and reduce adverse side effects. Liposomal encapsulation of PSA may achieve these goals especially with targeted liposomes, which can be brought into close contact with target cells enabling rapid transport of short-lived ROS from the irradiated PSA within the liposome to nearby target cells (Figure 2). To make these more effective light-triggerable platforms that release the agent have been designed.
5. Light-sensitive Liposomes-background
Light-sensitive liposomes (explored since early 1980's) have lately re-gained attention. Photoreactive lipid molecules provide an opportunity to generate stable nanodelivery vehicles for sustained release of drugs in circulation together with photo-triggerable systems for localized drug delivery. Triggerable liposomes are generally based on the principle of membrane destabilization (resulting in creation of local defects) for release of liposome-entrapped drugs and pharmaceuticals. Among other triggerable formulations, thermosensitive liposomes depend on transition release (PTR) properties of conventional phospholipids (or lipid mixtures), primarily phosphatidylcholines (PC), that are used to prepare liposomes. However, light-triggerable liposomes are developed by introducing photoreactive groups in the phospholipid molecule (“designer lipids”) usually by chemical synthesis. One exception to this notion is plasmalogen (a natural ether phospholipid, typically found in tissues such as heart) that reacts with reactive oxygen species (ROS) to generate lysolipid,, which promotes leakage from liposomes. There are several designer lipids synthesized to date, each of which utilizes a defined mechanism for photo-activation (Table I). The PC molecule can be divided into three major parts, head group, glycerol backbone and fatty acyl chains (Figure 3). Each of these regions have been modified either by introduction of additional groups or modification of existing chemical bonds such as polymerizable moieties [64][76] to produce light-sensitive liposomes. The fatty acyl chain length and degree of unsaturation are important factors that govern bilayer packing properties of liposomes. Fatty acyl chains of the phospholipids have been further modified with light-sensitive groups and the resulting phospholipids have yielded photoactivable liposomes [61] [72]. Currently available photoactivable liposome systems are discussed below:
Table I. A partial list of Synthetic photo-sensitive phospholipids.
| Lipid modification | wavelength | Mechanism | Triggering | Ref. |
|---|---|---|---|---|
| DC8,9PC | 514 nm | Entrapped sensitizer (Calcein, Doxorubicib) is required | On going work | Yavlovioch 2010 (Submitted) |
| DC8,9PC | 254 nm | Photo-polymerization | cross-linking lead to leakage between domain boundaries | Yavlovioch 2009 |
| Biz-azo PC | UV | Photo-isomerization transform from thermodynamically favored trans form to cis isomer | Bulky cis isomer interferes with bilayer packing resulting leakage | Bisby et al |
| 0-nitrobenzyl PC | 365 nm | Photo-cleavage upon radiation | --------- | Han X et al 2007 |
| NVOC-DOPE | 345 nm | Photo-cleavage upon radiation | leakage | Smith |
| plasmalogen | 630-820 nm | Photo-oxidation by bilayer sensitizer (Zinc phthalocyanine, tin ocabutoxyphthalocynine, bacterio-chlorophyll a) | Plasmenylcholine cleavage produced lyso-lipids and membrane permeability | Thompson et al |
| Dienoyl PC | yellow light(wavelength>500 nm) | photoactivation of a poly(PSLB) with incorporated Rho | ---------- | SaavedraSS et al2008 |
| Bis-Sorbyl PC | 550 nm | Photo-polymerization induced by Bilayer sensitizer (Dil) | Cross linking reaction and polymer domain boundaries | Muller A 2000 |
| Bis-Sorbyl PC | UV | Photo-polymerization | Bondurant et al | |
| Head-group polymerizable | UV | Radical initiation reaction | Crosslinking of head group, liposome intact | Lawson et al |
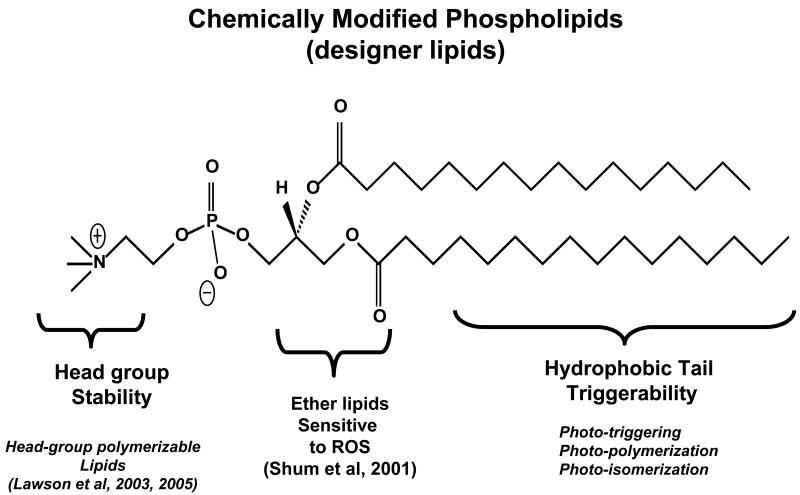
Figure 3. A schematic representation of chemically modified phospholipids (designer lipids).
Three major parts of phospholipidsthat can be chemical modification to induce photo activity. The lipid parts: lipid head group, glycerol backbone and fatty acyl chains the are described.:
5a. Head-group polymerizable liposomes as candidates for sustained drug delivery
In late 70's, the drug delivery potential of liposomes was realized. However, soon after, the issues related to the stability of liposomes became evident. The primary factors involved in destabilization of liposomes resulted from interaction of liposomal lipids with serum components [36]. In addition, preferential uptake of liposomes by the reticulo endothelial system (RES) limited their in vivo applications [28,50]. Research efforts to generate stable liposomes include formulating compositions including lipids containing poly(ethylene glycol) (PEG) in their headgroup [28,50]. This method is currently the most widely applicable method and has led to highly significant milestones in liposome drug delivery research (prolonged circulation in blood) (reviewed in Puri et al, 2009). Since the efficiency of pegylated liposomes is less than optimal in vivo, alternate approaches to generate stable liposomes are warranted. Towards these efforts, stabilization of liposomes by using the chemically synthesized photoreactive lipids have been reported recently [38,39]. The light-induced reaction in these liposomes involves photo-crosslinking (light-induced polymerization) under relatively mild conditions and often a water soluble free radical initiator is used to begin this reaction. The latter system is potentially versatile as the monomer-crosslinking methods (in preformed liposomes) may find applications for both biological and non-biological systems. The choice of monomer functionalities and the flexibility to place these monomers in the liposome membrane prior to cross-linking are attractive aspects of the polymerization approach to generate stable vesicles. However, an important consideration in developing the photoreactive monomers (such as photopolymerizable lipids) is to maintain the integrity of the liposome membrane as well as entrapped contents (such as pharmaceutical agents) during the photopolymerization step. Since the photo-activation of fatty acyl chains in the phospholipid molecules (Figure 3) will result in the perturbations in the liposome membrane, such modifications have been implicated for photo-triggering to accomplish localized drug delivery from liposome formulations (discussed in sections below). It can be envisioned that introduction of a photoreactive moiety in the head group of the phospholipid (see Figure 3) will be a smart choice; as such modifications will result in polymerized liposomes while sustaining their membrane properties. In the phospholipid realm, there are only a few examples of head-group photopolymerizable molecules (Figure 4).
Figure 4. A graphic illustration of light sensitive head-group polymerizable lipids.
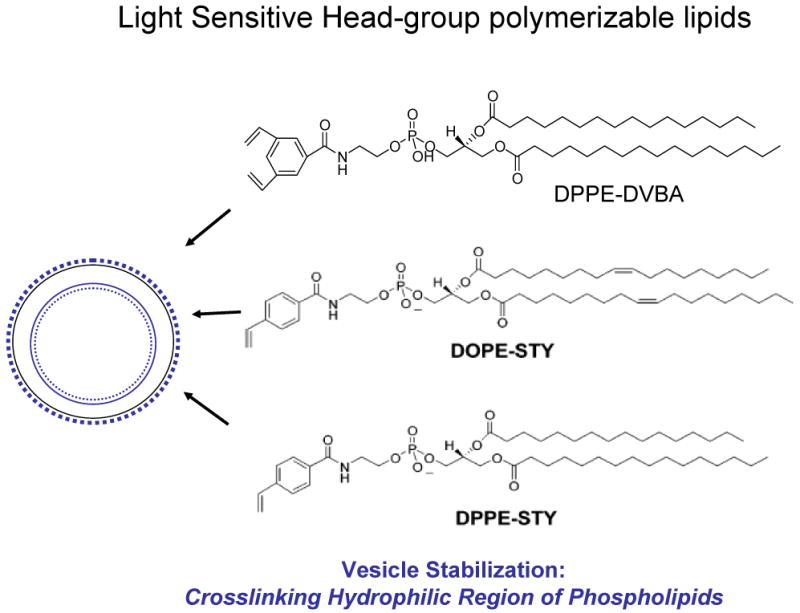
Liposomes formulation containing polymerizable phospholipids in the treatment of UV light has been cross linked leading to vesicle stabilization without affecting the activity of entrapped agents.
The synthesis of these phospholipids is based on the generation of N-acyl phosphatidylethanolamines (N-acyl PEs) that readily form liposomes. The chemical structures of PEs containing a 3, 5-divinylbenzoyl functionality[39] and N-(4-Vinylbenzoyl) head group [38] are shown in Figure 4. Polymerization in liposomes (prepared from these phospholipids) has been demonstrated to photo-crosslink in the presence of UV light without compromising the activity of entrapped enzymes. Although biophysical studies demonstrate that these head-group polymerizable lipids are potential candidates for generating stable liposomes [38], further studies are needed to evaluate the merit of these lipids for sustained drug delivery.
5b. Phototriggerable Liposomes
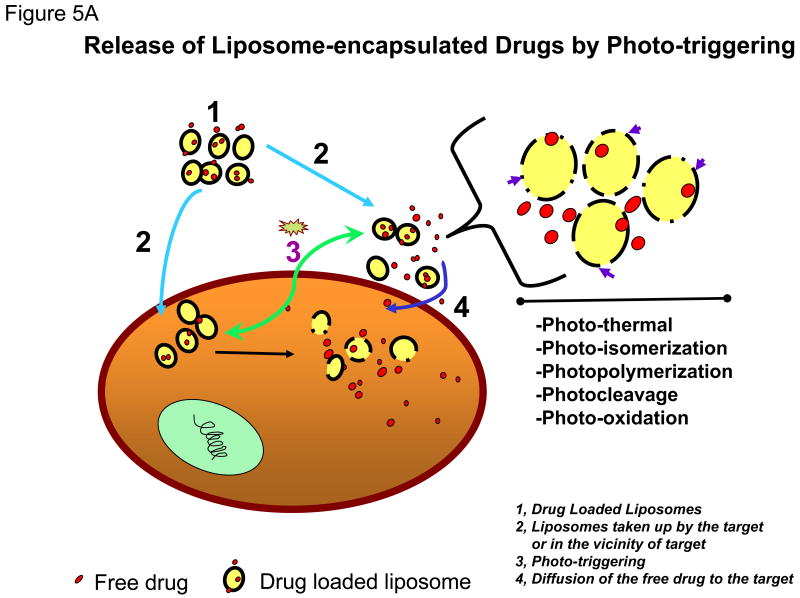
Photo-activation is an attractive option for triggering liposomal contents release since it provides a very broad range of adjustable parameters (e.g., wavelength, duration, intensity) that can be optimized to suit a given application. The range of light necessary for tissue penetration is discussed below. The cartoon presented in Figure 5A shows drug-loaded photo-triggerable liposomes (Figure 5A, 1), that either accumulate in the interior of the cells (tumor area) or in the vicinity of the disease cells/tissues (Figure 5A, 2). Treatment with a suitable light source (Figure 5A, 3) will trigger drug release from these liposomes upon photo-ctivation. The internalized liposomes will deliver drug directly in the interior of the cell. The liposomes in the vicinity of cells/tumors will also destabilize (upon light treatment), and the released drug will eventually be taken up by passive diffusion (Figure 5A, 4). The mechanism(s) by which light-triggered defects in the liposome membrane are created encompass(s) several modalities (shown in the zoomed version of cartoon, see arrows, Figure 5A, right).
Figure 5.
Figure 5A: Release of Liposome-encapsulated Drugs by Photo-triggering. The diagram describes the cellular process of treatment with drugs entrapped liposomes.
- Drug Loaded Liposomes
- Liposomes taken up by the target cells or in the vicinity of target cells
- Triggering release of entrapped agents after light radiation treatment and activation of photo-sensor lipids.
- Diffusion of the free drug to the target cells
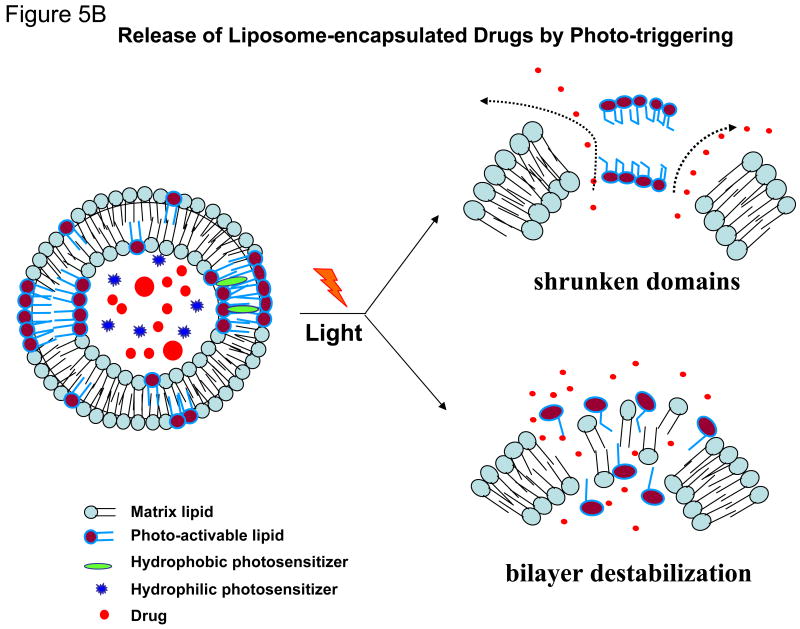
Figure 5B: A cartoon presenting the mechanisms of trigger release from photo-activated liposomes. Two known conformational changes in photo-reactive liposome segments due to light treatment are presented. Light radiation can result in a shrunken domain that leads to leakage between domain boundaries (right, top). Light radiation can also create changes in the liposome bilayer resulting in destabilization and the release of the liposomal contents (right, top).
Light-triggered release of liposomal drugs involves modified phospholipid molecules, that undergo either photopolymerization [54], photosensitization by membrane anchored hydrophobic (Chowdhary et al 1993) [10,13-15,37], or water soluble probes [76], photo-isomerization [47], photooxidation [61], or the degradation of photocleavable lipids [14,15]. Approaches to promote photo-induced drug delivery from liposomes also include the use of wavelength-specific photosensitizers (primarily lipidic and/or hydrophobic in nature) in conjunction with photoactivable lipids. The detailed mechanisms of light-triggered chemical changes in photo-reactive segments of some of these molecules were reviewed about 10 years ago [61], and are shown in Figure 5B in a cartoon form. Since then, some progress has been made in this field and here we will mainly focus on latest developments in the field. It may be noted that light-induced effects result in irreversible changes in the majority of liposome systems with the exception of molecules that under go cis-trans isomerization.
5b i. Photooxidation
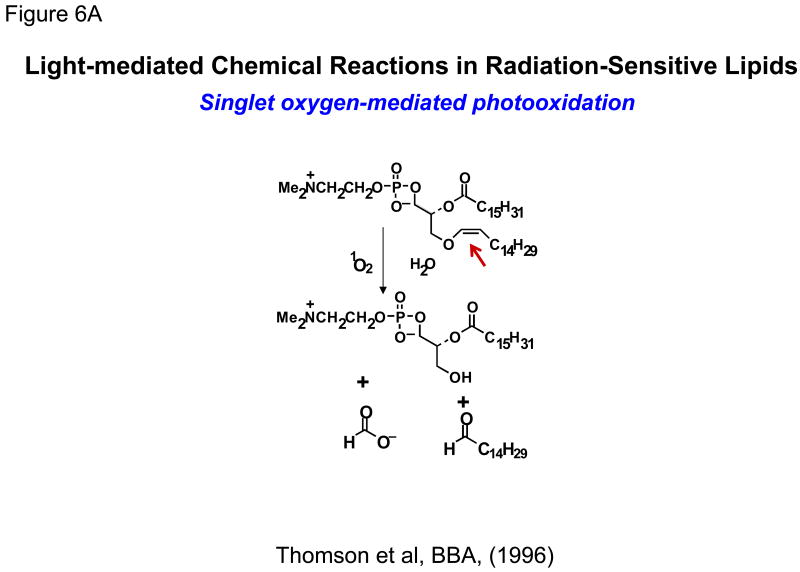
In early 1990's, researchers explored photo-oxidation of lipids containing vinyl ether linkages (bearing at least one double bond) by reactive oxygen species (generated by a series of photosensitizers) (Figure 6A). Significant efforts were put forward by Thompson and colleagues to design and synthesize plasmenylcholine molecules [56,57]. Photo-oxidation was initiated by several light-responsive molecules such as Zn-phthalocyanine, octabutoxyphthalocyanine, and bacterio-chlorophyll-a. These sensitizing agents absorb between 630-820 nm photo-triggering of liposomes and release of contents [67]. The use of the NIR sensitizer bacteriochlorophyll led to 100% calcein release in less than 20 min when irradiated at 800 nm, indicating that these formulations may have the advantage for deep tissue penetration depth for small animal model studies (for discussion, please see section 6 later in the article) [30,61]. Vinyl ether linkages in plasmenylcholine and diplasmenylcholine being susceptible to photooxidation were proposed as a novel targets for triggering.
Figure 6. Schematic diagrams of light-mediated chemical reactions in radiation-sensitive lipids.
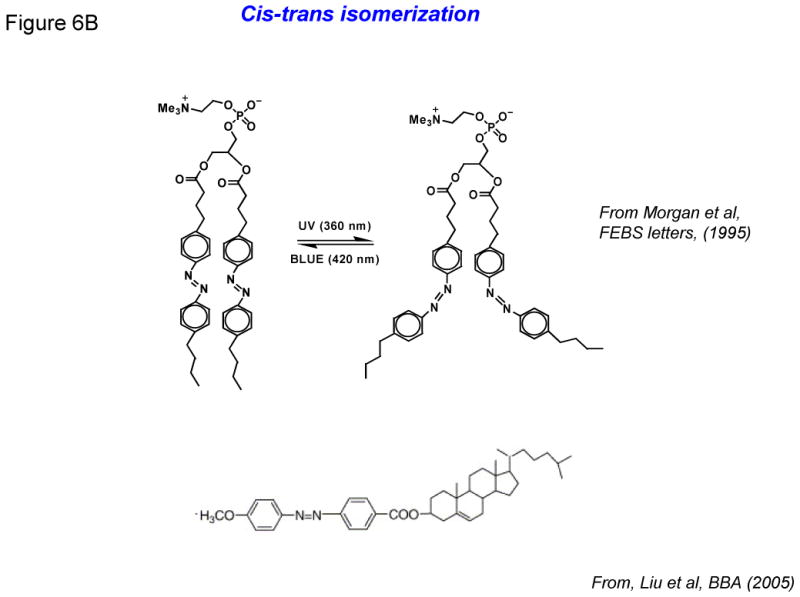
- Photo-oxidation of lipids containing vinyl ether linkages by reactive oxygen species (generated by a series of photo sensitizers).
- Photo isomerization of azobenzene groups undergo wavelength-specific manner radiation leading to cis-trans (420/360nm) conformational changes.
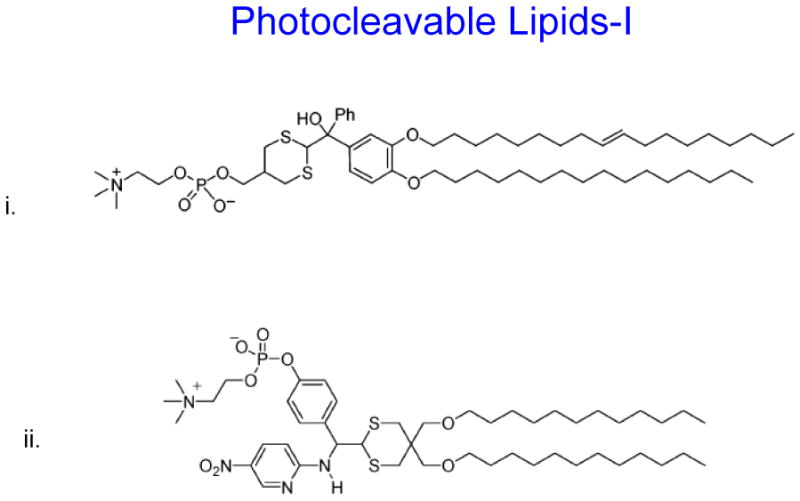
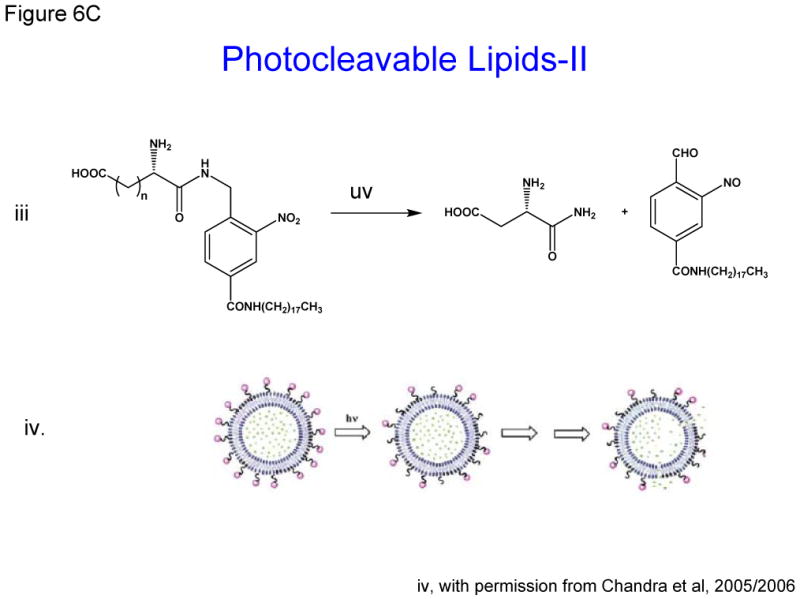
- i, ii and iii: Photo cleavable lipids that after light radiation their breakdown products destabilize liposome membrane
-
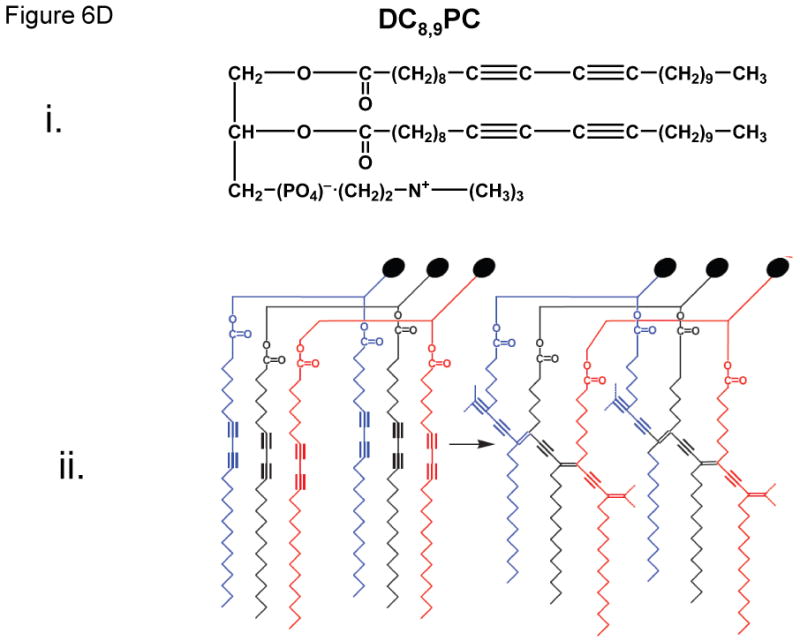
i: Photo-polymerizable phospholipid (DC8,9PC) graphic illustration.ii: Description of DC8,9PC chemical modifications and cross linking upon UV treatment
5bii. Cis-trans isomerization
As mentioned above, most of the light-activated liposome formulations result in irreversible changes in the phospholipids, and hence represent one-time delivery protocols. However, formulations with an ability to reversibly regulate light-triggered drug release will have significant advantage in the clinics. Keeping this in mind, phospholipids containing the azobenzene group were designed by Bisby and colleagues in 1990's. The azobenzene groups undergo cis-trans isomerization (420/360nm) in a wavelength-specifc manner (Figure 6B), resulting in transient/programmed release of the entrapped solutes from the liposomes. It was demonstrated that DPPC liposome containing a photochromic lipid “Bis-Azo PC” (6 %mol) with addition of cholesterol (up to 25 mol%) released their contents in response to visible light in the region of 470 nm. A photo stationary state is dominated by bulky cis form after photo isomerization created by UV radiation (360 nm) which interfere in the liposomes bilayer packing allowing rapid leakage of encapsulated agents. The acyl chains are compact and organized in closely packed liposomes in the trans form that is thermodynamically preferential [12,13]. In 2005, Liu and colleagues reported the synthesis of a series of photo-isomerizable cholesterol derivatives (Figure 6B); these molecules may have potential for light-triggered delivery, although no biological data has been reported [44].
5b iv. Photocleavable liposomes
The concept of photocleavable lipids has been in existence for decades [61,72](Zhang and Smith 1999). In recent years, photo-labile lipid molecules (other than phospholipids) have been reported for their potential applications in drug delivery [14,15,42]. These molecules (Figure 6C) were designed on the basis of their susceptibility to light (typically in the UV range) resulting in the breakdown products that will destabilize liposome membrane. Li et al synthesized the dihydroxybenzophenone-based amphiphiles as the photolabile lipids using the dithiane-based modular approach. The same group also synthesized nitropyridine-based self-sensitized photolabile amphiphiles (Figure 6C, i) [42]. These molecules also included the possibility of addition of hydrogen-bond elements for further modification of hydrophilic portion of the lipids. Biophysical studies revealed that these amphiphiles could be used in conventional liposome formulations for phototriggering. Chandra et al also aimed at synthesizing photocleavable amphiphilic lipids (Figure 6C, iii) by connecting stearyl amine (as the non-polar tail) and charged amino acids (as polar heads) via the o-nitrobenzyl derivatives [14,15]. Their efforts were focused on using the chemical approaches that will produce photocleavable lipids in high yields and easy purifications steps. Using these photocleavable lipids, the investigators showed that light-induced leakage occurred in a two step process, first the fast reaction of photolysis followed by a slow release of entrapped contents (shown in cartoon Figure 6C,iv, with permission from xx). Therefore, it is possible that reorganization of the lipids occurs before all entrapped dye is released. Incidentally photocleavable lipids described here are activated only by the short-wave lengths and their in vivo application remains to be examined.
5b iv. Photopolymerizable liposomes
The systems described above (5b, i-iii) are primarily based on the light-induced perturbations in the liposome membrane, either via the irreversible modification (photo-cleavage) of the photoreactive lipids (5b, i&iii) or by reversible conformational changes in the lipids (5b, ii). This mode of phototriggering is shown in Figure 5B (lower panel, right, membrane destabilization). However, it is possible to use photoreactive lipids that can undergo photo-crosslinking leading to polymerization within the liposome bilayer (photopolymerization). It can be assumed that the synergized molecular packing of photopolymerizable lipids is a crucial element to this effect. The phenomenon is shown in the cartoon in Figure 5B (top, right panel, shrunken domains). We will discuss the properties and drug delivery potential of two photopolymerizable phospholipid molecules namely bis-SorbPC and DC8,9PC.
Bis-SorbPC
Elegant studies by O'Brien and colleagues previously reported that UV light-induced photopolymerization of liposomes containing bis-sorbyl phosphatidylcholine (bis-SorbPC) results in leakage of liposome-entrapped contents (Bondurant et al 1998). Interestingly, inclusion of a cationic dye, 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, as a hydrophobic photo-sensitizer) in these liposomes also triggered destabilization of the liposomal membranes when treated with visible light (550 nm). The light at this wavelength is only absorbed by the DiI and not by the lipids. The photo-activation of DiI produces oxygen radicals in the presence of oxygen which can initiate the polymerization of bis-SorbPC resulting in creation of domain boundaries (shrunken domains, Figure 5B, top right) (Mueller A and O'Brien D 2000). One can predict that the packing properties of photoactivable lipid (such as bis-SorbPC) and the hydrophobic photosensitiser (such as DiI) in the liposome bilayer should be in concert to produce oxygen radical-mediated photoreaction. Therefore, lipid-photosensitizer packing will be a major determinant of this photopolymerization mechanism. Our recent studies show that entrapment of a water soluble photosensitizer in the liposomes containing a diacetylenic lipid promotes a similar outcome, but resulting from an independent mechanism unrelated to photopolymerization (unpublished). These results are discussed below.
DC8,9PC
The photopolymerizable phospholipid, (1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine (DC8,9PC, figure 6D,i), is present in lower organisms (Figure 6D,ii) [49], and has been shown to uniquely assemble into the lipid bilayer due to the presence of triple bonds in the fatty acyl chains. UV (254 nm)-induced photopolymerization of DC8,9PC, as well as the analysis of chemical modifications in this molecule upon UV treatment has been well documented since 1980's (Figure 6D,ii) [54,63]. Photo-crosslinking properties of DC8,9PC have been exploited for potential biological applications that include functionalized polymerized vesicles for vascular targeted molecular imaging[41], candidates for oral vaccine preparations [7], and DNA delivery [18,77].
Recently, we have examined in situ light-triggered drug release properties of DC8,9PC liposomes [76]. According to our hypothesis, DC8,9PC is likely to form aggregates (self-assemble) in the bilayer of phospholipids containing saturated acyl chains, and this packing is prone to create phase boundary defects in lipid model membranes (Figure 6D). In support of this hypothesis, we demonstrated that UV (254)-triggered calcein release occurs from liposomes containing a mixture of saturated phospholipids and DC8,9PC. The UV-triggered mechanism of calcein release was due to the photopolymerization of DC8,9PC.
We are currently pursuing DC8,9PC formulations for their drug delivery applications. We have demonstrated that visible light (514 nm) treatment of liposomes containing a photo-sensitizer results in contents release in a wave-length specifc manner. It is evident that the visible-light triggered mechanism of solute release is unrelated to photopolymerization. The DC8,9PC formulations appear promising candidates for drug delivery because 514-nm triggered release of doxorubicin (an anticancer drug) from these liposomes improves cytotoxicity in cell culture experiments. We are hopeful that our formulations can be considered as next-generation of light-sensitive liposomes for drug delivery applications in the clinic (discussed below).
5c. Limitations of Phototriggerable Liposomes for Biological Applications
Although a number of light-triggerable formulations have been examined to date (Figures 6A-D), none of the formulations developed so far have been successful for in vivo applications presumably due to the lack of adequate photon energy produced by the radiation source(s) or inability of radiation to penetrate into biological tissues. We believe that the development of innovative strategies to combine unique chemistry of photoactivable lipids with “the helper” components (such as metal ions) is needed to acquire high energy radiations. One should keep in mind that the light source(s) used should have minimal effect on the biology of normal cells and tissues. Alternate strategies to promote–light mediated drug release from the liposomes include use of metal ions with unique characteristics. One such metal, gold has been recently tested. The studies are summarized in next section below.
5d. Gold-Activated Liposomes
Nanostructures bearing noble metals are considered smart choices for targeting, and imaging because their properties deviate from the bulk materials [11] 12–15]. Among various metal ion-nanostructures, gold nanostructures (AuNSTs), have been extensively investigated as a model platform for biomedical research [2]. The size and shape of AuNSTs can be customized in the range of 2-100 nm and their surface properties are amenable to introduction of functional groups for conjugation of targeting ligands [3-9]. AuNSTs are also biocompatible and, therefore, may serve as good tools for in vivo cell imaging, biosensing, and drug and/or gene delivery, [14-20]. Currently studied AuNSTs can be categorized into gold nanoparticles (AuNPs) or gold nanoshells (HGNs).
Gold nanostructures (AuNP/HGNs) can be encapsulated into other nanoparticles such as liposomes [19–22] and polymeric matrices [16-18] making them suitable for biological applications. Recently it was reported that incorporation of hydrophobic stearylamine coated AuNPs (3–4 nm) into the lipid bilayer of thermosensitive liposomes (DPPC formulation) increased the fluidity of the membranes above their phase transition temperature (Tm). This effect correlated with a significant increase in efflux of entrapped contents in contrast to non-AuNP loaded liposomes [22]. The observed effect may be attributed to the altered packing properties of the liposomes. This observation indicates that the thermosensitive properties of liposomes coupled with an increase in AuNP-mediated fluidity may have an advantage to deliver payload of drugs at a particular temperature. These studies were however, conducted using only biophysical assays, and their application in biological systems remains to be evaluated.
AuNSTs exhibit surface plasmon resonance [26], as they absorb energy at a characteristic wavelength. Most of the absorbed energy is converted to heat while a part of it is emitted as photoluminescence [27,28]. Therefore, light-induced heating of AuNSTs -liposome systems by UV, visible and near-infrared (NIR) to release liposome-entrapped drugs and pharmaceuticals is drawing considerable interest [ ]. It may be noted that AuNSTs when irradiated serve as energy collectors and work as localized heat sources by absorbing the energy of radiation. The heat is then transferred in a highly selective manner to the immediate microenvironment, without dissipating heat to the bulk suspension. Therefore, light-triggered thermal properties of AuNST-liposomes are distinct than the “classical” thermosensitive liposomes (that use local hyperthermia). We will briefly discuss various AuNSTs/liposome systems that have been developed for light-triggered drug delivery using the UV, VIS or NIR (described below)
5d i. AuNP-Liposomes for triggered release
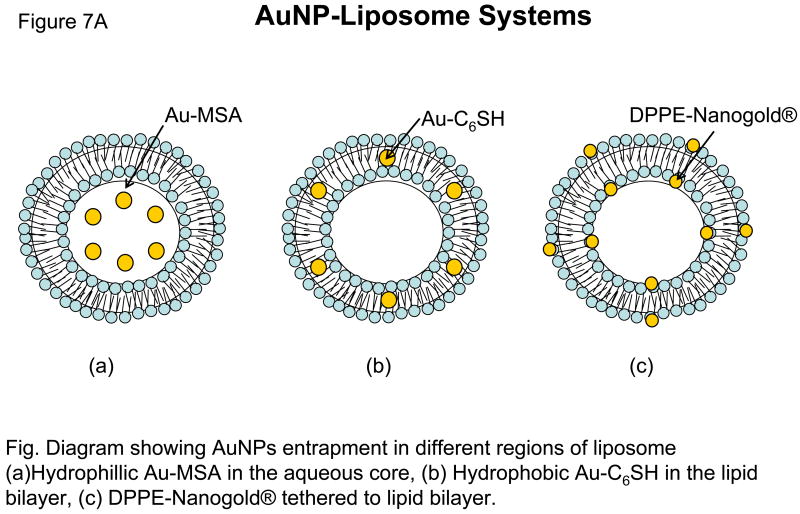
Paasonen et al, (ref) for the first time described a novel method for light-induced contents release from AuNPs-incorporated liposomes. Preferential partitioning of AuNP into the aqueous milieu, hydrophilic surface or the lipid bilayer of liposomes is modulated by physico-chemical properties of AuNPs being examined. In this study, hydrophobic hexanethiol-capped AuNPs (Au-C6SH) were embedded into the lipid bilayer, negatively charged hydrophilic mercaptosuccinic acid AuNPs (Au-MSA) were encapsulated in the core of DPPC liposomes, and lipid functionalized AuNPs (DPPE-Nanogold®) were localized on the inner and the outer surface of the liposomes (Figure 7). These AuNPs loaded liposomes released calcein, upon light irradiation of 250 nm. 2–3 nm small AuNPs were used because bigger particles could impede with the packing of the liposome bilayer and cause uninvited leak of contents.
Figure 7. Nanao Particls.
The proximity of AuNP to the lipid bilayer was an important parameter for the calcein release kinetics. Since Au-C6SH and DPPE-Nanogold® are in direct contact with the lipid bilayer, heat is conducted more efficiently to the lipid molecules, thereby inducing the phase transition and calcein release whereas Au-MSA particles located in the core of the liposomes exhibited very slow kinetics of calcein release upon UV treatment. Higher AuNP concentrations showed maximum calcein release in all the liposomal formulations in comparison to lower concentration, however, the stability of liposomes was compromised at higher AuNP concentrations. In all the formulations examined, longer treatments with UV light (up to 30 minutes) resulted in maximum calcein release (Figure 7). Although no biological data were reported, this system deserves a closer look especially in combination with photoactivable lipids (described in section 5b).
5d ii. HGNs as Nano-“Sonicator” for liposome triggering
UV-triggered release of liposomal contents (described above) is limited only for topical treatments (such as skin), therefore AuNP mediated triggering mechanisms using the light sources compatible with deep tissue penetrating wavelengths are desired. NIR light penetrates into the tissue up to 10 cm, and bears additional advantages (please see section 6 later for details). NIR-based drug release (using the AuNP) has been demonstrated for polymeric carriers (12–15), and these systems are currently in the developmental stage (16). However, NIR- based liposome-AuNP systems have not been fully explored presumably due to the technical limitations in synthesizing AuNPs particles susceptible to NIR light sources. (17)
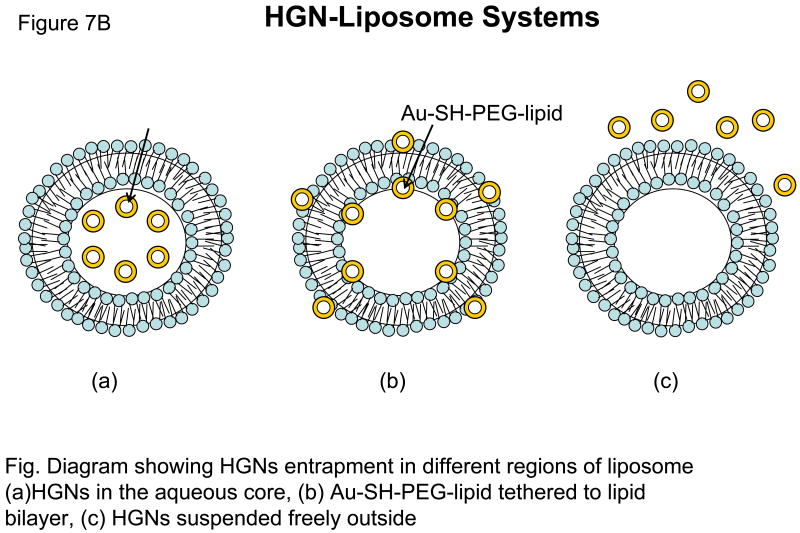
An alternate approach using the HGN was developed by Wu et al (ref). This group synthesized 33 nm HGNs, either tethered to the liposome bilayer with a Au-SH-PEG-lipid linker or encapsulated within liposomes by an interdigitation-fusion method 8. Almost complete contents release was achieved within seconds (“burst” kinetics) from liposomes by irradiating the liposomes tethered with Au-SH-PEG-lipid linker by femtosecond pulses of the NIR laser. Since irradiation of HGNs is likely to produce localized heating within the vicinity of HGN, this temperature gradient was proposed to produce unstable vapor microbubbles leading to transient cavitation by the mechanical and thermal effects (28, 30). Therefore, these laser-heated HGNs act as optically triggered nano-“sonicators” for triggered release. When HGN were encapsulated within the liposomes, the NIR light-triggered release was not significant.
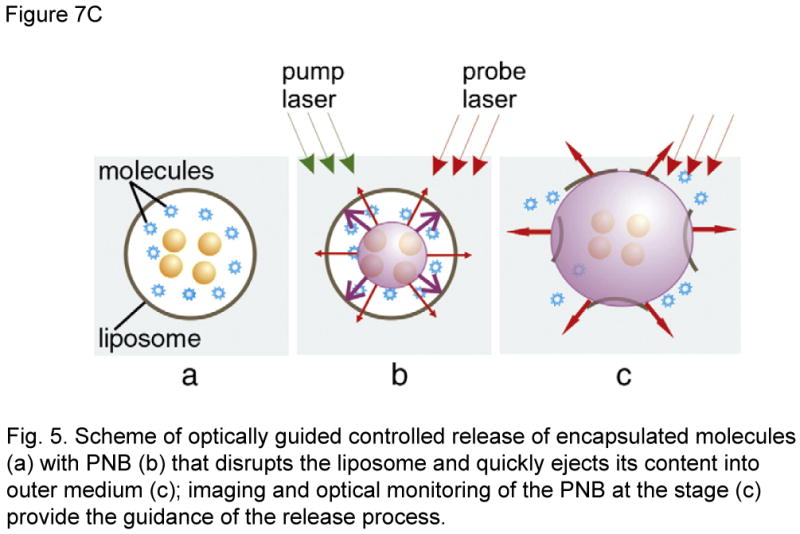
5bvii. AuNP-Liposomes, Plasmonic nanobubble triggered release
The principle of “nano-sonication” described pertains generation of microbubbles by NIR-induced local heating in HGNs. Anderson LJE et al (ref.) devised a new method of optically guided controlled release from liposomes AuNPs) by tunable plasmonic nanobubbles (PNBs). In this study, fluorescent proteins eg: R-phycoerythrin (PE) or allophycocyanin (APC) were released by PNBs generated by irradiating 80 nm AuNPs (supposingly trapped inside the liposome) with a single 532 nm or 633 nm, 0.5 ns short laser pulse. Upon absorption of a pulsed laser, AuNPs quickly evaporate the surrounding liquid medium to produce transient PNBs which can mechanically disrupt the liposome to release the entrapped content. PNBs besides inducing mechanical disruption contents release from liposomes, bears other significant advantages of being a non-thermal process in which the thermal energy is concentrated within PNBs [22,45], and thus protect the entrapped load in the liposome. PNBs have outstanding optical scattering properties that surpass those of AuNPs and fluorescent markers and can serve to guide the release process.
6. Electromagnetic Radiation: General considerations for therapy
Steady advancements in the laser-technology field have yielded devices with marked improvement in the wavelength range, output of light intensity, beam diameter regulation, and light responsiveness. These improvements have served as an added asset in the development of light-triggered treatment modalities including photo-triggerable nano-particles and PDT [74].
It is evident that light-guided therapy is subject to the use of adequate light sources that can penetrate the tissues for drug delivery and therapeutic applications. Wavelength sources below 700 nm cannot penetrate into the tissue, because of scattering and a high level of endogenous absorbers, such as oxy- and deoxy-hemoglobin, lipids and water [35]. Near-infrared wavelengths (700 nm to 2500 nm) penetrate more than 1 cm deep into human skin and blood and have a low absorption coefficient with water and lipids and hemoglobin (the principal absorber of visible light) [74]. Therefore current technologies have proved to be successful for skin-related diseases [23,46] as well for oral treatments [24], primarily because tissue depth was not a limitation. Since high energy/long wavelength sources are a requirement for treatment of deep tissues [1], currently available UV/visible light-photoactivable drug delivery systems (described in section 5b) are not suitable for clinical applications. Therefore, a key strategy will be to develop NIR-sensitive phototriggerable nanoparticles. NIR is considered innocuous and does not cause a significant heating in the area of its application, therefore, such light source bears merit for triggered drug delivery and PDT [11]. Interestingly, the type of organs (and tissues) as well as their location in the body also determines the success of light-guided therapies. For example, bladder is relatively more translucent than other human tissues and therefore has great potential for photodynamic therapy. Shackley and colleagues [59,60] reported that laser sources with wavelengths of 633-693 nm were potentially applicable for bladder PDT.
Table II. A Summary of AuNSTs-Liposome Systems.
| AuNSTs | Size | Localization in Liposomes | Light Source | Ref. |
|---|---|---|---|---|
| Au-C6SH | 2.5 nm | Lipid bilayer | UV 250 nm | |
| Au-MSA | 2.8 nm | Encapsulated | UV 250 nm | |
| Nanogold® | 1.4 nm | Tethered | UV 250 nm | |
| HGNs | 33 nm | Tethered, encapsulated, suspended freely | NIR 820 nm | |
| AuNPs | 80 nm | Trapped inside | Vis 532 nm or 633 nm |
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- Tm
Phase-transition
- DPPC
1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine
- DC8,9PC
Photo-polymerizable phospholipid: 1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine
- EPR
Enhanced permeability and retention effect
- DDS
Drug delivery system
- PDT
Photodynamic therapy
- PSA
Photosensitizing chemical agent
- NIR
Near infrared
- AuNP
Gold nanoparticle
- AuNSTs
Gold nanostructures
- HGNs
Gold hollow shells
- RES
Reticulo endothelial system
- ROS
Reactive oxygen species
- BPD-MA
Benzoporphyrin derivative monoacid ring A
- APRPG
peptide ??
- AlPcS4
Aluminum phthalocyanine tetrasulfonate
Reference List
- 1.Ackermann G, Hartmann M, Scherer K, Lang EW, Hohenleutner U, Landthaler M, Baumler W. Correlations between light penetration into skin and the therapeutic outcome following laser therapy of port-wine stains. Lasers in Medical Science. 2002;17:70–78. doi: 10.1007/s101030200013. [DOI] [PubMed] [Google Scholar]
- 2.Alaouie AM, Sofou S. Liposomes with Triggered Content Release for Cancer Therapy. Journal of Biomedical Nanotechnology. 2008;4:234–244. [Google Scholar]
- 3.Allen TM. Long-Circulating (Sterically Stabilized) Liposomes for Targeted Drug-Delivery. Trends in Pharmacological Sciences. 1994;15:215–220. doi: 10.1016/0165-6147(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 4.Allen TM. Liposomes. Opportunities in drug delivery. Drugs. 1997;54 4:8–14. doi: 10.2165/00003495-199700544-00004. [DOI] [PubMed] [Google Scholar]
- 5.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 6.Alonso MJ. Nanomedicines for overcoming biological barriers. Biomedicine & Pharmacotherapy. 2004;58:168–172. doi: 10.1016/j.biopha.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Romanowski S, Chiaramoni NS, Lioy VS, Gargini RA, Viera LI, Taira MC. Characterization of diacetylenic liposomes as carriers for oral vaccines. Chem Phys Lipids. 2003;122:191–203. doi: 10.1016/s0009-3084(02)00190-1. [DOI] [PubMed] [Google Scholar]
- 8.Bakri SJ, Pulido JS, Mukherjee P, Marler RJ, Mukhopadhyay D. Absence of histologic retinal toxicity of intravitreal nanogold in a rabbit model. Retina. 2008;28:147–149. doi: 10.1097/IAE.0b013e3180dc9360. [DOI] [PubMed] [Google Scholar]
- 9.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair MK, Hoelzer K, Tkaczuk K, Park YC, Lee LW. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 10.Bisby RH, Mead C, Mitchell AC, Morgan CG. Fast laser-induced solute release from liposomes sensitized with photochromic lipid: effects of temperature, lipid host, and sensitizer concentration. Biochem Biophys Res Commun. 1999;262:406–410. doi: 10.1006/bbrc.1999.1206. [DOI] [PubMed] [Google Scholar]
- 11.Bisby RH, Mead C, Morgan CG. Photosensitive liposomes as ‘cages’ for laser-triggered solute delivery: the effect of bilayer cholesterol on kinetics of solute release. FEBS Lett. 1999;463:165–168. doi: 10.1016/s0014-5793(99)01612-9. [DOI] [PubMed] [Google Scholar]
- 12.Bisby RH, Mead C, Morgan CG. Active uptake of drugs into photosensitive liposomes and rapid release on UV photolysis. Photochem Photobiol. 2000;72:57–61. doi: 10.1562/0031-8655(2000)072<0057:auodip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Bisby RH, Mead C, Morgan CG. Wavelength-programmed solute release from photosensitive liposomes. Biochem Biophys Res Commun. 2000;276:169–173. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 14.Chandra B, Mallik S, Srivastava DK. Design of photocleavable lipids and their application in liposomal “uncorking”. Chem Commun (Camb) 2005:3021–3023. doi: 10.1039/b503423j. [DOI] [PubMed] [Google Scholar]
- 15.Chandra B, Subramaniam R, Mallik S, Srivastava DK. Formulation of photocleavable liposomes and the mechanism of their content release. Org Biomol Chem. 2006;4:1730–1740. doi: 10.1039/b518359f. [DOI] [PubMed] [Google Scholar]
- 16.Chen YH, Gryshuk A, Achilefu S, Ohulchansky T, Potter W, Zhong TX, Morgan J, Chance B, Prasad PN, Henderson BW, Oseroff A, Pandey RK. A novel approach to a bifunctional photosensitizer for tumor imaging and phototherapy. Bioconjugate Chemistry. 2005;16:1264–1274. doi: 10.1021/bc050177o. [DOI] [PubMed] [Google Scholar]
- 17.Chen YH, Miclea R, Srikrishnan T, Balasubramanian S, Dougherty TJ, Pandey RK. Investigation of human serum albumin (HSA) binding specificity of certain photosensitizers related to pyropheophorbide-a and bacteriopurpurinimide by circular dichroism spectroscopy and its correlation with in vivo photosensitizing efficacy. Bioorganic & Medicinal Chemistry Letters. 2005;15:3189–3192. doi: 10.1016/j.bmcl.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Chiaramoni NS, Speroni L, Taira MC, Alonso SV. Liposome/DNA systems: correlation between association, hydrophobicity and cell viability. Biotechnol Lett. 2007;29:1637–1644. doi: 10.1007/s10529-007-9454-y. [DOI] [PubMed] [Google Scholar]
- 19.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29:992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 21.De Rosa FS, Bentley MV. Photodynamic therapy of skin cancers: sensitizers, clinical studies and future directives. Pharm Res. 2000;17:1447–1455. doi: 10.1023/a:1007612905378. [DOI] [PubMed] [Google Scholar]
- 22.Derycke AS, Kamuhabwa A, Gijsens A, Roskams T, De VD, Kasran A, Huwyler J, Missiaen L, De Witte PA. Transferrin-conjugated liposome targeting of photosensitizer AlPcS4 to rat bladder carcinoma cells. J Natl Cancer Inst. 2004;96:1620–1630. doi: 10.1093/jnci/djh314. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly RF, Ma LW, Juzenas P, Iani V, McCarron PA, Woolfson AD, Moan J. Topical bioadhesive patch systems enhance selectivity of protoporphyrin IX accumulation. Photochemistry and Photobiology. 2006;82:670–675. doi: 10.1562/2005-08-08-RA-641. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly RF, McCarron PA, Ma LW, Juzenas P, Iani V, Woolfson AD, Zawislak AA, Moan J. Facilitated delivery of ALA to inaccessible regions via bioadhesive patch systems. Journal of Environmental Pathology Toxicology and Oncology. 2006;25:389–402. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.240. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly RF, McCarron PA, Morrow DI, Sibani SA, Woolfson AD. Photosensitiser delivery for photodynamic therapy. Part 1: Topical carrier platforms. Expert Opinion on Drug Delivery. 2008;5:757–766. doi: 10.1517/17425247.5.7.757. [DOI] [PubMed] [Google Scholar]
- 26.Fenske DB, Chonn A, Cullis PR. Liposomal nanomedicines: an emerging field. Toxicol Pathol. 2008;36:21–29. doi: 10.1177/0192623307310960. [DOI] [PubMed] [Google Scholar]
- 27.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5:25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 28.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54 4:15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 29.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Gerasimov OV, Qualls M, Rui Y, Thompson DH. Intracellular drug delivery using pH- and light-activated diplasmenylcholine liposomes. Abstracts of Papers of the American Chemical Society. 1997;213:303-MSE. [Google Scholar]
- 31.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane Ammonium-Sulfate Gradients in Liposomes Produce Efficient and Stable Entrapment of Amphipathic Weak Bases (Vol 1151, Pg 201, 1993) Biochimica et Biophysica Acta-Biomembranes. 1994;1190:197. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 32.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane Ammonium-Sulfate Gradients in Liposomes Produce Efficient and Stable Entrapment of Amphipathic Weak Bases. Biochimica et Biophysica Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 33.Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 34.Juarranz A, Jaen P, Sanz-Rodriguez F, Cuevas J, Gonzalez S. Photodynamic therapy of cancer. Basic principles and applications. Clinical & Translational Oncology. 2008;10:148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 35.Klohs J, Wunder A, Licha K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Research in Cardiology. 2008;103:144–151. doi: 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- 36.Kronberg B, Dahlman A, Carlfors J, Karlsson J, Artursson P. Preparation and Evaluation of Sterically Stabilized Liposomes - Colloidal Stability, Serum Stability, Macrophage Uptake, and Toxicity. Journal of Pharmaceutical Sciences. 1990;79:667–671. doi: 10.1002/jps.2600790803. [DOI] [PubMed] [Google Scholar]
- 37.Lavi A, Weitman H, Holmes RT, Smith KM, Ehrenberg B. The depth of porphyrin in a membrane and the membrane's physical properties affect the photosensitizing efficiency. Biophysical Journal. 2002;82:2101–2110. doi: 10.1016/S0006-3495(02)75557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson GE, Lee Y, Singh A. Formation of stable nanocapsules from polymerizable phospholipids. Langmuir. 2003;19:6401–6407. [Google Scholar]
- 39.Lawson GW, Breen JJ, Marquez M, Singh A, Smith BD. Polymerization of vesicles composed of N-(4-inylbenzoyl)phosphatidylethanolamine. Langmuir. 2003;19:3557–3560. [Google Scholar]
- 40.Leamon CP, Low PS. Folate-mediated targeting: from diagnostics to drug and gene delivery. Drug Discov Today. 2001;6:44–51. doi: 10.1016/s1359-6446(00)01594-4. [DOI] [PubMed] [Google Scholar]
- 41.Li KC, Bednarski MD. Vascular-targeted molecular imaging using functionalized polymerized vesicles. J Magn Reson Imaging. 2002;16:388–393. doi: 10.1002/jmri.10174. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Wan Y, Kutateladze AG. Dithiane-Based Photolabile Amphiphiles ΓÇë Toward Photolabile Liposomes1,2. Langmuir. 2003;19:6381–6391. [Google Scholar]
- 43.Liao JY. Construction of nanogold hollow balls with dendritic surface as immobilized affinity support for protein adsorption. Colloids Surf B Biointerfaces. 2007;57:75–80. doi: 10.1016/j.colsurfb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Liu XM, Yang B, Wang YL, Wang JY. Photoisomerisable cholesterol derivatives as photo-trigger of liposomes: Effect of lipid polarity, temperature, incorporation ratio, and cholesterol. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2005;1720:28–34. doi: 10.1016/j.bbamem.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Majoros IJ, Williams CR, Baker JR., Jr Current dendrimer applications in cancer diagnosis and therapy. Curr Top Med Chem. 2008;8:1165–1179. doi: 10.2174/156802608785849049. [DOI] [PubMed] [Google Scholar]
- 46.Mccoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. Light-triggered molecule-scale drug dosing devices. Journal of the American Chemical Society. 2007;129:9572-+. doi: 10.1021/ja073053q. [DOI] [PubMed] [Google Scholar]
- 47.Morgan CG, Bisby RH, Johnson SA, Mitchell AC. Fast solute release from photosensitive liposomes: an alternative to ‘caged’ reagents for use in biological systems. FEBS Lett. 1995;375:113–116. doi: 10.1016/0014-5793(95)01193-i. [DOI] [PubMed] [Google Scholar]
- 48.Oku N, Ishii T. Antiangiogenic Photodynamic Therapy with Targeted Liposomes. Methods in Enzymology Liposomes. 2009;Pt G 465:313–330. doi: 10.1016/S0076-6879(09)65016-3. [DOI] [PubMed] [Google Scholar]
- 49.Pakhomov S, Hammer RP, Mishra BK, Thomas BN. Chiral tubule self-assembly from an achiral diynoic lipid. Proc Natl Acad Sci U S A. 2003;100:3040–3042. doi: 10.1073/pnas.0030051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H, Jung SH, Kim DC, Shin SC, Cho CW. Development and Validation of HPLC Assay of a New Molecule, 6-methyl-3-phenethyl-3, 4-dihydro-1H-quinazoline-2-thione from Solid Lipid Nanoparticles and its Topical Formulations. Journal of Liquid Chromatography & Related Technologies. 2009;32:512–525. [Google Scholar]
- 52.Peetla C, Stine A, Labhasetwar V. Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol Pharm. 2009;6:1264–1276. doi: 10.1021/mp9000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy JA, Low PS. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit Rev Ther Drug Carrier Syst. 1998;15:587–627. [PubMed] [Google Scholar]
- 54.Regen SL, Singh A, Oehme G, Singh M. Polymerized phosphatidyl choline vesicles. Stabilized and controllable time-release carriers. Biochem Biophys Res Commun. 1981;101:131–136. doi: 10.1016/s0006-291x(81)80020-4. [DOI] [PubMed] [Google Scholar]
- 55.Robertson CA, Evans DH, Abraharnse H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. Journal of Photochemistry and Photobiology B-Biology. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Rui YJ, Thompson DH. Stereocontrolled Synthesis of Plasmalogen-Type Lipids from Glyceryl Ester Precursors. Journal of Organic Chemistry. 1994;59:5758–5762. [Google Scholar]
- 57.Rui YJ, Thompson DH. Efficient stereoselective synthesis of plasmenylcholines. Chemistry-A European Journal. 1996;2:1505–1508. [Google Scholar]
- 58.Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: Perspectives on pharmacology and toxicology of quantum dots. Toxicology and Applied Pharmacology. 2009;238:280–288. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shackley DC, Whitehurst C, Clarke NM, Betts C, Moore JV. Photodynamic therapy. Journal of the Royal Society of Medicine. 1999;92:562–565. doi: 10.1177/014107689909201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shackley DC, Whitehurst C, Moore JV, George NJR, Betts CD, Clarke NW. Light penetration in bladder tissue: implications for the intravesical photodynamic therapy of bladder tumours. Bju International. 2000;86:638–643. doi: 10.1046/j.1464-410x.2000.00872.x. [DOI] [PubMed] [Google Scholar]
- 61.Shum P, Kim JM, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv Drug Deliv Rev. 2001;53:273–284. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 62.Sibani SA, McCarron PA, Woolfson AD, Donnelly RF. Photosensitiser delivery for photodynamic therapy. Part 2: systemic carrier platforms. Expert Opinion on Drug Delivery. 2008;5:1241–1254. doi: 10.1517/17425240802444673. [DOI] [PubMed] [Google Scholar]
- 63.Singh A. An efficient synthesis of phosphatidylcholines. J Lipid Res. 1990;31:1522–1525. [PubMed] [Google Scholar]
- 64.Singh A, Marchywka S. Synthesis and Characterization of Head Group Modified 1,3 Diacetylenic Phospholipids. Abstracts of Papers of the American Chemical Society. 1989;198:147-MSE. [Google Scholar]
- 65.Stover TC, Kim YS, Lowe TL, Kester M. Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials. 2008;29:359–369. doi: 10.1016/j.biomaterials.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 66.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11:3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 67.Thompson DH, Gerasimov OV, Wheeler JJ, Rui Y, Anderson VC. Triggerable plasmalogen liposomes: improvement of system efficiency. Biochim Biophys Acta. 1996;1279:25–34. doi: 10.1016/0005-2736(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 68.Tiwari SB, Amiji MM. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol. 2006;6:3215–3221. doi: 10.1166/jnn.2006.440. [DOI] [PubMed] [Google Scholar]
- 69.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35:61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 70.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 71.Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and Imaging. Aaps Journal. 2007;9:E128–E147. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.varez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive Intelligent Drug Delivery Systems. Photochemistry and Photobiology. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang SX, Bao A, Phillips WT, Goins B, Herrera SJ, Santoyo C, Miller FR, Otto RA. Intraoperative therapy with liposomal drug delivery: retention and distribution in human head and neck squamous cell carcinoma xenograft model. Int J Pharm. 2009;373:156–164. doi: 10.1016/j.ijpharm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 75.Yang T, Choi MK, Cui FD, Lee SJ, Chung SJ, Shim CK, Kim DD. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharmaceutical Research. 2007;24:2402–2411. doi: 10.1007/s11095-007-9425-y. [DOI] [PubMed] [Google Scholar]
- 76.Yavlovich A, Singh A, Tarasov S, Capala J, Blumenthal R, Puri A. Design of liposomes containing photopolymerizable phospholipids for triggered release of contents. Journal of Thermal Analysis and Calorimetry. 2009;98:97–104. doi: 10.1007/s10973-009-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zarif L. Elongated supramolecular assemblies in drug delivery. J Control Release. 2002;81:7–23. doi: 10.1016/s0168-3659(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Y. Lipid Nanotubes: Formation, Templating Nanostructures and Drug Nanocarriers. Critical Reviews in Solid State and Materials Sciences. 2008;33:183–196. [Google Scholar]