Abstract
Rationale
Hemodymic forces caused by the altered blood flow in response to an occlusion lead to the induction of collateral remodeling and arteriogenesis. Previous work showed that platelet endothelial cell adhesion molecule-1 (PECAM-1) is a component of a mechanosensorycomplex that mediates endothelial cell (EC) responses to shearstress.
Objective
We hypothesized that PECAM-1 plays an important role in arteriogenesis and collateral remodeling.
Methods and Results
PECAM-1 knockout (KO) and wild-type littermates underwent femoral artery ligation. Surprisingly, tissue perfusion and collateral-dependent blood flow were significantly increased in the KO mice immediately after surgery. Histology confirmed larger caliber of preexisting collaterals in the KO mice. Additionally, KO mice showed blunted recovery of perfusion from hindlimb ischemia and reduced collateral remodeling, due to deficits in shear stress-induced signaling, including activation of the NF-κB pathway and inflammatory cell accumulation. Partial recovery was associated with normal responses to circumferential wall tension in the absence of PECAM-1, as evidenced by the upregulation of ephrin B2 and MCP-1, two stretch-induced regulators of arteriogenesis, both in vitro and in vivo.
Conclusions
Our findings suggest a novel role for PECAM-1 in arteriogenesis and collateral remodeling. Furthermore, we identify PECAM-1 as the first molecule that determines preexisting collateral diameter.
Keywords: PECAM-1, arteriogenesis, collateral vessels, shear stress, cyclic stretch
Introduction
Arteriogenesis, the outward remodeling of arteriole-arteriole anastomoses, is necessary to restore blood flow to tissue distal to an occlusion. While angiogenesis, capillary growth from pre-existing capillaries, is important for distributing existing flow, it is the caliber, number and growth of conduit arteries (collaterals) by arteriogenesis that determines tissue viability. A number of studies have shown that hindlimb flow subsequent to femoral artery occlusion is primarily determined by these collaterals.1 Notably, the number and size of these anastomoses varies greatly between species and tissues, resulting in different degrees of protection after arterial occlusion.2 Additionally, genetic mouse strain differences in preexisting collaterals exist, which are of major importance for final recovery after femoral artery occlusion.3 Despite a growing number of putative arteriogenic factors, little is known about the exact mechanisms that regulate collateral remodeling and virtually nothing is known about the genetic program and signaling mechanisms that regulate preexisting collateral networks.4
Mechanistically, arteriogenesis is a complex process that requires proliferation of EC and mural cells; upregulation of adhesion molecules, like intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1); chemokines, like monocyte chemoattractant protein-1 (MCP-1); and membrane-associated ligands like ephrin B2 in collateral arterioles. 5, 6
Mechanical hemodynamic forces are pivotal triggers for arteriogenesis. As a consequence of a pressure drop distal to the site of occlusion, the pressure difference between the ends of the collaterals is enhanced, resulting in increased blood flow, and, consequently, a rise in both shear stress and circumferential wall tension.7 Although shear stress is thought to be responsible for collateral remodeling during arteriogenesis, the role of circumferential wall tension in arteriogenesis is less well defined.8, 9 In this context, the expression of several regulators of arteriogenesis, such as the transcription factor activator protein-1 (AP-1), MCP-1 and ephrin B2 is regulated by circumferential stretch.6, 10 The up-regulation of these genes promotes SMC migration and monocyte recruitment and activation, which are critical regulators of arteriogenesis and collateral vessel growth.11
Vascular ECs are ideally positioned to serve as transducers, to relay hemodynamic and biochemical changes into molecular events in the other layers of the vascular wall.12 EC surfaces are equipped with numerous mechanoreceptors capable of detecting and responding to shear stress, including caveolae, ion channels, integrins, receptor Tyr kinases, the apical glycocalyx, primarycilia, heterotrimeric G proteins, and intercellular junctions.13 In this context, we previously identified a mechanosensory complexcomprising PECAM-1, vascular endothelial cadherin (VE-cadherin),and vascular EC growth factor receptor-2 (VEGFR2) that mediatesEC responses to shear stress.14 Based on the significant role of PECAM-1 in transducing shear stress in ECs in vitro and flow-mediated vascular remodeling in vivo,15 we hypothesized that PECAM-1 plays an important role in arteriogenesis and collateral remodeling in ischemia.
Methods
PECAM-1 knockout (PECAM-1−/−) mice were kindly provided by Dr. P. Newman (Blood Research Institute, Blood Center of Wisconsin, Milwaukee). PECAM-1+/+ C57/BL6 mice and PECAM-1−/− mice hadbeen backcrossed for >12 generations onto a C57/BL6 background. Cell culture,16, 17 shear stress18 and mechanical stretch (15% strain, 1Hz for 24 hours)19 assays were performed as described.
Unilateral hindlimb ischemic surgery procedure was described previously.20 Laser-Doppler Imaging, postmortem angiography, morphometric analysis, blood vessel silver staining, measurement of leukocyte density and capillary density, quantitative real-time PCR, immunofluorescence, Western Blot assays and statistical analyses are described in detail in the expanded Methods section, available in the Online Data Supplement at http://circres.ahajournals.org.
Results
PECAM-1 regulates acute plantar perfusion and recovery after hindlimb ischemia
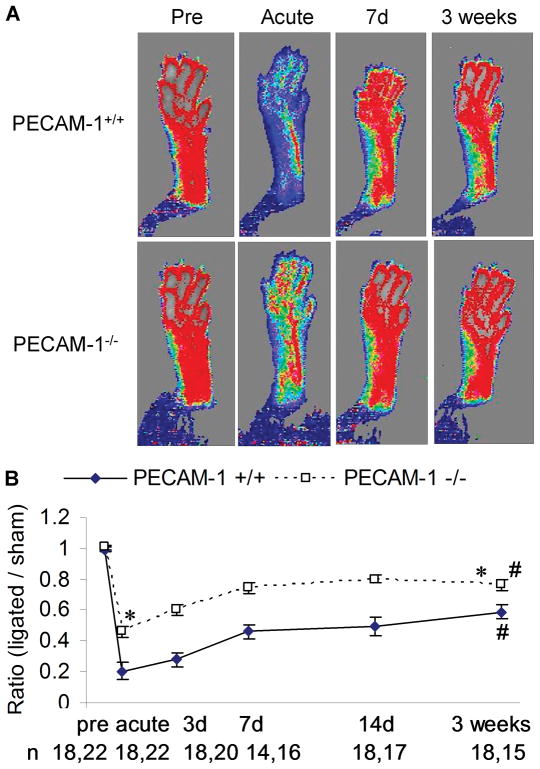
To determine the role of PECAM-1 in arteriogenesis, we subjected PECAM-1−/− (KO) and wild-type littermates (WT) to hindlimb ischemia by ligation of the superficial branch of the femoral artery, which triggers growth of preexisting collaterals from the deep femoral artery.20 Blood perfusion of hind paws (plantar) was monitored with a laser Doppler imaging system before surgery (pre), immediately after surgery (acute), 7 days (7d) and 3 weeks after surgery (Figure 1A). Plantar perfusion was quantified from the Doppler images and normalized to sham control side of the same animal for the comparisons of different time points. Surprisingly, we observed much higher acute plantar perfusion in PECAM-1−/− mice than in WT mice, while plantar perfusion was higher in both genotypes after 3 weeks (Figure 1B). The appearance and use scores of both genotypes were similar during the 3-week recovery stages (data not shown). Quantitation of recovery revealed a 65.5% increase in plantar perfusion in PECAM-1−/− mice from acute to 3 weeks after surgery vs. a 189.8% increase in WT mice (Figure 1B and Online Figure I), suggesting impaired recovery in the absence of PECAM-1.
Figure 1. Plantar perfusion after hindlimb ischemia.
(A) Doppler images of plantar perfusion before (Pre), immediately after (Acute), 7 days (7d) and 3 weeks after hindlimb ischemia. Pseudo-color scale: black=0, white=1000, in arbitrary units. (B) Ratio of plantar perfusion (ligated vs. sham control side) quantified from the Doppler images. Values are means ± SE. * p<0.05, compared with the respective time point of PECAM-1+/+; # p<0.05, compared with acute perfusion.
In addition to the less severe femoral artery ligation model, we also performed a more severe ischemia model, proximal lateral caudal femoral artery ligation, in a separate group of mice (Online Figure II). PECAM-1−/− mice showed blunted perfusion recovery compared to WT mice, similar to what we observed with the less severe model.
PECAM-1 regulates the lumen diameter of preexisting collateral vessels
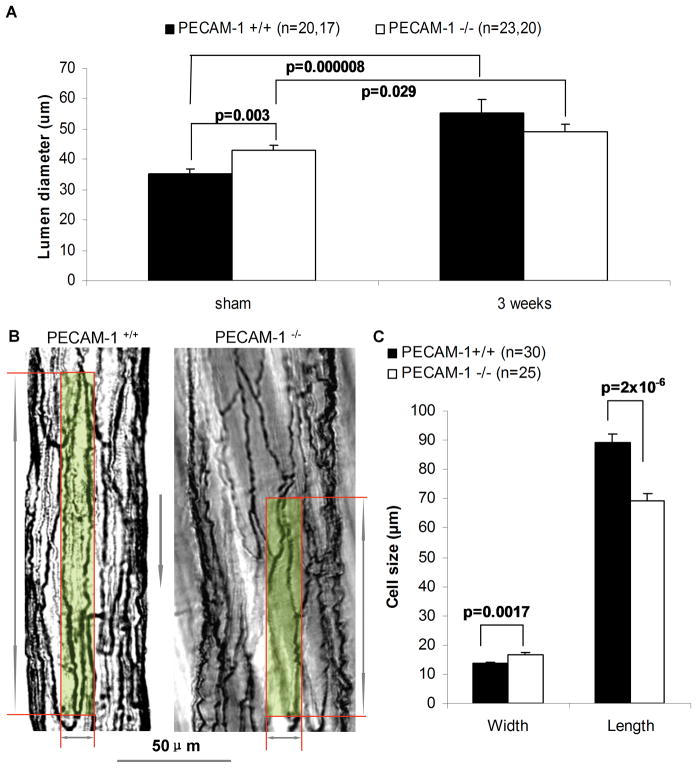
Because conductance of the native collateral circulation regulates plantar perfusion immediately after surgery, we hypothesized that there are anatomic differences in preexisting collaterals. We performed microangiography and counted the number of arterialvessels crossing a line drawn through the center of the thighcollateral zone to determine whether PECAM-1−/− mice have more preexisting collaterals and arterioles. Quantification revealed no differences between the two genotypes (not shown). Next, we measured the diameter of collaterals in the anterior and posterior gracilis muscles by histomorphometry (Figure 2A). Baseline diameter of PECAM-1−/−collaterals was significantly bigger compared to WT animals. This suggests that the higher perfusion in PECAM-1−/− mice immediately after surgery is not due to more collaterals, but reflects wider collaterals (Figure 1B). To determine if differences in caliber is seen in other vascular beds, we examined small intestine collaterals. Interestingly, quantitation revealed wider collaterals in the intestine of PECAM-1−/− mice (Online Figure III). Consistent with the recovery of plantar perfusion in the WT, collateral lumen diameter significantly increased 3 weeks after surgery. In contrast, the increase in collateral diameter was attenuated in PECAM-1−/− compared to WT (14.8% vs 58.1%, Online Figure I).
Figure 2. Lumen diameter and endothelial cell morphometry in collateral vessels.
(A) Collateral lumen diameter in sham and ligated side 3 weeks after hindlimb ischemic surgery. Values are means ± SE. (B) Silver staining of isolated collaterals from PECAM-1+/+ and PECAM-1−/− mice. A single EC is highlighted in each vessel. Gray arrow indicates flow direction. Scale bar: 50μm. The diagram arrows indicate the width and length of the highlighted ECs. (C) Width and length of collateral ECs. Values are means ± SE. The n number in panel C are cells measured in the collateral endothelium of PECAM-1+/+ and PECAM-1−/− animals.
The larger diameter of collateral vessels in the PECAM-1−/− mice may reflect differences in the number, size or orientation of ECs. We counted the number of ECs in collateral cross sections and found that the numbers were similar in both genotypes (data not shown). Next, we considered differences in size and orientation of ECs. For these studies, we isolated collateral vessels from gracilis muscles and EC borders were visualized by silver staining. In WT mice, collateral ECs displayed an elongated phenotype and were uniform in their orientation (Figure 2B). In contrast, PECAM-1−/− collateral ECs were shorter, wider, and less elongated. We measured the length and width of ECs in collateral intima, and confirmed that the EC width was significantly larger in PECAM-1−/− mice (16.6μm) than in WT mice (13.6μm), and the length was significantly shorter in PECAM-1−/− (69μm) than in WT mice (89μm, Figure 2C). Interestingly, the ratio of collateral EC width (16.6/13.6=1.22, KO vs. WT) is equal to the ratio of diameter of preexisting collaterals (42.8/35.0=1.22, KO vs. WT). These observations suggest that the differences in diameter of preexisting collaterals reflect differences in width and orientation of collateral ECs.
Angiogenesis in PECAM-1−/− mice
Although baseline collateral conductance and their subsequent enlargement are the primary determinants of plantar perfusion after surgery, baseline capillary density and ischemic angiogenesis could also contribute to plantar perfusion. Interestingly, recent reports have shown a role for PECAM-1 in angiogenesis, as matrigel implants, tumor angiogenesis and retinal angiogenesis were inhibited in PECAM-1−/− mice.21, 22 To address this possibility, we measured capillary density in the gastrocnemius muscle of sham or ligated animals (Online Figure IV A-B). The capillary density before ligation is similar in WT and PECAM-1−/− mice. This strengthens the suggestion from the preceding analyses that larger preexisting collateral diameter in PECAM-1−/−mice, rather than baseline capillary density, accounts for the smaller drop in perfusion immediately after surgery. After 3 weeks of ischemia, WT mice showed a significant increase in capillary density, while PECAM-1−/− mice exhibited a trend (P=0.085) to increase their capillary density relative to sham controls.
Collateral Remodeling in PECAM-1−/− mice
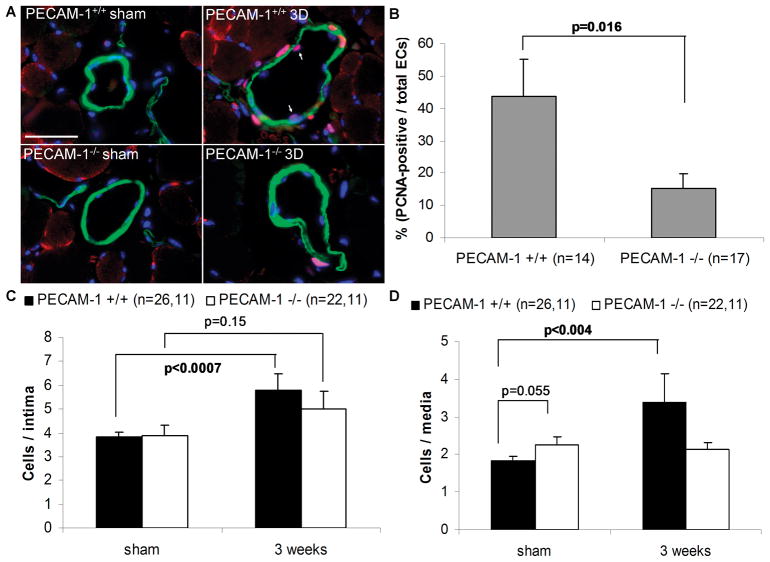
The attenuated increase in perfusion recovery (Figure 1B) and collateral diameter (Figure 2A) in PECAM-1−/− mice suggested a role for PECAM-1 in arteriogenesis. This was particularly interesting in light of the requirement for PECAM-1 in shear stress signaling14, 23–27. To gain further insight into the mechanisms that regulate arteriogenesis, we evaluated proliferation and activation of the inflammatory response, as both are known to play an important role in collateral remodeling. Figure 3A illustrates the degree of EC proliferation as determined by PCNA staining of collaterals. Quantification of PCNA-positive ECs showed that there are significantly more proliferating ECs in WT mice than in PECAM-1−/− mice 3 days after hindlimb ischemia (Figure 3B).
Figure 3. Cell proliferation during arteriogenesis.
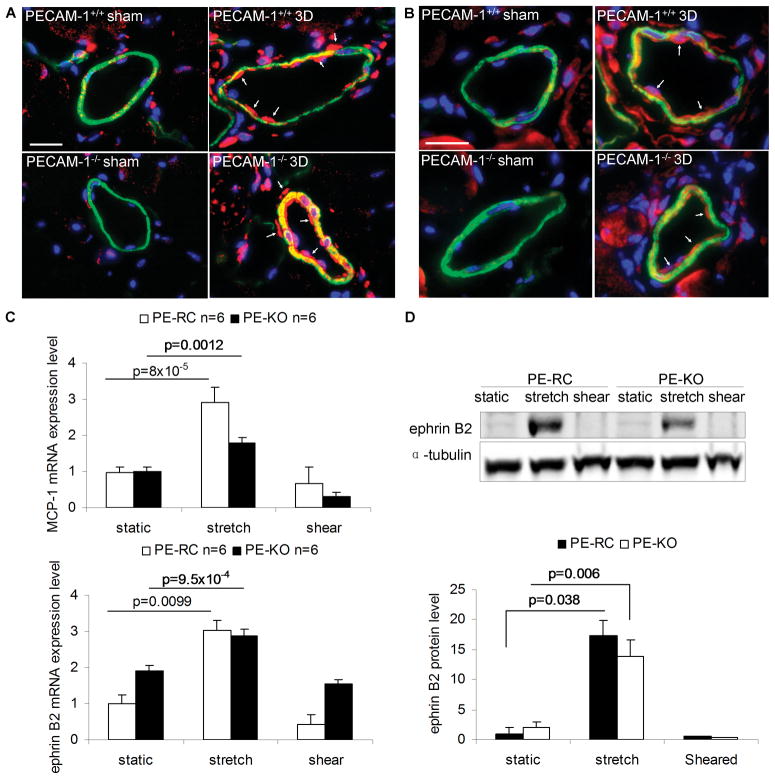
(A) PCNA-positive cells (red) in preexisting collaterals 3 days (3D) after surgery. Slides were co-stained for smooth muscle cell α-actin (green) and DAPI (blue). Bar, 20μm; (B) Percentage of PCNA-positive ECs; (C) Total number of ECs per collateral intima; and (D) Total number of smooth muscle cells per collateral media. Values are means ± SE.
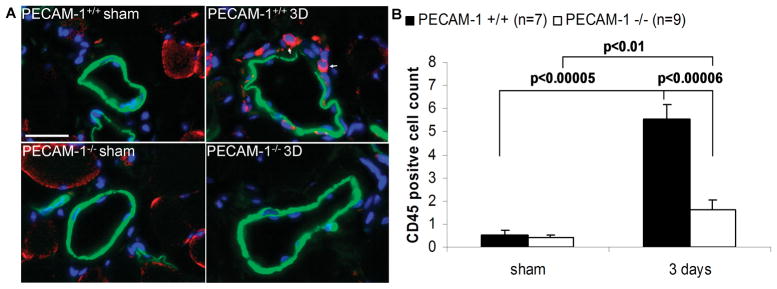
Because monocytes/macrophages are known to play an important role in hindlimb ischemia and PECAM-1 is known to facilitate leukocyte transendothelial migration28, we investigated the effects of PECAM-1 genetic deletion on leukocyte infiltration during hindlimb ischemia. Collaterals were immunostained for CD45, SMC α-actin and DAPI (Figure 4A). On day 3 after hindlimb ischemia,PECAM-1 deletion significantly decreased the number of CD45-positivecells as compared to wild-type littermates, suggesting that PECAM-1 deletion reducesinflammatory cell infiltration (Figure 4B).
Figure 4. Leukocyte accumulation in collaterals during arteriogenesis.
(A) CD45-positive cells (red) in preexisting collaterals 3 days (3D) after surgery. Slides were co-stained for smooth muscle cell α-actin (green) and DAPI (blue). Bar, 20μm; (B) CD45-positive cell count per collateral area. Values are means +/− SE
To identify a mechanism for the decrease in infiltration ofCD45+ cells in the ischemic tissue of PECAM-1−/− mice,we examined the NF-κB pathway. Importantly, the NF-κB pathway is activated by shear stress and PECAM-1 is important for flow-induced NF-κB activation.14, 15, 29 In particular, we examined activation of NF-κB by assaying nuclear translocation of the p65 subunit (Figure 5A) and downstream intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) upregulation. As shown in Figure 5B-C, ECs from WT collaterals showed nuclear accumulation for the p65 subunit of NF-κB, whereas ECs from PECAM-1−/− mice showed cytoplasmic localization for NF-κB. Additionally, while we did not observe any differences in VCAM-1or ICAM-1 staining in sham collaterals, expression of both cell adhesion molecules in ligated collaterals was much lower in PECAM-1−/− compared with WT animals (Figure 5B-C). Taken together, these results demonstrate that lack of PECAM-1 signaling leads to a disruption of the NF-κB pathway and a reduction in inflammatory cell accumulation during collateral remodeling.
Figure 5. Inflammatory signaling during arteriogenesis.
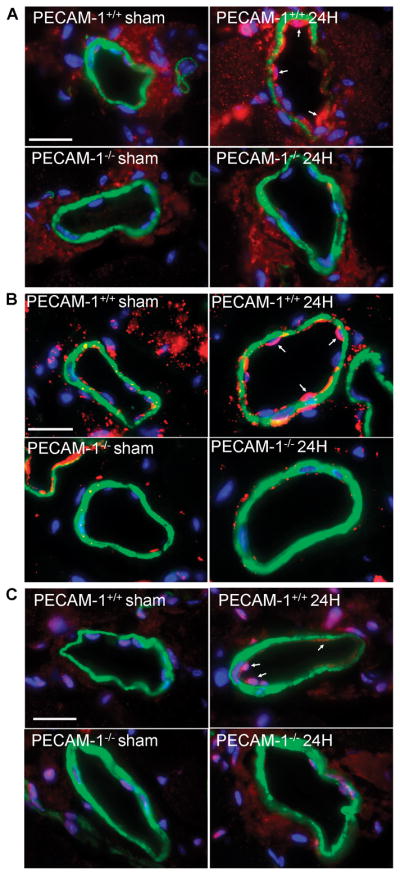
(A, B, C) Expression of NF-κB p65 subunit (red, A), ICAM-1 (red, B), or VCAM-1 (red, C) in preexisting collaterals 24 hours (24H) after surgery. Slides were co-stained for smooth muscle cell α-actin (green) and DAPI (blue). Bar, 20μm.
Responses to cyclic stretch
PECAM-1−/− mice have impaired collateral remodeling, yet show some perfusion recovery- albeit blunted compared to WT mice. Although we cannot exclude a role for other mechanosensors in the attenuated perfusion recovery in the PECAM-1−/− mice, we considered the complex hemodynamic environment in collaterals after ligation. Collective evidence suggests that shear stress and circumferential wall strain act together to alter the hemodynamics which govern arteriogenic remodelling.30 We therefore examined the role of PECAM-1 in mediating EC responses to circumferential stretch.
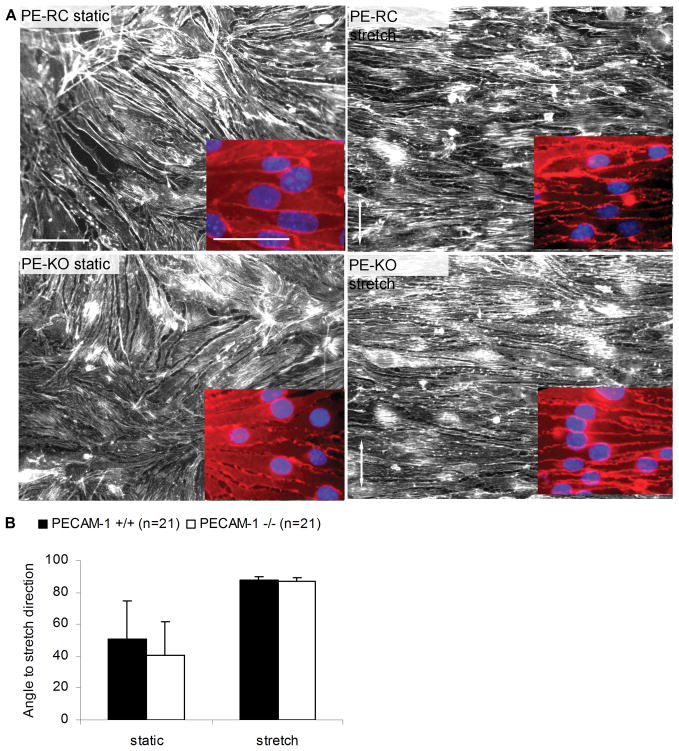
First, we used an in vitro system to apply cyclic stretch to ECs that express (PE-RC) or lack (PE-KO) PECAM-1. PE-RC and PE-KO ECs were exposed to cyclic stretch (1Hz, 15% strain for 24 hours) to allow alignment of actin stress fibers, which was assessed by staining with TRITC-phalloidin (Figure 6). These assays showed that ECs expressing PECAM-1 oriented their cytoskeletons perpendicular to the stretching direction, consistent with previous reports.31–33 Similarly, PECAM-1 null ECs were also oriented perpendicular to the stretch direction, suggesting that PECAM-1 null ECs are able to respond to stretch.
Figure 6. Effects of cyclic stretch on endothelial cell alignment in vitro.
(A) PECAM-1 reconstituted (PE-RC) or knockout (PE-KO) endothelial cells were exposed to cyclic stretch (15% strain, 1Hz, 24 hours) and stained with TRITC-phalloidin, or β-catenin (red) and DAPI (blue, inset images). Arrows show the direction of stretch. Bar: 50μm. (B) Quantification of orientation of F-actin stress fibers. Values are mean ± S.E.
We further tested this hypothesis by examining expression of ephrin B2 and MCP-1, two proteins previously shown to be upregulated during arteriogenesis due to circumferential wall strain.6, 10 MCP-1 and ephrin B2 abundance in sham collaterals was rather low; however, we noticed a marked increase in MCP-1 and ephrin B2 expression in response to ligation in collaterals from both genotypes (Figure 7A-B). Although some groups reported upregulation of MCP-1 and ephrin B2 by shear stress,34 others reported only a transient upregulation35 or even downregulation of their expression by shear stress36,37, 38. We examined MCP-1 and ephrin B2 expression at the mRNA and protein levels in ECs subjected to cyclic stretch (15% strain, 1Hz) or laminar shear stress (12 dynes/cm2) for 24 hours. We observed a robust increase in MCP-1 and ephrin2 mRNA levels in both PE-RC and PE-KO ECs in response to cyclic stretch compared with static controls (Figure 7C). Prolonged cyclic stretch also resulted in an increase in ephrin B2 protein levels in both cell types (Figure 7D); however, we did not observe changes in MCP-1 expression in cell lysates (data not shown), possibly due to secretion of MCP-1 in the culture media.39 Importantly, we did not observe upregulation of MCP-1 or ephrin B2 by shear stress in either cell type. These results therefore demonstrate that PECAM-1 is not required for expression of ephrin B2 and MCP-1 during arteriogenesis, possibly due to a lack of requirement of PECAM-1 for EC responses to circumferential stretch.
Figure 7. Expression of MCP-1 and ephrin B2 during arteriogenesis and in response to cyclic stretch or laminar shear stress in vitro.
(A, B) Expression of MCP-1 (A, red) or ephrin B2 (B, red) in preexisting collaterals 3 days (3D) after hindlimb ischemic surgery. Slides were co-stained for smooth muscle cell α-actin (green) and DAPI (blue). Bar, 20μm. (C) Relative mRNA expression levels of MCP-1 and ephrin B2 in ECs subjected to cyclic stretch or laminar shear stress. Static ECs in plates were controls. Values are expressed as mean ± S.E. (D) Western blots and quantification of ephrin B2 in cyclic stretch- or laminar shear stress-stimulated ECs. The protein blots were quantified and normalized to α-tubulin. Values are mean ± S.E.
Discussion
Physical forces are pivotal triggers for arteriogenesis.30 Mechanical forces caused by the altered blood flow in response to an occlusion lead to the induction of collateral remodeling. PECAM-1 is thought to be involved in flow mechanosensing or transduction, based on changes in its phosphorylation with altered flow and in vitro and ex vivo experiments showing PECAM-1-dependentactivation of flow-mediated intracellular signaling pathways.14, 23–27 More recently, our group demonstrated a role for PECAM-1 in flow-mediated vascular remodeling and intima-media thickening in vivo.15 In light of the view that arteriogenesis is a predominantly shear stress-mediated remodeling process and the importance of PECAM-1 in transduction of shear stress signaling, we hypothesized that PECAM-1−/− animals would have impaired ischemia-induced arteriogenesis. Our data show that PECAM-1 contributes to multiple steps involved in collateral remodeling, in particular, activation of the NF-κB pathway and downstream inflammatory cell accumulation. Consistent with these findings, PECAM-1 mediates shear stress-induced NF-κB activation in vitro and in vivo.14, 15, 29 A possible explanation for the impaired recovery might be deficits in shear stress-induced signaling in the PECAM-1−/− animals. Another equally plausible possibility is that the PECAM-1−/− mice already have higher perfusion acutely, thus meeting tissue perfusion demands even in the face of reduced collateral remodeling. Likewise, it is possible that PECAM-1−/− collaterals experience lower shear stress due to their larger size, thus lacking a key stimulus for remodeling. To address this, we created a shunt between the distal stump of the occluded femoral artery and the accompanying vein in order to increase shear stress.30 Importantly, although the higher shear stress greatly stimulated arteriogenesis in the WT, PECAM-1−/− mice showed reduced perfusion recovery (data no shown), suggesting impaired remodeling even in the presence of increased shear stress.
It is thought that hemodynamic forces and, in particular, fluid shear stress is the primary morphogenic physical factor that induces collateral remodeling.40 ECs are equipped with numerous mechanoreceptors capable of detecting and responding to shear stress, and, thus, regulating perfusion recovery in response to ischemic insult, including caveolae, ion channels, integrins, receptor Tyr kinases, the apical glycocalyx, primary cilia, heterotrimeric G proteins, and intercellular junctions. It is therefore possible that in the absence of PECAM-1, other mechanosensors kick in and facilitate the blunted, albeit significant, perfusion recovery. Although we cannot rule out this possibility, we considered that the vessel wall is exposed to both shear stress and circumferential stretch during arteriogenesis. Based on Poiseuille’s equation, progressive stenosis of the main artery will lead to an increase in resistance hence a significant drop in pressure distal to the site of occlusion. As a consequence, the pressure difference between both ends of the collateral arterioles is enhanced, resulting in increased flow 41 and, consequently, a rise in both shear stress and circumferential wall tension, which may act in concert on the collateral blood vessels. There is accumulating evidence that these altered hemodynamics to which the arteriolar vessel wall is exposed to initiate arteriogenesis.30 While a requirement for PECAM-1 in shear stress responses is well-established, its role in circumferential stretch is unknown. Our data show that PECAM-1 is not only dispensable for stretch-induced EC alignment, but also for stretch-induced expression of ephrin B2 and MCP-1, two proteins previously shown to be upregulated during arteriogenesis due to circumferential wall strain.
An unexpected finding of this study was that PECAM-1−/− animals show increased perfusion immediately after surgery. This increased perfusion is not due to more collaterals, but, instead reflects widercollaterals. Although virtually nothing is known about the genetic and environmental factors that specify collateral formation, recent reports have identified two genes that specify preexisting collateral numbers (VEGF and CLIC4).42, 43 Here, we identify PECAM-1 as the first molecule that determines preexisting collateral diameter in skeletal muscle. We hypothesized that the larger diameter of collateral vessels in the PECAM-1−/− mice may reflect differences in the morphology or orientation of ECs. We therefore developed a method to isolate collaterals and visualized EC morphology. Surprisingly, WT collateral ECs were elongated and uniformly oriented; whereas KO ECs were less elongated and wider, thus contributing to the larger lumen diameter. Importantly, it has been proposed that flow-evoked remodeling processes determine the number, and possibly diameter, of preexisting collaterals during embryonic development44. Our own work has pointed to PECAM-1 as an important regulator of shear stress-induced cell alignment.14 In contrast, ECs are able to align in response to cyclic stretch independently of PECAM-1. Importantly, PECAM-1 has also been shown to regulate the activity of Rho family GTPases,45 which are known to be master regulators of the cytoskeleton and, thus, regulate processes such as cell shape, adhesion and migration.46 Consistent with this, our own preliminary data show decreased Rho activation in the absence of PECAM-1 (Tzima, unpublished). It is therefore possible that the wider collateral diameter in the PECAM-1−/−mice is due to differential activation of Rho GTPases, which, in turn, regulate cell shape via their effects on the cytoskeleton. Alternatively, the eNOS/NO pathway might be worthy of consideration. An interesting recent study reported dilated vessels in the retinal vasculature of PECAM-1−/− mice, which mechanistically might be associated with dysregulation of eNOS.22 Moreover, PECAM-1 has been shown to regulateeNOS activity, which in turn affects vessel structure.26, 27, 47 Interestingly, it has been reported that the activity of Rho GTPases can be controlled by the level of NO via changes in their phosphorylation48, 49. Further studies are required to define when and how PECAM-1 regulates collateral formation, as well as the downstream pathways that stabilize and maintain collaterals.
Supplementary Material
What is known?
In the face of an arterial obstruction, small arteriole-arteriole anastomoses (collaterals) are recruited to function as alternate routes of blood supply.
Collateral density, diameter and growth (remodeling) varies greatly amongst species and tissues.
Mechanical hemodynamic forces, such as shear stress and circumferential stretch, are pivotal triggers for collateral remodeling.
Platelet endothelial cell adhesion molecule (PECAM)-1 is part of a mechanosensory complex expressed in endothelial cells that mediates endothelial cell responses to shear stress.
What new information does this article contribute?
PECAM-1 null mice show reduced collateral remodeling due to deficits in shear stress-induced signaling, including activation of the NF-κB pathway and inflammatory cell accumulation.
PECAM-1 regulates the caliber of preexisting collaterals, and is identified as the first molecule that determines preexisting collateral diameter.
Collaterals are small arterioles that serve as alternate routes of blood supply in the face of an obstruction by connecting two larger arteries. The density and diameter of preexisting collateral networks and their ability to grow and remodel are critical factors that determine restoration of blood flow. Mechanical forces (shear stress and circumferential stretch) caused by the altered blood flow in response to an occlusion are critical regulators of collateral remodeling. Vascular endothelial cells are equipped with numerous mechanoreceptors capable of detecting and responding to changes in blood flow, including PECAM-1. In this study, we demonstrate impaired perfusion recovery from femoral artery ligation in PECAM-1 deficient mice. This was associated with deficits in shear stress-induced signaling, such as activation of the NF-κB pathway and inflammatory cell accumulation, but normal responses to circumferential stretch. Unexpectedly, PECAM-1 deficient animals showed increased perfusion immediately after ligation, due to the presence of larger diameter collateral vessels. Further studies are required to define when and how PECAM-1 regulates collateral formation, as well as the downstream pathways that stabilize and maintain collaterals.
Acknowledgments
We gratefully acknowledge the help of Dr Mauricio Rojas with the flow measurements, surgery and laser Doppler Imaging. We thank Dr. Natasha Case for technical assistance with the cyclic stretch experiments. Kirk McNaughton kindly provided us with his expert histology assistance.
Source of funding
This work was supported in part by an NIH grant (HL088632) to E.T., and American Heart Association postdoctoral fellowship (0825442E) to Z.C. E.T. is an Ellison Medical Foundation New Scholar.
Non-standard Abbreviations and Acronyms
- PECAM-1
platelet endothelialcell adhesion molecule-1
- EC
endothelial cell
- SMC
smooth muscle cell
- KO
knockout
- WT
wild type
- PE-RC
PECAM-1 reconstituted
- PE-KO
PECAM-1 knockout
- DAPI
4’,6-diamidino-2-phenylindole dihydrochloride
- VEGFR2
vascular endothelial cell growth factor receptor 2
- VE-cadherin
vascular endothelial cadherin
- PCNA
proliferating cell nuclear antigen
- CD45
cluster of differentiation (CD) antigen 45
- NF-κB
nuclear factor-kappa B
- p65
NF-κB p65 subunit
- ICAM-1
intercellular adhesion molecule 1
- VCAM-1
vascular cell adhesion molecule-1
- AP-1
activator protein-1
- TRITC
tetramethylrhodamine isothiocyanate
- MCP-1
monocyte chemotactic protein-1
- VEGF
vascular endothelial cell growth factor
- CLIC4
chloride intracellular channel 4
- eNOS
endothelial nitric oxide synthase
- WGA
wheat germ agglutinin
- D
post-surgery day
- H
post-surgery hour
- n
number of animals (cells)
Footnotes
Disclosures
None.
Reference List
- 1.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–87. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 2.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 3.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–6. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 4.Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol. 2006;98:1194–7. doi: 10.1016/j.amjcard.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007;8:35–42. doi: 10.2174/138920107779941408. [DOI] [PubMed] [Google Scholar]
- 6.Korff T, Braun J, Pfaff D, Augustin HG, Hecker M. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration and monocyte recruitment. Blood. 2008;112:73–81. doi: 10.1182/blood-2007-12-128835. [DOI] [PubMed] [Google Scholar]
- 7.Scheel KW, Fitzgerald EM, Martin RO, Larsen RA. The possible role of mechanical stresses on coronary collateral development during gradual coronary occlusion. In: Schaper W, editor. The Pathophysiology of Myocardial Perfusion. Amsterdam: Elsevier North-Holland Biomedical Press; 1979. pp. 489–518. [Google Scholar]
- 8.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–51. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 9.Resnick N, Einav S, Chen-Konak L, Zilberman M, Yahav H, Shay-Salit A. Hemodynamic forces as a stimulus for arteriogenesis. Endothelium. 2003;10:197–206. doi: 10.1080/10623320390246289. [DOI] [PubMed] [Google Scholar]
- 10.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res. 2008;103:477–84. doi: 10.1161/CIRCRESAHA.108.177782. [DOI] [PubMed] [Google Scholar]
- 11.Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45 (Suppl A):A48–A56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–93. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–73. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CW, Wiedle G, Ballestrem C, Wehrle-Haller B, Etteldorf S, Bruckner M, Engelhardt B, Gisler RH, Imhof BA. PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin alphavbeta3 in Cis. Mol Biol Cell. 2000;11:3109–21. doi: 10.1091/mbc.11.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–92. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–47. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin J, Murphy TC, Rahnert J, Song H, Nanes MS, Greenfield EM, Jo H, Fan X. Mechanical inhibition of RANKL expression is regulated by H-Ras-GTPase. J Biol Chem. 2006;281:1412–8. doi: 10.1074/jbc.M508639200. [DOI] [PubMed] [Google Scholar]
- 20.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H947–H959. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- 21.Cao G, Fehrenbach ML, Williams JT, Finklestein JM, Zhu JX, DeLisser HM. Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am J Pathol. 2009;175:903–15. doi: 10.2353/ajpath.2009.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol. 2008;315:72–88. doi: 10.1016/j.ydbio.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–85. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182:753–63. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujiwara K, Masuda M, Osawa M, Kano Y, Katoh K. Is PECAM-1 a mechanoresponsive molecule? Cell Struct Funct. 2001;26:11–7. doi: 10.1247/csf.26.11. [DOI] [PubMed] [Google Scholar]
- 26.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–11. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 27.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–5. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–60. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- 29.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2003–8. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–8. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 31.Shirinsky VP, Antonov AS, Birukov KG, Sobolevsky AV, Romanov YA, Kabaeva NV, Antonova GN, Smirnov VN. Mechano-chemical control of human endothelium orientation and size. J Cell Biol. 1989;109:331–9. doi: 10.1083/jcb.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugimoto K, Fujii S, Takemasa T, Yamashita K. Factors inducing codistribution of marginal actin fibers and fibronectin in rat aortic endothelial cells. Am J Physiol. 1997;272:H2188–H2194. doi: 10.1152/ajpheart.1997.272.5.H2188. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Ip W, Boissy R, Grood ES. Cell orientation response to cyclically deformed substrates: experimental validation of a cell model. J Biomech. 1995;28:1543–52. doi: 10.1016/0021-9290(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 34.Cheng C, Tempel D, van HR, de Boer HC, Segers D, Huisman M, van Zonneveld AJ, Leenen PJ, van der SA, Serruys PW, de CR, Krams R. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J Clin Invest. 2007;117:616–26. doi: 10.1172/JCI28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A. 1994;91:4678–82. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deindl E, Buschmann I, Hoefer IE, Podzuweit T, Boengler K, Vogel S, van RN, Fernandez B, Schaper W. Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ Res. 2001;89:779–86. doi: 10.1161/hh2101.098613. [DOI] [PubMed] [Google Scholar]
- 37.Goettsch W, Augustin HG, Morawietz H. Down-regulation of endothelial ephrinB2 expression by laminar shear stress. Endothelium. 2004;11:259–65. doi: 10.1080/10623320490904151. [DOI] [PubMed] [Google Scholar]
- 38.Melchionna R, Porcelli D, Mangoni A, Carlini D, Liuzzo G, Spinetti G, Antonini A, Capogrossi MC, Napolitano M. Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J. 2005;19:629–31. doi: 10.1096/fj.04-2219fje. [DOI] [PubMed] [Google Scholar]
- 39.Okada M, Matsumori A, Ono K, Furukawa Y, Shioi T, Iwasaki A, Matsushima K, Sasayama S. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:894–901. doi: 10.1161/01.atv.18.6.894. [DOI] [PubMed] [Google Scholar]
- 40.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–58. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 42.Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105:89–98. doi: 10.1161/CIRCRESAHA.109.197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–36. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.le NF, Fleury V, Pries A, Corvol P, Eichmann A, Reneman RS. Control of arterial branching morphogenesis in embryogenesis: go with the flow. Cardiovasc Res. 2005;65:619–28. doi: 10.1016/j.cardiores.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J. 2003;17:1458–69. doi: 10.1096/fj.02-1040com. [DOI] [PubMed] [Google Scholar]
- 46.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–22. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Bubolz AH, Shi Y, Newman PJ, Newman DK, Gutterman DD. Peroxynitrite reduces the endothelium-derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R57–R65. doi: 10.1152/ajpregu.00424.2005. [DOI] [PubMed] [Google Scholar]
- 48.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem. 2003;278:19023–31. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- 49.Loirand G, Guilluy C, Pacaud P. Regulation of Rho proteins by phosphorylation in the cardiovascular system. Trends Cardiovasc Med. 2006;16:199–204. doi: 10.1016/j.tcm.2006.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.