Abstract
Nearly 7% of men are afflicted by male infertility worldwide, and genetic factors are suspected to play a significant role in the majority of these patients. Although sperm morphology is an important parameter measured in the semen analysis, only a few genetic causes of teratozoospermia are currently known. The objective of this study was to define the association between alterations in the genes encoding the Golgi-associated PDZ- and coiled-coil motif containing protein (GOPC), the protein interacting with C kinase 1 (PICK1) and the acrosomal protein zona pellucida binding protein 1 (ZPBP1/sp38) with abnormal sperm head morphology in infertile men. Previous reports demonstrated that mice lacking Gopc, Pick1 and Zpbp1 are infertile due to abnormal head morphology. Herein, using our validated RNA-based method, we studied spermatozoal cDNA encoding the human GOPC, PICK1 and ZPBP1 genes in 381 teratozoospermic and 240 controls patients via direct sequencing. Among these genes, we identified missense and splicing mutations in the sperm cDNA encoding ZPBP1 in 3.9% (15/381) of men with abnormal sperm head morphology. These mutations were not observed in 240 matched controls and the dbSNP database (χ2 = 9.3, P = 0.002). In contrast, statistically significant and functionally relevant mutations were not discovered in the GOPC and PICK1 genes. In our study ZPBP1 mutations are associated with abnormal sperm head morphology, defined according to strict criteria, resembling the mouse Zpbp1 null phenotype. We hypothesize that missense mutations exert a dominant-negative effect due to altered ZPBP1 protein folding and protein:protein interactions in the acrosome.
Keywords: male infertility, teratozoospermia, abnormal head morphology, ZPBP1 cDNA mutations, sp38
Introduction
Male infertility is a multifactorial condition encompassing a variety of disorders (Matzuk and Lamb, 2002, 2008). A plethora of genetic and environmental factors can cause spermatogenic deficiencies leading to a block in spermatogenesis, defects in spermiogenesis and/or defective function of mature spermatozoa. Ultimately, structural and/or functional sperm defects may result in male infertility. In the majority of infertile men, the etiology of impaired spermatogenesis remains unknown (Guzick et al., 2001). Clinical laboratory diagnosis of infertile men employs a routine semen analysis to assess the semen parameters of the ejaculate such as sperm count, motility and morphology (WHO, 2010). Sperm morphology (SM) provides important information regarding the process of spermiogenesis (the differentiation steps in spermatogenesis culminating in the production of the spermatozoa) that may ultimately impact sperm function. In general, while sperm with abnormal morphology frequently function abnormally, the converse is not true; namely, sperm with normal morphology may function abnormally. Furthermore, in the clinical andrology laboratory the molecular cause of an identified sperm defect is almost never defined. Nevertheless, defects in the sperm cell structure or function clearly underlie some types of male infertility (Barratt et al., 2011).
Teratozoospermia (abnormal SM) is characterized by morphological defects in spermatozoa and is defined as an ejaculate with <4% normal spermatozoa (WHO, 2010) as defined according to strict morphologic criteria first described by Kruger et al. (1987, 1988). Assessment of SM has become increasingly strict since the 1950s. Routine classification criteria and evaluation procedures were developed by Eliasson in 1971 (Eliasson and Treichl, 1971) and these criteria have changed with the successive editions of the publication on semen evaluation by the World Health Organization (WHO, 1992, 2010). According to the strict criteria defined by Kruger et al. (1988), a normal spermatozoon has specific dimensions with an oval head configuration featuring a smooth shape and no defects in midpiece and tail. SM defects are generally classified as head, neck, midpiece and/or tail defects. Examples of teratozoospermia include globozoospermia (round-headed sperm lacking an acrosome), macrocephaly (large-headed sperm), two-headed or two-tailed sperm, bent midpiece or the presence of a cytoplasmic droplet (Dam et al., 2007a; WHO, 2010).
Genetic studies of infertile men designed to detect familial segregation of subfertility markers using linkage analyses are hindered by the absence of large affected pedigrees and a high degree of clinical and genetic heterogeneity (Hackstein et al., 2000; Matzuk and Lamb, 2008). Accordingly, much of our understanding of the genes required for male fertility comes from studies of mouse models. Targeted gene disruption strategies in mice have revealed hundreds of genes causing abnormal spermatogenesis and/or fertility defects; however, translation of these findings to define a specific etiologies of infertility is not always successful (Matzuk and Lamb, 2008; Yatsenko et al., 2010). A genetic cause of teratozoospermia is suggested by the discovery of this phenotype in several mouse models (Matzuk and Lamb, 2008). Mutations in the aurora kinase C, protamine 1 and SPATA16 genes are examples of gene mutations associated with teratozoospermia in humans (Iguchi et al., 2006; Dieterich et al., 2007, 2009; Dam et al., 2007b).
Golgi-associated PDZ- and coiled-coil motif containing protein (GOPC) is involved in Golgi vesicle trafficking and is essential for acrosome formation in developing spermatids localizing to the proacrosomal vesicles (Yao et al., 2002; Ito et al., 2004). Mice homozygous for a null mutation in Gopc are infertile as a result of acrosome formation defects and abnormalities with mitochondrial arrangement. Gopc null mouse sperm are characterized by rounded head morphology and occasional tail defects similar to that seen in teratozoospermic men (Yao et al., 2002). Protein interacting with C kinase 1 (PICK1) is associated with the Golgi and membrane proteins and is necessary for normal acrosome formation (Xu and Xia, 2006; Xiao et al., 2009). PICK1 encodes a protein with PDZ and BAR domains and interacts with over 40 proteins (Xu and Xia, 2006). PICK1 is expressed in various tissues, demonstrating the highest protein expression in the brain and testis (Xia et al., 1999). Pick1−/− mice are infertile (Xiao et al., 2009), and their reproductive phenotype is similar to Gopc−/− mice (Yao et al., 2002).
Zona pellucida binding protein 1 (ZPBP1/sp38), which localizes to the acrosomal membrane and likely interacts with multiple acrosomal matrix proteins, was named for its function—binding to the oocyte zona pellucida (ZP) protein following the acrosome reaction. Using an in silico search for germ cell-specific genes in mice, we discovered Zpbp1 and its novel paralog, Zpbp2 (sp17) (Katoh, 2003; Lin et al., 2007). Indeed, the mouse ortholog ZPBP1 (sp38), is required for sperm acrosome formation, compaction and sperm:oocyte binding (Mori et al., 1993, 1995; Lin et al., 2007). Mice homozygous for a null mutation in Zpbp1 have acrosome fragmentation, disrupted Sertoli-spermatid junctions and a defective sperm head morphology with characteristics reminiscent of teratozoospermia in infertile men with a type of head defect (Lin et al., 2007).
Using a non-invasive approach that exploits mRNA from germ cells, and is capable of efficiently detecting splicing and missense mutations in a high-throughput fashion (Yatsenko et al., 2006), we tested the hypothesis that GOPC, PICK1 and ZPBP1 gene defects are associated with abnormal SM. We screened 381 teratozoospermic patients and 240 controls for mutations in the GOPC, PICK1 and ZPBP1 genes. Novel mutations were found in some infertile men in ZPBP1, but not in GOPC and PICK1. These mutations were associated with defects in sperm head morphology in infertile men.
Materials and Methods
Patient recruitment and classification
The study was approved and oversight provided by the Institutional Review Board at Baylor College of Medicine (IRB # H-12083 and H-19753). Male patients in this study were evaluated for infertility by Larry I. Lipshultz, M.D. in the Division of Male Reproductive Medicine and Surgery in the Scott Department of Urology at Baylor College of Medicine. Residual excess semen samples to be discarded were collected for research after the samples were de-identified. The patients were anonymous to the investigators. Semen samples were classified as teratozoospermic based upon the semen analysis performed according to WHO guidelines (WHO, 1999). Using Kruger's strict criteria, abnormal SM is defined as semen in which ≤4.4% of the sperm show normal head, tail and/or midpiece/neck morphology. For this study, this lower limit is the statistically determined value obtained for men with proved fertility in the clinical andrology laboratory, but this lower limit is also consistent with the new fifth edition of the WHO guidelines for semen analysis (WHO, 2010).
When multiple semen analyses were performed, the semen parameters (i.e. count, motility, etc.) represent the single result closest to the mean value. The control population in this study (n = 240) is comprised of infertile men with a normal SM of ≥4.4% (n = 90) and infertile normozoospermic men with a sperm count >60 × 106/ml some of whom were tested for SM (n = 150; Supplementary data, Table S1). These normozoospermic men with randomly tested strict morphology (≥4.4%) served as the morphology controls (Supplementary data, Table S1). In addition, we analyzed results in dbSNP database from the 1000 genomes project (1000 Genomes Project Consortium, 2010). Procedures for semen analysis (e.g. cell count, motility and strict morphological analysis of spermatozoa) are described in the WHO guidelines (WHO, 1999). All patients underwent a complete history and physical, including evaluation of possible endocrine causes of male infertility. Teratozoospermic men were not tested for chromosomal aberrations and Y chromosome aberrations in accordance with the AUA/ASRM guidelines for the evaluation of the infertile male (AUA, 2010). Men with known causes of infertility, such as endocrine defects, cryptorchidism, chromosome abnormalities and Y chromosome deletions, were excluded from the study. Because patients' samples were de-identified, information on IVF/ICSI outcome could not be obtained.
RNA and DNA extraction, RT–PCR, sequencing and sequence analysis
Semen RNA and DNA extraction was performed as previously described (Yatsenko et al., 2006). Briefly, freshly ejaculated semen was liquefied for 30 min at 37°C. After all clinical testing was completed and the samples de-identified, the semen was thoroughly mixed with equal volume of Sperm Washing Medium (Irvine Scientific, Santa Ana, CA, USA), and the suspension was centrifuged for 10 min at 652g at room temperature. The sperm were resuspended and lysed with 1 ml of TriZOL reagent (Invitrogen, Carlsbad, CA, USA). Total RNA and DNA were extracted and resuspended in DEPC-treated water and sterile 10 mM Tris 1 mM EDTA solution, pH 7.0 (Ambion, Austin, TX, USA), respectively.
For first strand cDNA synthesis, we used M-MLV Reverse Transcriptase (Invitrogen) with random primers and total spermatozoal RNA (Yatsenko et al., 2006). PCR for GOPC and PICK1 were performed with KAPA HiFi HotStart DNA Polymerase and 40 ng of cDNA according to the manufacturer's protocol (Kapa Biosystems, Woburn, MA, USA). PCR for ZPBP1 was performed with JumpStart RedTaq DNA polymerase and 40 ng of cDNA or DNA according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO, USA), we used 37 cycles and Ta 60°C. Three PCR primers were designed for amplification of three cDNA fragments of GOPC (NM_020399.3) and three cDNA fragments of PICK1 (NM_012407.3) each, and two PCR primers were designed for amplification of two cDNA fragments of ZPBP1 (NM_001159878; Supplementary data, Table S2). Primer sequences for amplifying ZPBP1 exons and PCR conditions are shown in Supplementary data, Table S3. The PCR products were sequenced with BigDye V3.1 sequencing reagent and run on an ABI Prism Sequencer 3130XL (Applied Biosystems, Foster City, CA, USA). Sequence analyses were performed with Sequencher 4.2 (Gene Codes, Ann Arbor, MI, USA). Multiple protein alignments were made via ClustalW (http://www.ebi.ac.uk/clustalw). Novel single nucleotide polymorphisms (SNPs) were crosschecked against dbSNP database (build 37.1).
To test for an association between teratozoospermia in patients and the presence of SNPs in ZPBP1, the frequencies of the alleles were compared between the group with abnormal SM and the control group (normozoospermia and normal SM) separately and combined, using a likelihood ratio χ2 test. All tests were two-sided with an alpha level of 0.05 considered to indicate statistical significance, and unadjusted P values were reported. All statistical analyses were performed using JMP Start Statistics software (SAS Institute Inc., Cary, NC, USA).
Results
To test the hypothesis that GOPC, PICK1 and ZPBP1 gene defects are associated with abnormal SM, we screened for GOPC, PICK1 and ZPBP1 gene mutations in 381 teratozoospermic patients (≤4.4% of sperm with normal morphology) and 240 controls. We used our non-invasive and cost-effective approach that exploits mRNA isolated from ejaculated spermatozoa. This method allows detection of both splicing and missense mutations in a high-throughput fashion (Yatsenko et al., 2006).
We amplified the GOPC and PICK1 open reading frames and flanking regions, covering 1389 and 1248 bp, respectively, using three overlapping PCR fragments. Sequence analysis identified one novel GOPC heterozygous alteration (p.L123V) and one novel PICK1 heterozygous alteration (p.Q26E) in 381 patients (Table I). Statistical estimation for the frequencies of each novel SNP [0.3% (1/381)] evaluated, indicated a lack of statistical significance for the identified changes. Further computational analysis indicated that the amino acid alterations resulting in these changes will have no effect on the structure and function of the GOPC and PICK1 proteins. We also identified two frequent non-coding SNPs: in GOPC, a cDNA change c.695G>A (p.K147K, rs41302045, 3/381, 0.78%) and in PICK1, a cDNA change c.1435C>T (p.Y349Y, novel SNP, 3/381, 0.78%) (Table I). The frequency of these non-synonymous alterations was not tested in a control population.
Table I.
GOPC and PICK1 SNPs identified by analysis of 381 teratozoospermic patients.
| Patient | Vol. (ml) | Count ×106/ml | Mot % | FP | SM % normal | Head % defects | Neck % defects | Tail % defects | cDNA change | Protein | Zygocity | dbSNP Acc. # |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GOPC | ||||||||||||
| 1 | 2.5 | 4.5 | 40 | 2.5 | 1 | 98 | 1 | 0 | 621C>G | L123V | hetero | novel |
| 2 | 1 | 34 | 55 | 2.5 | 3 | 90 | 6 | 1 | 695G>A | K147K | hetero | rs41302045 |
| 3 | 4.6 | 11 | 60 | 2.5 | 0 | 100 | 0 | 0 | 695G>A | K147K | hetero | rs41302045 |
| 4 | 3.5 | 6.5 | 50 | 2.5 | 0.5 | 89.5 | 10 | 0 | 695G>A | K147K | hetero | rs41302045 |
| PICK1 | ||||||||||||
| 5 | 2.5 | 47.5 | 75 | 3 | 1 | 95.5 | 2.5 | 1 | 466C>G | Q26E | hetero | novel |
| 6 | 4 | 50 | 45 | 2.5 | 0 | 100 | 0 | 0 | 573C>T | D61D | hetero | rs117707976 |
| 7 | 3 | 55 | 75 | 3 | 0 | 88 | 12 | 0 | 789C>T | T133T | hetero | novel |
| 8 | 5.5 | 14 | 70 | 2.5 | 3 | 86 | 9.5 | 1.5 | 1437C>T | Y349Y | hetero | novel |
| 9 | 2 | 25.5 | 60 | 2.5 | 3 | 91 | 6 | 0 | 1437C>T | Y349Y | hetero | novel |
| 10 | 2 | 21 | 60 | 2.5 | 1 | 92 | 7 | 0 | 1437C>T | Y349Y | hetero | novel |
Count, sperm count; Mot %, percent motile; FP, forward progression; SM, strict morphology (percent of spermatozoa with normal morphology defined by Kruger's strict criteria); hetero, heterozygous; homo, homozygous.
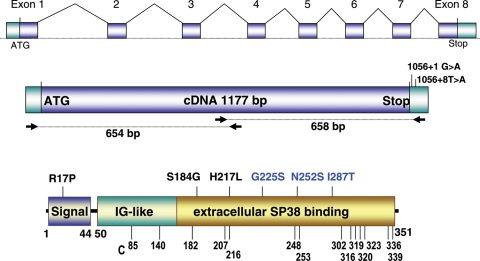
We efficiently amplified the ZPBP1 1177 bp open reading frame (NM_001159878.1) via two overlapping cDNA fragments (Fig. 1). Sequence analysis identified 16 ZPBP1 alterations in 3.9% (15/381) of the patients (Table II, Fig. 1). Analysis of the gene in the dbSNP database did not identify these ZPBP1 changes as polymorphic, and these alterations were not present in the 240 control men, providing strong statistical evidence for these mutations to be deleterious (χ2 = 9.3, P = 0.002). These teratozoospermic patients are characterized by abnormal head morphology (for these men, ≥90% sperm analyzed were abnormal) (Table II), resembling the abnormal head morphology phenotype of Zpbp1 mouse knockout model. Six of the 15 patients with novel mutations were oligoteratozoospermic.
Figure 1.
ZPBP1 gene and cDNA and point mutations identified in teratozoospermic patients. ZPBP1 gene, cDNA amplification scheme and mutations in the 3′UTR are shown in the upper two diagrams. The position of the missense mutations in the ZPBP1 protein is shown in the lower diagram. The predicted protein structure is based on Interproscan, Prosite, Pfam and Blocks algorithms. ZPBP1 has a signal peptide (aa 1–44) and an extracellular region consisting of two predicted overlapping domains, an immunoglobulin-like (IG; aa 50–156) domain and an sp38 domain (aa 86–351). Homozygous mutations are depicted in blue, and heterozygous mutations are depicted in black. The positions of the cysteines (C) are shown at the bottom.
Table II.
ZPBP1 mutations identified by analysis 381 teratozoospermic men.
| Patient | Vol. (ml) | Count ×106/ml | Mot % | FP | SM % normal | Head % defects | Neck % defects | Tail % defects | cDNA change | Protein | Zygocity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.5 | 225 | 45 | 3 | 2 | 64 | 7 | 27 | c.50G>C | R17P | hetero |
| 2 | 4 | 55 | 35 | 2.5 | 3 | 95 | 2 | 0 | c.50G>C | R17P | hetero |
| 3 | 4 | 44 | 75 | 3 | 4 | 90 | 4 | 2 | c.550A>G | S184G | hetero |
| 4 | 1 | 28 | 55 | 3 | 1 | 96 | 2 | 1 | c.650 A>T | H217L | hetero |
| 5 | 4 | 13 | 35 | 2 | 1 | 90 | 3 | 6 | c.673G>A | G225S | homo |
| 5 | 4 | 13 | 35 | 2 | 1 | 90 | 3 | 6 | c.860T>C | I287T | homo |
| 6 | 3.5 | 5.5 | 35 | 2.5 | 0 | 98 | 1 | 1 | c.755A>G | N252S | homo |
| 7 | 3 | 52.5 | 75 | 3 | 1.5 | 92.5 | 6 | 0 | c.1056+1G>A | 3′UTR | hetero |
| 8 | 5.5 | 31 | 70 | 2.5 | 2 | 94.5 | 2.5 | 1 | c.1056+8T>A | 3′UTR | hetero |
| 9 | 3.5 | 43 | 60 | 3 | 4 | 89 | 3 | 4 | spl ex5–7 del | del158 aa | ∼50% |
| 10 | 5 | 19 | 55 | 2.5 | 1 | 95.5 | 3 | 0.5 | spl ex6–7 del | del 85 aa | ∼90% |
| 11 | 3 | 13 | 65 | 2.5 | 2 | 98 | 0 | 0 | spl. ex7 del | FS | ∼90% |
| 12 | 2 | 7 | 55 | 2.5 | 3.5 | 95.5 | 1 | 0 | spl. ex7 del | FS | ∼50% |
| 13 | 7 | 14 | 40 | 2.5 | 3.5 | 83.5 | 12 | 1 | spl. ex7 del | FS | ∼50% |
| 14 | 1 | 15 | 15 | 2 | 0.5 | 97 | 1 | 1.5 | spl.ex7a, ins144 (Alu) | ins 48aa | ∼50% |
| 15 | 2 | 27.5 | 60 | 2.5 | 0 | 93 | 7 | 0 | spl.ex7a, ins144 (Alu) | ins 48aa | ∼50% |
Mutations shown in bold were analyzed and confirmed by analysis of genomic DNA from sperm. Semen analysis (WHO, 1999) results for teratozoospermic patients with identified ZPBP1 genetic defects are shown. This analysis includes sperm count, motility and strict morphology with three subcategories (percentile of head, neck and tail defects). Count, sperm count; FP, forward progression; SM, strict morphology (percent of spermatozoa with normal morphology defined by Kruger's strict criteria); nt, nucleotides; hetero, heterozygous; homo, homozygous.
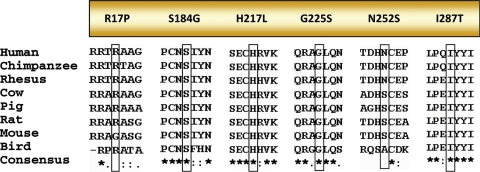
Missense mutations were present in six patients. Seven patients had gene defects predicted to cause splicing defects in the ZPBP1 mRNA, and two infertile men had mutations in the 3′UTR (Table II and Figs 1 and 2). The missense changes were distributed throughout the ZPBP1 coding region. Two patients showed a predicted p.R17P change in the ZPBP1 signal peptide; however, prediction programs suggest that the ZPBP1 signal peptide would continue to be cleaved after amino acid 44. In addition, the arginine at position 17 is not 100% conserved between several species (Fig. 3). Likewise, at position 252, an asparagine is present in primates, whereas a serine is present in other mammals and birds suggesting that the p.N252S change does not impart a major effect on the protein structure despite its ‘homozygous’ presence in patient #5.
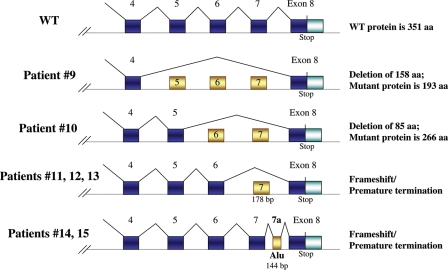
Figure 2.
Schematic diagrams for ZPBP1 splicing mutations identified in teratozoospermic patients. The upper diagram depicts the normal splicing pattern; the middle schematics show abnormal splicing defects of ZPBP1 exons 5–7 (patient 9), exons 6–7 (patient 10) and exon 7 (patients 11, 12, and 13) resulting in in-frame deletions or frameshifts as shown. The bottom schematic shows inclusion of exon 7A that includes partial retention of an Alu repeat.
Figure 3.
Evolutionary conservation of amino acids affected by missense mutations. Alignments of species' ZPBP1s reveal evolutionary conservation of amino acids affected by missense mutations among mammalian and bird orthologs. The mutant alleles are boxed.
The other four missense mutations identified (p.S184G, p.H217L, p.G225S and p.I287T) alter amino acids that are 100% conserved in mammals and birds. In the mature (processed) portion of the ZPBP1 sequence, there are 15 cysteines (Fig. 1) and 3 putative N-linked glycosylation sites (N-X-S/T) at asparagine located at positions 114, 187 and 340. Using two independent prediction algorithms, SIFT 2.0 and PolyPhen, the missense amino acid alterations in the mature region are predicted to cause significant effects on the ZPBP1 protein structure by at least one algorithm.
The p.G225S and p.I287T mutations (present as a double mutation in one patient) are observed as ‘homozygous’ mutations indicating that these are true homozygous defects in the genome or that they are present in one ZPBP1 allele and the second ZPBP1 allele is not expressed or absent. In contrast, the p.H217L and p.S184G mutations are heterozygous mutations, each in one patient. These mutations are present in the cysteine-rich C-terminus of the ZPBP1 protein with the p.H217L mutation adjacent to the fifth cysteine in the ZPBP1 sequence (position 216) and the p.S184G change in close proximity to cysteine 182; these mutations are predicted to disrupt intramolecular disulfide bond formation of the ZPBP1 protein, an effect that is expected to disrupt protein function (Fig. 1). These patients exhibit high levels of sperm head defects with minimal or no neck and tail defects. Accordingly, these missense mutations may act in a dominant-negative manner and/or as null mutations in the four men who express these alleles.
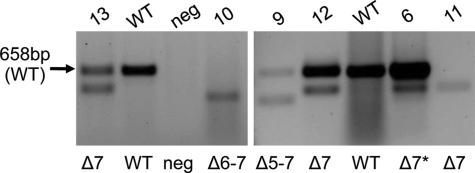
We also identified seven teratozoospermic patients with splicing defects (≥50% abnormal product relative to the normal RT–PCR product) that are predicted to lead to large disruptions of the protein (Table II, Fig. 4). Skipping ZPBP1 exons 5, 6 and 7 is predicted to result in in-frame deletions of 158 amino acids (17 kDa), skipping of exons 6 and 7 is predicted to cause an 85 amino acid deletion (9 kDa) and omission of exon 7 is predicted to cause a frameshift with truncation of the protein (Fig. 2). An insertion of abnormal exon 7a, containing 144 bp segment of an Alu repeat, is predicted to introduce a premature stop codon (Fig. 2). Since these splicing defects delete regions of the cysteine-rich region of ZPBP1, we anticipate that these mutations would not permit ZPBP1 disulfide bond formation and protein folding and would impair ZPBP1 function. With the exception of patient 13, the other 7 patients with splicing defects demonstrate ≥89% sperm head defects and ≤8% neck and tail defects (Table II).
Figure 4.
Detection of splicing defects in the ZPBP1 gene in teratozoospermic patients. Gel electrophoresis of RT–PCR products are shown. WT denotes wild-type sample and neg denotes negative control. Δ5–7, Δ6–7 and Δ7 denote splicing defects in the patients as shown that result in omission of exons 5–7, 6–7 and 7, respectively. * denotes patient with a mild abnormal splicing product.
Two teratozoospermic patients have single heterozygous nucleotide alterations in the ZPBP1 3′UTR of unknown significance (Fig. 1).
Lastly, we identified several rare ZPBP1 SNPs in patients and controls (Table III). We also found a low-abundance splicing alterations in some patients and controls at comparable frequencies [i.e. low expression of the exon 7 deletion (25–30% of the wild-type RT–PCR product) was detected in ∼14% of teratozoospermic patients and ∼16% of controls (Table III)].
Table III.
Rare ZPBP1 SNPs and polymorphic splicing variants identified in teratozoospermic patients and controls.
| # | DNA, nt | Protein, aa | Zygosity | Patients freq | Controls freq | dbSNP |
|---|---|---|---|---|---|---|
| 1 | 74G>C | R25P | hetero | 1/381 (0.3%) | 0 | rs61696422 |
| 2 | 312G>A | G104G | hetero | 1/381 (0.3%) | 0 | |
| 3 | 375T>C | L125L | hetero | 1/381 (0.3%) | 1/240 (0.4%) | rs76027725 |
| 4 | 498G>A | E166E | hetero | 1/381 (0.3%) | 1/240 (0.4%) | |
| 5 | 645T>C | L215L | homo | 1/381 (0.3%) | 0 | |
| 6 | 732A>G | G244G | homo | 2/381 (0.5%) | 0 | |
| 7 | 735C>T | P245P | hetero | 0 | 1/240 (0.4%) | |
| 8 | 1047C>A | T349K | hetero | 0 | 1/240 (0.4%) | |
| 9 | ex7 del | Spl | low level | 52/381 (14%) | 39/240 (16%) | |
| 10 | ex6–7 del | Spl | low level | 8/381 (2%) | 6/240 (2.5%) | |
| 11 | ex5–7 del | Spl | low level | 4/381 (1%) | 2/240 (0.8%) |
Discussion
Male infertility affects ∼4 million men in the USA (Abma et al., 1997; Anderson et al., 2009). Today, investigators suspect that perhaps 50% or more of these infertile men have a genetic cause for their infertility (Matzuk and Lamb, 2008). While some infertile men have cytogenetic defects (Retief et al., 1984; Bourrouillou et al., 1985), a majority of these genetic factors that underlie male infertility remain undiagnosed. Another issue to consider is that while infertile men can be normozoospermic, closer laboratory inspection can reveal the presence of subtle sperm defects (i.e. ultrastructural morphology defects causing immotile cilia, globozoospermia causing failure of the acrosome reactions, etc.). These defects and other as yet unidentified genetic defects are treated with ICSI together with in vitro fertilization. ICSI is associated with an increase in congenital birth defects, perinatal complications, growth delay, general health problems and disturbed normal genomic imprinting (Hansen et al., 2002; Olson et al., 2005; Knoester et al., 2008; Wen et al., 2010; Yan et al., 2011). This fact, along with the limitations of current methods for diagnosis of male infertility, suggests that affected males may be transmitting unrecognized male infertility-causing mutations to their offspring. Accordingly, the development of improved diagnostic tools is paramount for couples seeking treatment for their infertility (WHO, 1987; Lipshultz and Lamb, 2007).
Here, we tested the hypothesis that mutations in GOPC, PICK1 and ZPBP1 are associated with abnormal SM and specifically head defects. We did not identify any homozygous mutations in PICK1 or GOPC in 381 teratozoospermic patients. A previous study of three globozoospermic infertile men, identified a single homozygous mutation in one patient in PICK1 (p.G393R) and no mutations in GOPC. Both parents were heterozygous for the PICK1 mutation and were fertile (Liu et al., 2010). This previous study and our current study indicate that mutations in PICK1 and GOPC are rare in the infertile population. Alternatively, PICK1 and GOPC may be critical for human spermatozoon maturation; aberrations in these genes may not be tolerated. Our studies and those of others suggest that high-throughput technologies (e.g. next generation sequencing) should be applied for such heterogeneous conditions such as male infertility.
Previously, we showed that disruption of Zpbp1 led to male sterility secondary to abnormal sperm head morphology and no progressive motility (Lin et al., 2007). Ultrastructural studies demonstrated that absence of ZPBP1 prevents proper acrosome formation, resulting in acrosome fragmentation as well as disruption of the Sertoli-spermatid junctions (Lin et al., 2007). Recent studies suggest that ZPBP1 and other yet unknown acrosomal proteins play important roles in the intricate fertilization process including the acrosome reaction, ZP recognition, ZP binding and ZP penetration (Tardif et al., 2010; Jin et al., 2011). In this study, we identified homozygous and heterozygous missense mRNA alterations and high-level splicing mutations of ZPBP1 mutations in 15 out of 381 (3.9%) teratozoospermic men tested (P ≤ 0.002). ZPBP1 alterations in teratozoospermic patients may contribute to the dysmorphic shape of the sperm head. Although the null ZPBP1 mouse model demonstrates head morphology and forward progression defects, we observed that teratozoospermic patients have abnormal sperm head morphology, implying a different effect of presumably ‘hypomorphic’ mutations on protein function.
We noted that a patient with the splicing defect causing the absence of exon 7 (#13) has milder morphology defects (84% sperm head defects). This observation could be explained by a residual (∼50%) level of normal functional ZPBP1 that partially compensates for the abnormal protein. In contrast to the teratozoospermic patients, ZPBP1 point mutations were not present in control men or the dbSNP database; we identified several rare ZPBP1 SNPs and polymorphic weakly expressed splicing mutations in controls and believe that they have mild or negligent/neutral effect on ZPBP protein function and SM phenotype. In addition, weakly expressed splicing mutations in 14–16% of patients and controls suggest that abnormal morphology is a quantitative trait.
The low frequency of ZPBP1 mutations in the teratozoospermic population (∼4%) is not unexpected given the predicted complexity and heterogeneous nature of this defect. Importantly, ∼90% of germ cells with mutations in the human ZPBP1 gene have a dysmorphic appearance of the sperm head. The abnormal head morphology in these cases may be due to absence of functional ZPBP1 proteins or due to dominant-negative effects of the mutant ZPBP1 proteins with other acrosomal proteins that are required for formation of the sperm head. The mutations identified may also have a dominant-negative effect. The mechanism by which disruption of ZPBP1 leads to teratozoospermia in men is under further investigation. Importantly, other proteins involved in spermiogenesis may be responsible for synergistic effects as well.
In summary, analysis of full-length mRNAs isolated from transcriptionally inert human spermatozoa revealed multiple, novel germline splicing and missense mutations in the ZPBP1 gene but no novel mutations in PICK1 or GOPC. The ZPBP1 variants were associated with sperm head morphology defects in 3.9% of teratozoospermic men. These findings are reminiscent of the phenotype observed in our Zpbp1 knockout mouse model, which displays male infertility due to abnormal sperm head morphology (Lin et al., 2007). Therefore, we hypothesize that ZPBP1 is important for spermiogenesis and sperm:egg binding in mammals. Both mouse and human studies suggest that mutations in ZPBP1 are associated with teratozoospermia, providing a previously unrecognized underlying etiology for a subgroup of infertile men with sperm head morphology defects. Genetic diagnosis of men with defective human spermiogenesis might provide new insights into the cause of abnormal sperm head morphology in men with teratozoospermia.
Accession numbers
GenBank accession number for the human GOPC gene is NM_020399.3, for the PICK1 gene is NM_012407.3, and for the ZPBP1 gene is NM_001159878.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
A.N.Y., D.S.O., A.R., D.J.L. and M.M.M. designed the study and prepared the manuscript, to which other authors added their comments. Laboratory work was undertaken by A.N.Y., D.S.O., A.R., P.A.A., R.C. and L.J.M.; R.C. and L.J.M. collected and processed samples.
Funding
These studies were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD36289 to D.J.L. and MMM), a Mentored Clinical Scientist Development Award from NICHD (K08HD058073 to A.N.Y.), and the National Institute of General Medical Sciences (T32 GM088129-01 to D.S.O.).
Supplementary Material
Acknowledgements
We thank Dr Larry I. Lipshultz for clinical evaluation of the patients.
References
- 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. doi:10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women's health: new data from the 1995 National Survey of Family Growth. Vital Health Stat. 1997;23:1–114. [PubMed] [Google Scholar]
- Anderson JE, Farr SL, Jamieson DJ, Warner L, Macaluso M. Infertility services reported by men in the United States: national survey data. Fertil Steril. 2009;91:2466–2470. doi: 10.1016/j.fertnstert.2008.03.022. doi:10.1016/j.fertnstert.2008.03.022. [DOI] [PubMed] [Google Scholar]
- AUA. The Evaluation of the Azoospermic Male: Best Practice Statement. Revised. Male Infertility. AUA. 2010:4–25. http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm . [Google Scholar]
- Barratt CL, Mansell S, Beaton C, Tardif S, Oxenham SK. Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl. 2011;13:53–58. doi: 10.1038/aja.2010.63. doi:10.1038/aja.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourrouillou G, Dastugue N, Colombies P. Chromosome studies in 952 infertile males with a sperm count below 10 million/ml. Hum Genet. 1985;71:366–367. doi: 10.1007/BF00388466. doi:10.1007/BF00388466. [DOI] [PubMed] [Google Scholar]
- Dam AH, Feenstra I, Westphal JR, Ramos L, van Golde RJ, Kremer JA. Globozoospermia revisited. Hum Reprod Update. 2007a;13:63–75. doi: 10.1093/humupd/dml047. doi:10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- Dam AH, Koscinski I, Kremer JA, Moutou C, Jaeger AS, Oudakker AR, Tournaye H, Charlet N, Lagier-Tourenne C, van Bokhoven H, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007b;81:813–820. doi: 10.1086/521314. doi:10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich K, Soto Rifo R, Faure AK, Hennebicq S, Ben Amar B, Zahi M, Perrin J, Martinez D, Sele B, Jouk PS, et al. Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nature genetics. 2007;39:661–665. doi: 10.1038/ng2027. doi:10.1038/ng2027. [DOI] [PubMed] [Google Scholar]
- Dieterich K, Zouari R, Harbuz R, Vialard F, Martinez D, Bellayou H, Prisant N, Zoghmar A, Guichaoua MR, Koscinski I, et al. The Aurora Kinase C c.144delC mutation causes meiosis I arrest in men and is frequent in the North African population. Hum Mol Genet. 2009;18:1301–1309. doi: 10.1093/hmg/ddp029. doi:10.1093/hmg/ddp029. [DOI] [PubMed] [Google Scholar]
- Eliasson R, Treichl L. Supravital staining of human spermatozoa. Fertil Steril. 1971;22:134–137. doi: 10.1016/s0015-0282(16)38049-9. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. doi:10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- Hackstein JH, Hochstenbach R, Pearson PL. Towards an understanding of the genetics of human male infertility: lessons from flies. Trends Genet. 2000;16:565–572. doi: 10.1016/s0168-9525(00)02140-5. doi:10.1016/S0168-9525(00)02140-5. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. doi:10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- Iguchi N, Yang S, Lamb DJ, Hecht NB. An SNP in protamine 1: a possible genetic cause of male infertility. J Med Genet. 2006;43:382–384. doi: 10.1136/jmg.2005.037168. doi:10.1136/jmg.2005.037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito C, Suzuki-Toyota F, Maekawa M, Toyama Y, Yao R, Noda T, Toshimori K. Failure to assemble the peri-nuclear structures in GOPC deficient spermatids as found in round-headed spermatozoa. Arch Histol Cytol. 2004;67:349–360. doi: 10.1679/aohc.67.349. doi:10.1679/aohc.67.349. [DOI] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. doi:10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Identification and characterization of human ZPBP-like gene in silico. Int J Mol Med. 2003;12:399–404. [PubMed] [Google Scholar]
- Knoester M, Helmerhorst FM, Vandenbroucke JP, van der Westerlaken LA, Walther FJ, Veen S. Perinatal outcome, health, growth, and medical care utilization of 5- to 8-year-old intracytoplasmic sperm injection singletons. Fertil Steril. 2008;89:1133–1146. doi: 10.1016/j.fertnstert.2007.04.049. doi:10.1016/j.fertnstert.2007.04.049. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Veeck LL, Morshedi M, Brugo S. New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology. 1987;30:248–251. doi: 10.1016/0090-4295(87)90246-9. doi:10.1016/0090-4295(87)90246-9. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- Lin YN, Roy A, Yan W, Burns KH, Matzuk MM. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol. 2007;27:6794–6805. doi: 10.1128/MCB.01029-07. doi:10.1128/MCB.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz LI, Lamb DJ. Risk of transmission of genetic diseases by assisted reproduction. Nat Clin Pract Urol. 2007;4:460–461. doi: 10.1038/ncpuro0879. doi:10.1038/ncpuro0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Shi QW, Lu GX. A newly discovered mutation in PICK1 in a human with globozoospermia. Asian J Androl. 2010;12:556–560. doi: 10.1038/aja.2010.47. doi:10.1038/aja.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–s49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. doi:10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori E, Baba T, Iwamatsu A, Mori T. Purification and characterization of a 38-kDa protein, sp38, with zona pellucida-binding property from porcine epididymal sperm. Biochem Biophys Res Commun. 1993;196:196–202. doi: 10.1006/bbrc.1993.2234. doi:10.1006/bbrc.1993.2234. [DOI] [PubMed] [Google Scholar]
- Mori E, Kashiwabara S, Baba T, Inagaki Y, Mori T. Amino acid sequences of porcine Sp38 and proacrosin required for binding to the zona pellucida. Dev Biol. 1995;168:575–583. doi: 10.1006/dbio.1995.1103. doi:10.1006/dbio.1995.1103. [DOI] [PubMed] [Google Scholar]
- Olson CK, Keppler-Noreuil KM, Romitti PA, Budelier WT, Ryan G, Sparks AE, Van Voorhis BJ. In vitro fertilization is associated with an increase in major birth defects. Fertil Steril. 2005;84:1308–1315. doi: 10.1016/j.fertnstert.2005.03.086. doi:10.1016/j.fertnstert.2005.03.086. [DOI] [PubMed] [Google Scholar]
- Retief AE, Van Zyl JA, Menkveld R, Fox MF, Kotze GM, Brusnicky J. Chromosome studies in 496 infertile males with a sperm count below 10 million/ml. Hum Genet. 1984;66:162–164. doi: 10.1007/BF00286592. doi:10.1007/BF00286592. [DOI] [PubMed] [Google Scholar]
- Tardif S, Wilson MD, Wagner R, Hunt P, Gertsenstein M, Nagy A, Lobe C, Koop BF, Hardy DM. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J Biol Chem. 2010;285:24863–24870. doi: 10.1074/jbc.M110.123125. doi:10.1074/jbc.M110.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen SW, Leader A, White RR, Leveille MC, Wilkie V, Zhou J, Walker MC. A comprehensive assessment of outcomes in pregnancies conceived by in vitro fertilization/intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2010;150:160–165. doi: 10.1016/j.ejogrb.2010.02.028. doi:10.1016/j.ejogrb.2010.02.028. [DOI] [PubMed] [Google Scholar]
- WHO. Towards more objectivity in diagnosis and management of male infertility. Int J Androl. 1987;7(suppl):1–53. [Google Scholar]
- WHO. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Special Programme of Research Development and Research Training in Human Reproduction. 3rd edn. Cambridge, England: Published on behalf of the World Health Organization by Cambridge University Press; 1992. New York, NY, USA. [Google Scholar]
- WHO. World Health Organization Laboratory Manual for Human Semen and Sperm Cervical-Mucus Interaction. 4th edn. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- WHO. WHO Laboratory Manual for Examination and Processing of Human Semen. 5th edn. Geneva, Switzerland: WHO Press; 2010. pp. 56–102. [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. doi:10.1016/S0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Xiao N, Kam C, Shen C, Jin W, Wang J, Lee KM, Jiang L, Xia J. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest. 2009;119:802–812. doi: 10.1172/JCI36230. doi:10.1172/JCI36230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xia J. Structure and function of PICK1. Neurosignals. 2006;15:190–201. doi: 10.1159/000098482. doi:10.1159/000098482. [DOI] [PubMed] [Google Scholar]
- Yan J, Huang G, Sun Y, Zhao X, Chen S, Zou S, Hao C, Quan S, Chen ZJ. Birth defects after assisted reproductive technologies in China: analysis of 15,405 offspring in seven centers (2004 to 2008) Fertil Steril. 2011;95:458–460. doi: 10.1016/j.fertnstert.2010.08.024. doi:10.1016/j.fertnstert.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Yao R, Ito C, Natsume Y, Sugitani Y, Yamanaka H, Kuretake S, Yanagida K, Sato A, Toshimori K, Noda T. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci USA. 2002;99:11211–11216. doi: 10.1073/pnas.162027899. doi:10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsenko AN, Roy A, Chen R, Ma L, Murthy LJ, Yan W, Lamb DJ, Matzuk MM. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15:3411–3419. doi: 10.1093/hmg/ddl417. doi:10.1093/hmg/ddl417. [DOI] [PubMed] [Google Scholar]
- Yatsenko AN, Iwamori N, Iwamori T, Matzuk MM. The power of mouse genetics to study spermatogenesis. J Androl. 2010;31:34–44. doi: 10.2164/jandrol.109.008227. doi:10.2164/jandrol.109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.