Abstract
Context
Serotonergic dysfunction is implicated in the pathogenesis of posttraumatic stress disorder (PTSD), and recent animal models suggest that disturbances in serotonin type 1B receptor function, in particular, may contribute to chronic anxiety. However, the specific role of the serotonin type 1B receptor has not been studied in patients with PTSD.
Objective
To investigate in vivo serotonin type 1B receptor expression in individuals with PTSD, trauma-exposed control participants without PTSD (TC), and healthy (non–trauma-exposed) control participants (HC) using positron emission tomography and the recently developed serotonin type 1B receptor selective radiotracer [11C]P943.
Design
Cross-sectional positron emission tomography study under resting conditions.
Setting
Academic and Veterans Affairs medical centers.
Participants
Ninety-six individuals in 3 study groups: PTSD (n=49), TC (n=20), and HC (n=27).
Main Outcome Measure
Regional [11C]P943 binding potential (BPND) values in an a priori–defined limbic corticostriatal circuit investigated using multivariate analysis of variance and multiple regression analysis.
Results
A history of severe trauma exposure in the PTSD and TC groups was associated with marked reductions in [11C]P943 BPND in the caudate, the amygdala, and the anterior cingulate cortex. Participant age at first trauma exposure was strongly associated with low [11C]P943 BPND. Developmentally earlier trauma exposure also was associated with greater PTSD symptom severity and major depression comorbidity.
Conclusions
These data suggest an enduring effect of trauma history on brain function and the phenotype of PTSD. The association of early age at first trauma and more pronounced neurobiological and behavioral alterations in PTSD suggests a developmental component in the cause of PTSD.
Traumatic events and extreme stress exposure can elicit a variety of psychiatric syndromes.1 However, relatively little is known regarding the human neurobiological mechanisms that underlie adaptive or pathologic responses to traumatic stress.2,3 A subset of individuals exposed to trauma1 develop posttraumatic stress disorder (PTSD), a disabling clinical syndrome characterized by recurrent intrusive memories of a traumatic event, repeated avoidance of reminders of the trauma, and high levels of arousal and anxiety.4 The neurobiological mechanisms through which specific features of stress, including the number, severity, and duration of stress exposure or the developmental timing of traumatization, modulate PTSD risk and severity are unknown.5–9 Consequently, treatment options for these often chronically symptomatic patients remain limited.10,11
The serotonin system plays a critical role in behavioral and emotional regulation,12–14 and serotonin dysfunction has been implicated repeatedly in the neurobiological processes of PTSD.3,15–17 Administration of m-chlorophenylpiperazine, a serotonin agonist, can evoke specific symptoms of PTSD, but these effects are not observed when m-chlorophenylpiperazine is administered to patients with other psychiatric disorders.18–20 Also, the serotonin system is the target of the only 2 medications approved by the US Food and Drug Administration for the treatment of PTSD.21–24 However, defining the specific role of the serotonin system in PTSD has been challenging in the face of a wide and complex diversity of receptor subtypes and their functions.25 To date, relatively few in vivo molecular imaging studies of the serotonin system in PTSD have been undertaken. A small study26 of the serotonin type 1A receptor in a heterogeneous sample of patients with PTSD found no regional binding differences compared with a control group, and no other serotonin receptors or the serotonin transporter have been studied in PTSD. Recent studies27–33 have begun to highlight the role of the serotonin type 1B receptor subtype specifically in anxious and depressive phenotypes in animals. Of particular relevance to PTSD, the serotonin type 1B receptor is stress responsive such that stress exposure reduces receptor function in animals, resulting in behaviors that resemble aspects of chronic anxiety.34,35
Functional neuroimaging research36–38 has identified a circuit of cortical and subcortical brain regions that regulates stress-related behaviors that may be dysfunctional in individuals with PTSD. This circuit includes the anterior cingulate cortex (ACC), the striatum, the amygdala, and the hippocampus36,39,40 and is innervated extensively by serotonin-containing neurons arising from raphe nuclei in the brainstem.14 Maladaptive behavioral responses to trauma exposure and clinical symptoms of PTSD are believed to emerge from neurochemical and neurophysiologic dysfunction of this circuit; evidence suggests that serotonergic mechanisms play a key role in this process.38,41
The recent development of the serotonin type 1B receptor selective radiotracer, designated [11C]P943,42 now makes it possible to conduct an in vivo assessment of serotonin type 1B receptors in humans in brain regions associated with stress regulation and PTSD. Following the animal models, we predicted that individuals with PTSD would have lower levels of regional [11C]P943 binding potential (BPND) in the limbic corticostriatal circuit compared with non–trauma-exposed control participants. In light of the clinical and epidemiologic data suggesting a more severe clinical course of PTSD and higher rates of comorbid depression with PTSD after higher levels of traumatic stress exposure and early onset of stress exposure (eg, trauma exposure in adolescence),5–9,43 we specifically investigated the effect of these variables on [11C]P943 BPND. To test the hypotheses, we studied patients with PTSD, trauma-exposed control participants free of lifetime psychiatric illness (TC), and healthy (ie, non–trauma-exposed) control participants (HC). We included the TC group to assess the effects of trauma exposure per se on [11C]P943 BPND, independent of PTSD symptoms, to clarify whether serotonin type 1B receptor alterations are explained by a history of trauma exposure or whether they result from the presence of PTSD symptoms.
METHODS
PARTICIPANTS
Ninety-six participants in 3 study groups (PTSD, n=49; TC, n=20; and HC, n=27) were recruited through public advertisements. Participants with PTSD were free of comorbid psychiatric disorders except for major depressive disorder (MDD) if the primary diagnosis was determined to be PTSD (which was defined by PTSD being the dominant clinical syndrome) and if the onset of MDD occurred after the onset of PTSD. Participants with PTSD were largely treatment naive (ie, 1 of 49 participants had a history of treatment with a serotonin-selective reuptake inhibitor and was not taking medication for >4 weeks before the scan via positron emission tomography [PET]). None of the participants were undergoing psychotherapy at the time of scanning. After providing written informed consent, participants underwent a thorough medical and psychiatric evaluation followed by magnetic resonance imaging (MRI) and a resting-state scan via PET with the serotonin type 1B receptor antagonist radiotracer [11C]P943. Psychiatric diagnoses were made using DSM-IV-TR criteria and the Structured Clinical Interview for DSM-IV administered by an experienced physician.4,44 Symptom severity of PTSD was measured using the Clinician-Administered PTSD Scale for DSM-IV (CAPS),45 and trauma history was quantified using the Traumatic Life Events Questionnaire.46 Only traumatic events that met DSM-IV-TR PTSD criterion A1 for trauma exposure and criterion A2, which confirms the emotional response to the trauma, were counted toward participants’ trauma history in this study. Additional measurements included the Hamilton Rating Scale for Anxiety (HAM-A),47 the Montgomery-Asberg Depression Rating Scale (MADRS),48 the Fagerström Test for Nicotine Dependence,49 and the Wechsler Abbreviated Scale of Intelligence.50 To meet the TC inclusion criteria, individuals must have been exposed to at least 1 potentially traumatic event that met DSM-IV-TR criteria A1 and A2 but have no lifetime PTSD or other Axis I diagnosis.
All the participants were evaluated by physical examination; electrocardiographic, standard blood chemistry, hematology laboratory, and toxicologic testing; and urinalysis. Participants with significant medical or neurologic conditions, with substance abuse within 12 months of the scan via PET or a lifetime history of substance dependence, or with history of head injury with loss of consciousness were excluded from the study. The absence of substance use was determined by self-report and confirmed by the results of urine toxicologic and breathalyzer tests at screening and on the days when scans via MRI and PET were administered. This study was approved by the Yale University School of Medicine Human Investigation Committee, the Mount Sinai School of Medicine Institutional Review Board, the Human Subjects Subcommittee of the Veterans Affairs Connecticut Healthcare System, the Yale University Magnetic Resonance Research Center, and the Yale–New Haven Hospital Radiation Safety Committee.
ACQUISITION AND ANALYSIS OF SCANS VIA [11C]P943 PET
Participant preparation for the scan via PET consisted of indwelling venous catheter placement across all diagnostic groups. Also, radial arterial catheter placement was performed in an initial subgroup of individuals (n=39) for arterial blood collection and blood input function establishment to validate the kinetic modeling method. After a robust analysis method was established, placement of the arterial catheter was discontinued. A transmission scan using a 137Cs point source was obtained before the emission scan. The scans via PET were acquired for 120 minutes at rest using a single intravenous injection of the high–specific activity selective serotonin type 1B receptor antagonist radiotracer [11C]P94342 and a high-resolution research PET scanner (207 slices, resolution <3-mm full-width at half maximum in 3-dimensional acquisition mode). Dynamic scan data were reconstructed with corrections (ie, attenuation, normalization, scatter, randoms, and dead time). Motion correction of data obtained via PET was performed by coregistering each reconstructed frame to an early summed image (0–10 minutes after injection) using a 6-parameter mutual information algorithm and Functional Magnetic Resonance Imaging of the Brain (FMRIB)’s Linear Image Registration Tool (FLIRT, FSL 3.2; FMRIB Analysis Group, Oxford, United Kingdom).
The MRI results were obtained for each participant using a Siemens 3T Trio system (Siemens Medical Solutions USA, Inc, Malvern, Pennsylvania) to exclude individuals with anatomical abnormalities and for coregistration. A second summed image (0–10 minutes after injection) was created from the motion-corrected scan via PET and registered to the participant’s MRI results, which, in turn, was registered (via 12-parameter affine transformation) to an MRI template (a Montreal Neurological Institute space). The regions of interest (ROIs) were taken from the template for SPM2 (via anatomical automatic labeling) and applied to the image obtained via PET to produce time-activity curves for each ROI in reference to the cerebellum.51 Pixel-by-pixel analysis was performed using the multilinear reference tissue model MRTM252 to produce images of BPND.53 The interpretation of BPND is fND*Bavail/Kd, in which fND is the tracer-free fraction in a region without specific binding, Bavail is the unoccupied receptor concentration, and Kd is the dissociation equilibrium constant of the tracer. The cerebellum was used as the reference region because it is essentially devoid of serotonin type 1B receptors.54 Assuming that no change is observed in affinity or nonspecific binding between participant groups, changes in BPND were interpreted as changes in receptor concentration. The BPND values from MRTM2 have provided highly comparable results (R2=0.83–0.94 across the ROIs) with those obtained with arterial input functions.55 Mean (SD) cerebellum distribution volume data collected in the initial subgroup of study participants were not different among the 3 diagnostic groups (HC: 4.48 [1.09]; TC: 4.89 [0.52]; and PTSD: 4.47 [0.46]; HC vs TC: P=.43; HC vs PTSD: P=.98; and TC vs PTSD: P=.34), validating its use as a reference region in modeling analysis.
The [11C]P943 BPND values were measured in a prespecified limbic corticostriatal circuit that includes the amygdala, the hippocampus, the ACC, the caudate, and the globus pallidus. These regions constitute a neural circuit consistently implicated in the pathogenesis of PTSD36,39,40 and are known to contain high levels of serotonin type 1B receptors.54
STATISTICAL ANALYSIS
Analyses of variance (ANOVAs) were used to compare continuous clinical and demographic variables of the PTSD, TC, and HC groups. Continuous variables were examined for normality using normal probability plots and Kolmogorov-Smirnov test statistics by group and region. No transformations were necessary. In the case of dichotomous variables, χ2 tests were used. Multivariate ANOVA (MANOVA) of [11C]P943 BPND values from the 5 ROIs in the a priori limbic corticostriatal circuit model across the 3 groups was used to test for group differences in [11C]P943 BPND in the circuit. Subsequently, separate ANOVAs for each region were used to determine the location of between-group differences in [11C]P943 BPND, followed by post hoc Tukey honestly significant difference t tests among groups. Also, MANOVA was used to assess the effects of age, sex, race, body mass index, and injected dose of [11C]P943 BPND.
Associations among continuous demographic, clinical, and [11C]P943 BPND variables were assessed using Pearson product-moment correlations using a 2-sided .05 significance threshold. Follow-up stepwise multiple regression was performed to further clarify the effect of demographic and clinical variables on [11C]P943 BPND. In the stepwise regression, we evaluated the following variables for entry into the model: age, age at first traumatic experience, number of traumatic experiences, MADRS score, CAPS score, sex, and body mass index. The variable associated with the lowest P value below the threshold of .05 was entered into the model first. Then, we attempted to add additional variables 1 at a time using a significance level of .05 until no more variables could be added. We used R2 to assess what percentage of the variability in the serotonin type 1B measures the predictors of the model explained. We also performed an additional stepwise regression in which group was entered into the model regardless of significance and additional predictors were added as indicated. Each of the potentially influential variables was considered 1 at a time, and at each inclusion step, the predictor with the lowest significance level less than .05 was entered. The model building stopped when no more predictors could be entered into the model. In the last step, we reexamined the significance of the group effect to assess whether group was a significant predictor of [11C]P943 BPND after accounting for the other significant variables.
Mean (SD) is reported unless otherwise noted, and significance was set at 2-tailed α =.05. Statistical analyses were conducted using commercially available software programs (SPSS, version 16.0; SPSS, Inc, Chicago, Illinois, and SAS, version 9.1.3l; SAS Institute, Inc, Cary, North Carolina).
RESULTS
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF THE SAMPLE
Participants with PTSD experienced clinically significant symptoms of PTSD (mean [SD] CAPS score: 66.5 [19.7]; range, 27.0–97.0), anxiety (mean [SD] HAM-A score: 19.3 [7.2]; range, 13.0–31.0), and depression (mean [SD] MADRS score: 25.6 [8.2]; range, 14.0–41.0). In comparison, participants in the TC group had very low scores on the CAPS (mean [SD], 3.3 [5.2]; range, 0–17.0), HAM-A (2.5 [3.1]; 0–10.0), and MADRS ( 3.7 [3.2]; 0–11.0). Both trauma-exposed groups experienced multiple lifetime traumas (PTSD: mean [SD], 4.7 [2.5]; range, 1.0–11.0; TC: 3.6 [2.5]; 1.0–11.0), and the numbers did not differ between groups (t67=−1.6, P =.11). The mean (SD) age of participants at their first trauma exposure was 15.1 (5.5) years (range, 8.0–25.0 years) in the PTSD group and 16.5 (7.0) years (range, 3.0–27.0 years) in the TC group (t67=0.86, P=.39) (Table 1). In the PTSD group, 15 patients met the criteria for current MDD at the time of scanning via PET and 34 were free of lifetime or current MDD.
Table 1.
Demographic, Clinical, and Positron Emission Tomographic Procedural Characteristics of the 96 Study Participants
| Characteristic | PTSD Group (n=49) | TC Group (n=20) | HC Group (n=27) | P Valuea |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) [range], y | 32.0 (9.3) [19.0–54.0] | 30.7 (9.6) [20.0–55.0] | 30.4 (8.9) [18.0–54.0] | .74 |
| Sex, M/F, No. | 26/23 | 14/6 | 17/10 | .39 |
| Ethnicity, No. | ||||
| White | 23 | 10 | 22 | NA |
| African American | 19 | 9 | 4 | NA |
| Hispanic | 7 | 1 | 1 | NA |
| BMI, mean (SD) | 28.4 (5.0) | 27.2 (4.4) | 24.2 (4.6) | .003b |
| Smoking status, No. | .95 | |||
| Yes | 4 | 2 | 2 | NA |
| No | 45 | 18 | 25 | NA |
| Description of index trauma, No. (%) | ||||
| Physical assaultc | 38 (77.6) | 16 (80.0) | NA | NA |
| Combat related | 9 (18.4) | 4 (20.0) | NA | NA |
| MVC | 2 (4.1) | 0 | NA | NA |
| Indices of lifetime trauma burden, mean (SD) | ||||
| Age at first trauma, y | 15.1 (5.5) | 16.5 (7.0) | NA | .39 |
| No. of lifetime traumatic experiencesd | 4.7 (2.5) | 3.6 (2.5) | NA | .11 |
| Clinical measures, mean (SD) | ||||
| HAM-A score | 19.3 (7.2) | 2.5 (3.1) | 3.3 (4.3) | <.001e |
| MADRS score | 25.6 (8.2) | 3.7 (3.2) | 4.1 (4.5) | <.001e |
| CAPS total score | 66.5 (19.7) | 3.3 (5.2) | NA | <.001e |
| CAPS reexperiencing cluster score | 17.7 (7.4) | 0.56 (1.4) | NA | <.001e |
| CAPS avoidance cluster score | 27.6 (9.9) | 0.89 (1.9) | NA | <.001e |
| CAPS hyperarousal cluster score | 21.2 (6.7) | 1.9 (3.1) | NA | <.001e |
| Injection variables, mean (SD) | ||||
| Injected dose, MBq | 596 (135.00) | 637 (99.00) | 625 (140.00) | .41 |
| Specific activity, MBq/nmol | 165 (69.08) | 176 (83.00) | 176 (67.00) | .77 |
| Injected mass, μg | 1.89 (1.35) | 1.89 (1.02) | 1.73 (0.77) | .83 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAPS, Clinician-Administered PTSD Scale for DSM-IV; F, female; HAM-A, Hamilton Rating Scale for Anxiety; HC, healthy (ie, non–trauma-exposed) control participant; M, male; MADRS, Montgomery-Asberg Depression Rating Scale; MVC, motor vehicle crash; NA, not applicable; PTSD, posttraumatic stress disorder; TC, trauma-exposed control participant free of lifetime psychiatric illness.
Determined by independent-samples t tests or analyses of variance followed by the Tukey post hoc test for continuous variables or by the χ2 test for dichotomous variables.
Post hoc PTSD>HC, P=.002.
Includes sexual assault, domestic violence, and other non–combat-related physical violence.
Those included meet DSM-IV-TR criterion A for PTSD.
Post hoc PTSD>HC, P<.001; PTSD>TC, P<.001.
MEASURES OF [11C]P943 BPND VIA PET
Analysis of [11C]P943 BPND in a Limbic Corticostriatal Circuit
The full factorial group×ROI MANOVA was significant, indicating [11C]P943 BPND differences between groups in the proposed limbic corticostriatal circuit (F10,178=2.18, P =.02). Univariate tests of between-group differences in each ROI yielded significant regional effects in the caudate (F2,93=7.23, P =.001), the amygdala (F2,93=4.12, P =.02), and the ACC (F2,93=3.34, P =.04). The hippocampus and globus pallidus ANOVAs were not significant (Table 2).
Table 2.
Regional [11C]P943 BPND in the PTSD, TC, and HC Study Groups in a Limbic Corticostriatal Circuita
| Region | [11C]P943 BPND, Mean (SD)
|
F2,93 | P Value | ||
|---|---|---|---|---|---|
| PTSD Group (n=49) | TC Group (n=20) | HC Group (n=27) | |||
| ACC | 0.909 (0.143) | 0.902 (0.104) | 1.00 (0.216) | 3.34 | .04 |
| Amygdala | 0.692 (0.166) | 0.663 (0.115) | 0.777 (0.133) | 4.12 | .02 |
| Caudate | 0.465 (0.147) | 0.425 (0.156) | 0.573 (0.130) | 7.23 | .001 |
| Hippocampus | 0.395 (0.099) | 0.366 (0.103) | 0.401 (0.106) | 0.94 | .39 |
| Pallidum | 1.583 (0.338) | 1.579 (0.271) | 1.722 (0.291) | 1.96 | .15 |
Abbreviations: ACC, anterior cingulate cortex; BPND, binding potential; HC, healthy (ie, non–trauma-exposed) control participant; PTSD, posttraumatic stress disorder; TC, trauma-exposed control individual free of lifetime psychiatric illness.
Significant group differences in [11C]P943 BPND in a limbic corticostriatal circuit initially established by multivariate analysis of variance, including the 5 a priori regions of interest (Wilks λ F10,18=2.18; P =.02). The F statistics and P values were determined by subsequent 1-way analysis of variance for each region of interest. The Tukey honestly significant difference post hoc t tests and percentage differences were as follows: anterior cingulate cortex: PTSD<HC (9.1%, P=.048), TC=HC (P=.10), PTSD=TC (P=.98); amygdala: PTSD<HC (10.9%, P=.047), TC<HC (14.7%, P=.027), PTSD=TC (P=.74); and caudate: PTSD<HC (18.8%, P=.006), TC<HC (25.8%, P=.002), PTSD=TC (P=.56).
Primary Hypothesis Testing: [11C]P943 BPND Comparison Between PTSD and HC
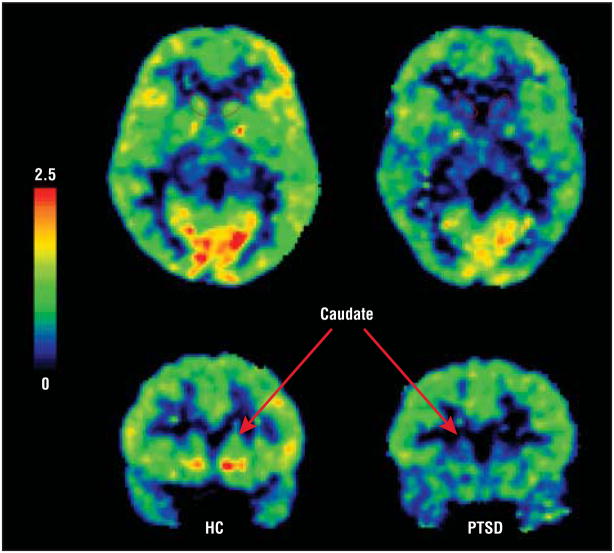
To test the primary hypothesis of serotonin type 1B receptor dysfunction in PTSD, we performed Tukey honestly significant difference post hoc t tests on [11C]P943 BPND values between the PTSD and HC groups in each of the 3 ROIs that demonstrated significance by NOVA (ie, the caudate, the amygdala, and the ACC) after the primary MANOVA. In the caudate, [11C]P943 BPND was reduced by 18.8% in the PTSD group compared with the HC group (P = .006) (Figure 1). In the amygdala, [11C]P943 BPND was reduced by 10.9% in the PTSD group compared with the HC group (P=.047). In the ACC, [11C]P943 BPND was reduced by 9.1% in the PTSD group compared with the HC group (P=.048) (Table 2). These results were driven by the PTSD+MDD subgroup, which, in these regions, differed significantly from the group with PTSD only, as will be described in detail herein.
Figure 1.
Regional [11C]P943 MRTM2 binding potential (BPND ) scans via positron emission tomography coregistered to magnetic resonance images illustrate reduced caudate BPND in the posttraumatic stress disorder (PTSD) group (right) relative to the healthy control (HC) group (left). The color scale represents relative BPND values (as reported in the Results section of the text). The caudate region of interest is outlined in the axial section and is indicated by an arrow in the corresponding coronal view.
Effects of Trauma on [11C]P943 BPND in the TC Group
We compared [11C]P943 BPND values between the TC and HC groups and between the TC and PTSD groups. Again, we restricted the analysis to the 3 brain regions that demonstrated group significances by ANOVA (ie, the caudate, the amygdala, and the ACC) after the primary MANOVA. In the caudate, [11C]P943 BPND was reduced by 25.8% in the TC group compared with the HC group (post hoc Tukey comparison, P=.002); the PTSD and TC groups did not differ (post hoc Tukey comparison, P=.56). In the amygdala, [11C]P943 BPND was reduced by 14.7% in the TC group compared with the HC group (post hoc Tukey comparison, P=.03); the PTSD and TC groups did not differ (post hoc Tukey comparison, P=.74). In the ACC, [11C]P943 BPND values were not significantly different between the TC and HC groups (post hoc Tukey comparison, P=.10) (Table 2).
Associations Among [11C]P943 BPND, Trauma History, and Clinical Characteristics
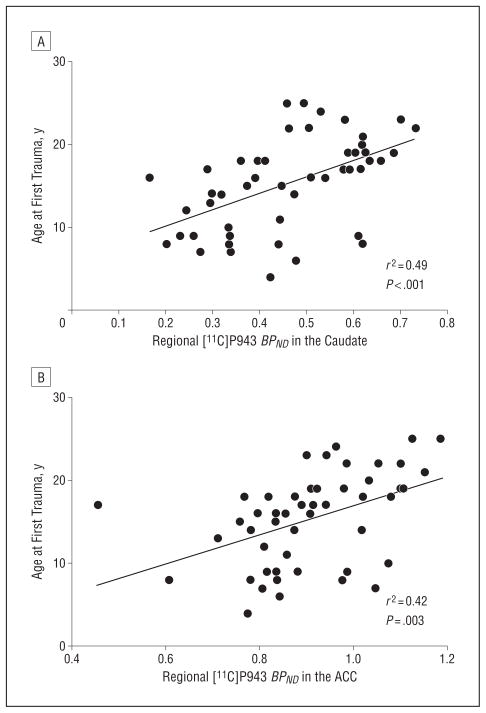
To further characterize the relationship between [11C]P943 BPND and trauma history, we first performed bivariate correlation analyses between [11C]P943 BPND values and trauma history variables in each significant ROI (ie, the caudate, the amygdala, and the ACC) in the PTSD and TC groups. In the PTSD group, [11C]P943 BPND was correlated with participant age at first trauma in the caudate (r=0.49, P<.001) and the ACC (r=0.42, P=.003) such that the earlier the first trauma exposure, the lower the [11C]P943 BPND (Figure 2). This association was not found in the amygdala. Participant age at first trauma was negatively correlated with CAPS score (r=−0.36, P=.02) (but not with HAM-A or MADRS scores) such that the younger the participant age at first trauma, the higher the level of PTSD symptom severity. Also, the total number of lifetime traumatic experiences was positively correlated with the CAPS score (r=0.32, P=.04). In the TC group, [11C]P943 BPND values were similarly correlated with age at first trauma in the caudate (r=0.75, P<.001) and the amygdala (r=0.47, P=.04).
Figure 2.
Association between participant age at first trauma exposure and regional [11C]P943 binding potential (BPND ) in the caudate (A) and the anterior cingulate cortex (ACC) (B) in posttraumatic stress disorder. The younger the age at first trauma exposure, the lower the [11C]P943 BPND in the caudate and the ACC.
To determine the effects of MDD comorbidity on [11C]P943 BPND, we first divided the participants with PTSD into those with (n=15) and those without (n=34) lifetime or current MDD. Participants with PTSD+MDD were older than were participants with PTSD only (mean [SD] age, 36.7 [9.6] vs 29.8 [8.3] years; t46=−2.54; P =.02), had greater PTSD symptom severity (mean [SD] CAPS score, 75.3 [16.1] vs 62.2 [20.1]; t41=−2.13; P =.04), had a significantly earlier onset of trauma (9.9 [4.1] vs 17.4 [4.4] years; t47=5.62; P < .001), and had a significantly greater number of lifetime traumatic experiences (5.9 [1.9] vs 4.1 [2.5]; t47=−2.52; P =.02). The [11C]P943 BPND was significantly reduced in the caudate in the PTSD+MDD group compared with the group with PTSD only (27.0% reduction, t47=3.27, P =.002); the same was true in the ACC (11.0% reduction, t47=2.44, P =.02), and the hippocampus (15.4% reduction, t47=2.19, P =.03).
To clarify the effects of trauma history, symptom severity, and MDD comorbidity on [11C]P943 BPND, we performed stepwise multiple regression analysis. In the PTSD group, only participant age at first trauma was significantly associated with the caudate [11C]P943 BPND (F1,40=15.44, P < .001), explaining 28.0% of the variance, and ACC [11C]P943 BPND (F1,40=9.71, P =.003), explaining 20.0% of the variance. The [11C]P943 BPND difference between the PTSD and PTSD+MDD subgroups was no longer statistically significant after entering age at first trauma into the model, indicating that age at first trauma accounts for the observed reductions in [11C]P943 BPND. Neither the group nor any of the other variables was significantly associated with amygdala [11C]P943 BPND.
Regression analysis performed in the combined sample of all trauma-exposed participants (PTSD and TC, n=67) further confirmed that only participant age at first trauma was significantly associated with caudate [11C]P943 BPND (F1,58=17.09, P < .001), explaining 23.0% of the variance, and amygdala [11C]P943 BPND (F1,58=8.72, P =.005), explaining 13.0% of the variance.
COMMENT
In this study, we demonstrated a robust association between in vivo regional reductions in [11C]P943 BPND, a measure of serotonin type 1B receptor expression, and trauma history in patients with PTSD and in TC participants. Lower [11C]P943 BPND in PTSD was associated with an earlier age at first trauma exposure, a greater number of lifetime trauma exposures, more severe PTSD symptoms, and comorbidity with MDD. Regression analyses clarified that participant age at onset of the first trauma was the strongest predictor of [11C]P943 BPND reductions in individuals with a history of severe trauma.
The findings that low serotonin type 1B receptor expression is associated with greater symptom severity, co-morbidity with MDD, earlier onset of trauma exposure, and greater total lifetime trauma burden are consistent with the results of numerous epidemiologic studies documenting elevated risk of PTSD and more severe symptoms associated with childhood trauma and higher trauma load.5–9,43 In particular, early life stress has been repeatedly implicated as a risk factor for adult trauma exposure56,57 and for the development of PTSD.7,58,59 Although most research has focused on neuroendocrine function and the hypothalamic-pituitary-adrenal axis as mediating biological factors between early trauma and risk of PTSD,43,60 the findings of the present study highlight the serotonin system as being potentially important as well. The present findings are consistent with the lasting behavioral effects resulting from early perturbations of the serotonin system leading to anxiety-related phenotypes in animals.61,62
The strong association between trauma exposure and reduced serotonin type 1B receptor level found in the trauma control group further demonstrates the specific effects of trauma on molecular adaptations in neuronal networks that are dysfunctional in PTSD. However, low [11C]P943 BPND in the PTSD and TC groups suggests that abnormal serotonin type 1B receptor expression does not sufficiently explain the phenotype of PTSD. Thus, the data more strongly support the hypothesis that the extent of serotonin type 1B receptor alteration reflects features of trauma exposure (eg, age at exposure and the intensity, number, or perhaps other features of the trauma history) rather than the nature of the response to the trauma (eg, whether the individual did or did not develop PTSD). Individuals who proceed to develop PTSD would be expected to possess an additional vulnerability factor resulting from genetic or environmental factors or to lack a protective factor that may characterize resistance to the pathologic effects of trauma.
Autoradiographic studies demonstrate high levels of serotonin type 1B receptors localized to the basal ganglia,27,63–66 with somewhat lower levels localized to the neocortex and the amygdala,27,67 consistent with the findings of the present study. The observed [11C]P943 BPND reductions in the trauma-exposed cohorts were in the amygdala, the ACC, and the caudate, in line with current neurocircuitry hypotheses of PTSD.36,39,40 The largest [11C]P943 BPND reductions were in the caudate, a region that has been implicated in normal emotional behavior and emotional disorders68–70; however, it has received relatively little attention in neurobiological studies of PTSD (although several studies71–74 do address it). Of note, Cohen et al73 reported volumetric reductions in the caudate and the ACC in adults with a history of high levels of early life stress in the absence of psychiatric illness. The researchers found that higher levels of childhood and adolescent stress were associated with a larger magnitude of volumetric reduction, paralleling the findings of the present study. However, the [11C]P943 BPND data reported in the present study are corrected for any volumetric variation in the sample so that our serotonin type 1B receptor findings represent an additional neurobiological abnormality rather than being explained by any potential volumetric variations.
Functionally, serotonin type 1B receptors are G proteins negatively coupled to adenylyl cyclase that seem to regulate limbic corticostriatal signaling primarily as axon terminal heteroreceptors on nonserotonergic neurons (eg, γ-aminobutyric acid–, glutamate-, and dopamine-containing neurons).75–78 Although the behavioral functions of this receptor are incompletely understood, numerous preclinical studies30–35,79–81 implicate the receptor in emotional behavior, stress reactivity, and anxiety states. The present findings of in vivo reductions in serotonin type 1B receptors associated with trauma are consistent with those of animal studies34,35 demonstrating reductions in serotonin type 1B receptor messenger RNA transcription after stress exposure. A study33 of serotonin type 1B receptor overexpression in the dorsal raphe nucleus demonstrates decreased anxiety in animals (ie, fear-potentiated startle) in a stress-dependent manner. Loss of serotonin type 1B receptor–mediated regulation of glutamate and γ-aminobutyric acid systems has the potential to lead to downstream reductions in neurotrophic signaling and neurogenesis and loss of dendritic spines and branches in neurocircuitry relevant to PTSD.82
The serotonin system likely plays a role in multiple psychiatric disorders, and the degree of specificity of the observed [11C]P943 BPND reductions related to trauma exposure in the present study remains uncertain. Some of us recently reported reduced ventral striatal [11C]P943 BPND in individuals with MDD compared with HC participants.83 In a second study,84 some of us found elevated ventral striatal [11C]P943 BPND in individuals with alcohol dependence. Notably, MDD and alcohol dependence are often comorbid with PTSD. However, no ventral striatal between-group differences were observed in the present study. Instead, we found reduced [11C]P943 BPND in the caudate in trauma-exposed individuals. Although the causative implications of these differential binding patterns are not yet fully understood, these initial data suggest potential diagnostic specificity.
Some difficulty can occur in interpreting BPND data from human neuroimaging studies. Between-group differences in ligand binding could represent differences in receptor number as a result of true downregulation, which requires receptor degradation and perhaps decreased synthesis. Alternately, increased serotonin release, enhanced neuronal activity in the dorsal raphe nuclei, and increased serotonin synthesis and turnover in response to stress85,86 could lead to agonist-induced internalization of serotonin type 1B receptors.87 Finally, assuming a displacement model, differences in ligand binding could be explained by changes in transmitter release in which serotonin and the radioligand may compete directly for occupancy of the same receptor binding site,88 although this possibility has not yet been sufficiently addressed in human studies.
Several limitations to the present study deserve comment. Inherent limitations exist regarding the reliance on retrospective participant reports of trauma history. We also chose to include a broad range of trauma histories and individuals who had experienced multiple different trauma types. Therefore, the specific contribution of different types of trauma cannot be evaluated reliably in this study owing to the presence of multiple traumas of mixed types in many individuals in the cohort. However, the inclusion of a range of trauma histories allowed for observations of correlations between the developmental timing of trauma, trauma load, and serotonin type 1B receptor measures. The present study design does not address the important question of whether trauma exposure directly reduces serotonin type 1B receptor expression. Preclinical data suggest serotonin type 1B receptor modulation by environmental stress. However, other explanations for the observed findings include premorbid low levels of serotonin type 1B receptor expression resulting from genetic or environmental factors in individuals predisposed to trauma exposure or to the development of PTSD. Perhaps most important, this study did not allow us to fully clarify the potential pathologic role of reduced serotonin type 1B receptor expression in PTSD.
The findings of the present study are consistent with the premise that exposure to early trauma produces long-lasting neurobiological changes in the human brain and suggests a potential neurodevelopmental component in the cause of PTSD. Future studies are needed to clarify molecular factors in addition to the serotonin type 1B receptor that may characterize individuals who are vulnerable to the potentially pathogenic effects of trauma.
Acknowledgments
Funding/Support: This study was supported by awards R21 MH081103 (through the American Recovery and Reinvestment Act), R21 MH085627, and RL1 AA017540 from the National Institutes of Health; the Department of Veterans Affairs (VA) through its support of the Clinical Neurosciences Division of the VA National Center for PTSD; a VA merit review grant; and a Brain and Behavior Research Foundation (formerly the National Alliance for Research on Schizophrenia and Depression) 2007 Independent Investigator Award (Dr Neumeister). Dr Murrough receives research support from the Brain and Behavior Research Foundation and salary support through a Mount Sinai School of Medicine research fellowship funded with an educational grant from AstraZeneca Pharmaceuticals.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health of the National Institutes of Health, the VA, or the Brain and Behavior Research Foundation.
Previous Presentation: This study was presented in part at the Society of Biological Psychiatry 66th Annual Meeting; May 13, 2011; San Francisco, California.
Financial Disclosure: Dr Czermak has received salary support from the Max Kade Foundation, Inc. Dr Krystal has been a consultant to Aisling Capital LLC; AstraZeneca Pharmaceuticals; Brintnall & Nicolini, Inc; Easton Associates, LLC; Gilead Sciences, Inc; GlaxoSmithKline plc; Ortho-McNeil-Janssen Pharmaceuticals, Inc; Lundbeck Research USA, Inc; Merz Pharmaceuticals GmbH; MK Medical Communications; Pfizer, Inc; F. Hoffmann–La Roche Ltd; SK Holdings Co Ltd; Takeda Pharmaceutical Company Limited; Teva Pharmaceutical Industries Ltd; and Transcept Pharmaceuticals, Inc; and has the following patents and inventions: with Seibyl JP, Krystal JH, and Charney DS, dopamine and noradrenergic reuptake inhibitors in the treatment of schizophrenia, patent No. 5 447 948, September 5, 1995; and a coinventor on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (Patent Application No. PCTWO06108055A1).
Additional Contributions: We thank the staff of the Yale PET Center; Sue Kasserman, RN, for her help in recruitment and patient care; Brenda Breault, RN, BSN, for her contributions to patient care during the scans via PET; and the Yale-Pfizer Bioimaging Alliance for support in the development of [11C]P943.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52 (12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- 5.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48(3):216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz MJ, Wilner N, Kaltreider N, Alvarez W. Signs and symptoms of post-traumatic stress disorder. Arch Gen Psychiatry. 1980;37(1):85–92. doi: 10.1001/archpsyc.1980.01780140087010. [DOI] [PubMed] [Google Scholar]
- 7.Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993;150(2):235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- 8.Silva RR, Alpert M, Munoz DM, Singh S, Matzner F, Dummit S. Stress and vulnerability to posttraumatic stress disorder in children and adolescents. Am J Psychiatry. 2000;157(8):1229–1235. doi: 10.1176/appi.ajp.157.8.1229. [DOI] [PubMed] [Google Scholar]
- 9.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156(8):1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 10.Stein DJ, Ipser JC, Seedat S. Cochrane Database Syst Rev. 1. 2006. Pharmacotherapy for post traumatic stress disorder (PTSD) p. CD002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, Figueira I. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169–180. doi: 10.1016/j.pnpbp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 suppl):28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 13.Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev. 1993;18(1):1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 14.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 15.Southwick SM, Krystal JH, Bremner JD, Morgan CA, III, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54(8):749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 16.Davis LL, Clark DM, Kramer GL, Moeller FG, Petty F. D-fenfluramine challenge in posttraumatic stress disorder. Biol Psychiatry. 1999;45(7):928–930. doi: 10.1016/s0006-3223(98)00215-7. [DOI] [PubMed] [Google Scholar]
- 17.Corchs F, Nutt DJ, Hood S, Bernik M. Serotonin and sensitivity to trauma-related exposure in selective serotonin reuptake inhibitors—recovered posttraumatic stress disorder. Biol Psychiatry. 2009;66(1):17–24. doi: 10.1016/j.biopsych.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Charney DS, Goodman WK, Price LH, Woods SW, Rasmussen SA, Heninger GR. Serotonin function in obsessive-compulsive disorder: a comparison of the effects of tryptophan and m-chlorophenylpiperazine in patients and healthy subjects. Arch Gen Psychiatry. 1988;45(2):177–185. doi: 10.1001/archpsyc.1988.01800260095012. [DOI] [PubMed] [Google Scholar]
- 19.Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, Charney DS. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153(1):83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- 20.Price LH, Malison RT, McDougle CJ, McCance-Katz EF, Owen KR, Heninger GR. Neurobiology of tryptophan depletion in depression: effects of m-chlorophenyl-piperazine (mCPP) Neuropsychopharmacology. 1997;17(5):342–350. doi: 10.1016/S0893-133X(97)00084-5. [DOI] [PubMed] [Google Scholar]
- 21.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 22.Davidson JRT, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multi-center, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58(5):485–492. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- 23.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158(12):1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 24.Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62(11):860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 25.Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33(3–4):275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 26.Bonne O, Bain E, Neumeister A, Nugent AC, Vythilingam M, Carson RE, Luck-enbaugh DA, Eckelman W, Herscovitch P, Drevets WC, Charney DS. No change in serotonin type 1A receptor binding in patients with posttraumatic stress disorder. Am J Psychiatry. 2005;162(2):383–385. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- 27.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28(6):565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Ruf BM, Bhagwagar Z. The 5-HT1B receptor: a novel target for the pathophysiology of depression. Curr Drug Targets. 2009;10(11):1118–1138. doi: 10.2174/138945009789735192. [DOI] [PubMed] [Google Scholar]
- 29.Lin D, Parsons LH. Anxiogenic-like effect of serotonin1B receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav. 2002;71(4):581–587. doi: 10.1016/s0091-3057(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 30.Critchley MA, Handley SL. Effects in the X-maze anxiety model of agents acting at 5-HT1 and 5-HT2 receptors. Psychopharmacology (Berl) 1987;93(4):502–506. doi: 10.1007/BF00207243. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin D, Lal H, Meyerson LR. The effects of 5-HT1B characterizing agents in the mouse elevated plus-maze. Life Sci. 1990;47(3):195–203. doi: 10.1016/0024-3205(90)90320-q. [DOI] [PubMed] [Google Scholar]
- 32.Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using herpes simplex virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22(11):4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007(1–2):86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 34.Bolanos-Jimenez F, Manhaes de Castro RM, Seguin L, Cloez-Tayarani I, Monneret V, Drieu K, Fillion G. Effects of stress on the functional properties of pre- and post-synaptic 5-HT1B receptors in the rat brain. Eur J Pharmacol. 1995;294(2–3):531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- 35.Bibancos T, Jardim DL, Aneas I, Chiavegatto S. Social isolation and expression of serotonergic neurotransmission-related genes in several brain areas of male mice. Genes Brain Behav. 2007;6(6):529–539. doi: 10.1111/j.1601-183X.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- 36.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 38.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54 (5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 39.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 40.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 41.Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11(10):413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Nabulsi N, Huang Y, Weinzimmer D, Ropchan J, Frost JJ, McCarthy T, Carson RE, Ding Y-S. High-resolution imaging of brain 5-HT1B receptors in the rhesus monkey using [11C]P943. Nucl Med Biol. 2010;37(2):205–214. doi: 10.1016/j.nucmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl) 2010;212(3):405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 45.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 46.Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad–spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess. 2000;12(2):210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 48.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 49.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 50.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 51.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 52.Ichise M, Liow J-S, Lu J-Q, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23(9):1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- 53.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 54.Varnäs K, Hurd YL, Hall H. Regional expression of 5-HT1B receptor mRNA in the human brain. Synapse. 2005;56(1):21–28. doi: 10.1002/syn.20128. [DOI] [PubMed] [Google Scholar]
- 55.Gallezot J-D, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams WA, Singhal T, Kim S, Maguire RP, McCarthy T, Frost JJ, Huang Y, Ding Y-S, Carson RE. Kinetic modeling of the serotonin 5-HT1B receptor radioligand [11C]P943 in humans. J Cereb Blood Flow Metab. 2010;30(1):196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68(5):748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 57.Nishith P, Mechanic MB, Resick PA. Prior interpersonal trauma: the contribution to current PTSD symptoms in female rape victims. J Abnorm Psychol. 2000;109(1):20–25. [PMC free article] [PubMed] [Google Scholar]
- 58.Cloitre M, Scarvalone P, Difede JA. Posttraumatic stress disorder, self- and interpersonal dysfunction among sexually retraumatized women. J Trauma Stress. 1997;10(3):437–452. doi: 10.1023/a:1024893305226. [DOI] [PubMed] [Google Scholar]
- 59.Lang AJ, Aarons GA, Gearity J, Laffaye C, Satz L, Dresselhaus TR, Stein MB. Direct and indirect links between childhood maltreatment, posttraumatic stress disorder, and women’s health. Behav Med. 2008;33(4):125–135. doi: 10.3200/BMED.33.4.125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meewisse M-L, Reitsma JB, de Vries G-J, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 61.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306(5697):879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 62.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28(1):199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonaventure P, Schotte A, Cras P, Leysen JE. Autoradiographic mapping of 5-HT1B-and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Receptors Channels. 1997;5(3–4):225–230. [PubMed] [Google Scholar]
- 64.Boulenguez P, Segu L, Chauveau J, Morel A, Lanoir J, Delaage M. Biochemical and pharmacological characterization of serotonin-O-carboxymethylgly-cyl[125I]iodotyrosinamide, a new radioiodinated probe for 5-HT1B and 5-HT1D binding sites. J Neurochem. 1992;58(3):951–959. doi: 10.1111/j.1471-4159.1992.tb09348.x. [DOI] [PubMed] [Google Scholar]
- 65.Varnäs K, Hall H, Bonaventure P, Sedvall G. Autoradiographic mapping of 5-HT1B and 5-HT1D receptors in the post mortem human brain using [3H]GR 125743. Brain Res. 2001;915(1):47–57. doi: 10.1016/s0006-8993(01)02823-2. [DOI] [PubMed] [Google Scholar]
- 66.Sari Y, Miquel M-C, Brisorgueil M-J, Ruiz G, Doucet E, Hamon M, Vergé D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88(3):899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 67.Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain, I: serotonin-1 receptors. Brain Res. 1985;346(2):205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 68.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 69.Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30 (11):3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lucey JV, Costa DC, Adshead G, Deahl M, Busatto G, Gacinovic S, Travis M, Pi-lowsky L, Ell PJ, Marks IM, Kerwin RW. Brain blood flow in anxiety disorders: OCD, panic disorder with agoraphobia, and post-traumatic stress disorder on 99mTcHMPAO single photon emission tomography (SPET) Br J Psychiatry. 1997;171:346–350. doi: 10.1192/bjp.171.4.346. [DOI] [PubMed] [Google Scholar]
- 72.Hull AM. Neuroimaging findings in post-traumatic stress disorder: systematic review. Br J Psychiatry. 2002;181:102–110. [PubMed] [Google Scholar]
- 73.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei [published correction appears in Biol Psychiatry. 2006;60(9):1023] Biol Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Looi JCL, Maller JJ, Pagani M, Högberg G, Lindberg O, Liberg B, Botes L, Engman E-L, Zhang Y, Svensson L, Wahlund L-O. Caudate volumes in public transportation workers exposed to trauma in the Stockholm train system. Psychiatry Res. 2009;171(2):138–143. doi: 10.1016/j.pscychresns.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 75.Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D α, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33(3–4):367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 76.Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58 (1):167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 77.Pranzatelli MR, Durkin MM, Farmer M. Plastic responses of neonatal 5-hydroxy-tryptamine1B receptors to 5,7-dihydroxytryptamine lesions mapped by quantitative autoradiography. Int J Dev Neurosci. 1996;14(5):621–629. [PubMed] [Google Scholar]
- 78.Vergé D, Daval G, Marcinkiewicz M, Patey A, El Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6(12):3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellow S, Johnston AL, File SE. Selective agonists and antagonists for 5-hydroxytryptamine receptor subtypes, and interactions with yohimbine and FG 7142 using the elevated plus-maze test in the rat. J Pharm Pharmacol. 1987;39(11):917–928. doi: 10.1111/j.2042-7158.1987.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 80.Kaiyala KJ, Vincow ES, Sexton TJ, Neumaier JF. 5-HT1B receptor mRNA levels in dorsal raphe nucleus: inverse association with anxiety behavior in the elevated plus maze. Pharmacol Biochem Behav. 2003;75(4):769–776. doi: 10.1016/s0091-3057(03)00152-7. [DOI] [PubMed] [Google Scholar]
- 81.de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526(1–3):125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 82.Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7(6):617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murrough JW, Henry S, Hu J, Gallezot J-D, Planeta-Wilson B, Neumaier JF, Neumeister A. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology (Berl) 2011;213(2–3):547–553. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu J, Henry S, Gallezot J-D, Ropchan J, Neumaier JF, Potenza MN, Sinha R, Krystal JH, Huang Y, Ding Y-S, Carson RE, Neumeister A. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;67(9):800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 suppl):28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 86.Dunn AJ. Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J Neurochem. 1988;51(2):406–412. doi: 10.1111/j.1471-4159.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 87.Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, Manivet P, Launay J-M, Maroteaux L. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol. 2007;71(6):1463–1474. doi: 10.1124/mol.106.032656. [DOI] [PubMed] [Google Scholar]
- 88.Finnema SJ, Varrone A, Hwang TJ, Gulyás B, Pierson ME, Halldin C, Farde L. Fenfluramine-induced serotonin release decreases [11C]AZ10419369 binding to 5-HT1B-receptors in the primate brain. Synapse. 2010;64(7):573–577. doi: 10.1002/syn.20780. [DOI] [PubMed] [Google Scholar]