Abstract
The auditory CNS is influenced profoundly by sounds heard during development. Auditory deprivation and augmented sound exposure can each perturb the maturation of neural computations as well as their underlying synaptic properties. However, we have learned little about the emergence of perceptual skills in these same model systems, and especially how perception is influenced by early acoustic experience. Here, we argue that developmental studies must take greater advantage of behavioral benchmarks. We discuss quantitative measures of perceptual development, and suggest how they can play a much larger role in guiding experimental design. Most importantly, including behavioral measures will allow us to establish empirical connections among environment, neural development, and perception.
Keywords: hearing, perception, experience, deafness, auditory cortex, sensory coding, auditory processing, synaptic plasticity
Most toddlers are enthusiastic talkers, so it may come as a surprise that humans do not attain the full complement of mature auditory perceptual skills for over a decade. Such a long period for maturation of sensory perception implies that experience may have a significant impact on the outcome. In fact, the number and strength of synapses are quite malleable when animals begin to interact with the environment, after most sensory connections have formed rather accurately. During this phase of life, central nervous system structure and function can be modified profoundly by auditory experience, in a way that has long term effects on perception.
Support for the role of experience in auditory development emerges from studies showing that sound exposure or deprivation can affect central form and function. For example, the acoustic rearing environment can shape frequency coding (Sanes and Constantine-Paton, 1985) tonotopic maps (Norena et al., 2006; Yu et al; 2007; Kandler et al., 2009; Barkat et al., 2011), spatial processing (Knudsen, 1999; Popescu and Polley, 2010), and vocalization coding (Razak et al, 2008; George et al., 2010; Woolley et al., 2010). Despite these compelling demonstrations, direct correlations between the neurophysiological and perceptual effects of experience are uncommon. Moreover, we know little about the development of auditory perception for any species other than humans.
Here, we argue that the interpretation of neural development and plasticity findings must take greater advantage of an essential benchmark: behavioral relevance. For our purposes, behaviorally relevant neural mechanisms are defined as those that correlate closely with, or are causally related to the perception of sound. We first describe what is known about the development of auditory perceptual skills, and then examine its relationship to central auditory processing. We next evaluate evidence that the acoustic rearing environment alters neural properties in such a way that perceptual skills are likely to be affected. Along the way, we suggest research opportunities that can bridge our understanding of experience-dependent developmental plasticity in the auditory system and the natural development of perceptual skills.
AUDITORY PERCEPTUAL SKILLS EMERGE SLOWLY & ASYNCHRONOUSLY
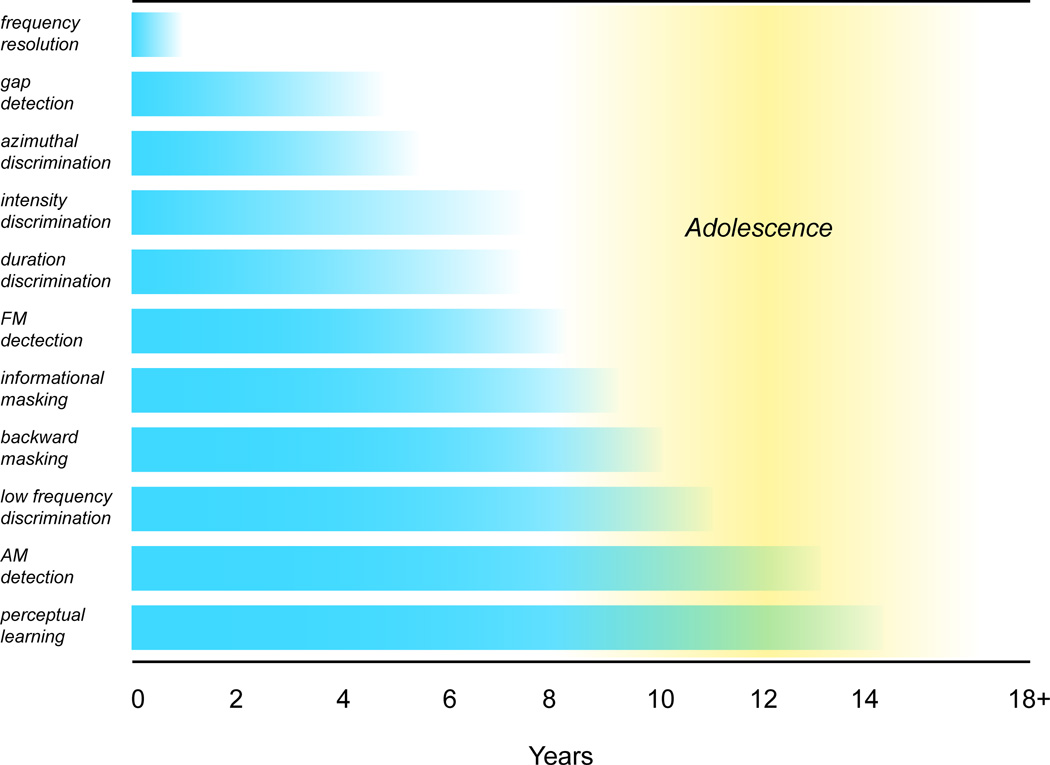
The awkward truth is that our understanding of auditory perceptual development draws largely from studying one species, us. Two broad principles to emerge from human studies are that perceptual maturation extends through adolescence and the age at which adult-like performance is attained varies tremendously across skills (Figure 1). In this section, we emphasize the research studies that support this conclusion, and then consider how sensory and non-sensory factors might account for the findings.
Figure 1.
Human auditory perceptual skills develop over at least 10 years. The schematic summarizes the age at which different perceptual skills reach a mature state (see text for description of each percept). Note that frequency resolution matures earliest, reflecting the development of cochlear function. At the other extreme, AM detection thresholds and perceptual learning are not adult-like until well after 10 years of age. (frequency resolution: Abdala and Keefe, 2012; gap detection: Wightman et al., 1989; azimuthal discrimination: Litovsky, 1997; intensity discrimination: Maxon and Hochberg, 1982; duration discrimination: Jensen and Neff, 1993; FM detection: Banai et al., 2011; informational masking: Wightman et al., 2010; backward masking: Wright and Zecker, 2004; low frequency discrimination: Moore et al., 2011; AM detection: Banai et al., 2011; auditory perceptual learning: Huyck and Wright, 2011)
Human perception of frequency and intensity
Frequency resolution (tone detection in the presence of a second nearby tone) matures first for low frequencies, but is adult-like by 6 months at all frequencies tested (Spetner and Olsho, 1990; Schneider et al., 1990; Hall and Grose, 1991). This corresponds to cochlear development, and is consistent with functional measures suggesting that the low frequency region of the cochlea matures somewhat earlier (reviews: Rübsamen and Lippe, 1998; Abdala and Keefe, 2012). In contrast, frequency discrimination (i.e. hearing a difference between two tones presented sequentially) does not mature until roughly 10 years of age for low frequency tones (Maxon and Hochberg, 1982; Olsho, 1984; Sinnott and Aslin, 1985; Olsho et al., 1987; Jensen and Neff, 1993; Thompson et al., 1999; Moore et al., 2011). To detect a difference in intensity between two sounds, infants require about a 6 dB increase; this declines to 2 dB by 4 years of age for sounds of sufficient duration, but may not be fully mature until 10 years (Sinnott and Aslin, 1985; Maxon and Hochberg, 1982). Thus, even for the most basic auditory percepts, human performance emerges gradually over nearly a decade.
Human perception of temporal cues
Temporal processing displays a range of developmental time courses. For example, juveniles (those who have passed infancy, and have adult-like cochlear processing, but who have not yet reached sexual maturity) and adults display differences in temporal integration, the process whereby information is summed over time, resulting in the best possible detection or discrimination thresholds (Maxon and Hochberg, 1982; Berg and Boswell, 1995; Moore et al., 2011). Figure 2 shows two experiments in which tone threshold was determined at both a short and a long duration. In both cases, the young subjects display greater improvement (blue Δ) than adults (red Δ). This is because their performance is exceptionally poor at the short stimulus durations. The ability to discriminate duration differences matures later, dropping from 80 to 20 ms between 6 years and adulthood (Elfenbein et al., 1993; Jensen and Neff, 1993).
Figure 2.
Young subjects are especially poor at processing very brief sounds. These two examples illustrate the performance of young and adult subjects on a perceptual task. In each case, the x-axis displays the stimulus duration, and the y-axis displays the detection threshold for a tone. (A) Detection thresholds for infants versus adults. Infants have much poorer detection thresholds at both durations, but display a much greater improvement (blue Δ) as duration increases from 10 to 100 ms, as compared to adults (red Δ). (B) Detection thresholds for 6–7 year old children versus adults. Children have poorer detection thresholds at the short duration, but improve more with duration (blue Δ) than adults (red Δ), and display about the same threshold as adults at 200 ms. (panel A is adapted from Berg and Boswell, 1995; panel B is adapted from Moore et al., 2011)
Some temporal processing skills such as the detection of amplitude and frequency modulations (AM & FM) are exceptionally slow to mature. These cues are a predominant component of communication sounds, including speech (Rosen, 1992; Shannon et al., 1995; Singh and Theunissen, 2003). In humans, the detection threshold for AM stimuli continues to mature beyond 12 years (Banai et al., 2011). Interestingly, sound-evoked cortical potentials mature over the first 10 years of life in humans, as do axonal arbors in supragranular cortical layers (Ceponiene et al., 2002; Moore and Guan, 2001). These findings illustrate that perceptual skills related to temporal processing mature at different ages, and suggest that the underlying neural representations will also mature at different rates.
Sensitivity and the psychometric function slope
To this point, we have stressed the lower limits of detection for many auditory tasks, but these are just convenient measures plucked from parametric analyses. Thus, when we say that adults detect smaller sound intensity differences than children, performance has actually been quantified across a range of sound levels. Plots that relate an observer's performance (y-axis) to some physical measure of the signal (x-axis) are generally called psychometric functions. The slope of a psychometric function is thought to reflect “internal noise,” a broad term that could encompass many neural inaccuracies, including stimulus encoding. In fact, when electrical stimulation is applied to a visual cortical area that contributes to motion processing while animals perform a motion discrimination task, their psychometric functions become shallow; that is, the electrical stimulation appears to increase internal noise (i.e., spikes that are unrelated to the stimulus) and reduce discrimination (Murasugi et al., 1993). Children often have much shallower psychometric functions than adults. For example, performance improves more gradually as the intensity difference between two sounds increases (Figure 3A; Buss et al., 2006; Buss et al., 2009). A similar pattern emerges from measurements of tone threshold in the presence of a noise; again, children have psychometric functions with shallower slopes (Figure 3B; Allen and Wightman, 1994). We return to this characteristic when discussing neural processing (below).
Figure 3.
Psychometric functions are shallower in young subjects than in adults. These two examples illustrate the performance of young and adult subjects on a perceptual task. In each case, the x-axis displays the stimulus, ranging from hard to easy to detect and the y-axis displays performance from chance to excellent detection of the stimulus. (A) The subjects are asked to determine whether two tones are different in level. Both the child and the adult reach excellent performance at a 12 dB difference, but the child has a higher threshold and improves more gradually. (B) The subjects are asked to detect the presence of a tone during a noise masker. Both the child and the adult reach excellent performance at a tone level of 90 dB SPL, but the child has a higher threshold and improves more gradually. (panel A is adapted from Buss et al., 2009; panel B is adapted from Allen and Wightman, 1994)
Distinguishing sensory and cognitive development
Of consequence to neurophysiologists who study central mechanisms that support perceptual maturation is whether these mechanisms can be detected at the level of encoding (i.e., sensory factors), or whether they operate at a more cognitive level (i.e., non-sensory factors such as attention, memory, or motivation). A key argument favoring non-sensory factors as an explanation for immature perception is the finding that diminished sensitivity is often accompanied by less consistent performance. That is, if young animals display more variable performance on a task, as compared to adults, then it is thought that they cannot rally as much attention (Allen et al., 1989; Wightman et al., 1989, Moore et al., 2010). The validity of this hypothesis can be addressed, in part, by measuring proxies for attention (e.g., inter-session variance, response to catch trials, false alarm rate, asymptotic performance), and asking whether they correlate with improvement in perceptual thresholds. In fact, measures of attention during performance on an auditory task can remain stable during development, even though perceptual performance improves. For example, measures of attention do not have the same developmental trajectory as performance on tasks such as AM detection, FM detection, or intensity discrimination (Dawes and Bishop 2008; Sarro and Sanes, 2010; Buss et al., 2009; Banai et al., 2011). Furthermore, the maturation rates for different auditory tasks are not correlated (Figure 1), as would be expected if a non-sensory factor (e.g., selective attention) had a uniform influence on performance (Jensen and Neff, 1993; Hartley et al., 2000; Werner and Boike, 2001; Wright and Zecker, 2004; Dawes and Bishop, 2008; Moore et al., 2011; Banai et al., 2011). This is not to deny the certain influence of attention on juvenile performance (Gomes et al., 2000). However, our conclusion is that immature sensory processing does limit perceptual skills, and is a logical target for neurophysiological research.
Even if young animals are attentive to the task, they may listen with a different strategy. Adults are much better at detecting a sound frequency, duration, or presentation time that is expected, a phenomenon called selective listening (Greenberg and Larkin, 1968; Dai and Wright, 1995; Wright and Fitzgerald, 2004). However, young animals appear to listen more broadly, as illustrated in Figure 4. Adults are excellent at detecting a tone that is presented on 75% of trials, but poor at detecting an adjacent tone that is presented on only 25% of trials (i.e., unexpected). In contrast, infants are excellent at detecting both the high and low probability signals; that is, they do not listen selectively (Bargones and Werner, 1994). The listening strategy of children has also been explored with distracting stimuli that interfere with detection of a signal, a phenomenon called informational masking. When children are asked to recognize speech through one ear, while distracting speech sounds are presented to the other ear, they perform poorly. An adult capacity for overcoming the distraction of the masker is not reached until ~10 years (Wightman et al., 2010). Since descending control has been implicated both in selective listening and auditory maturation (Scharf et al., 1997; Walsh et al., 1998; Lauer and May, 2011), developmental studies of efferent mechanisms may be of special interest to neurophysiologists.
Figure 4.
Young and adult subjects may use different listening strategies. (A) Subjects were asked to detect a tone, but one frequency was presented on 75% of the trials, and other frequencies occurred much less frequently. (B) The plot illustrates performance (y-axis) for infants (blue) and adults (red) as a function of the stimulus frequency. Adults listen selectively for the stimulus that occurs with a high probability (i.e., 1000 Hz), and perform poorly when low probability tones are delivered. In contrast, infants do not show this listening strategy; they appear to listen broadly across the frequency spectrum and perform quite well on stimuli that occur with a low probability. (adapted from Bargones and Werner, 1994)
Opportunities await
Human behavior studies suggest that it is reasonable to search for immature CNS encoding mechanisms, and it seems axiomatic that animal behavior studies can guide neurophysiologists toward the most fruitful opportunities to identify the neural bases of perceptual maturation (discussed below). The few non-human studies on perceptual development suggest that perception is quite immature initially (Kerr et al., 1979; Gray and Rubel, 1985; Kelly and Potash 1986; Gray, 1991; Gray, 1992; Gray, 1993a; Gray, 1993b). However, direct quantitative comparisons of juvenile and adult performance are seldom made simply because young animals are tested using a behavior that is not displayed in older animals (e.g., approach to a maternal call). There are behavioral procedures that can be used to test perception in both juvenile and adult animals, such as operant training. When procedures of this sort are employed, clear age-dependent improvements in perception are observed (Ehret, 1976; Sarro and Sanes, 2010). If perceptual development can be characterized in non-humans, using modern techniques and high resolution analyses, then neurophysiologists will, at last, have phenotypes against which to compare their findings and models.
EARLY AUDITORY EXPERIENCE INFLUENCES PERCEPTUAL SKILLS
Diminished auditory experience impairs perceptual development
During development, the sounds that are heard - and those that are not heard - have the potential to shape adult perceptual skills. A prolonged period of developmental hearing loss can lead to persistent deficiencies in human auditory processing skills. These include the ability to locate sounds, detect signals in noise, and discriminate frequency or amplitude modulations (Hall and Grose, 1994b; Hall et al., 1995; Wilmington et al., 1994; Kidd et al., 2002; Rance et al., 2004; Halliday and Bishop, 2005; Halliday and Bishop, 2006). More importantly, developmental hearing loss in humans may lead to delays in speech acquisition and perception (Schonweiler et al., 1998; Psarommatis et al., 2001; Svirsky et al., 2004; Pittman et al., 2005; Whitton and Polley, 2011).
Experimental studies of auditory deprivation have focused almost exclusively on binaural hearing and sound localization. These studies ask whether a period of monaural sound attenuation influences the maturation of binaural processing. In fact, plugging one ear, even transiently during development, profoundly impairs the ability to localize sounds after the plug is removed (Clements and Kelly, 1978; Knudsen et al., 1984a; Parsons et al., 1999; Moore et al., 1999; King et al., 2000). The effect depends on the age at which monaural hearing loss occurs. Young owls that are reared with one ear plugged can gradually adjust, and eventually display normal sound localization behavior, while older owls cannot adjust to the ear plug and make large errors in localization (Knudsen et al. 1984a). That is, there is a sensitive period during which the developing animal can learn to accommodate to the unilateral hearing loss. This sensitive period also applies to the restoration of normal hearing. For owls reared with one ear plugged, accurate sound localization does not develop when the plug is removed after the animal is 40 weeks old (Knudsen et al. 1984b). Evidence for a sensitive period has also been found in humans born with unilateral conductive hearing loss due to atresia to one ear (i.e., the absence of an ear canal and malformation of the middle ear). The ability to understand speech in the presence of noise, a task that takes advantage of binaural processing, improves following surgery to reverse the atresia. However, the improvement declines with age at the time of surgery (Gray et al., 2009). This result suggests that auditory perceptual skills, and the underlying neural mechanisms, are most vulnerable to auditory deprivation during a developmental sensitive period.
Even when hearing is intact, the development of perceptual skills can be impaired by removing specific acoustic features from the rearing environment. For example, perceptual deficits are found in songbirds that have been deprived only of song exposure during the juvenile period. When evaluated as adults, birds reared in the absence of adult songs exhibit frequency discrimination deficits. Furthermore, birds reared without hearing sibling or adult vocalizations show poor frequency discrimination and song note recognition (Njegovan and Weisman, 1997; Sturdy et al., 2001). These studies suggest that the maturation of auditory perception is not simply a matter of hearing, but requires experience with specific features of the acoustic environment.
Exposure to novel acoustic environments influences perceptual development
If the loss of early auditory experience degrades behavioral performance, then the opposite manipulation (augmented sound exposure) might be expected to improve perception. Studies that address this issue commonly expose developing animals to a specific acoustic environment, often for a prolonged period. However, the effects are usually assessed by recording from the nervous system (below), and the behavioral impact is not well understood. When developing rats are exposed to a single frequency for 3 weeks, and then tested on a frequency discrimination task in adulthood, their perceptual skills display an intricate set of changes. As adults, these animals actually display poor discrimination at the exposed frequency, yet their discrimination of adjacent frequencies is significantly better than controls (Han et al., 2007). In contrast, when young animals are exposed to noise for days or weeks, behavioral measures typically reveal diminished or delayed capacities (Philbin et al., 1994; Zhang et al., 2008; Zhou and Merzenich, 2009; Sun et al., 2011). Because the tone or noise levels used in these experiments appear to be too low to injure the cochlea, the behavioral impact is likely attributable to central changes (below).
Learning during development can improve adult perceptual skills
A second approach to evaluate how acoustic stimulation influences development is to assess auditory learning. An exceptional series of studies by Gilbert Gottlieb (1975a, 1975b, 1978, 1980, 1981, 1983), used a biologically relevant form of learning, called vocal imprinting, to examine the role of early auditory experience in behavioral responses to sound. Devocalized and isolated ducklings do not develop accurate sensitivity to maternal calls, but this perceptual skill is rescued by stimulating the ducklings with natural vocalizations. Preference for the natural call note repetition rate and frequency modulation must be induced or maintained by experiencing those acoustic features. However, merely hearing the right sound may not be sufficient to influence perceptual development; it is often gated by non-auditory factors, such as the state of arousal (Gottlieb, 1993; Sleigh et al., 1996; Markham et al., 2006). Even very brief periods of sound exposure can induce new perceptual skills when the acoustic features have a reliable statistical structure (e.g., a high probability that two sounds occur sequentially). After only 2 minutes of experience, infants can discriminate familiar syllable sequences from novel ones, including those from a natural language (Saffran et al., 1996; Pelucchi et al., 2009). This process of statistical learning may require a certain degree of attention and social interaction (Toro et al., 2005; Kuhl, 2007).
Studies focused on the emergence of vocal behavior in songbirds have demonstrated the importance of early sensory exposure to natural communication sounds on adult perception. In zebra finches, hearing vocalizations begins to influence auditory perception and vocal behavior shortly after auditory brainstem thresholds mature (Amin et al., 2007). Starting at post-hatch day 20, juveniles memorize the songs of adult tutors. This period of auditory learning generates the perceptual templates used for motor learning of vocal production by males. In addition to vocal learning, both males and females remember the songs that they hear frequently during development, and are attracted to similar sounds in adulthood. For example, zebra finches develop preferences for hearing conspecific song over heterospecific song based on juvenile early experience, and females sexually imprint on the songs that they hear as juveniles (Miller, 1979; Peters et al., 1980; Clayton, 1988; Clayton and Prove, 1989; Nagle and Kreutzer, 1997; Riebel et al., 2002; Lauay et al., 2004).
Cross-fostering studies provide additional support for the idea that perceptual preferences are shaped by hearing communication vocalizations during development. Both males and females that are raised by adults of another species or subspecies fail to show consistent preferences for conspecific songs as adults, and show increased attraction to heterospecific songs (Immelmann, 1969; Clayton, 1988; Clayton, 1990; Campbell & Hauber, 2009). These studies suggest that juvenile exposure to adult communication sounds influences auditory system maturation, but we do not yet know how to relate the effects of vocal experience on behavior to the functional development of auditory circuits and cellular properties (below).
In adults, auditory training on a variety of perceptual tasks inevitably leads to improvement in performance (Recanzone et al., 1993; Wright et al., 1997; Ari-Even Roth et al., 2003; Beitel et al., 2003; Brown et al., 2004; Sakai and Kudoh, 2005; Rutkowski and Weinberger, 2005; Mossbridge et al., 2006; Polley et al., 2006; Blake et al., 2006; van Wassenhove and Nagarajan, 2007; Draganova et al., 2009; Ilango et al., 2010; Bieszczad and Weinberger 2010; Comins and Gentner, 2010). In contrast, adolescent humans and juvenile gerbils do not display adult-like perceptual learning, even when they begin training with adult-like perceptual skills and display adult-like measures of attention (Sarro and Sanes, 2010; Huyck and Wright, 2011). When trained on a duration discrimination task for 10 days, 11-year-old children exhibit no perceptual learning, whereas adults improve significantly when trained on the identical task. Of course, it is possible that some other training regimen might lead to performance improvement in young animals, but the key point is that they do not display an adult form of learning.
If auditory training during development does not yield an immediate change in performance, then perhaps it provides some advantages for future performance, and this only becomes evident in adulthood. Only a few experiments have asked how learning during development influences adult perceptual skills. For example, when gerbils are trained on an AM detection task during development, the experience exerts a unique improvement on adult perceptual skills; the identical training in adults does not result in the same improvement (Sarro and Sanes, 2011). In humans, musical training is associated with a broad range of perceptual skills in adulthood (Kraus and Chandrasekaran, 2010). Therefore, auditory experience can produce distinct behavioral outcomes, depending on the specific balance of acoustic stimulation and learning.
The need for innovative environmental manipulations
To summarize, even very brief exposure to specific sounds may enhance perceptual skills. This is best illustrated by experiments in which animals are actively engaged in learning, and especially when natural communication sounds are involved. In contrast, the behavioral impact of prolonged exposure to arbitrary waveforms is poorly understood (for a cogent valuation of controlled rearing experiments and perceptual development, see Chapter 12 in Gibson, 1969). Studies in which the rearing environment is chronically biased to one sound (e.g., tones or clicks) demonstrate convincingly that the environment can influence neural processing (below). However, it has been challenging to interpret this data in the absence of behavioral phenotypes.
Moving forward, we suggest that environmental manipulations can be optimized to address questions concerning both normal development and pathology. To understand the natural activity-dependent mechanisms that regulate nervous system development, neurophysiologists should embrace paradigms that more closely resemble the exposure and learning that animals experience in the natural world. Thus, to understand how auditory perception might mature through unsupervised, statistical learning mechanisms, future sound rearing studies may exploit a novel set of statistical relationships of modest complexity (McDermott and Simoncelli, 2011). They can also address the impact of statistical relationships that are available at irregular and unpredictable times during the day. Moreover, perceptual learning paradigms should play a more prominent role in developmental manipulations since this approach does lead to dramatic changes in auditory detection and discrimination, including arbitrary waveforms. In contrast, to understand the pathological consequences of an unnatural acoustic environment, neurophysiologists should step up their assessment of behavioral deficits that accompany developmental hearing loss or chronically noisy environments (Lauer and May, 2011; Pienkowski and Eggermont, 2011).
NORMAL MATURATION OF CENTRAL AUDITORY COMPUTATIONS
A common assumption is that central auditory coding properties that diverge from those displayed by control adults must be associated with diminished perceptual skills. However, establishing a quantifiable relationship between function at the cellular and circuit level and perception is challenging. Furthermore, most development and plasticity studies are based on recordings from anesthetized animals, leading to some uncertainty about their relationship to the processing that occurs during behavior. Below, we provide abridged reviews of developmental physiology in normal animals, and suggest opportunities that would be afforded by incorporating behavioral observations.
Development of frequency processing
The maturation of neural coding is most often assessed along the same three acoustic parameters (frequency, level, time) that are discussed above with reference to human perceptual development. Measures of frequency processing include single neuron tuning curves (a plot of the minimum sound level that drives the neuron as a function of sound frequency), and tonotopic maps (the regular progression of characteristic frequency along one axis of a neural structure). By each measure, frequency processing appears to mature at a relatively early age. For example, rodent brainstem and cortical tuning curves and tonotopic maps appear to be mature within days of hearing onset (Sanes et al., 1989; Romand and Ehret, 1990; Ehret and Romand, 1992; de Villers-Sidani et al., 2007; Bonham et al., 2004). For precocial mammals, the tonotopic map is mature at birth (Pienkowski and Harrison, 2005).
In contrast to frequency tuning curves and maps, the presumptive basis for discrimination of low frequencies (below ~2kHz), phase-locking (temporally precise discharge at the same phase of each period) matures more slowly in cochlear nucleus than auditory nerve, becoming adult-like at ~4 weeks in cats (Brugge et al, 1978; Kettner et al., 1985). The rapid maturation of tuning curves and tonotopic maps suggests that perceptual discrimination of high frequencies should mature before discrimination of low frequencies. Human behavioral studies indicate that frequency discrimination is late to mature, particularly at low frequencies. Therefore, if sensory factors limit perceptual skills, then we would expect the neural mechanisms that support discrimination of high frequencies (e.g., tuning curves) and perceptual discrimination of high frequencies to mature before mechanisms that support discrimination of low frequencies (e.g., phase-locking) and behavioral discrimination of low frequencies. This question can only be tackled when behavioral data become available in developing animals from which these recordings can be obtained.
Development of sound level processing
Measures of sound level coding also mature rapidly. By way of comparison with phase-locking (see previous paragraph), cat cochlear nucleus neurons display a mature dynamic range (the dB range across which spike rate increases) and maximum spike rate by ~3 weeks (Brugge et al., 1981; Walsh and McGee, 1987). Therefore, the resolution of level coding (spikes per change in dB) is fully developed long before adulthood. In principle, this would permit mature intensity discrimination from an early age. If there is a relative order to the appearance of mature coding that reflects perceptual development, then we would expect adult-like level coding at a time when amplitude modulation coding remains immature. Recordings from single neurons in awake gerbils are consistent with this idea. As a population, cortical neurons display a mature distribution of dynamic range and maximum discharge rate during late juvenile development. However, they do not display adult-like sensitivity to AM depth (Rosen et al., 2010). The delayed maturation of AM encoding is consistent with behavioral measures showing that juveniles are less sensitive to AM depth (Sarro and Sanes, 2010), but there is no comparable data set on intensity discrimination. Neuronal responses to frequency modulated (FM) stimuli can also display a relatively prolonged period of maturation, depending on the stimulus attribute. In bats, the selectivity of cortical neurons for FM rate is mature within 2 weeks of birth, but selectivity for FM direction continues to improve for over 12 weeks (Razak and Fuzessery, 2007). FM direction selectivity also matures relatively late in precocial animals (Brown and Harrison, 2010).
Concepts emerging from studies of normal development
To recap, behavioral evidence (primarily from humans) and electrophysiological evidence (primarily from non-humans) lead to the hypothesis that central auditory system development is responsible for much of the age-dependent improvement in perceptual performance, even for relatively simple percepts (Figure 1). This idea is based on the observation that frequency resolution, a proxy for cochlear processing, is mature by 6 months in humans (Hall and Grose, 1991; Spetner and Olsho, 1990). In fact, functional measurements of frequency resolution and dynamic range do indicate that the cochlea is mature by ~6 months (for review, see Abdala and Keefe, 2012), while auditory brainstem and cortical evoked potentials mature at ≈4 years and late adolescence, respectively (Ponton et al., 1996; McGee and Kraus, 1996; Johnson et al., 2008; Sussman et al., 2008; Müller et al., 2009). A similar case can be made for rodents such as gerbils; the cochlear amplifier is mature by postnatal day (P) 30, while cortical evoked potentials do not mature until ≈P60 (Mills and Rubel, 1996; Kraus et al., 1987a; Kraus et al., 1987b). Thus, the maturation of central processing is delayed relative to peripheral processing, and is, in many cases, more closely correlated in time with perceptual development.
Using the kinetics of auditory processing
The relationship between the stimulus duration and optimal performance on an auditory task, called temporal integration, differs with age. The most common assay of temporal integration measures a subject’s thresholds for detecting a sound as its duration increases. Temporal integration differs markedly in infants and adults; to determine whether a sound is coming from the right or left side, infants must listen for about 1 second, whereas adults need only a few milliseconds (Clarkson et al. 1989). Studies suggest that children are particularly poor when stimulus duration is <20 ms, although temporal integration is adult-like above this duration (Maxon and Hochberg, 1982; Berg and Boswell, 1995; He et al., 2010; Moore et al., 2011). Using behavioral findings like this to guide experiments, a relatively simple comparison of the time constant for optimal performance could be used to correlate perceptual skills to neural coding over the course of development.
Using the profile of psychometric functions
Children often display a more gradual improvement in performance as stimulus magnitude increases (Figure 3). The position and shape of these functions can be used to make inferences about the underlying neural processing. Figure 5A (top) shows a hypothetical psychometric function for an adult (red dashed), and a second for a juvenile with a shallower slope (dashed blue). This behavioral data can be used to generate hypothetical internal neural responses that represent the target stimuli using signal detection theory (see Figure legend for details). Using this framework, Figure 5A (bottom) illustrates one simple model to account for poor performance in developing animals: for a given stimulus magnitude, the Juvenile mean internal response has a larger variance (blue distributions), and overlaps more with the mean internal signal when no stimulus is present (gray distributions), as compared to the Adult (red distributions). That is, the Juvenile internal responses are more difficult to discriminate from one another. For comparison, Figure 5B presents an alternative model that could account for poor performance in developing animals: for a given stimulus magnitude, the Juvenile mean internal responses (blue distributions) increase less as stimulus magnitude gets larger, as compared to the Adult (red distribution). If psychometric functions were available from developing and adult animals, then credible comparisons could be made to neurometric analyses of the putative internal signal (solid blue and red lines). Of course, the more rigorous the experiment (e.g., recording responses while animals perform the task), the more plausible the analysis. The key point is that one can construct models to motivate specific hypotheses about the development of auditory processing.
Figure 5.
The relationship between developmental changes in perceptual skills and a theoretical neural response. (A) The plot shows psychometric functions for an adult (red dashed) and a juvenile (blue dashed) subject. For both subjects, performance improves (y-axis) as stimulus magnitude increases (x-axis). However, the juvenile’s psychometric function displays a shallower slope, as illustrated in Figure 3. From these psychometric functions, we can make inferences about the underlying neural processing. Shown beneath the plot are a set of hypothetical internal neural responses that represent the target stimuli, one for an adult (red) and one for a juvenile (blue). Sensitivity to the target stimulus increases as the mean internal response becomes larger, but variance of this internal signal also has a profound influence. For a given difference in mean internal response, sensitivity is lower if variance is larger. Here, the mean internal response has a larger variance for the juvenile (blue normal distributions), and overlaps more with the mean internal signal when no stimulus is present (gray normal distributions), as compared to the adult (red normal distributions). That is, the juvenile internal responses with stimulus present are more difficult to discriminate from those without a stimulus (i.e., lower sensitivity), as compared to the adult. If an internal signal of this sort could be identified in juvenile and adult animals, then we would expect the neurometric functions (solid red and blue lines) to mirror the psychometric functions. (B) For the purpose of discussion, we illustrate a second possibility in which the juvenile and adult psychometric functions have the same slope, but the juvenile function is shifted to the right. Here, the juvenile’s mean internal responses (blue distributions) increase less as stimulus magnitude increases, as compared to the adult (red distribution).
DEVELOPMENTAL PLASTICITY OF CENTRAL AUDITORY COMPUTATIONS
The descriptive studies of normal development, discussed above, establish a framework for deductive research that seeks to understand how early auditory experience influences adult perceptual skills and their underlying central auditory computations. Again, the fundamental premise is that experience-dependent changes in CNS coding properties are causally related to certain perceptual skills. In this section, we emphasize research studies that have considered this relationship, especially those that explore the impact of natural acoustic stimuli.
Behaviorally relevant neural changes induced by experience
The idea that auditory coding properties do not mature properly in the absence of acoustic experience receives its strongest endorsement from studies in barn owls showing that monaural deprivation induces altered connectivity and binaural coding properties of midbrain neurons, and these changes correlate closely to abnormalities in sound localization (Knudsen et al., 1984; Mogdans et al., 1993, 1994; DeBello et al., 2001). The neural effects of unilateral hearing loss depend on the age at which the manipulation occurs. For example, when rats are reared with one ear ligated, stimulation through the open ear is subsequently found to elicit a stronger than normal cortical response in adulthood. However, when the same manipulation is performed on adults, this augmented response does not occur (Popescu and Polley, 2010). This indicates that there is a sensitive period during which one can observe correlated changes in both neural coding and behavior. Furthermore, the results offer a mechanistic explanation for the perceptual deficits that may follow periods of conductive hearing loss in children (Whitton and Polley, 2011).
There is some evidence that early acoustic stimulation leads to correlated neural and behavioral changes as well. For example, noise pulse exposure beginning when the auditory system is not yet mature can delay the behavioral and neural signs of high frequency hearing loss in several mouse strains (Willott et al., 2000; Willott and Turner, 2000). Continuous exposure of rat pups to pure tone pulses leads to an enlarged cortical representation of that frequency, and reduces the representation of adjacent frequencies. This functional effect is closely correlated with impaired discrimination near the exposure frequency, but improved performance at neighboring frequencies (Han et al., 2007). Even 3 days of pure tone exposure, initiated soon after the onset of hearing, can disturb the tonotopic projection from auditory thalamus to cortex (Barkat et al., 2011). This finding implies that adult auditory skills could be impacted by relatively brief periods of augmented experience. Therefore, the few studies to have examined the relationship between neural and behavioral changes support the strength of this approach.
Late-developing neural properties may be more influenced by experience
As with normal development, those interested in central plasticity may find the greatest opportunity in focusing on late-developing perceptual skills and their associated neural representation, thus minimizing conflation with cochlear maturation. Based on human research (Figure 1), we speculate that perceptual skills that rely on temporal processing (e.g., low frequency discrimination, FM detection, AM detection) would serve this goal. Coupled with the fact that natural sounds are composed of temporal correlations and amplitude modulations (Singh and Theunissen, 2003), it is plausible that experience-dependent plasticity plays a prominent role in the maturation of temporal processing circuits. One approach is to examine the plasticity of amplitude modulation (AM) coding. Central coding of AM is well characterized in adults of several species (Krishna and Semple, 2000; Liang et al., 2002; Joris et al., 2004; Woolley and Casseday, 2005; Wang et al., 2008; Woolley et al., 2009), and animals, including juveniles, can be operantly conditioned to indicate when they detect AM signals (Schultz and Scheich, 1999; Sarro and Sanes, 2010, 2011) or temporal gaps in continuous sounds (Wang et al., 2009). Therefore, the behavioral relevance of experimentally-induced shifts in pattern discrimination could provide fertile ground for developmental plasticity studies. For example, exposing rat pups to FM stimuli has a differential effect on cortical physiology, depending on the age of exposure (Insanally et al., 2009). The behavioral impact remains to be determined, but the physiology results predict that the effects of FM exposure on perception will vary with the age of the experience.
Correlating early-stage changes in physiology with perceptual development is plausible given that both young and older animals exhibit some similar reflexive behaviors such as paired pulse inhibition. Habituation generalization may also serve as a successful assay for early perceptual development. However, the fundamental challenge to identifying causal relationships between neonatal physiology and perception is that the entire system, from pinna shape to descending motor pathways, is immature. While experimental paradigms that examine early development have proven fruitful, we suggest that late-developing perceptual skills are most favorable for the purpose of identifying direct associations between neurophysiological mechanisms and behavior.
Experiential effects of natural sounds
Coding selectivity for the frequency modulations (FM) inherent to many vocalizations is firmly established for midbrain, thalamus and ACx neurons (Suga, 1965; Heil et al., 1992; Mendelson et al., 1993; Rauschecker and Tian, 2000; Nelken and Versnel, 2000). For example, bat midbrain and ACx neurons respond selectively to downward FM sweeps that mimic their sonar calls (Pollak et al., 2011; Fuzessary et al., 2011). This “direction selectivity” develops months after the onset of hearing in the echolocation range and the use of sonar signals, suggesting that experience plays a role in its development. Indeed, if young bats are prevented from hearing normal echolocation calls, the development of FM direction selectivity is compromised (Figure 6; Razak and Fuzessary, 2008). Additionally, development of FM direction selectivity in the rat ACx is influenced by exposure to FM sweeps (Insanally et al., 2009). An added advantage of studying FM processing is that the underlying circuit and cellular mechanisms have, in part, been worked out (Ye et al., 2010; Pollak et al., 2011; Fuzessary et al., 2011).
Figure 6.
Early exposure to sonar vocalizations affects neuronal directional selectivity. (A) Young pallid bats can be reared vocalizing and hearing the sonar calls of other bats (left) or vocally impaired and deprived of exposure to the sonar calls of other bats (right). (B) Frequency modulated (FM) sweeps mimicking sonar calls that differ in direction. Downward sweeps (red) mimic the natural call while upward sweeps (blue) are unnatural. (C) Some single ACx neurons are selectively responsive to downward FM sweeps (left) while other neurons are non-selective and respond to both downward and upward FM sweeps (right). (D) The percent of recorded neurons with low and high directional selectivity is similar in normal and vocally deprived 30-day-old bats but is biased toward low selectivity in 90 day old bats that were reared without normal vocal experience. (panels C and D are adapted from Razak et al., 2008)
The manner in which experience-based plasticity influences perception of communication sounds may provide a unique opportunity to establish the relationship between neural properties and behavior. The acoustic rearing environment of songbirds is rich in complex vocalizations, and provides a paradigm to test whether early exposure to natural sounds can influence auditory coding in juveniles and adults. Because juvenile forebrain neurons display weaker selectivity for song and lower responsiveness compared to adults (Amin et al., 2007), it is plausible that auditory coding develops under the influence of song experience. Indeed, early deprivation of song experience results in abnormally large auditory responsive regions in ACx and compromised neural selectivity in groups of neurons recorded simultaneously, as depicted in Figure 7 (Cousillas et al., 2004; Hauber et al., 2007; George et al., 2010; Maul et al., 2010). For example, the number of physiological recording sites that respond to songs is lower and the response selectivity of recorded sites is higher in European starlings caught in the wild (and presumably exposed to a rich diversity of songs) than in starlings reared without exposure to adult song (George et al., 2010). A general hypothesis that emerges from these studies is that normal ACx development in animals that show vocal learning depends on juvenile exposure to adult communication sounds.
Figure 7.
Early exposure to communication vocalizations may influence neuronal selectivity. (A) Early song experience may influence the development of response selectivity of auditory forebrain neurons for vocal sound features. Neural recordings in songbirds reared with exposure to adult song may show more selectivity and fewer responsive recording sites compared to neural recordings in songbirds that were reared in isolation from adult song. (B) Blood oxygenation level difference (BOLD) to presentation of a song and a song syllable differ in the auditory forebrains of adult male zebra finches reared with song experience and those reared in isolation from song. (panel B adapted from Maul et al., 2010)
Songbirds, including juveniles, can be trained to discriminate among complex sounds and to demonstrate their perceptual preferences for sounds by approaching or eliciting the presentation of some sounds over others (Miller, 1979; Dent and Dooling, 2004; Braaten et al., 2006; Gess et al., 2011). Therefore, one can test for experience-dependent changes in perceptual skills over the course of postnatal development, and search for neural coding correlates (Grace et al., 2003; Woolley et al., 2005; Hauber et al., 2007; Phan and Vicario, 2006; Woolley et al., 2010; Schneider and Woolley, 2010). For example, the role of experience in development of response selectivity for vocalization types in the songbird ACx (e.g. Figure 7) can be examined in parallel with perceptual tests, staggered over ages, to test how perceptual behavior and neural representations of vocal sounds co-vary with age and experience. Here, the opportunity is to test hypotheses about how experience-dependent plasticity drives the development of both neural coding strategies and complex perceptual abilities that are clearly related to natural behavior.
To further test the idea that exposure to natural sounds influences the maturation of songbird auditory circuits, songbirds can be reared and tutored with either conspecific or heterospecific song. The intent of this manipulation is to alter the acoustics rather than the amount of auditory experience. One study has shown that the responses of single central auditory neurons display higher information coding capacities and firing rates in male birds tutored with conspecific song, as compared to birds tutored with heterospecific song, demonstrating that experience and vocal learning are linked with auditory development (Figure 8; Woolley et al., 2010). The influence of this kind of experience on development of perceptual skills such as discriminating among the unique songs of individual birds is still unknown.
Figure 8.
Behavioral and neural coding outcomes of manipulating early vocal experience. (A) Spectrograms of songs show that juvenile zebra finches learn Bengalese finch song when tutored by adult Bengalese finches. Top: Song of the genetic father of the zebra finch tutee. Middle: Song of the foster father, a Bengalese finch. Bottom: Song of the tutee. The tutee’s song is similar to the heterospecific tutor’s song and dissimilar to the genetic father’s song. (B) Spike rates of single midbrain (left) and forebrain (right) neurons in response to presentation of zebra finch songs. Spike rates are higher in neurons from zebra finches tutored by zebra finches than in neurons from Bengalese finches and from zebra finches tutored by Bengalese finches. Error bars are standard deviations. ** is p < 0.01 and *** is p < 0.001. (panel B adapted from Woolley et al., 2010)
The maturation of learning, itself, provides another unique opportunity to assess auditory coding properties. As discussed above, auditory training commonly improves adult performance, but perceptual learning is poor in young animals (Sarro and Sanes, 2010; Huyck and Wright, 2011). Although the effects of learning have yet to be assessed in the developing auditory CNS, a study in finches has shown that, at the level of sound production, forebrain motor neurons display increased temporal precision and rate as finches practice their songs (Crandall et al., 2007).
SYNAPTIC CORRELATES OF CENTRAL AUDITORY DEVELOPMENT & PLASTICITY
Neural maturation is influenced by cochlear spontaneous activity
Even before hair cells are activated by sound, they discharge spontaneously and release transmitter, thereby eliciting bursts of action potentials in primary auditory neurons, which then excites central circuits (Tritsch et al., 2010; Johnson et al., 2011). The presence of spontaneous activity prior to sensory activation has been implicated in the maturation of synaptic connections in the visual system (Feller, 2009), and may serve a similar role in auditory development. For example, inhibitory projections from the medial nucleus of the trapezoid body (MNTB) to the lateral superior olive (LSO) undergo a dramatic period of functional refinement before the onset of hearing (Kim and Kandler, 2003). During this period, MNTB afferents release three transmitters (GABA, glycine, glutamate), and disruption of glutamate release prevents functional refinement (Gillespie et al., 2005; Noh et al., 2010). A current model suggests that the pre-hearing period of spontaneous activity-dependent synapse maturation may lead to a second phase during which silenced synapses are anatomically eliminated (Kandler et al., 2009). Thus, activity-dependent plasticity occurs well in advance of acoustic experience.
Many synaptic properties develop rapidly after hearing onset
Our emphasis on the prolonged time course of perceptual maturation implies that certain synaptic or biophysical properties must also be late-developing. However, studies that chart the maturation of these cellular properties generally report that maturation occurs rapidly, usually within 7–14 days of hearing onset, in rodents. The development of synaptic potentials and membrane properties has been particularly well-studied in the gerbil medial superior olivary (MSO) nucleus (Scott et al., 2005; Magnusson et al., 2005; Chirila et al., 2007). MSO neurons are extremely sensitive to the coincident arrival of excitatory events, and they encode the sound localization cue called interaural time difference. In the two weeks following hearing onset at P12, inhibitory (IPSP) and excitatory postsynaptic potentials (EPSP) become much faster, low threshold-activating potassium currents increase, and the AP threshold current rises (Figure 9A). These results are broadly consistent with developmental findings from several other auditory brainstem nuclei (Sanes, 1993; Kandler and Friauf, 1995; Chuhma and Ohmori, 1998; Taschenberger and von Gersdorff, 2000; Brenowitz and Trussell, 2001; Balakrishnan et al., 2003; Nakamura and Takahashi, 2007; Gao and Lu, 2008; Sanchez et al., 2010). The rapid functional development of synapses throughout the auditory neuraxis must surely have interesting correlates in auditory perception; however, it will be tricky to disentangle the relative contribution of CNS changes from those occurring concomitantly in the middle ear and cochlea. Since auditory deprivation appears to delay CNS maturation (below), it may be possible to ascertain the contribution of central properties by comparing the perceptual abilities of control animals to those reared with moderate hearing loss.
Figure 9.
Rapid development of synaptic and membrane properties in the rodent auditory CNS. (A) Intracellular recordings were obtained from gerbil medial superior olivary (MSO) neurons in brain slices obtained at several ages. The top graph plots the half-width of inhibitory (IPSP, blue) and excitatory postsynaptic potentials (EPSP, green) as a function of age. Whereas the duration of these events is quite long at the onset of hearing (~postnatal day 12), the half-width decreases rapidly over 10–14 days. At the same time, the current required to elicit an action potential and the magnitude of a critical potassium current also display a swift maturation. (B) Intracellular recordings from ACx neurons in mouse brain slices (left) and in anesthetized rats (right) also suggest that development is rapid. By recording from pairs of connected neurons in vitro, the amplitude of individual synaptic events can be measured. Here, the recordings indicate that there is a modest reduction in both IPSPs (blue dashed) and EPSPs (green dashed) over the first two weeks after hearing onset. When sound evoked events are measured in vivo, the latency of inhibitory (IPSC, blue dashed) and excitatory currents (EPSC, green dashed) decline rapidly within two weeks of hearing onset. (C) Spontaneous IPSCs recorded from ACx neurons in a gerbil brain slice display a faster decay time with development. Animals are sexually mature at ~P80 and the sIPSC kinetics are not mature until this age. (panel A adapted from Scott et al., 2005; Magnusson et al., 2005; panel B adapted from Oswald and Reyes, 2008; Oswald and Reyes, 2011; Sun et al., 2010; panel C adapted from Takesian et al., 2011)
It is possible that there are late-developing synaptic properties which help to explain limitations in juvenile perceptual skills, but these properties are found at higher levels of the CNS. However, intracellular recordings in brain slices and in anesthetized animals suggest that synaptic transmission matures rapidly in cortex as well. When neuron pairs are recorded in mouse auditory cortex brain slices, such that synaptic potentials can be quantified for individual connections, the IPSP and EPSP amplitudes decline by about 30% during the two weeks after hearing onset (Figure 9B). This decline may be explained by a 50% reduction in the postsynaptic neurons’ input resistances (Oswald and Reyes, 2008; Oswald and Reyes, 2011). In fact, some inhibitory synaptic currents display a dramatic increase in amplitude during this same period, suggesting that they are compensating for the drop in resistance (Takesian et al., 2010). When whole-cell voltage-clamp recordings are obtained in vivo from the auditory cortex of anesthetized rat, the amplitudes of sound-evoked IPSPs and EPSPs do not change significantly between the onset of hearing and adulthood (Sun et al., 2010; Dorrn et al., 2010). These synaptic events do display age-dependent alterations such as frequency selectivity and response latency, but these changes also tend to occur soon after hearing onset.
Identifying late-developing cellular properties
Because few late-developing cellular properties have been described, one possibility is that immaturities are only observable within the context of a network. For example, coding sparsity and efficiency are likely to have developmental time courses that depend on synaptic properties. If this is the case, then single neuron coding properties will provide the most sensitive assay for synaptic maturation. Before accepting this option, we must toil a bit harder to measure synaptic and ionic properties in tissue from mature adults. Whole cell recordings obtained from adult tissue are typically low in yield, and this may explain why so few studies have included an assessment of cellular properties from the latter period of development. A second possibility is that the brain slice experiments that we conduct to examine synaptic properties are not challenging enough to reveal age-dependent changes. That is, the brief trains of current pulses delivered in vitro are not a realistic assessment for the afferent activity patterns elicited by acoustic stimuli in vivo. Therefore, it seems plausible that a thorough search for late-developing synaptic and membrane properties will be worthwhile. In fact, IPSC decay time in auditory cortex continues to mature until sexual maturation (Figure 9C; Takesian et al., 2011) Along this line, it is worth noting that the expression of two GABAA receptor alpha subunits continues to change in human visual cortex over the first decade of life (Pinto et al., 2010).
Experience influences synaptic function
Synapse function remains quite sensitive to environmental manipulations after the onset of sound-evoked responses, but there is little direct evidence on the precise critical period. One recent study examined the effect of a brief period of acoustic stimulation on the amplitude of EPSPs and IPSPs recorded in the auditory cortex of anesthetized rats (Dorrn et al., 2010). As shown in Figure 10A, there is a strong effect of stimulation within about 10 days of hearing onset, but not thereafter. This demonstrates a fairly brief critical period for use-dependent strengthening of synaptic connections. A larger set of studies has reported that a period of developmental deprivation (i.e., hearing loss) can lead to profound changes in synaptic strength along the entire auditory CNS (Takesian et al., 2009). Figure 10B shows one set of examples from the gerbil auditory cortex following about 1 week of hearing loss (Takesian et al., 2010). One type of inhibitory synapse made by fast spiking interneurons displays a dramatic reduction in strength, while a second class of inhibitory synapses displays an increase in short-term depression. Although these examples of synaptic plasticity are presumed to account for specific changes in auditory processing and perception, we do not yet have a clear correlate, or even an experimental design that will yield one.
Figure 10.
Use-dependent synaptic plasticity during development. (A) Whole cell recordings were obtained from ACx neurons in anesthetized rats, and tone pulses were delivered for 3–5 minutes. The sound-evoked inhibitory (blue) and excitatory (green) synaptic conductance were each enhanced when this procedure was applied to young neurons (<P22), but did not induce an effect thereafter. (B) Whole-cell voltage clamp recordings were obtained from pairs of neurons in the auditory cortex in brain slices from control animals and those that were deafened just before hearing onset. The inhibitory synaptic currents produced by fast-spiking (FS) inhibitory interneurons at pyramidal neurons are dramatically smaller following hearing loss. The inhibitory synaptic currents produced by a different class of inhibitory interneuron (LTS, low threshold spiking) do not decline in amplitude. However, whereas the IPSCs from control LTS neurons do not display short-term depression in response to a train of stimuli, hearing loss causes them to exhibit synaptic depression. This is calculated as the ratio of the 1st IPSC to the 10th IPSC. (panel A adapted from Dorrn et al., 2010; panel B adapted from Takesian et al., 2010)
SUMMARY
The significance of experience-based plasticity depends, ultimately, on its behavioral relevance. Furthermore, to properly characterize and interpret a phenotypic change in behavior, one typically requires an accurate description of the ‘wild type’. Here, we have argued that the behavioral phenotype is too often missing from our approach to auditory development and plasticity; put another way, significant opportunities can be leveraged by taking advantage of the information provided by studying normal behavioral development. A compelling description of auditory development in children suggests that perceptual skills mature at different rates and over a prolonged period, long after cochlear processing is adult-like. If quantitative behavioral measures can be obtained from non-human animals during development, we can then use these phenotypes to establish the relationship between neural processing and normal perceptual maturation. Additionally, we can ask whether perceptual skills that remain immature are relatively more vulnerable to experience manipulations, including vocal learning and hearing loss. If specific postnatal experiences can be tied to distinct alterations to a behavioral phenotype, there emerges a second set of opportunities to relate neural processing to perception.
Progress toward linking early experience to neural plasticity will require that measures of neural plasticity in developing animals take advantage of accompanying measures of perceptual development. Challenges in this pursuit include finding measures of perception that are consistent between juveniles and adults, disambiguating the effects of cochlear and CNS development on skill acquisition, considering cognitive and attentional changes over development and identifying specific neural mechanisms that underlie specific percepts. The opportunities described here are potential starting points to capitalize on aspects of auditory processing and model systems for which there is already good evidence that changes in neural processing parallel perceptual development.
ACKNOWLEDGEMENTS
We thank George Pollak, Beverly Wright, David Schneider, Emma Sarro, Carolina Abdala, Huanping Dai, Virginia Wohl, and the anonymous referees for their helpful comments. This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (DC009237 and DC011284, D.H.S.; DC009810, S.M.N.W), and the National Science Foundation (IOS-0920081, S.M.N.W.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdala C, Keefe DH. Morphological and Functional Development of the Ear. In: Werner L, editor. Springer Handbook of Auditory Research: Human Development. New York: Springer-Verlag; 2012. [Google Scholar]
- Allen P, Wightman F. Psychometric functions for children's detection of tones in noise. J Speech Hear Res. 1994;37:205–215. doi: 10.1044/jshr.3701.205. [DOI] [PubMed] [Google Scholar]
- Allen P, Wightman F, Kistler D, Dolan T. Frequency resolution in children. J Speech Hear Res. 1989;32:317–322. doi: 10.1044/jshr.3202.317. [DOI] [PubMed] [Google Scholar]
- Amin N, Doupe A, Theunissen FE. Development of selectivity for natural sounds in the songbird auditory forebrain. J Neurophysiol. 2007;97:3517–3531. doi: 10.1152/jn.01066.2006. [DOI] [PubMed] [Google Scholar]
- Ari-Even Roth D, Amir O, Alaluf L, Buchsenspanner S, Kishon-Rabin L. The effect of training on frequency discrimination: generalization to untrained frequencies and to the untrained ear. J Basic Clin Physiol Pharmacol. 2003;15:137–150. doi: 10.1515/jbcpp.2003.14.2.137. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Sabin AT, Wright BA. Separable developmental trajectories for the abilities to detect auditory amplitude and frequency modulation. Hear Res. 2011 doi: 10.1016/j.heares.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargones JY, Werner LA. Adults listen selectively; adults do not. Psychol Sci. 1994;5:170–174. [Google Scholar]
- Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci U S A. 2003;100:11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Boswell AE. Temporal summation of 500-Hz tones and octave-band noise bursts in infants and adults. Percept Psychophys. 1995;57:183–190. doi: 10.3758/bf03206504. [DOI] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Remodeling the cortex in memory: Increased use of a learning strategy increases the representational area of relevant acoustic cues. Neurobiol Learn Mem. 2010;94:127–144. doi: 10.1016/j.nlm.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham BH, Cheung SW, Godey B, Schreiner CE. Spatial organization of frequency response areas and rate/level functions in the developing AI. J Neurophysiol. 2004;91:841–854. doi: 10.1152/jn.00017.2003. [DOI] [PubMed] [Google Scholar]
- Braaten RF, Petzoldt M, Colbath A. Song perception during the sensitive period of song learning in zebra finches (Taeniopygia guttata) J Comp Psychol. 2006;120:79–88. doi: 10.1037/0735-7036.120.2.79. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci. 2001;21:9487–9498. doi: 10.1523/JNEUROSCI.21-23-09487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Irvine DR, Park VN. Perceptual learning on an auditory frequency discrimination task by cats: association with changes in primary auditory cortex. Cereb Cortex. 2004;14:952–965. doi: 10.1093/cercor/bhh056. [DOI] [PubMed] [Google Scholar]
- Brown TA, Harrison RV. Postnatal development of neuronal responses to frequency-modulated tones in chinchilla auditory cortex. Brain Res. 2010;1309:29–39. doi: 10.1016/j.brainres.2009.10.053. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Javel E, Kitzes LM. Signs of functional maturation of peripheral auditory system in discharge patterns of neurons in anteroventral cochlear nucleus of kitten. J Neurophysiol. 1978;41:1557–1559. doi: 10.1152/jn.1978.41.6.1557. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Kitzes LM, Javel E. Postnatal development of frequency and intensity sensitivity of neurons in the anteroventral cochlear nucleus of kittens. Hear Res. 1981;5:217–229. doi: 10.1016/0378-5955(81)90047-2. [DOI] [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH. Development and the role of internal noise in detection and discrimination thresholds with narrow band stimuli. J Acoust Soc Am. 2006;120:2777–2788. doi: 10.1121/1.2354024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH. Psychometric functions for pure tone intensity discrimination: slope differences in school-aged children and adults. J Acoust Soc Am. 2009;125:1050–1058. doi: 10.1121/1.3050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DLM, Hauber ME. Cross-fostering diminishes song discrimination in zebra finches (Taeniopygia guttata) Animal Cognition. 2009;12:481–490. doi: 10.1007/s10071-008-0209-5. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Naatanen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Chirila FV, Rowland KC, Thompson JM, Spirou GA. Development of gerbil medial superior olive: integration of temporally delayed excitation and inhibition at physiological temperature. J Physiol. 2007;584:167–190. doi: 10.1113/jphysiol.2007.137976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Ohmori H. Postnatal development of phase-locked high-fidelity synaptic transmission in the medial nucleus of the trapezoid body of the rat. J Neurosci. 1998;18:512–520. doi: 10.1523/JNEUROSCI.18-01-00512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson MG, Clifton RK, Swain IU, Perris EE. Stimulus duration and repetition rate influence newborns' head orientation toward sound. Dev Psychobiol. 1989;22:683–705. doi: 10.1002/dev.420220704. [DOI] [PubMed] [Google Scholar]
- Clayton NS. Song learning and mate choice in estrildid finches raised by 2 species. Anim Behav. 1988;36:1589–1600. [Google Scholar]
- Clayton NS. The effects of cross-fostering on assortative mating between zebra finch subspecies. Anim Behav. 1990;40:1102–1110. [Google Scholar]
- Clayton NS, Prove L. Song discrimination in female zebra finches and Bengalese finches. Anim Behav. 1989;38:352–353. [Google Scholar]
- Clements M, Kelly JB. Auditory spatial responses of young guinea pigs (Cavia porcellus) during and after ear blocking. J Comp Physiol Psychol. 1978;92:34–44. doi: 10.1037/h0077424. [DOI] [PubMed] [Google Scholar]
- Cline H. Sperry and Hebb: oil and vinegar? Trends Neurosci. 2003;26:655–661. doi: 10.1016/j.tins.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Comins JA, Gentner TQ. Working memory for patterned sequences of auditory objects in a songbird. Cognition. 2010;117:38–53. doi: 10.1016/j.cognition.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Cousillas H, Richard JP, Mathelier M, Henry L, George I, Hausberger M. Experience-dependent neuronal specialization and functional organization in the central auditory area of a songbird. Eur J Neurosci. 2004;19:3343–3352. doi: 10.1111/j.0953-816X.2004.03376.x. [DOI] [PubMed] [Google Scholar]
- Crandall SR, Aoki N, Nick TA. Developmental modulation of the temporal relationship between brain and behavior. J Neurophysiol. 2007;97:806–816. doi: 10.1152/jn.00907.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Wright BA. Detecting signals of unexpected or uncertain durations. J Acoust Soc Am. 1995;98:798–806. doi: 10.1121/1.413572. [DOI] [PubMed] [Google Scholar]
- Dawes P, Bishop DV. Maturation of visual and auditory temporal processing in school-aged children. J Speech Lang Hear Res. 2008;51:1002–1015. doi: 10.1044/1092-4388(2008/073). [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBello WM, Feldman DE, Knudsen EI. Adaptive axonal remodeling in the midbrain auditory space map. J Neurosci. 2001;21:3161–3174. doi: 10.1523/JNEUROSCI.21-09-03161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent ML, Dooling RJ. The precedence effect in three species of birds (Melopsittacus undulatus, Serinus canaria, and Taeniopygia guttata) J Comp Psychol. 2004;118:325–331. doi: 10.1037/0735-7036.118.3.325. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganova R, Wollbrink A, Schulz M, Okamoto H, Pantev C. Modulation of auditory evoked responses to spectral and temporal changes by behavioral discrimination training. BMC Neurosci. 2009;10:143. doi: 10.1186/1471-2202-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eales LA. Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1:179–184. [PubMed] [Google Scholar]
- Ehret G, Romand R. Development of tone response thresholds, latencies and tuning in the mouse inferior colliculus. Brain Res Dev Brain Res. 1992;67:317–326. doi: 10.1016/0165-3806(92)90233-m. [DOI] [PubMed] [Google Scholar]
- Elfenbein JL, Small AM, Davis JM. Developmental patterns of duration discrimination. J Speech Hear Res. 1993;36:842–849. doi: 10.1044/jshr.3604.842. [DOI] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzessery ZM, Razak KA, Williams AJ. Multiple mechanisms shape selectivity for FM sweep rate and direction in the pallid bat inferior colliculus and auditory cortex. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197:615–623. doi: 10.1007/s00359-010-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Lu Y. Early development of intrinsic and synaptic properties of chicken nucleus laminaris neurons. Neuroscience. 2008;153:131–143. doi: 10.1016/j.neuroscience.2008.01.059. [DOI] [PubMed] [Google Scholar]
- George I, Alcaix S, Henry L, Richard JP, Cousillas H, Hausberger M. Neural correlates of experience-induced deficits in learned vocal communication. PLoS One. 2010;5:e14347. doi: 10.1371/journal.pone.0014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gess A, Schneider DM, Vyas A, Woolley SM. Automated auditory recognition training and testing. Anim Behav. 2011;82:285–293. doi: 10.1016/j.anbehav.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EJ. Principles of perceptual learning and development. Englewood Cliffs, NJ: Prentice-Hall; 1969. [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Gomes H, Molholm S, Christodoulou C, Ritter W, Cowan N. The development of auditory attention in children. Front Biosci. 2000;5:D108–D120. doi: 10.2741/gomes. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: I. Nature of perceptual deficit caused by embryonic auditory deprivation. J Comp Physiol Psychol. 1975a;89:387–399. doi: 10.1037/h0077068. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: II. Experimental prevention of perceptual deficit caused by embryonic auditory deprivation. J Comp Physiol Psychol. 1975b;89:675–684. doi: 10.1037/h0077067. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: IV. Change in species-specific perception caused by auditory deprivation. J Comp Physiol Psychol. 1978;92:375–387. doi: 10.1037/h0077473. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: VI. Specific embryonic experience required to maintain species-typical perception in ducklings. J Comp Physiol Psychol. 1980;94:579–587. doi: 10.1037/h0077691. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: VIII. Embryonic versus postnatal critical period for the maintenance of species-typical perception. J Comp Physiol Psychol. 1981;95:540–547. doi: 10.1037/h0077793. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: X. Perceptual specificity in the wood duck embryo requires sib stimulation for maintenance. Dev Psychobiol. 1983;16:323–333. doi: 10.1002/dev.420160407. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Social induction of malleability in ducklings: Sensory basis and psychological mechanism. Anim Behav. 1993;45:707–719. [Google Scholar]
- Grace JA, Amin N, Singh NC, Theunissen FE. Selectivity for conspecific song in the zebra finch auditory forebrain. J Neurophysiol. 2003;89:472–487. doi: 10.1152/jn.00088.2002. [DOI] [PubMed] [Google Scholar]
- Gray L. Development of a frequency dimension in chickens (Gallus gallus) J Comp Psychol. 1991;105:85–88. doi: 10.1037/0735-7036.105.1.85. [DOI] [PubMed] [Google Scholar]
- Gray L. An auditory psychometric function from newborn chicks. J Acoust Soc Am. 1992;91:1608–1615. doi: 10.1121/1.402441. [DOI] [PubMed] [Google Scholar]
- Gray L. Developmental changes in chickens' masked thresholds. Dev Psychobiol. 1993a;26:447–457. doi: 10.1002/dev.420260803. [DOI] [PubMed] [Google Scholar]
- Gray L. Simultaneous masking in newborn chickens. Hear Res. 1993b;69:83–90. doi: 10.1016/0378-5955(93)90095-i. [DOI] [PubMed] [Google Scholar]
- Gray L, Kesser B, Cole E. Understanding speech in noise after correction of congenital unilateral aural atresia: effects of age in the emergence of binaural squelch but not in use of head-shadow. Int J Pediatr Otorhinolaryngol. 2009;73:1281–1287. doi: 10.1016/j.ijporl.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Gray L, Rubel EW. Development of absolute thresholds in chickens. J Acoust Soc Am. 1985;77:1162–1172. doi: 10.1121/1.392180. [DOI] [PubMed] [Google Scholar]
- Greenberg GZ, Larkin WD. Frequency-response characteristic of auditory observers detecting signals of a single frequency in noise: the probe-signal method. J Acoust Soc Am. 1968;44:1513–1523. doi: 10.1121/1.1911290. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH. Notched-noise measures of frequency selectivity in adults and children using fixed-masker-level and fixed-signal-level presentation. J Speech Hear Res. 1991;34:651–660. doi: 10.1044/jshr.3403.651. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH. The effect of conductive hearing loss on the masking-level difference: insert versus standard earphones. J Acoust Soc Am. 1994b;95:2652–2657. doi: 10.1121/1.409834. [DOI] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH, Pillsbury HC. Long-term effects of chronic otitis media on binaural hearing in children. Arch Otolaryngol Head Neck Surg. 1995;121:847–852. doi: 10.1001/archotol.1995.01890080017003. [DOI] [PubMed] [Google Scholar]
- Halliday LF, Bishop DV. Frequency discrimination and literacy skills in children with mild to moderate sensorineural hearing loss. J Speech Lang Hear Res. 2005;48:1187–1203. doi: 10.1044/1092-4388(2005/083). [DOI] [PubMed] [Google Scholar]
- Halliday LF, Bishop DV. Is poor frequency modulation detection linked to literacy problems? A comparison of specific reading disability and mild to moderate sensorineural hearing loss. Brain Lang. 2006;97:200–213. doi: 10.1016/j.bandl.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Han YK, Kover H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Hartley DE, Wright BA, Hogan SC, Moore DR. Age-related improvements in auditory backward and simultaneous masking in 6- to 10-year-old children. J Speech Lang Hear Res. 2000;43:1402–1415. doi: 10.1044/jslhr.4306.1402. [DOI] [PubMed] [Google Scholar]
- Hauber ME, Woolley SMN, Theunissen FE. Experience-dependence of neural responses to social. vs. isolate conspecific songs in the forebrain of female zebra finches. J. Ornithol. 2007 in press. [Google Scholar]
- He S, Buss E, Hall JW., 3rd Monaural temporal integration and temporally selective listening in children and adults. J Acoust Soc Am. 2010;127:3643–3653. doi: 10.1121/1.3397464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil P, Langner G, Scheich H. Processing of frequency-modulated stimuli in the chick auditory cortex analogue: evidence for topographic representations and possible mechanisms of rate and directional sensitivity. J Comp Physiol A. 1992;171:583–600. doi: 10.1007/BF00194107. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Huyck JJ, Wright BA. Late maturation of auditory perceptual learning. Dev Sci. 2011;14:614–621. doi: 10.1111/j.1467-7687.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Wetzel W, Scheich H, Ohl FW. The combination of appetitive and aversive reinforcers and the nature of their interaction during auditory learning. Neuroscience. 2010;166:752–762. doi: 10.1016/j.neuroscience.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge UP: Cambridge; 1969. pp. 61–77. [Google Scholar]
- Insanally MN, Kover H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JK, Neff DL. Development of basic auditory discrimination in preschool children. Psychol Sci. 1993;4:104–107. [Google Scholar]
- Johnson KL, Nicol T, Zecker SG, Kraus N. Developmental plasticity in the human auditory brainstem. J Neurosci. 2008;28:4000–4007. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, Roberts TP, Masetto S, Knipper M, Kros CJ, Marcotti W. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci. 2011;14:711–717. doi: 10.1038/nn.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Judge PW, Fraser IH. Development of the auditory orientation response in the albino rat (Rattus norvegicus) J Comp Psychol. 1987;101:60–66. [PubMed] [Google Scholar]
- Kelly JB, Potash M. Directional responses to sounds in young gerbils (Meriones unguiculatus) J Comp Psychol. 1986;100:37–45. [PubMed] [Google Scholar]
- Kerr LM, Ostapoff EM, Rubel EW. Influence of acoustic experience on the ontogeny of frequency generalization gradients in the chicken. J Exp Psychol Anim Behav Process. 1979;5:97–115. doi: 10.1037//0097-7403.5.2.97. [DOI] [PubMed] [Google Scholar]
- Kettner RE, Feng JZ, Brugge JF. Postnatal development of the phase-locked response to low frequency tones of auditory nerve fibers in the cat. J Neurosci. 1985;5:275–283. doi: 10.1523/JNEUROSCI.05-02-00275.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G, Jr, Arbogast TL, Mason CR, Walsh M. Informational masking in listeners with sensorineural hearing loss. J Assoc Res Otolaryngol. 2002;3:107–119. doi: 10.1007/s101620010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- King AJ, Parsons CH, Moore DR. Plasticity in the neural coding of auditory space in the mammalian brain. Proc Natl Acad Sci U S A. 2000;97:11821–11828. doi: 10.1073/pnas.97.22.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Esterly SD, Knudsen PF. Monaural occlusion alters sound localization during a sensitive period in the barn owl. J Neurosci. 1984a;4:1001–1011. doi: 10.1523/JNEUROSCI.04-04-01001.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF, Esterly SD. A critical period for the recovery of sound localization accuracy following monaural occlusion in the barn owl. J Neurosci. 1984b;4:1012–1020. doi: 10.1523/JNEUROSCI.04-04-01012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Mechanisms of experience-dependent plasticity in the auditory localization pathway of the barn owl. J Comp Physiol A. 1999;185:305–321. doi: 10.1007/s003590050391. [DOI] [PubMed] [Google Scholar]
- Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nat Rev Neurosci. 2010;11:599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- Kraus N, Smith DI, McGee T. Rate and filter effects on the developing middle-latency response. Audiology. 1987a;26:257–268. doi: 10.3109/00206098709081554. [DOI] [PubMed] [Google Scholar]
- Kraus N, Smith DI, McGee T, Stein L, Cartee C. Development of the middle latency response in an animal model and its relation to the human response. Hear Res. 1987b;27:165–176. doi: 10.1016/0378-5955(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J Neurophysiol. 2000;84:255–273. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Is speech learning 'gated' by the social brain? Dev Sci. 2007;10:110–120. doi: 10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Lauay C, Gerlach NM, Adkins-Regan E, Devoogd TJ. Female zebra finches require early song exposure to prefer high-quality song as adults. Animal Behaviour. 2004:68. [Google Scholar]
- Lauer AM, May BJ. The medial olivocochlear system attenuates the developmental impact of early noise exposure. J Assoc Res Otolaryngol. 2011;12:329–343. doi: 10.1007/s10162-011-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol. 2002;87:2237–2261. doi: 10.1152/jn.2002.87.5.2237. [DOI] [PubMed] [Google Scholar]
- Litovsky RY. Developmental changes in the precedence effect: estimates of minimum audible angle. J Acoust Soc Am. 1997;102:1739–1745. doi: 10.1121/1.420106. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Kapfer C, Grothe B, Koch U. Maturation of glycinergic inhibition in the gerbil medial superior olive after hearing onset. J Physiol. 2005;568:497–512. doi: 10.1113/jphysiol.2005.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham RG, Toth G, Lickliter R. Prenatally elevated physiological arousal interferes with perceptual learning in bobwhite quail (Colinus virginianus) embryos. Behav Neurosci. 2006;120:1315–1325. doi: 10.1037/0735-7044.120.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul KK, Voss HU, Parra LC, Salgado-Commissariat D, Ballon D, Tchernichovski O, Helekar SA. The development of stimulus-specific auditory responses requires song exposure in male but not female zebra finches. Dev Neurobiol. 2010;70:28–40. doi: 10.1002/dneu.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon AB, Hochberg I. Development of psychoacoustic behavior: sensitivity and discrimination. Ear Hear. 1982;3:301–308. doi: 10.1097/00003446-198211000-00003. [DOI] [PubMed] [Google Scholar]
- McDermott JH, Simoncelli EP. Sound texture perception via statistics of the auditory periphery: evidence from sound synthesis. Neuron. 2011;71:926–940. doi: 10.1016/j.neuron.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee T, Kraus N. Auditory development reflected by middle latency response. Ear Hear. 1996;17:419–429. doi: 10.1097/00003446-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Schreiner CE, Sutter ML, Grasse KL. Functional topography of cat primary auditory cortex: responses to frequency-modulated sweeps. Exp Brain Res. 1993;94:65–87. doi: 10.1007/BF00230471. [DOI] [PubMed] [Google Scholar]
- Miller DB. The acoustic basis of mate recognition by female zebra finches (Taeniopygia guttata) Anim Behav. 1979;27:376–380. [Google Scholar]
- Mills DM, Rubel EW. Development of the cochlear amplifier. J Acoust Soc Am. 1996;100:428–441. doi: 10.1121/1.415857. [DOI] [PubMed] [Google Scholar]
- Mogdans J, Knudsen EI. Early monaural occlusion alters the neural map of interaural level differences in the inferior colliculus of the barn owl. Brain Res. 1993;619:29–38. doi: 10.1016/0006-8993(93)91593-h. [DOI] [PubMed] [Google Scholar]
- Mogdans J, Knudsen EI. Site of auditory plasticity in the brain stem (VLVp) of the owl revealed by early monaural occlusion. J Neurophysiol. 1994;72:2875–2891. doi: 10.1152/jn.1994.72.6.2875. [DOI] [PubMed] [Google Scholar]
- Moore DR, Cowan JA, Riley A, Edmondson-Jones AM, Ferguson MA. Development of auditory processing in 6- to 11-yr-old children. Ear Hear. 2011;32:269–285. doi: 10.1097/AUD.0b013e318201c468. [DOI] [PubMed] [Google Scholar]
- Moore DR, Ferguson MA, Edmondson-Jones AM, Ratib S, Riley A. Nature of auditory processing disorder in children. Pediatrics. 2010;126:e382–e390. doi: 10.1542/peds.2009-2826. [DOI] [PubMed] [Google Scholar]
- Moore DR, Hine JE, Jiang ZD, Matsuda H, Parsons CH, King AJ. Conductive hearing loss produces a reversible binaural hearing impairment. J Neurosci. 1999;19:8704–8711. doi: 10.1523/JNEUROSCI.19-19-08704.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Guan YL. Cytoarchitectural and axonal maturation in human auditory cortex. J Assoc Res Otolaryngol. 2001;2:297–311. doi: 10.1007/s101620010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossbridge JA, Fitzgerald MB, O'Connor ES, Wright BA. Perceptual-learning evidence for separate processing of asynchrony and order tasks. J Neurosci. 2006;26:12708–12716. doi: 10.1523/JNEUROSCI.2254-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller V, Gruber W, Klimesch W, Lindenberger U. Lifespan differences in cortical dynamics of auditory perception. Dev Sci. 2009;12:839–853. doi: 10.1111/j.1467-7687.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- Murasugi CM, Salzman CD, Newsome WT. Microstimulation in visual area MT: effects of varying pulse amplitude and frequency. J Neurosci. 1993;13:1719–1729. doi: 10.1523/JNEUROSCI.13-04-01719.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle L, Kreutzer MI. Song tutoring influences female song preferences in domesticated canaries. Behaviour. 1997;134:89–104. [Google Scholar]
- Nakamura Y, Takahashi T. Developmental changes in potassium currents at the rat calyx of Held presynaptic terminal. J Physiol. 2007;581:1101–1112. doi: 10.1113/jphysiol.2007.128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken I, Versnel H. Responses to linear and logarithmic frequency-modulated sweeps in ferret primary auditory cortex. Eur J Neurosci. 2000;12:549–562. doi: 10.1046/j.1460-9568.2000.00935.x. [DOI] [PubMed] [Google Scholar]
- Njegovan M, Weisman R. Pitch discrimination in field- and isolation-reared black capped chickadees Parus atricapillus. J Comp Psychol. 1997;111:294–301. [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, Gourevitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- Olsho LW. Infant frequency discrimination. Infant Behav Dev. 1984;7:27–35. [Google Scholar]
- Olsho LW, Koch EG, Halpin CF. Level and age effects in infant frequency discrimination. J Acoust Soc Am. 1987;82:454–464. doi: 10.1121/1.395446. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Development of inhibitory timescales in auditory cortex. Cereb Cortex. 2011;21:1351–1361. doi: 10.1093/cercor/bhq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CH, Lanyon RG, Schnupp JW, King AJ. Effects of altering spectral cues in infancy on horizontal and vertical sound localization by adult ferrets. J Neurophysiol. 1999;82:2294–2309. doi: 10.1152/jn.1999.82.5.2294. [DOI] [PubMed] [Google Scholar]
- Pelucchi B, Hay JF, Saffran JR. Statistical learning in a natural language by 8-month-old infants. Child Dev. 2009;80:674–685. doi: 10.1111/j.1467-8624.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Searcy WA, Marler P. Species song discrimination in choice experiments with territorial male swamp and song sparrows. Anim Behav. 1980;28:393–404. [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbin MK, Ballweg DD, Gray L. The effect of an intensive care unit sound environment on the development of habituation in healthy avian neonates. Dev Psychobiol. 1994;27:11–21. doi: 10.1002/dev.420270103. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Harrison RV. Tone frequency maps and receptive fields in the developing chinchilla auditory cortex. J Neurophysiol. 2005;93:454–466. doi: 10.1152/jn.00569.2004. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ. Cortical tonotopic map plasticity and behavior. Neurosci Biobehav Rev. 2011;35:2117–2128. doi: 10.1016/j.neubiorev.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Pinto JG, Hornby KR, Jones DG, Murphy KM. Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front Cell Neurosci. 2010;4:16. doi: 10.3389/fncel.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman AL, Lewis DE, Hoover BM, Stelmachowicz PG. Rapid word-learning in normal-hearing and hearing-impaired children: effects of age, receptive vocabulary, and high-frequency amplification. Ear Hear. 2005;26:619–629. doi: 10.1097/01.aud.0000189921.34322.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD, Xie R, Gittelman JX, Andoni S, Li N. The dominance of inhibition in the inferior colliculus. Hear Res. 2011;274:27–39. doi: 10.1016/j.heares.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton CW, Moore JK, Eggermont JJ. Auditory brain stem response generation by parallel pathways: differential maturation of axonal conduction time and synaptic transmission. Ear Hear. 1996;17:402–410. doi: 10.1097/00003446-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarommatis IM, Goritsa E, Douniadakis D, Tsakanikos M, Kontrogianni AD, Apostolopoulos N. Hearing loss in speech-language delayed children. Int J Pediatr Otorhinolaryngol. 2001;58:205–210. doi: 10.1016/s0165-5876(01)00430-x. [DOI] [PubMed] [Google Scholar]
- Rance G, McKay C, Grayden D. Perceptual characterization of children with auditory neuropathy. Ear Hear. 2004;25:34–46. doi: 10.1097/01.AUD.0000111259.59690.B8. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of "what" and "where" in auditory cortex. Proc Natl Acad Sci U S A. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of inhibitory mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurosci. 2007;27:1769–1781. doi: 10.1523/JNEUROSCI.3851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci U S A. 2008;105:4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebel K, Smallgange IM, Terpstra NJ, Bolhuis JJ. Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc Roy Soc Lond. 2002;269:729–733. doi: 10.1098/rspb.2001.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R. Development in the frequency selectivity of auditory nerve fibers in the kitten. Neurosci Lett. 1983;35:271–276. doi: 10.1016/0304-3940(83)90329-4. [DOI] [PubMed] [Google Scholar]
- Romand R, Ehret G. Development of tonotopy in the inferior colliculus. I. Electrophysiological mapping in house mice. Brain Res Dev Brain Res. 1990;54:221–234. doi: 10.1016/0165-3806(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Rosen MJ, Semple MN, Sanes DH. Exploiting development to evaluate auditory encoding of amplitude modulation. J Neurosci. 2010;30:15509–15520. doi: 10.1523/JNEUROSCI.3340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci. 1992;336:367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Rübsamen R, Lippe W. Functional development of the cochlea. In: Rubel EW, Popper AN, Fay RR, editors. Development of the mammalian auditory system. New York: Springer Handbook of Auditory research, Springer Verlag; 1998. [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Sakai M, Kudoh M. Characteristics of sound discrimination enhancement after sound exposure in adult rats. Behav Neurosci. 2005;119:961–973. doi: 10.1037/0735-7044.119.4.961. [DOI] [PubMed] [Google Scholar]
- Sanchez JT, Wang Y, Rubel EW, Barria A. Development of glutamatergic synaptic transmission in binaural auditory neurons. J Neurophysiol. 2010;104:1774–1789. doi: 10.1152/jn.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH. The development of synaptic function and integration in the central auditory system. J Neurosci. 1993;13:2627–2637. doi: 10.1523/JNEUROSCI.13-06-02627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Constantine-Paton M. The sharpening of frequency tuning curves requires patterned activity during development in the mouse, Mus musculus. J Neurosci. 1985;5:1152–1166. doi: 10.1523/JNEUROSCI.05-05-01152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Merickel M, Rubel EW. Evidence for an alteration of the tonotopic map in the gerbil cochlea during development. J Comp Neurol. 1989;279:436–444. doi: 10.1002/cne.902790308. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Sanes DH. Prolonged maturation of auditory perception and learning in gerbils. Dev Neurobiol. 2010;70:636–648. doi: 10.1002/dneu.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Sanes DH. The cost and benefit of juvenile training on adult perceptual skill. J Neurosci. 2011;31:5383–5391. doi: 10.1523/JNEUROSCI.6137-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC, Dolgin KG, Lowry LD. The maturation of frequency selectivity in C57BL/6J mice studied with auditory evoked response tuning curves. Brain Res. 1980;187:69–79. doi: 10.1016/0006-8993(80)90495-3. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Chays A. On the role of the olivocochlear bundle in hearing: 16 case studies. Hear Res. 1997;103:101–122. doi: 10.1016/s0378-5955(96)00168-2. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Morrongiello BA, Trehub SE. Size of critical band in infants, children, and adults. J Exp Psychol Hum Percept Perform. 1990;16:642–652. doi: 10.1037//0096-1523.16.3.642. [DOI] [PubMed] [Google Scholar]
- Schneider DM, Woolley SMN. Discrimination of Communication Vocalizations by Single Neurons and Groups of Neurons in the Auditory Midbrain. J Neurophysiol. 2010;103:3248–3265. doi: 10.1152/jn.01131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonweiler R, Ptok M, Radu HJ. A cross-sectional study of speech- and language-abilities of children with normal hearing, mild fluctuating conductive hearing loss, or moderate to profound sensoneurinal hearing loss. Int J Pediatr Otorhinolaryngol. 1998;44:251–258. doi: 10.1016/s0165-5876(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Schulze H, Scheich H. Discrimination learning of amplitude modulated tones in Mongolian gerbils. Neurosci Lett. 1999;261:13–16. doi: 10.1016/s0304-3940(98)00991-4. [DOI] [PubMed] [Google Scholar]
- Scott LL, Mathews PJ, Golding NL. Posthearing developmental refinement of temporal processing in principal neurons of the medial superior olive. J Neurosci. 2005;25:7887–7895. doi: 10.1523/JNEUROSCI.1016-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Shnerson A, Pujol R. Age-related changes in the C57BL/6J mouse cochlea. I. Physiological findings. Brain Res. 1981;254:65–75. doi: 10.1016/0165-3806(81)90059-6. [DOI] [PubMed] [Google Scholar]
- Singh NC, Theunissen FE. Modulation spectra of natural sounds and ethological theories of auditory processing. J Acoust Soc Am. 2003;114:3394–3411. doi: 10.1121/1.1624067. [DOI] [PubMed] [Google Scholar]
- Sinnott JM, Aslin RN. Frequency and intensity discrimination in human infants and adults. J Acoust Soc Am. 1985;78:1986–1992. doi: 10.1121/1.392655. [DOI] [PubMed] [Google Scholar]
- Sleigh MJ, Columbus RF, Lickliter R. Type of prenatal sensory experience affects prenatal auditory learning in bobwhite quail (Colinus virginianus) J Comp Psychol. 1996;110:233–242. doi: 10.1037/0735-7036.110.3.233. [DOI] [PubMed] [Google Scholar]
- Spetner NB, Olsho LW. Auditory frequency resolution in human infancy. Child Dev. 1990;61:632–652. [PubMed] [Google Scholar]
- Sturdy CB, Phillmore LS, Sartor JJ, Weisman RG. Reduced social contact causes auditory perceptual deficits in zebra finches, Taeniopygia guttata. Anim Behav. 2001;62:1207–1218. [Google Scholar]
- Suga N. Responses of cortical auditory neurones to frequency modulated sounds in echo-locating bats. Nature. 1965;206:890–891. doi: 10.1038/206890a0. [DOI] [PubMed] [Google Scholar]
- Sun W, Tang L, Allman BL. Environmental noise affects auditory temporal processing development and NMDA-2B receptor expression in auditory cortex. Behav Brain Res. 2011;218:15–20. doi: 10.1016/j.bbr.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman E, Steinschneider M, Gumenyuk V, Grushko J, Lawson K. The maturation of human evoked brain potentials to sounds presented at different stimulus rates. Hear Res. 2008;236:61–79. doi: 10.1016/j.heares.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9:224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurol. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Presynaptic GABA(B) receptors regulate experience-dependent development of inhibitory short-term plasticity. J Neurosci. 2010;30:2716–2727. doi: 10.1523/JNEUROSCI.3903-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Critical period for experience-dependent development of inhibitory synaptic function in auditory cortex. J Neurophysiol. 2011. 2011 Nov 16; [Epub ahead of print] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson NC, Cranford JL, Hoyer E. Brief-tone frequency discrimination by children. J Speech Lang Hear Res. 1999;42:1061–1068. doi: 10.1044/jslhr.4205.1061. [DOI] [PubMed] [Google Scholar]
- Toro JM, Sinnett S, Soto-Faraco S. Speech segmentation by statistical learning depends on attention. Cognition. 2005;97:B25–B34. doi: 10.1016/j.cognition.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci. 2010;13:1050–1052. doi: 10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wassenhove V, Nagarajan SS. Auditory cortical plasticity in learning to discriminate modulation rate. J Neurosci. 2007;27:2663–2672. doi: 10.1523/JNEUROSCI.4844-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EJ, McGee J. Postnatal development of auditory nerve and cochlear nucleus neuronal responses in kittens. Hear Res. 1987;28:97–116. doi: 10.1016/0378-5955(87)90157-2. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, McFadden SL, Liberman MC. Long-term effects of sectioning the olivocochlear bundle in neonatal cats. J Neurosci. 1998;18:3859–3869. doi: 10.1523/JNEUROSCI.18-10-03859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D, Bartlett E. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience. 2008;157:484–494. doi: 10.1016/j.neuroscience.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Werner LA, Boike K. Infants' sensitivity to broadband noise. J Acoust Soc Am. 2001;109:2103–2111. doi: 10.1121/1.1365112. [DOI] [PubMed] [Google Scholar]
- Whitton JP, Polley DB. Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies. J Assoc Res Otolaryngol. 2011;12:535–547. doi: 10.1007/s10162-011-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman FL, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Dev. 1989;60:611–624. [PubMed] [Google Scholar]
- Willott JF, Turner JG. Neural plasticity in the mouse inferior colliculus: relationship to hearing loss, augmented acoustic stimulation, and prepulse inhibition. Hear Res. 2000;147:275–281. doi: 10.1016/s0378-5955(00)00137-4. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG, Sundin VS. Effects of exposure to an augmented acoustic environment on auditory function in mice: roles of hearing loss and age during treatment. Hear Res. 2000;142:79–88. doi: 10.1016/s0378-5955(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Wilmington D, Gray L, Jahrsdoerfer R. Binaural processing after corrected congenital unilateral conductive hearing loss. Hear Res. 1994;74:99–114. doi: 10.1016/0378-5955(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Casseday JH. Processing of modulated sounds in the zebra finch auditory midbrain: responses to noise, frequency sweeps, and sinusoidal amplitude modulations. J Neurophysiol. 2005;94:1143–1157. doi: 10.1152/jn.01064.2004. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci. 2005;8:1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Gill PR, Fremouw T, Theunissen FE. Functional groups in the avian auditory system. J Neurosci. 2009;29:2780–2793. doi: 10.1523/JNEUROSCI.2042-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Hauber ME, Theunissen FE. Developmental experience alters information coding in auditory midbrain and forebrain neurons. Dev Neurobiol. 2010;70:235–252. doi: 10.1002/dneu.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Buonomano DV, Mahncke HW, Merzenich MM. Learning and generalization of auditory temporal-interval discrimination in humans. J Neurosci. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Fitzgerald MB. The time course of attention in a simple auditory detection task. Percept Psychophys. 2004;66:508–516. doi: 10.3758/bf03194897. [DOI] [PubMed] [Google Scholar]
- Wright BA, Zecker SG. Learning problems, delayed development, and puberty. Proc Natl Acad Sci U S A. 2004;101:9942–9946. doi: 10.1073/pnas.0401825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye CQ, Poo MM, Dan Y, Zhang XH. Synaptic mechanisms of direction selectivity in primary auditory cortex. J Neurosci. 2010;30:1861–1868. doi: 10.1523/JNEUROSCI.3088-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Sanes DH, Zou J, Aristizabal O, Wadghiri YZ, Turnbull DH. Large-scale reorganization of the tonotopic map in mouse auditory midbrain revealed by MRI. Proc Natl Acad Sci USA. 2007;104:12193–12198. doi: 10.1073/pnas.0700960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen L, Gao F, Pu Q, Sun X. Noise exposure at young age impairs the auditory object exploration behavior of rats in adulthood. Physiol Behav. 2008;95:229–234. doi: 10.1016/j.physbeh.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Developmentally degraded cortical temporal processing restored by training. Nat Neurosci. 2009;12:26–28. doi: 10.1038/nn.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]