Abstract
Aim
Bangladesh has the highest level of incidence and mortality rates due to cervical cancer among women. The prevalence of cervical cancer in Bangladeshi women is 25–30/100 000. Human papillomavirus is an important cause of cervical cancer. The study was conducted to assess the immunogenicity and safety profile of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccines in healthy Bangladeshi girls aged 9–13 years.
Procedure
This was a randomized (3:1) controlled trial with two parallel groups, the vaccine and control groups, that included 67 participants in Bangladesh. Subjects were given GlaxoSmithKline human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine (and controls no vaccine) at the first day of vaccination (Day 0), at 1- and 6-month schedule and followed up until 7 months. Blood samples were taken for human papillomavirus antibody at enrollment and 1 month post-schedule at Month 7 from both subjects and controls. Safety data were gathered throughout the study period.
Results
Fifty subjects received vaccine at Day 0, 1 month and 6 months. All subjects were initially sero-negative in the vaccine group, and developed sero-conversion for human papillomavirus-16 and -18 antibodies except for one at Month 7. Seventeen controls did not receive vaccine. Clients were followed up for serious medically important events and blood samples were taken for human papillomavirus antibody detection at Day 0 and Month 7. Sero-conversion was found in 97.5% of subjects and no sero-conversion was found in the controls. Bivalent human papillomavirus vaccine was generally well tolerated, with no vaccine-related serious adverse experiences.
Conclusions
The human papillomavirus-16/18 AS04-adjuvanted vaccine was generally well tolerated and highly immunogenic when administered to young adolescent females and could be a promising tool for the prevention and control of cervical cancer in Bangladesh.
Keywords: cervical cancer prevention, AS04-adjuvanted vaccine, HPV-16/18, immunogenicity, safety, randomized trial
INTRODUCTION
Almost half a million women develop cervical cancer and >288 000 women die each year worldwide, the disease disproportionately affecting the poorest, most vulnerable women. At least 80% of cervical cancer deaths occur in developing countries, with most occurring in the poorest regions—South Asia, Sub-Saharan Africa and parts of Latin America, where cervical cancer accounts for 15% of all cancer deaths but which have only 5% of the world's cancer resources (1).
Health-care providers in developing countries regularly see women with advanced, incurable cervical cancer, and around 28.9% incidence and 17.9% mortality of all cancer cases have been reported in Bangladesh (2). An Indian study showed that nearly 70% of cervical cancer patients present at Stages III and IV of the disease. Around 20% of women who develop cervical cancer die within the first year of diagnosis and the 5-year relative survival rate is 50% (3). At this late stage, there is little to do to save women's lives. Even drugs designed to ease cancer pain often are unavailable. Yet cervical cancer can be readily prevented, even in women at high risk for the disease, through screening and treatment using relatively simple technologies. When pre-cancerous changes in cervical tissue are found and the abnormal tissue is successfully treated, a woman will not develop cancer.
Human papillomavirus (HPV) infection is one of the most common sexually transmitted diseases worldwide. Up to 70% of sexually active women worldwide will become infected with HPV during their lifetime (4). HPV, particularly the two strains HPV-16 and -18, accounts for most cervical cancer cases. Genital HPV is usually transmitted via the vaginal and anal route. Infection is common within a few years of onset of intercourse. The prevalence of HPV infection among women in Bangladesh has been reported to be 4.1% (5). Importantly, the rate of HPV infection in women younger than 25 years was roughly double that in women older than 25 years of age in the same city in a recent study (33%) (6). Moreover, HPV prevalence has been reported to be 38% in a cohort of sexually active female college students in Busan (7).The sexual transmission of HPV is an important factor to consider for vaccination strategies, including the optimal age of vaccination. Persistent HPV infection is associated with the development of cervical and other ano-genital cancers (8–10). In a study regarding HPV genotype distribution in invasive cervical carcinoma, it was found that HPV-16 and -18 were the major type in all continents (11). For example, >50% of college-age women acquired an HPV infection within 4 years of first intercourse (12).
There is a huge discrepancy in incidence between developed and developing countries largely due to the availability of screening and treatment facilities in industrialized countries. Although widespread and organized screening programs using Papanicolaou (Pap) testing may reduce the mortality associated with cervical cancer worldwide, screening does not prevent HPV infection or the development of pre-cancerous lesions that may require treatment such as cervical intraepithelial neoplasia cases or carcinoma in situ. Therefore, a mechanism for primary prevention of HPV infection is desirable.
A vaccine to prevent oncogenic HPV infection, or premalignant cervical lesions from progressing to cancer, would clearly offer a cost-effective long-term strategy to reduce the cervical cancer burden, particularly for developing countries where effective screening programs are not available. Recombinant DNA technology is being used to produce vaccines against HPV, and both prophylactic and therapeutic vaccines are in use. Virus-like particles (VLPs) as recombinant L1 capsid protein from HPV contain no viral DNA and are therefore non-infectious and stimulate the production of antibodies that bind and neutralize the infectious virus. Live recombinant vectors such as recombinant vaccinia viruses, engineered to express genes from HPV-16 and -18, the most common viruses associated with cervical cancer, have been tested in therapeutic settings. The vaccine prevented 100% of high-grade cervical pre-cancers and non-invasive cervical cancers that were associated with HPV-16 and -18 (13). Given the long record of prophylactic viral vaccines as a cost-effective approach to prevent infection or modify disease, an effective vaccine against oncogenic types of HPV could have a tremendous impact on the global cervical cancer burden. Therefore, vaccination against HPV-16 and -18 will benefit the women of poor countries such as Bangladesh, who have limited access to testing and treatment.
In this study, we assessed the immunogenicity and safety profile of HPV-16/18 AS04-adjuvanted cervical cancer vaccine in adolescent girls aged between 9 and 13 years enrolled from Bangladesh in a randomized, controlled trial and compared the occurrence of serious adverse events (SAEs) between the two groups. HPV-16 and -18 antibody titers were assessed by enzyme-linked immunosorbent assay post-vaccination.
PATIENTS AND METHODS
Vaccine and Control Groups
This randomized (3:1) controlled clinical trial with two parallel groups, the vaccine and control groups, included 67 participants from Bangabandhu Sheikh Mujib Medical University and from two private hospitals in Bangladesh. The study was conducted from March 2008 to July 2009. GlaxoSmithKline's HPV-16/18 AS04-adjuvanted cervical cancer vaccine was given first day of vaccination (Day 0), at the 1- and 6-month schedule and followed up until Month 7. Controls were not given any vaccine. Serum samples were drawn at pre-vaccination and at Month 7 from both groups. Safety data were collected throughout the study. Main outcome measures were HPV-16/18 sero-conversion rates at Month 7 (in HPV-16/18 recipients) and safety.
Inclusion criteria of the vaccine and control groups were unmarried and sexually unexposed girls aged 9–13 years. Exclusion criteria were febrile illness at the time of vaccination, enrollment in another study with investigational agents, history of allergy to vaccine compounds (including aluminum, yeast), thrombo-cytopenia, history of another vaccination within 14 days of enrollment (previous 21 days for live vaccine), transfusion of blood or blood-derived products within 6 months preceding injection, immuno-suppression, diarrhea, vomiting and bleeding disorders. The vaccinated girls agreed to have no sexual exposure during the study period. As the girls were unmarried and sexually unexposed before enrollment into the study, Pap test, HPV identification and visual inspection with acetic acid were not done.
Ethics
The protocol and materials were approved by the ethical review committee of Bangladesh Medical Research Council and Bangabandhu Sheikh Mujib Medical University. Written informed consent was obtained from all participants or their parents or legal guardian or both after informing about the vaccination procedure.
Randomization
We randomly assigned participants in a 3:1 ratio to either the vaccination group or the control group.
Immunogenicity Assessments
CervarixTM is a bivalent HPV-16/18 LI VLP vaccine developed by GlaxoSmithKline (Cervarix, GlaxoSmithKline Biologicals, Rixensart, Belgium). In this preparation, the L1 protein of each HPV type is expressed via a recombinant baculo virus vector. The VLPs of each HPV type are produced separately and consist of purified L1 VLPs of HPV-16/18 at 20/20-g per dose formulated on AS04 adjuvant comprising 500 gm of aluminum hydroxide and 50 gm of 3-deacylated monopods phage lipid A.
Each subject received three doses of vaccine (0.5 ml) at Day 0, 1 month and 6 months. Blood samples were obtained from all subjects before vaccination, at the first day of vaccination (Day 0) and at Month 7. HPV IgG antibodies to HPV-16/18 were detected by capture enzyme-linked immunosorbent assay with a commercially available kit. A cut-off value was calculated from the mean optical density (OD) of the negative control at 450 nm. A cut-off value permits us to transform the OD values detected in positive or negative results due to the presence or absence of anti-HPV IgG. Samples with an OD of 450 nm lower than the cut-off value were considered as non-reactive for IgG anti-HPV. Samples with an OD of 450 nm higher than the cut-off value were considered as positive for IgG anti-HPV. The control group did not receive any vaccine. Sero-conversion/sero-positivity rates for anti-HPV-16 and anti-HPV-18 antibodies were calculated with a 95% confidence interval (CI).
Safety profile assessments of local symptoms (pain, redness and swelling at the injection site) and general symptoms (fever, headache, fatigue, gastrointestinal symptoms that included nausea, vomiting, diarrhea and/or abdominal pain, arthralgia, myalgia, rash and urticaria) were recorded for five consecutive days after each dose. The intensity of each symptom was graded on a non-quantifiable scale from mild, moderate and severe based on the extent of discomfort that subjects experienced. Unwanted events were followed for 14 days after each vaccination. SAE was classified by the Medical Dictionary for Regulatory Activities (14).
Statistical Analysis
Evaluation of the vaccine-induced serum anti-HPV-16 and anti-HPV-18 responses following administration of a three-dose regimen of bivalent HPV vaccine in female subjects aged 9–13 years in Bangladesh was done. Evaluation of the vaccine-induced serum anti-HPV responses following administration of three doses was done by the exact binomial test and 95% CIs were calculated. Risk differences in the percentage of subjects with one or more adverse experiences were estimated and their 95% CIs were provided.
A three-dose regimen of bivalent HPV vaccine is generally well tolerated in female subjects 9–13 aged years in Bangladesh. Specific adverse experiences, the incidence of injection-site adverse experiences and systemic clinical adverse experiences occurring on Days 1–15 of the post-vaccination period, were tabulated for the vaccination group. Risk differences in the percentage of subjects with one or more adverse experiences were estimated and their 95% two-sided CIs were provided.
RESULTS
Socio-demographic Characteristics
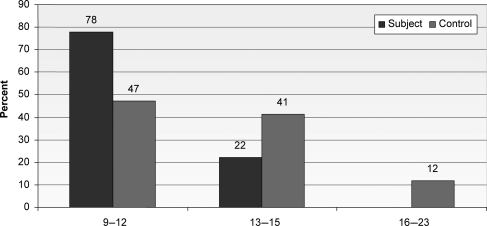
A total of 67 healthy subjects consented to participate and were enrolled and randomized (Fig. 1). Of these subjects, 50 received vaccine, and among these 50 subjects, 49 (98%) received all of the three-dose planned vaccination. Only 17 developed some adverse effects and were followed up for any medically important disease. Forty (80%) attended all visits and completed the study up to 7 months. Dose 3 of vaccination of one subject was done at a later date, as the recipient was absent. One subject from the vaccine group received only two vaccines because of shifting from Dhaka to a peripheral district. Demographic data are presented in Table 1. It was found that the mean age of vaccinated girls was 11.1 years with SD + 1.4 (range: 9–13 years) and the mean age of the control group was 12.9 + 206.The mean age of menarche was 11.2 in the vaccinated group and 12.5 in the control group. All the adolescent girls (100%) in both groups were unmarried.
Figure 1.
Age distribution of vaccine and control groups.
Table 1.
Summary of anti-HPV antibody after vaccination
| Subject |
Control |
P-value | ||||
|---|---|---|---|---|---|---|
| N (%) | 95% CI | N (%) | 95% CI | |||
| Anti-HPV | positive | 39/40 (97.5) | 86.8–99.9 | 0/15 (0.0) | 0.0–21.8 | 0.001 |
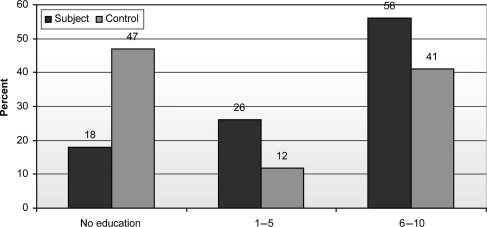
Eighteen percent of the vaccinated group and 47% of the control group were illiterate. Twenty-six percent of the vaccinated group and 11.8% of the control group had a primary level of education (1–5 years of schooling). Fifty-six percent of the vaccinated group and 41.2% of the control group had a secondary level of education (6–10 years of schooling; Fig. 2).
Figure 2.
Distribution of level of education of vaccinated and control groups.
Immunogenicity
Vaccine-induced immune responses were assessed in both the vaccine/subject and control groups (Table 1). Among 49 vaccinated subjects, 40 were available for detection of antibody to HPV. Among them, vaccine-induced sero-conversion was found in all subjects except 1. Nine subjects were not available for antibody detection at 7 months. Inoculation of Dose 3 of the sero-non-converted subject was done after 4 days due to absence.
Vaccine-induced antibody titer was high. Sero-conversion was not found among the control group. Two girls from the control group did not give blood samples for antibody detection (Table 1).
Safety Profile
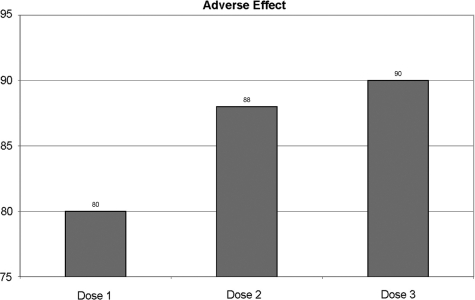
In general, the bivalent HPV vaccine was well tolerated with no reports of serious vaccine-related adverse experiences between enrollment and Month 7. Adverse effect after different doses of vaccine is shown in Figs 3–6.
Figure 3.
Percentage of one or more adverse effects by doses.
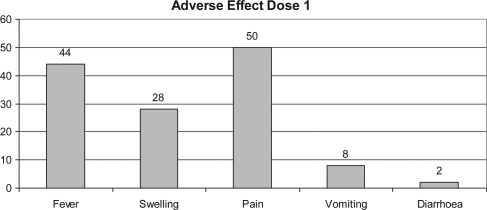
Figure 4.
Percentage of adverse effect in subjects after first dose.
Figure 5.
Percentage of adverse effect in subjects after second dose.
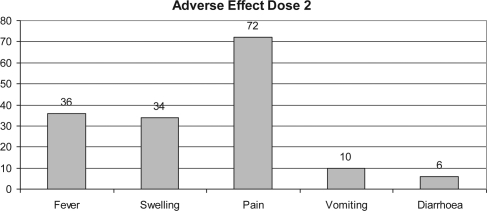
Figure 6.
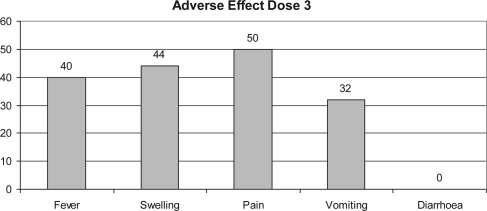
Percentage of adverse effect in subjects after third dose.
Among the vaccinated group, about 80% after the first dose, 88% after the second dose and 90% after the third dose of vaccination had at least one adverse experience. Among the vaccinated group, 20% after the first dose, 12% after the second dose and 10% after the third dose had no medical illness (Fig. 3).
Fever and injection-site-related adverse reaction were seen in those who had received vaccine (44 and 50%, respectively) after the first dose of vaccination, though most were mild in intensity. Injection-site pain was the most common vaccine-related adverse experience seen in those who had received vaccines (50, 72 and 50% after the first, second and third dose of vaccination, respectively). Vomiting was reported in four subjects after the first dose, five subjects after the second dose and 16 subjects after the third dose of vaccination (Figs 4–6).
No severe adverse experience was observed. Though vaccine related some adverse effects were observed in the vaccinated group but these are statistically not significant. Fever and injection-site adverse reaction was subsided by taking acetaminophen 500 mg three times daily for 1 or 2 days.
DISCUSSION
A range of studies conducted globally with efficacy trials with bivalent and quadrivalent HPV vaccine demonstrated its high efficacy and immunogenicity. Nevertheless, concerns exist regarding the effect of racial or ethnic difference on immunogenicity and safety of the vaccine. A recent report indicates that racial differences may affect the neutralizing antibody response to an investigational HIV vaccine (15).
The present study showed high immunogenicity of the HPV-16/18 AS04-adjuvanted cervical cancer vaccine among Bangladeshi adolescent girls. We found that all initially sero-negative subjects in the vaccine group sero-converted for both antibodies at the 7-month post-third dose, while the initially sero-negative control recipients had no change from the pre-vaccination titers in anti-HPV-16 and anti-HPV-18.
Therefore, the bivalent HPV VLP vaccine is generally tolerable and highly immunogenic in Bangladeshi girls aged 9–13 years. This vaccine can possibly protect women who have not been previously sexually exposed. The AS04 adjuvant was found to be key in inducing and maintaining an enhanced immune response (16). The induction of high immunogenicity by the AS04-adjuvanted vaccine has also been confirmed in a recent study (17).
The clinical importance of the data presented herein stems from their applicability to the South-East Asian population as a whole, where the incidence of cervical cancer is still high despite the presence of cervical cancer screening programs. Bangladesh is a developing country and HPV infection remains a high risk for cervical cancer. The age-adjusted rate of cervical cancer in Busan was reported to be 21.8 cases per 100 000 women, a rate higher than most Western countries (18). In Bangladesh, though we have no population-based cancer statistics, different hospital statistics show that the rate of cervical cancer is 21.5% among all malignancies of women, a rate higher than any developing country (19). However, according to Globocan (2), it is higher, around 28.9% incidence and 17.9% mortality of all cancer cases in Bangladesh. Therefore, due to lack of access to appropriate screening facilities, there is an obvious public health need to discover various approaches for preventing cervical cancer. Vaccination is one of the approaches and has the potential to play a crucial role in the prevention of cervical cancer in Bangladesh (20,21).
Safety results in this study showed no significant occurrence of adverse events in vaccine recipients, which is in line with the data from large previous studies conducted globally (22,23). The solicited general symptoms occurred in the vaccine group. Despite local injection site reactions being reported at a higher frequency in the vaccine group, there was similar conformity to the three-dose vaccination schedule and follow-up 1 month post-third dose, as has also been observed in other studies (22,24).
While the current study had a mean age of 11.1 years, the mean age of women enrolled in the prior study from the USA, Brazil and Europe was 20.0 years. Age was directly related to HPV antibody response seen in a combined analysis of data from two large pivotal studies for the quadrivalent HPV vaccine conducted internationally (FUTURE I and FUTURE II) (25,26). In combined data from these two trials, subjects aged 9–15 years had a significantly higher antibody response as measured by geometric mean titer against all vaccine-related HPV types when compared with chosen subjects who were 16–26 years of age (27).
In Bangladesh, opportunistic screening sometimes practised for cervical cancer may be helpful in increasing awareness of the disease. Nevertheless, it does not seem to have made any significant transformation on the mortality and morbidity burden of the disease (28). Therefore, due to a higher cervical cancer burden in Bangladesh and a deficient planned mass screening program for early detection, an effective prophylactic HPV vaccination program combined with effective screening could facilitate the control and prevention of cervical cancer in Bangladesh (29).
CONCLUSION
Among women aged 9–13 years not previously infected with vaccine-type HPV strains, prophylactic HPV-16/18 AS04-adjuvanted vaccine appears to be highly immunogenic, safe and generally well tolerated in adolescent girls from Bangladesh. Immunization of girls against oncogenic HPV before sexual debut could be a potential tool for the prevention and control of cervical cancer in Bangladesh.
Funding
Grameenphone Ltd, Bangladesh provided financial support.
Conflict of interest statement
None declared.
Acknowledgement
Oncology Club, Dhaka, Bangladesh provided added support for implementation of the research work.
References
- 1.Boyle P, Levin B. International Agency for Research on Cancer; 2008. World Cancer Report 2008; pp. 128–9. [Google Scholar]
- 2.Globocan. International Agency for Research on Cancer; 2008. Bangladeshdata.iarc.factsheets.pdf . [Google Scholar]
- 3.Dinshaw K, Mishra G, Shastri S. Determinants of compliance in a cluster randomised controlled trial on screening of breast and cervix cancer in Mumbai, India.1. Compliance to screening. Oncology. 2007;73:145–53. doi: 10.1159/000126497. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, de Sanjose S. Chapter 1: human papilloma virus and cervical cancer—burden and assessment of causality. J Natl cancer Inst Monogr. 2003;1:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafunnessa, Khatun SS, Chowdhury TA, Shamsuddin L, Islam MN, Hassan MS, et al. Human papilloma virus in cervical intraepithelial neoplasia in Bangladesh. Bangladesh J Obstet Gynaecol. 2005;20:13–8. [Google Scholar]
- 6.Akter M. 2010. Prevalence of HPV infection in apparently healthy woman. Dissertation of FCPS Part II Examination of BCPS. [Google Scholar]
- 7.Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papilloma virus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–76. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 8.Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, et al. Persistence genital human papilloma virus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Int. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 9.Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, Rozendaal L, Remmink AJ, Risse EK, et al. Relation of human papilloma virus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20–5. doi: 10.1016/S0140-6736(98)12490-X. [DOI] [PubMed] [Google Scholar]
- 10.Wallin KL, Wiklund F, Angstrom T, Bergman F, Stendahl U, Wadell G, et al. Type-specific persistence of human papilloma virus DNA before the development of invasive cervical cancer. N Engl J Med. 1999;341:1633–8. doi: 10.1056/NEJM199911253412201. [DOI] [PubMed] [Google Scholar]
- 11.Zur Hausen H. Immortalization of human cells and their malignant conversion by high risk human papilloma virus genotypes. Semin Cancer Biol. 1999;9:405–11. doi: 10.1006/scbi.1999.0144. [DOI] [PubMed] [Google Scholar]
- 12.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA, et al. Genital human papilloma virus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papilloma virus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325–39. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 14.Bhatla N, Suri V, Basu P, Shastri S, Datta SK, Bi D, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine in healthy Indian women. J Obstet Gynaecol Res. 2010;36:123–32. doi: 10.1111/j.1447-0756.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 15.Montefiori DC, Match B, McElrath MJ, Self S, Weinhold KJ, Corey L, et al. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infec Dis. 2004;190:1962–9. doi: 10.1086/425518. [DOI] [PubMed] [Google Scholar]
- 16.Giannini SL, Hanon E, Moris P, Mechelen MV, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV 16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–49. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and GardasilR human papilloma virus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccine. 2009;5:705–19. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 18.Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res. 2002;89:285–93. doi: 10.1016/s0168-1702(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Cancer Research and Hospital, World Health Organization, Country Office for Bangladesh; 2005. Cancer Registry Report; p. 15. 2007. [Google Scholar]
- 20.Das BC, Hussain S, Nasare V, Bharadwaj M. Prospects and prejudices of human papilloma virus vaccines in India. Vaccine. 2008;26:2669–79. doi: 10.1016/j.vaccine.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Ghim SJ, Basu PS, Jenson A. Cervical cancer: etiology, pathogenesis, treatment, and future vaccines. Asian Pac J Cancer Prev. 2002;3:207–12. [PubMed] [Google Scholar]
- 22.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA Study Group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papilloma virus types 16 and 18 in young women: an interim analysis of a phase III double-blind randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 23.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papilloma virus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 24.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjosé S, Hammouda D, et al. Against which human papilloma virus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 25.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 26.FUTURE II Study Group. Quadrivalent vaccine against human papilloma virus to prevent high-grade cervical lesions. New Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 27.Paavonen J The FUTURE II Study Group. he impact of baseline subject characteristics on the efficacy, safety and immunogenicity of a quadrivalent (types 6,11,16,8) human papillomavirus (HPV) L1 virus like particle vaccine. Presented at the 46th Interscience Conference on Antimicrobbial Agents and Chemotherapy (ICAAC); San Francisco, CA. 2006. 27 September. [Google Scholar]
- 28.Vallikad E. Cervical cancer: the Indian perspective. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S215–33. doi: 10.1016/S0020-7292(06)60037-4. [DOI] [PubMed] [Google Scholar]
- 29.Diaz M, Kim JJ, Albero G, de Sanjosé S, Clifford G, Bosch FX, et al. Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India. Br J Cancer. 2008;99:230–38. doi: 10.1038/sj.bjc.6604462. [DOI] [PMC free article] [PubMed] [Google Scholar]