Abstract
Background
Cerebral edema is one physical change associated with brain injury and decreased survival after cardiac arrest. Edema appears on computed tomography (CT) scan of the brain as decreased x-ray attenuation by gray matter. This study tested whether the gray matter attenuation to white matter attenuation ratio (GWR) was associated with survival and functional recovery.
Methods
Subjects were patients hospitalized after cardiac arrest at a single institution between 1/1/2005 and 7/30/2010. Subjects were included if they had non-traumatic cardiac arrest and a non-contrast CT scan within 24 hours after cardiac arrest. Attenuation (Hounsfield Units) was measured in gray matter (caudate nucleus, putamen, thalamus, and cortex) and in white matter (internal capsule, corpus callosum and centrum semiovale). The GWR was calculated for basal ganglia and cerebrum. Outcomes included survival and functional status at hospital discharge.
Results
For 680 patients, 258 CT scans were available, but 18 were excluded because of hemorrhage (10), intravenous contrast (3) or technical artifact (5), leaving 240 CT scans for analysis. Lower GWR values were associated with lower initial Glasgow Coma Scale motor score. Overall survival was 36%, but decreased with decreasing GWR. The average of basal ganglia and cerebrum GWR provided the best discrimination. Only 2/58 subjects with average GWR<1.20 survived and both were treated with hypothermia. The association of GWR with functional outcome was completely explained by mortality when GWR<1.20.
Conclusions
Subjects with severe cerebral edema, defined by GWR<1.20, have very low survival with conventional care, including hypothermia. GWR estimates pre-treatment likelihood of survival after cardiac arrest.
INTRODUCTION
Cardiac arrest is a significant public health problem, with an annual incidence of over 300,000 cases of out-of-hospital cardiac arrest in North America and a case-fatality rate approaching 95% [1]. Brain injury is a significant contributor to mortality after cardiac arrest. As many as 68% of out-of-hospital cardiac arrest patients and 23% of in-hospital cardiac arrest patients die from brain injury [2.3]. Furthermore, post-arrest brain injury may reduce quality of life in long-term survivors [4-7]. However, the precise mechanisms of post-cardiac arrest brain injury are poorly understood [2]. A reliable early indicator of neurological injury after cardiac arrest would be desirable because it might allow titration of early interventions, improve stratification of patients in clinical trials, and facilitate ongoing prognostication efforts during intensive care. No clinical sign or test performed shortly after restoration of pulses has been reliably associated with outcome.
Cerebral edema contributes to the pathology of post-cardiac arrest brain injury. This edema appears as a loss of gray matter to white matter differentiation on a cranial computed tomography (CT) scan. For comparison, decreased x-ray attenuation in gray matter is observed during stroke, and this decreased attenuation is correlated with decreased apparent diffusion coefficients on diffusion-weighted MRI [8]. Two previous studies found that the gray matter to white matter differentiation was significantly lower in post-cardiac arrest patients with poor outcomes (comatose and Glasgow Outcome Scale 1-2, respectively) relative to patients with good outcomes (awake and GOS 3-5, respectively) [9,10]. A third study found that attenuation in the putamen and cerebral cortex were decreased in patients with poor outcomes (CPC 4-5) compared to patients with good outcomes (CPC 1-3) [11]. A separate study found that cerebral edema was associated with poor outcome in pediatric drowning patients [12]. However, the prior studies were of insufficient size to determine the performance characteristics of cranial CT in this population.
Because cranial CT scans are easily obtained in comatose cardiac arrest patients, cerebral edema is a potentially useful early marker for brain injury. A method to stratify patients according to cerebral injury early after cardiac arrest would be useful in clinical trials or in titrating therapy. This study tested the hypothesis that gray matter to white matter differentiation on the initial cranial CT scan was associated with hospital outcome after cardiac arrest. Gray matter to white matter differentiation was quantified by the attenuation in gray matter to the attenuation in white matter ratio (GWR). Outcomes included survival, Cerebral Performance Category (CPC) and Modified Rankin Score (MRS) at hospital discharge.
METHODS
Patient Selection
All patients admitted to UPMC-Presbyterian hospital after in-hospital or out-of-hospital cardiac arrests were entered into a prospective quality improvement database. The Institutional Review Board of the University of Pittsburgh approved retrospective analysis of this database and associated CT scans under a waiver of the requirement to obtain informed consent for a minimal risk study. Inclusion criteria were age >18 years, cardiac arrest and return of spontaneous circulation (ROSC). We defined cardiac arrest as having received a rescue shock and/or chest compressions initiated by a health professional. Using this database, we identified subjects who received a cranial CT scan <24 hours after cardiac arrest between January 1, 2005 and June 30, 2010. Usual clinical care for this cohort has been detailed previously, and included increasing use of cardiac catheterization and therapeutic hypothermia after 2006 [13,14]. For patients receiving hypothermia treatment, this intervention began as soon as possible using bags of ice and was monitored using portable thermometers, in parallel with other procedures including CT scans. However, this institution recommended no specific treatment for post-cardiac arrest cerebral edema.
CT Measurements
We used only non-contrast CT scans of the head for the study. We acquired CT scans on a GE LightSpeed VCT scanner (GE Healthcare, Little Chalfont, UK) with 5 mm slices. An investigator blinded to clinical information opened CT scans for each patient using commercial image viewing software (Stentor iSite, South San Francisco, CA) with windowing adjusted to “brain,” and identified comparable brain slices at the level of the basal ganglia, and two levels of the superior cortex as depicted in prior reports [9,10]. Circular regions of measurement (0.1-0.15 cm2) were placed over these regions of interest, and average attenuation in Hounsfield Units (HU) was recorded. At the basal ganglia level, values were recorded bilaterally for caudate nucleus (CN), putamen (PU), corpus callosum (CC), thalamus (THL), and posterior limb of internal capsule (PIC). Gray matter to white matter ratio (GWR) in the basal ganglia was calculated according to previously reported methods as:
We recorded values bilaterally for the medial cortex and medial white matter at the level of the centrum semiovale (MC1 and MWM1, respectively) and high convexity area (MC2 and MWM2, respectively). Cerebrum GWR was calculated as:
We calculated Average GWR as the mean of the Basal Ganglia GWR and Cerebrum GWR. Increasing cerebral edema results in lower attenuation by gray matter and a lower GWR.
Outcomes
We determined survival to hospital discharge and key clinical variables from chart review. An attending intensivist or a resuscitation consultant performed the baseline neurological examination within 6 hours of return of pulses. Whenever possible, blood pressure was optimized prior to examination, and the incidence of hypotension in this cohort is similar across levels of coma. We considered the examination missing if not recorded by an attending physician within 6 hours. A trained occupational therapist determined Cerebral Performance Category (CPC) and Modified Rankin Score (MRS) at hospital discharge by review of physician, nursing, physical therapy and occupational therapy notes using a standard instrument.
Statistical Analysis
We tested the distributions of raw HU measurements and GWR for each region of interest for normality by visual inspection and the Shapiro-Wilk test. We summarized all raw measurements and GWR using median and interquartile range (IQR) because they were non-normally distributed. Pearson’s correlation coefficient described correlations between basal ganglia and cerebrum GWR. Mann-Whitney U tested whether attenuation and GWR differed between survivors and non-survivors. We tested associations between attenuation, GWR, and survival using binary logistic regression. We examined differences in GWR between strata of GCS Motor Score, CPC, and MRS using Kruskal-Wallis test. When a difference was found, we used a post-hoc Mann-Whitney U to determine which particular strata differed. Linear regression tested for association of GWR and the time interval from cardiac arrest to CT scan. Finally, we examined receiver operating characteristic (ROC) curves to determine the optimal (maximum specificity) threshold value of GWR for predicting mortality. We expressed performance of each measure for predicting mortality as the area under the curve (AUC) for ROC curves. We determined sensitivity, specificity, positive predictive value and negative predictive value at the optimal cutoff selected from ROC curves. Statistics were completed using SPSS v. 18 (SPSS, Chicago, IL, USA).
RESULTS
Patient Demographics
Between January 2005 and July 2010, we treated 680 subjects with in-hospital or out-of-hospital cardiac arrest (Figure 1). We identified 151 subjects who were awake on presentation and thus did not receive a CT scan (GCS motor score=6). We excluded subjects with surgical or traumatic causes of arrest (N=11), withdrawal of care or failure to sustain pulses long enough to receive CT scan (N=20), and current incarceration (N=3). If a patient had two cardiac arrests within 6 months, we excluded the second cardiac arrest (N=4). A cranial CT was not performed at this hospital within 24 hours of cardiac arrest for 233 comatose patients. This latter group included patients with CT scan performed at another hospital, CT scan performed >24 hours after arrest, and in-hospital cardiac arrests for whom CT scanning was less common practice. Finally, we did not analyze CT scans for subjects with intracranial hemorrhage (N=10), intravenous contrast from prior studies (N=3), and large scanning artifacts (N=5). A total of 240 CT scans remained for analysis. Out-of-hospital cardiac arrest and therapeutic hypothermia were more common in the study cohort than in the entire cardiac arrest population (Table 1).
Figure 1.
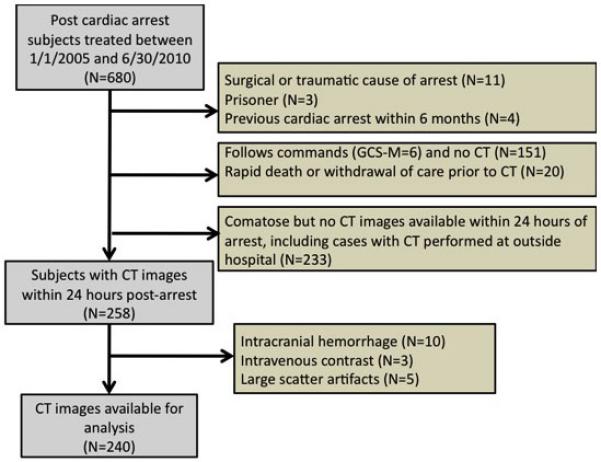
Subjects included in the study.
Table 1.
Patient demographics for study cohort, comatose patients without CT, all excluded patients, and entire database.
| Study cohort (N=240) |
Comatose but no CT available (N=233) |
All excluded patients (N=440) |
All cardiac arrest patients (N=680) |
|
|---|---|---|---|---|
| Mean Age (SD) | 60 (17) | 60 (16) | 60 (16) | 60 (16) |
| % Male | 128 (53%) | 131 (56%) | 258 (59%) | 386 (57%) |
| Initial Rhythm - VF - PEA - Asystole - Unknown |
75 (31%) 75 (31%) 53 (22%) 36 (15%) |
99 (43%) 66 (28%) 39 (17%) 28 (12%) |
* 191 (43%) 119 (27%) 69 (16%) 50 (11%) |
266 (39%) 194 (29%) 122 (18%) 86 (13%) |
| Initial GCS Motor -1 -2 -3 -4 -5 -6 |
113 (47%) 12 (5%) 19 (8%) 53 (22%) 11 (5%) 29 (12%) |
* 99 (43%) 8 (3%) 19 (8%) 61 (26%) 37 (16%) 0 (0%) |
* 124 (28%) 8 (2%) 25 (6%) 69 (16%) 39 (9%) 157 (36%) |
237 (35%) 20 (3%) 44 (6%) 122 (18%) 50 (7%) 186 (27%) |
| In-hospital arrest Out-of-hospital arrest |
64 (27%) 176 (73%) |
132 (57%) * 101 (43%) |
260 (59%) * 177 (40%) |
324 (48%) 353 (52%) |
| Hypothermia treatment Hypothermia and GCS-M<6 |
167 (70%) 166 (80%) |
115 (49%) * 115 (51%) |
131 (30%) * 128 (29%) |
298 (44%) 294 (62%) |
| Arrest-to-CT Time Interval - 0-6 hours - 6-12 hours - 12-18 hours - 18-24 hours |
161 (67%) 47 (20%) 16 (7%) 12 (5%) |
|||
| Survival to hospital discharge |
87 (36%) | 92 (40%) | 221 (50%) * | 308 (45%) |
indicates p<0.05 versus study cohort.
Attenuation Measurements
Attenuation measurements for all regions of interest were not normally distributed (Table 2A). Gray matter attenuation in the basal ganglia level (CN, PU, THL) tended to be higher than in the high convexity levels (MC1, MC2). White matter attenuation also tended to be higher in the basal ganglia level (CC, PIC) than in the centrum semiovale and high convexity levels (MWM1, MWM2). Gray matter attenuation in all regions (CN, PU, THL, MC1, MC2) was significantly different between survivors and non-survivors (p<0.005). White matter attenuation (CC, PIC, MWM1, MWM2) did not differ between survivors and non-survivors. Gray matter attenuation values were associated with survival (p<0.02), whereas white matter values were not associated with survival. In ROC curves, gray matter attenuation in each region was a weak predictor of survival (AUC for CN=0.62, PU=0.63, THL=0.65, MC1=0.63, and MC2=0.61).
Table 2.
A. Median (IQR) attenuation values for survivors and non-survivors. (CN=caudate nucleus, PU=putamen, THL=thalamus, PIC=posterior limb of internal capsule, MC=medial cortex, MWM=medial white matter) B. Median (IQR) GWR for survivors and non-survivors.
| A. | |||||
|---|---|---|---|---|---|
| Survivors | Non-survivors | P-value | |||
|
Basal
Ganglia |
Gray
Matter |
CN | 33.5 (31.8-34.9) | 32.6 (30.8-34) | 0.002 |
| PU | 34.3 (32.6-35.9) | 33.3 (31.2-34.9) | 0.001 | ||
| THL | 33.1 (32.1-34.6) | 32.3 (30.5-33.8) | <0.001 | ||
|
White
Matter |
CC | 26.2 (25.1-27.5) | 26.7 (25.4-28.1) | 0.213 | |
| PIC | 26.6 (24.8-27.5) | 26.4 (25.2-27.8) | 0.410 | ||
| Cerebrum |
Gray
Matter |
MC1 | 31.9 (30.6-33.4) | 30.9 (29.3-32.3) | 0.001 |
| MC2 | 31.4 (30.5-33.6) | 30.8 (28.8-32.3) | 0.004 | ||
|
White
Matter |
MWM1 | 25.6 (24.4-26.8) | 25.7 (24.3-27) | 0.771 | |
| MWM2 | 25.5 (23.9-26.8) | 25.8 (24.5-26.9) | 0.516 | ||
| B. | ||||
|---|---|---|---|---|
| Survivors | Non-survivors | P-value | ||
| GWR |
Basal
Ganglia |
1.29 (1.26-1.33) | 1.24 (1.18-1.29) | <0.001 |
| Cerebrum | 1.24 (1.21-1.29) | 1.21 (1.15-1.26) | <0.001 | |
| Average | 1.26 (1.24-1.3) | 1.22 (1.16-1.27) | <0.001 | |
GWR Measurements
GWR ranged from 0.98 to 1.77 in cerebrum, 0.79 to 1.57 in basal ganglia and 0.95 to 1.58 for average (Table 2B). In the 29 subjects who were awake on presentation (GCS-motor=6), median GWR was 1.24 (IQR 1.20-1.32) for cerebrum, 1.30 (IQR 1.28-1.35) for basal ganglia, and 1.27 (IQR 1.24-1.33) for average. GWR measured in cerebrum and basal ganglia were correlated (Pearson’s r=0.448; p<0.01). Cerebrum GWR tended to be lower, with the majority of data points falling below the identity line.
Association of GWR with Initial GCS Motor Score
Average, basal ganglia, and cerebral GWR were associated with initial GCS Motor Score (p<0.001, Figure 2). The majority of patients with GWR<1.20 had initial GCS motor scores of 1.
Figure 2.
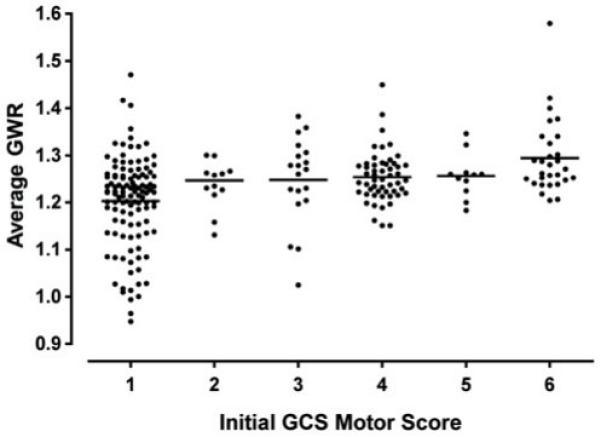
Average GWR decreases with initial GCS-motor score. Subjects with the lowest GWR had GCS-motor scores = 1.
To measure inter-rater reliability of GWR estimates, a second investigator measured GWR from a randomly generated sample of 5% of the study cohort. Ratios between investigators had a Pearson’s correlation coefficient of 0.642, indicating good agreement. Test-retest reliability was measured from a randomly generated sample of 10% of the study cohort and also was extremely consistent, with a Pearson’s correlation coefficient of 0.931.
Influence of Arrest-to-CT Time Interval
The time elapsed between the cardiac arrest and cranial CT (arrest-to-CT Interval) varied from 9 minutes to 23.8 hours (median 4.2 hours, IQR 2.7-6.9). Arrest-to-CT interval was 6 hours or less in the majority of cases (67%) (Table 1). There was no association between arrest-to-CT interval and gray matter attenuation in any measured region. Likewise, arrest-to-CT interval was not associated with basal ganglia GWR (p=0.24), cerebrum GWR (p=0.08), or average GWR (p=0.09).
Association of GWR with Survival and Outcome
Average, basal ganglia, and cerebral GWR were significantly higher in survivors than in non-survivors (p<0.005), and all were positively associated with survival (p<0.005). Average GWR was the strongest predictor of mortality as assessed using ROC curves (basal ganglia AUC=0.69; cerebrum AUC=0.67; average AUC = 0.72). Average GWR < 1.20 predicted death with a sensitivity of 36% (CI 29-45%), a specificity of 98% (CI 91-100%), a positive predictive value of 97% (CI 87-99%), and a negative predictive value of 46% (CI 39-54%). The false positive rate for predicting death with this cutoff was 2/58, or 3% (CI 1-13%).
Average, basal ganglia, and cerebral GWR were statistically associated with CPC and MRS (p≤0.001, Kruskal-Wallis) (Figure 3). However, inspection of the data reveals that this association merely reflected the low GWR in patients who died (MRS=6; CPC=5).
Figure 3.
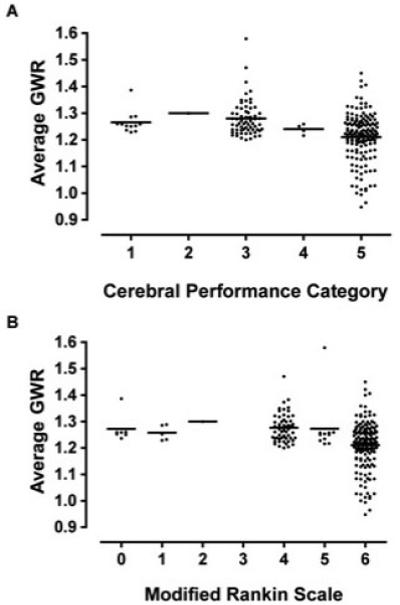
Average GWR on initial CT scan is related to functional outcome at hospital discharge measured by a) Cerebral Performance Category and b) Modified Rankin Scale. Lower GWR was associated with CPC and mRS, but this association results from the fact that subjects with lower GWR died (CPC=5, mRS=6).
Hypothermia-treated Cohort
Figure 4 shows mortality as a function of average GWR for patients receiving hypothermia (N=167) and patients not receiving hypothermia (N=72). Average and basal ganglia GWR were associated with survival in both groups (p<0.05, binary logistic regression), but cerebral GWR was only associated with survival in the hypothermia group (hypothermia p<0.001; no-hypothermia p=0.12). Both groups show near 100% mortality with average GWR< 1.2, although 2 patients with GWR=1.17 and GWR =1.15 who were treated with hypothermia did survive to hospital discharge.
Figure 4.
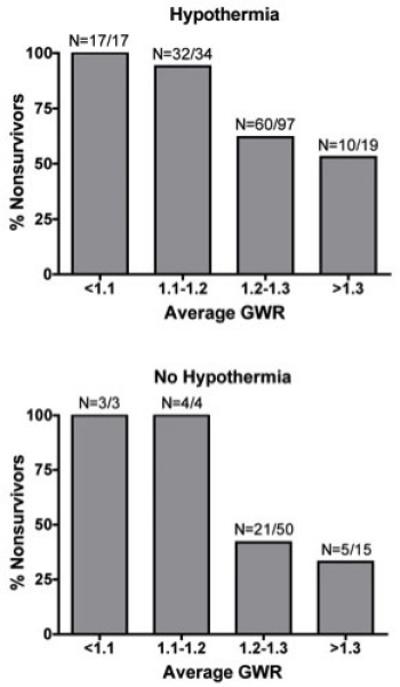
Mortality increases in subjects with lower average GWR receiving hypothermia (top) and patients not receiving hypothermia (bottom).
DISCUSSION
This study confirms prior studies that the ratio of gray matter to white matter attenuation on cranial CT images obtained within 24 hours after cardiac arrest is associated with survival [9-11]. Extending prior studies, this larger cohort provides more precise description of the performance characteristics of this test. An average GWR less than 1.20 had specificity of 98% for predicting mortality, similar to previous study cutoffs of 1.18 and 1.22, which yielded specificities of 100% [9,10]. Even in patients treated with therapeutic hypothermia there was a reliable association between GWR and survival. These data suggest that the average GWR can estimate risk of mortality for patients after cardiac arrest prior to treatment with or without hypothermia in a modern intensive care unit.
Changes in GWR reflect edema and decreased x-ray attenuation in gray matter. The selective vulnerability of gray matter to ischemia has been described in the literature and results from its higher metabolic rate, greater blood flow, and susceptibility to excitotoxicity [16,17]. Increased vascular permeability leading to extravasation of fluid (vasogenic edema) and changes in ion flow due to excitotoxicity (cytotoxic edema) contribute to a preferential accumulation of edema in gray matter compared to white matter. The tissue attenuation of white matter was not significantly different between survivors and nonsurvivors. Nevertheless, GWR was more closely associated with survival than gray matter attenuation alone. This fact may indicate that white matter attenuation adjusts for minor technical differences between CT scans.
Basal ganglia and cerebrum GWR varied independently of each other. Cerebral GWR tended to be lower on average than basal ganglia GWR, suggesting that the cerebrum may be more prone to edema than the basal ganglia. These regional differences may explain why average GWR is a better predictor of survival than basal ganglia or cerebrum GWR alone. Edema throughout the brain has a higher likelihood for mortality than edema in a single region.
Interestingly, the time from arrest to CT was not related to the GWR or attenuation values for any brain region. The only way to determine whether cerebral edema after cardiac arrest evolves over time similar to edema after other cerebral pathologies such as stroke would be to obtain serial images from the same patients [15]. Unfortunately, these data were not available in this cohort. The fact that significant edema is apparent in some CT scans at very short times after cardiac arrest could indicate a vasogenic contribution to the edema. There are several potential reasons why the time course of edema appearance after global ischemia may be faster than after stroke. For example, venous thrombosis may develop during complete circulatory arrest and compromise venous drainage after reperfusion. The tissue ischemia during cardiac arrest is complete whereas stroke may have trickle or low-flow from collaterals. Furthermore, reperfusion after cardiac arrest may differ qualitatively from stroke because of high levels of exogenous catecholamines, hypoxia and hypoventilation. Future studies could explore these potential mechanisms.
Of 58 patients with average GWR less than 1.20, only two survived. The first (GWR 1.17) was a 47-year old man with out-of-hospital VF cardiac arrest, witnessed collapse and immediate CPR who received therapeutic hypothermia. After 22 hospital days, he was discharged awake to rehabilitation. The second case (GWR 1.15) was a 70-year old woman who experienced out-of-hospital cardiac arrest after environmental exposure and severe hypothermia (initial temperature = 25.3°C). This patient was re-warmed to 33°C for 24 hours. After a prolonged ICU course complicated by frostbite, renal failure, and ventilator-associated pneumonia, she was discharged to a skilled nursing facility. Her mental status was conversant.
Limitations of this study include the large number of subjects who did not receive a CT scan despite being comatose on arrival. Some subjects might have had prognosis already deemed to be poor, which could be a source of bias in the results. This limitation is a result of practice variation among providers, who did not routinely order CT scans after out-of-hospital cardiac arrest until recent years and who still vary in the ordering of this test among in-hospital cardiac arrests. However, the survival and other clinical characteristics of comatose patients for whom CT scan was not performed were similar to the study cohort. Also, since this study excluded patients who were awake or following commands and did not receive at CT scan, the results can only be applied to patients who are comatose after cardiac arrest. Confirmation of these results in larger cohorts in which all post-cardiac arrest patients receive cranial CT scan will provide improved estimates of the value of GWR and confirm that the results can be generalized.
CONCLUSIONS
A low GWR (<1.20) measured from early cranial CT scans after cardiac arrest is associated with mortality. The ease of measurement makes GWR a potentially useful tool for predicting lower likelihood of survival in a subset of patients after cardiac arrest when treated conventionally.
Acknowledgements
Dr. Rittenberger is supported by Grant Number 1 KL2 RR024154-02 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr. Rittenberger is also supported by an unrestricted grant from the National Association of EMS Physicians/Zoll EMS Resuscitation Research Fellowship.
REFERENCES
- 1.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I, Resuscitation Outcomes Consortium Investigators Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 3.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 4.van Alem AP, Waalewijn RA, Koster RW, de Vos R. Assessment of quality of life and cognitive function after out-of-hospital cardiac arrest with successful resuscitation. Am J Cardiol. 2004;93:131–135. doi: 10.1016/j.amjcard.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Pusswald G, Fertl E, Faltl M, Auff E. Neurological rehabilitation of severely disabled cardiac arrest survivors. Part II. Life situation of patients and families after treatment. Resuscitation. 2000;47:241–248. doi: 10.1016/s0300-9572(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 6.Granja C, Cabral G, Pinto AT, Costa-Pereira A. Quality of life 6-months after cardiac arrest. Resuscitation. 2002;55:37–44. doi: 10.1016/s0300-9572(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 7.Cronberg T, Lilja G, Rundgren M, Friberg H, Widner H. Long-term neurological outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2009;80:1119–1123. doi: 10.1016/j.resuscitation.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Kucinsky T, Väterlein O, Glauche V, Fiehler J, Klotz E, Eckert B, Koch C, Röther J, Zeumer H. Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke. 2002;33:1786–1791. doi: 10.1161/01.str.0000019125.80118.99. [DOI] [PubMed] [Google Scholar]
- 9.Torbey MT, Selim M, Knorr J, Bigelow C, Recht L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke. 2000;31:2163–2167. doi: 10.1161/01.str.31.9.2163. [DOI] [PubMed] [Google Scholar]
- 10.Choi SP, Park HK, Park KN, Kim YM, Ahn KJ, Choi KH, Lee WJ, Jeong SK. The density ratio of grey to white matter on computed tomography as an early predictor of vegetative state or death after cardiac arrest. Emerg Med J. 2008;25:666–669. doi: 10.1136/emj.2007.053306. [DOI] [PubMed] [Google Scholar]
- 11.Yanagawa Y, Yasushi U, Sakamoto T, Okada Y. Cerebral density on CT immediately after a successful resuscitation of cardiopulmonary arrest correlates with outcome. Resuscitation. 2005;64:97–101. doi: 10.1016/j.resuscitation.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Rafaat KT, Spear RM, Kuelbs C, Parsapour K, Peterson B. Cranial computed tomographic findings in a large group of children with drowning: diagnostic, prognostic, and forensic implications. Pediatr Crit Care Med. 2008;9:567–572. doi: 10.1097/PCC.0b013e31818c8955. [DOI] [PubMed] [Google Scholar]
- 13.Rittenberger JC, Guyette FX, Tisherman SA, DeVita MA, Alvarez RJ, Callaway CW. Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008;79:198–204. doi: 10.1016/j.resuscitation.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds JC, Callaway CW, El Khoudary SR, Moore CG, Alvarez RJ, Rittenberger JC. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med. 2009;24:179–186. doi: 10.1177/0885066609332725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwamm LH, Koroshetz WJ, Sorensen AG, Wang B, Copen WA, Budzik R, Rordorf G, Buonanno FS, Schaefer PW, Gonzalez RG. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29:2268–76. doi: 10.1161/01.str.29.11.2268. [DOI] [PubMed] [Google Scholar]
- 16.Arbelaez A, Castillo M, Mukherji SK. Diffusion-weighted MR imaging of global cerebral anoxia. AJNR Am J Neuroradiol. 1999;20:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez LG, Rovira A, Portela LA, Leite Cda C, Lucato LT. CT and MR in non-neonatal hypoxic-ischemic encephalopathy: radiological findings with pathophysiological correlations. Neuroradiology. 2010;52:949–976. doi: 10.1007/s00234-010-0728-z. [DOI] [PubMed] [Google Scholar]


