Abstract
Growth and morphogenesis during embryonic development, asexual reproduction and regeneration require extensive remodeling of the extracellular matrix (ECM). We used the simple metazoan Hydra to examine the fate of ECM during tissue morphogenesis and asexual budding. In growing Hydra, epithelial cells constantly move towards the extremities of the animal and into outgrowing buds. It is not known, whether these tissue movements involve epithelial migration relative to the underlying matrix or whether cells and ECM are displaced as a composite structure. Furthermore, it is unclear, how the ECM is remodeled to adapt to the shape of developing buds and tentacles. To address these questions, we used a new in vivo labeling technique for Hydra collagen-1 and laminin, and tracked the fate of ECM in all body regions of the animal. Our results reveal that Hydra ‘tissue movements’ are largely displacements of epithelial cells together with associated ECM. By contrast, during the evagination of buds and tentacles, extensive movement of epithelial cells relative to the matrix is observed, together with local ECM remodeling. These findings provide new insights into the nature of growth and morphogenesis in epithelial tissues.
Key words: Extracellular matrix, ECM, Hydra, Epithelium, Morphogenesis, Epithelial migration, Tissue remodeling
Introduction
Tissues of multicellular animals are composed of cells mounted on scaffolds of extracellular matrix (ECM). The first ECM is laid down early in development and gives rise to basic epithelial organization (Tyler, 2003). This usually happens before tissue folding and morphogenesis (Ingber, 2006). Although we have considerable understanding of how different cellular behaviours contribute to the creation of shape in animal tissues, little is known about the dynamic organisation of ECM during this process.
Cell movements are one of the most important mechanisms driving animal morphogenesis. Single cells or isolated groups of cells can migrate based on invasive activity and cell–substrate interactions, as in vertebrate neural crest cells, Hydra nematocyte precursors, the lateral line in zebrafish, or Drosophila border cells. Morphogenetic movements can also include large areas of a tissue, such as vertebrate gastrulation and neurulation or Hydra ‘tissue movements’. For most of these large-scale tissue rearrangements, it is not yet clear whether cells move actively or whether they are passively displaced by global tissue deformations, which include cells and associated ECM. One way to address this question is to track the fate of cells and at the same time track changes in the associated ECM, as has been done in vivo in the avian embryo (Benazeraf et al., 2010; Zamir et al., 2006; Zamir et al., 2008).
Tissue of the freshwater polyp Hydra is organized as an epithelial double layer; the outer epithelium is the ectoderm and the inner the endoderm. These are separated by an intervening ECM, which is called the mesoglea. The mesoglea has the structural and molecular organisation of basement membranes in higher animals and is synthesized by epithelial cells of both layers (Epp et al., 1986; Sarras and Deutzmann, 2001). In regularly fed polyps, epithelial cells in the body column continually undergo cell division. This does not lead to an increase in body size because, under steady state conditions, tissue growth is balanced by tissue loss at the ends of the body column and in developing buds. These tissue movements have been systematically investigated with the help of various in vivo markers for ectodermal and endodermal epithelial cells (Campbell, 1967b; Campbell, 1973; Otto and Campbell, 1977a; Shostak and Kankel, 1967; Shostak et al., 1965; Wittlieb et al., 2006). However, the role of the mesoglea has remained unclear. Two opposing views are possible: (1) the mesoglea is a stationary structure that serves as a substratum for active epithelial motility (Shostak and Globus, 1966; Shostak et al., 1965), or (2) tissue movements are the result of continuous tissue expansion that includes both epithelia and the mesoglea (Campbell, 1973; Campbell, 1974).
During bud formation an initially flat area of the body wall in the lower gastric region evaginates and forms a small new animal. Although it has been shown that bud outgrowth is based on movement of epithelial cells from the mother polyp (Otto and Campbell, 1977a) and involves lateral intercalation of epithelial cells (Philipp et al., 2009), little is known about how the ECM in the bud is formed. Elevated levels of mesoglea synthesis in the bud (Hausman and Burnett, 1971; Zhang et al., 2007; Zhang et al., 2002) indicate the need for new ECM material, but it remains unknown whether the mesoglea, like the epithelial cells, is recruited from the parent, and how the mesoglea is reorganised during bud outgrowth while maintaining a functional epithelial basement membrane at the same time.
To address these questions we used a method to label major components of Hydra mesoglea in the living animal. The method was originally developed for the avian embryo and uses fluorescently tagged primary antibodies to target ECM components (Czirok et al., 2004; Rongish et al., 1998). The antibodies were microinjected into the mesoglea where they bound stably to their epitopes without disturbing physiological functions. Grafting of labeled tissue fragments into unlabeled animals and precise local injections of antibody allowed us to track the fate of mesoglea in live animals. Our findings show that the mesoglea is a surprisingly dynamic structure. Only in the head region did it remain stationary. In the adjacent body column and tentacles it was continuously displaced toward the ends of the animal. During bud evagination, it was stretched and remodeled to create the bud morphology. The dynamic displacement of the mesoglea along the body column and the tentacles largely overlapped with the movement of epithelial cells. These tissue movements, which might superficially appear as active cellular migrations, are largely passive tissue displacements. At sites of tissue evagination, however, the mesoglea was dramatically remodeled and epithelial cells moved relative to the mesoglea.
Our results, together with findings obtained in vertebrate systems (Czirok et al., 2004; Davidson et al., 2008; Larsen et al., 2006; Zamir et al., 2006; Zamir et al., 2008), suggest that our understanding of tissue movements and morphogenesis needs to be re-evaluated with respect to the role of ECM remodeling.
Results
In vivo labeling of Hydra ECM with non-interfering antibodies
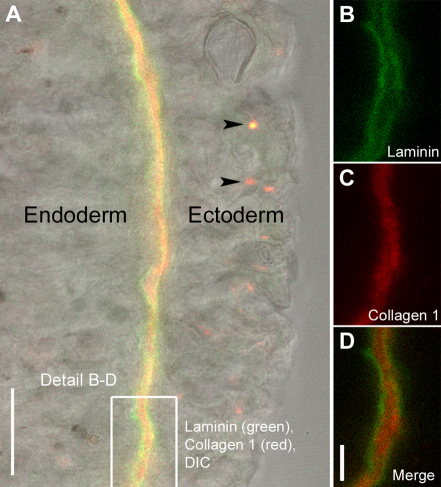
We succeeded in labeling two Hydra ECM components, collagen-1 and laminin, by injecting fluorescently tagged monoclonal antibodies into the mesoglea of living animals. To test the specificity of the labeling, animals were injected with both antibodies at the same time, fixed after 24 hours, and examined with confocal microscopy (Fig. 1A–D). Antibodies against Hydra laminin localized to the subepithelial basal lamina (Fig. 1A,B,D), whereas those against Hydra collagen-1 localized to the central interstitial matrix of the mesoglea (Fig. 1A,C,D). This is consistent with data obtained from previous studies (Sarras et al., 1993; Shimizu et al., 2008). Unbound antibody was taken up by epithelial cells and accumulated in terminal lysosomes (Fig. 1A, arrowheads) where it could be clearly distinguished from labeled mesoglea.
Fig. 1.
Localization of injected antibodies to the three layers of Hydra mesoglea. (A) Optical section through Hydra body wall showing ectoderm, endoderm and the intervening mesoglea in differential interference contrast (DIC) and immunofluorescent labeling. The mesoglea was labeled by injection of monoclonal antibodies mAb52 raised against Hydra laminin (green) and mAb39 raised against Hydra collagen-1 (red). Excess antibody is taken up by ectodermal cells and sequestered in terminal lysosomes (arrowheads). (B–D) Labeled mesoglea at higher magnification showing laminin localization in the two subepithelial basal laminae (B,D) and collagen-1 localization in the central interstitial matrix (C,D). Scale bars: 20 μm (A), 5 μm (D).
Budding rate and bud development were not different between labeled and control polyps. Head and foot regeneration proceeded at the same rate as in unlabeled animals. Finally, labeled polyps could not be distinguished from control animals by morphology, contraction–elongation behaviour or feeding behaviour. Taken together, in vivo labeling of mesoglea components in Hydra is specific and does not interfere with major developmental and physiological processes.
Hydra mesoglea is displaced along the body column of the animal
Small grafts containing labeled mesoglea were displaced down the body column over the course of several weeks (Fig. 2A–C). Both collagen-1 and laminin labels overlapped completely during these displacements and also during tissue evagination (n=12, data not shown), indicating that basal lamina and interstitial matrix function as a unit. Since label stability and the label to background ratio were better in specimens labeled only with collagen-1, we used it in all subsequent experiments.
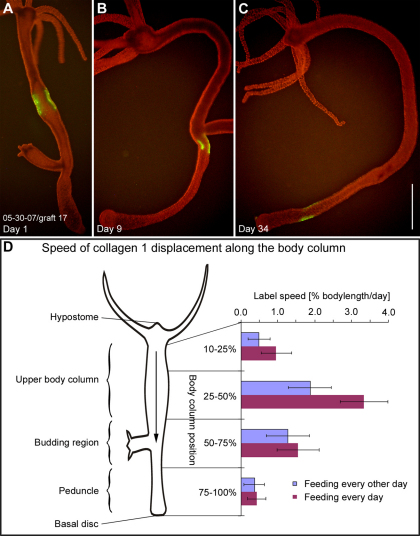
Fig. 2.
Mesoglea displacement along the body column. (A–C) A typical graft in the body column labeled for collagen-1 (green) on day 1, day 9 and day 34 after grafting. The labeled area is progressively displaced toward the aboral end of the animal. (D) Mean label displacement speed (x-axis) in different regions of the body column (y-axis). Tip of the hypostome, body column position 0%; basal disc, body column position 100%. Animals fed daily (purple bars) and animals fed every other day (blue bars) were examined. Each bar represents the mean value of individual label velocities (n=10–14) for each body region at each feeding regime. Scale bar: 1 mm.
The displacement speed of labeled grafts along the body column varied considerably in different regions of the body column (Fig. 2D). In the region from 0–9% body length, which contains the hypostome, the mesoglea was stationary (see below). Graft displacement was slow from 10–25% body length and reached a peak between 25–50% body length. From 50–75% body length, where the majority of budding events happen, the displacement slowed again and reached its lowest level in the peduncle region (75–100% body length). Previous studies have shown that Hydra epithelial movements are dependent on cell proliferation rates, which can be manipulated by different feeding regimes (Bosch and David, 1984; Holstein et al., 1991; Otto and Campbell, 1977b). Mesoglea displacement speed also depended on feeding. Animals fed daily (Fig. 2D, purple bars) showed a higher speed in all body regions compared with animals fed every other day (Fig. 2D, blue bars).
Mesoglea increases in size in the upper body column, decreases in size in the budding region and is degraded at the basal disc
The size of labeled mesoglea grafts increased in the upper body column (Fig. 3A–D). This increase could be observed starting from ~10% body length until the label reached the budding zone. In the budding region, labeled mesoglea grafts decreased in size (Fig. 3E–F, arrowheads). Because the decrease in size of the labeled graft depended on the number of budding events it was involved in, it appeared to be due to recruitment of labeled mesoglea into the evaginating buds (see below). However, among a large number of grafts in our record (n>50), five moved through the budding area without being directly involved in a budding event. Nonetheless, these grafts also exhibited a decrease in size ranging from 43% to 72% of their labeled area, suggesting that label loss into buds is not the only reason for the decrease in label size.
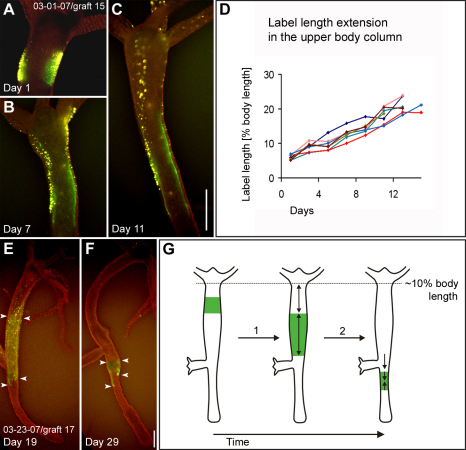
Fig. 3.
Size changes in mesoglea grafts moving down the body column. (A–C) A graft labeled for collagen-1 (green) and ectodermal epithelial cells (yellow) in the upper body column at three different time points. The length of the labeled graft increases from 6.6% body length on day 1 to 21% body length on day 11. (D) Length (y-axis) of seven individual grafts labeled for collagen-1 during a time period of 2 weeks (x-axis). The initial length of all grafts is between 5–7% body length and the initial graft position between 10–20% body length on day 1. The length of the labeled grafts was measured every other day. (E,F) A graft labeled for collagen-1 (green) before (E) and after moving through the budding region (F). Note the decrease in size of the labeled area (arrowheads). A decrease in size was typical for all grafts that moved through the budding region. (G) Schematic drawing of labeled graft expansion (1) in the upper body column and labeled graft shrinkage (2) in the budding region during graft displacement along the body column. Scale bar: 500 μm.
Increases and decreases in labeled mesoglea size (Fig. 3G) paralleled variations in the displacement speed of mesoglea along the body column (Fig. 2D). During displacement along the peduncle, the size of labeled grafts did not change. Only when labeled mesoglea reached the aboral end of the animal, did it decrease in size and ultimately disappear (data not shown). In some animals, we observed fragments of labeled mesoglea in the lumen, suggesting that at least part of the mesoglea, which reached the end of the animal is digested.
Mesoglea is displaced toward the tip of the tentacles, but is stationary in the head region
Labeled mesoglea in the tentacles was displaced toward the tip of the tentacle (Fig. 4A,B, star). The displacement took about 7 days to move from the base of the tentacle to the tip (n=20) as shown in Fig. 4A,B. No differences in label displacement were observed between animals fed daily compared with animals fed every other day (data not shown). Once the labeled mesoglea reached the tip of the tentacle, it shrank in size and disappeared (data not shown). Similarly to the situation in the basal disc, we observed fluorescent material in the endodermal cells of the tentacles, which suggests internalization of degraded mesoglea by the cells.
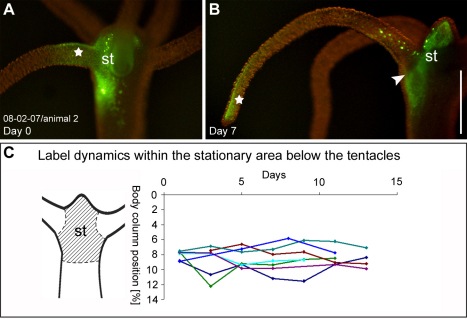
Fig. 4.
Mesoglea fate in the tentacles and the head region. (A,B) Labeled collagen-1 (green) in the head region and the tentacle base 3 hours after injection and 7 days after injection. The label in the tentacle (star) moves to the tip of the tentacle by day 7, the label in the head region (st) remains stationary. (C) Position (y-axis) of the border between labeled and unlabeled tissue of seven individual head grafts over the course of 11 and 13 days (x-axis). No displacement is observed. Scale bar: 500 μm.
Labeled mesoglea in the head region remained stationary (Fig. 4A,B, st) and a sharp border could be observed between the stationary head mesoglea and the mesoglea, which was displaced outward along the tentacle (Fig. 4B, arrowhead). The stationary region included the hypostome of the animal and extended to about 9% body column length: when we grafted heads of labeled donor animals, cut off at 9% (or less) body length, onto gastric columns of non-labeled acceptor animals (or vice versa), the label showed no displacement (Fig. 4C). Grafts at body column position lower than 9%, however, were subject to displacement (Fig. 2D). Labeled mesoglea within the stationary head region faded without visible lateral displacement, which suggests local turnover.
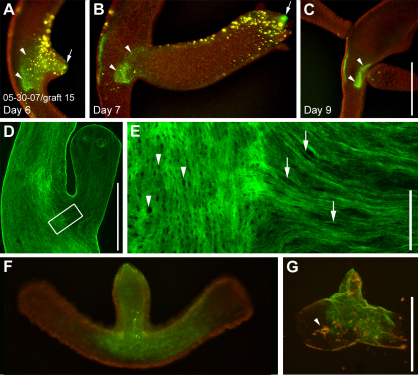
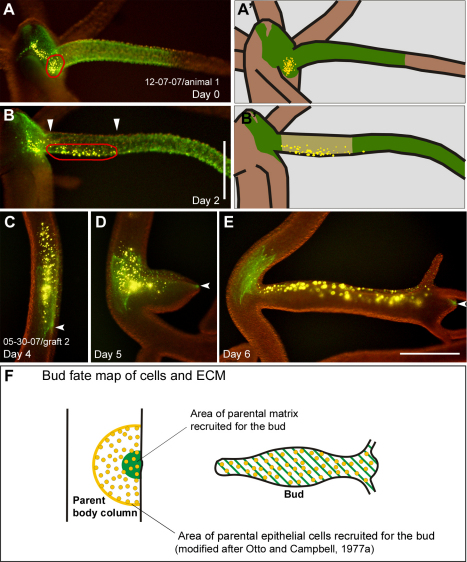
Parental mesoglea is recruited and stretched along evaginating buds
Fig. 5A–C shows a typical example of mesoglea recruitment from the parental body column into a bud. At an early stage of evagination, the labeled mesoglea formed a contiguous fluorescent patch (Fig. 5A, green label). Twenty-four hours later, the fluorescent signal was retained only at the very tip (Fig. 5B, arrow) and at the base of the developing bud (Fig. 5B, arrowheads). As a result of extensive stretching, intervening mesoglea appeared to have lost a significant amount of its immunofluorescence signal. The stretched state of bud mesoglea was also clearly detectable in fixed animals immunostained for collagen-1. Trans-mesogleal pores (Shimizu et al., 2008), which are circular in the parent animal (Fig. 5E, arrowheads) became elongated along the oral–aboral axis in the developing bud (Fig. 5E, arrows). Furthermore, fixed and immunostained animals showed higher collagen concentrations in the tip of the bud (supplementary material Fig. S1; Fig. 6B), confirming our in vivo observations, that this region was excluded from stretching.
Fig. 5.
Mesoglea remodeling during bud formation. (A–C) A graft labeled for collagen-1 (green) and epithelial cells (yellow) during bud formation. Tissue labeling preceded bud evagination. At an early stage of evagination, the labeled mesoglea forms a continuous fluorescent patch (A). 24 hours later fluorescence is only visible at the tip (B, arrow) and the base of the bud (B, arrowheads), whereas fluorescence in the intervening region has disappeared. Note the decrease in size of the labeled mesoglea, which remains in the parent body column (A–C, arrowheads). (D) Fixed stage-6 bud stained for collagen-1. (E) Detail from D showing the border region between parent and bud. The collagen-1 matrix in the bud has a clearly stretched appearance: mesoglea pores, which are circular in the parent animal (arrowheads) appear stretched along the oral–aboral axis of the developing bud (arrows). (F) A living bud stage 4–5 labeled for collagen-1 (green). (G) Isolated mesoglea of the same bud as in F. Some endodermal cell debris (arrowhead) remains trapped in the ECM. Scale bars: 500 μm (C,G), 50 μm (E).
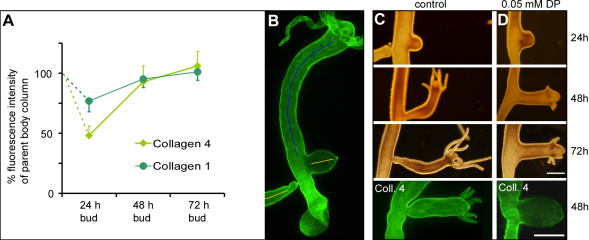
Fig. 6.
The role of collagen synthesis during bud formation. (A) Relative protein concentration of collagen-1 and collagen-4 in different bud stages. The graph shows average fluorescence intensities of collagen-1 and collagen-4 in 24 hour, 48 hour and 72 hour buds (n=10 for each time point). (B) For the values in the graph, the fluorescence intensity of immunocytochemically labeled specimens was measured along a line in the center of the bud (yellow lines) and compared with the fluorescence intensity of the upper body column of the parent animal (blue line). (C) Control bud development at 24, 48 and 72 hours and a 48 hour control bud stained for collagen-4. (D) A typical example of bud development under continuous treatment with 0.05 mM DP and a 48-hour-treated bud stained for collagen-4. Scale bar: 500 μm.
Native mesoglea washed free of cells (Day and Lenhoff, 1981) can be stretched mechanically and snaps back to resting length when released from the deforming force (Hausman and Burnett, 1969; Wainwright et al., 1976), indicating a high inherent elasticity (Sarras and Deutzmann, 2001). The conspicuous stretched appearance and the lowered collagen content of mesoglea in early bud stages (Fig. 5D,E, Fig. 6A,B) raised the question of whether the ECM is simply in a state of elastic stretching imposed by morphogenetic forces from the overlying epithelia or whether the ECM of the early bud is already stably deformed. To answer these questions, we selected intact early buds (stage 3–5), isolated their mesoglea and compared the shape of the bud before and after isolation (Fig. 5F,G). In all cases (n=10), the isolated mesoglea shortened about 40–60% in length along the oral–aboral axis of the animal, suggesting that it is under tension when integrated in tissue. Little change perpendicular to this axis was observed (Fig. 5F,G). However, in all observed specimens, the tube shape of the evaginated bud was preserved, indicating that the mesoglea of early buds is already stably deformed.
Biosynthesis of new collagens is not required for early stages of bud evagination
Transcription of collagens and laminin is upregulated in buds (supplementary material Fig. S2) (Deutzmann et al., 2000; Fowler et al., 2000; Zhang et al., 2007; Zhang et al., 2002), indicating that the developing bud is a site of extensive mesogleal biosynthesis. Nevertheless, owing to rapid bud outgrowth during early stages, collagen levels drop compared with levels in the parent (Fig. 6A). The lowest collagen levels were observed in 24 hour buds. By 48 hours, buds exhibited collagen levels only slightly lower than those in the adult body column, and no difference could be detected between parent and 72 hour buds. Changes in the fluorescence intensity were more pronounced for collagen-4 than for collagen-1 (Fig. 6A). The reason for this effect remains unclear; however, the results clearly indicate that the concentrations of both collagens drop during early bud stages and they only return to original levels in later stages.
To investigate the importance of collagen synthesis for bud evagination we applied the chemical inhibitor 2,2 Dipyridyl (DP). DP is a competitive inhibitor of prolyl-hydroxylase, which catalyses a critical step required for formation of the collagen triple helix (Kivirikko et al., 1989). DP interferes with collagen biosynthesis in vertebrate and invertebrate models, including Hydra (Kawano et al., 2005; Mizoguchi and Yasumasu, 1983; Sarras et al., 1991b; Sarras et al., 1993). Two sets of experiments were performed to test the effect of DP on bud formation. In the first experiments, animals were treated with 0.05 mM DP starting at the first visible sign of bud formation (stage 1–2). Bud development was normal until the first tentacles appeared, but later developmental stages were blocked or severely inhibited (Fig. 6C,D). By 72 hours, control buds had formed a basal disk and were ready to detach from the parent animal. In treated animals, formation of the basal disk at the border to the parent animal was severely inhibited (Fig. 6C,D): 76% of the buds did not form a basal disk, 11% formed an incomplete disk and only 8% formed a complete basal disk (n=83). In addition, tentacle outgrowth was greatly reduced in treated animals. Whereas the tentacles in control buds had grown out to an average length of 12.1 (±3.5) times their diameter at 72 hours, the length of tentacles of treated animals was only 2.1 (±0.8) times their diameter (n=30 tentacles in 15 animals per measurement, compare Fig. 6C and 6D). Washout experiments were performed to determine whether the effects of the inhibitor were reversible. Although tentacle outgrowth recommenced in all treated animals, foot differentiation did not occur, and the majority of inhibited buds did not detach from the parent animal (not shown). Immunostaining of buds after 48 hours of treatment revealed that collagen-4 (Fig. 6C,D) and collagen-1 (not shown) were greatly reduced in comparison to controls. In a second set of experiments, we used synchronized animals (Sudhop et al., 2004) to determine the effects of DP on bud initiation. Budding animals were synchronized by a weekly regime of feeding for 5 days and starving for 2 days. After resumption of feeding, about 50% of large, budless polyps started to form a bud by day 3. We preincubated budless animals starting on day 2 with DP and monitored budding events over the next 36 hours. Treated animals initiated buds and we observed no difference in the budding rate of treated versus untreated animals (0.1 mM DP, 56%, n=54; 0.05 mM DP, 48%, n=58; control 0.1% DMSO, 53%, n=55). The effects of the drug on later bud development were analogous to those described above. Hence we conclude that DP does not affect the initial phase of tissue evagination during budding.
Mesoglea is subject to different levels of proteolytic digestion depending on the body region
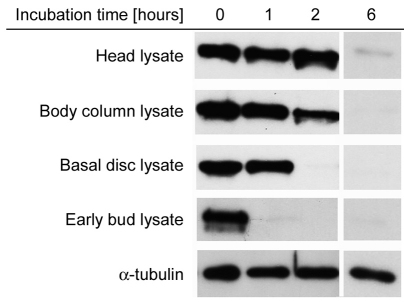
Stable deformation of mesoglea, as occurs during bud formation, requires remodeling of the collagen and laminin network. To investigate mesoglea turnover at these sites, we prepared extracts of appropriate tissue pieces from different body regions and assayed their ability to degrade Hydra laminin. Laminin was chosen as a marker for mesoglea integrity because western blotting for Hydra laminin, in contrast to collagen-1, yielded a stronger and more reproducible signal. Our antibody against Hydra laminin (mAb52) produced a clear band at about 200 kDa. As shown in Fig. 7, Hydra laminin was rapidly degraded by early bud extracts and basal disc tissue. Body column extracts and head extracts also degraded laminin, but at a slower rate. α-Tubulin, which was also present in all tissue lysates, was not significantly degraded under the conditions used. This suggests that the laminin degradation observed is not the result of non-specific protease activity present in tissue lysates. Thus, the body-part-specific differences in laminin proteolysis appear to reflect the different levels of mesoglea remodeling observed in our in vivo tracking experiments.
Fig. 7.
Mesoglea turnover in different body regions estimated as laminin stability in tissue lysates. Isolated mesoglea samples containing laminin were incubated in tissue lysates from different body regions of the animal as indicated. For head lysate, only the head without tentacle tissue was used (compare Fig. 4C, st). Bud lysate was taken from bud stages 3–5. Samples taken at different time points were analyzed by western blotting using antibodies against laminin and α-tubulin. The α-tubulin is not significantly degraded in any of the lysate samples; the α-tubulin blot shown corresponds to the basal disc lysate.
Mesoglea and overlying epithelial cells move at similar rates in most regions of the animal
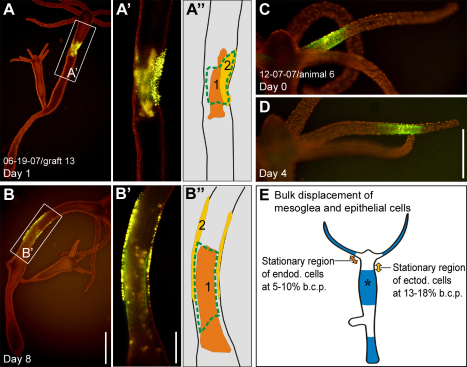
The apparent similarity between previously reported cell movement patterns and the mesogleal displacement patterns, which we observed in the current study, suggested that epithelial cells move in concert with their associated mesoglea. To address this question directly, we labeled epithelial cells and the mesoglea in the same region and compared their displacement dynamics. Epithelial cells move in both directions along the body column: up toward the tip of the tentacles and down toward the basal disc (Campbell, 1967b). The dividing line between upward and downward movement, the ‘stationary region’, is located in the upper body column. In our experiments, the stationary region was located in the upper body column at ~5–10% body length in the endoderm and at ~13–18% body length in the ectoderm (Fig. 8E), which is consistent with previous reports (Campbell, 1967b; Campbell, 1973; Otto and Campbell, 1977b). In the body column below ~18% body length, all three layers, mesoglea, endoderm and ectoderm, moved towards the basal disc. Furthermore, longitudinal expansion of all three tissue elements was observed concomitant with body column motion (Fig. 8A,B,A′,B′). Mesogleal and endodermal markers exhibited virtually complete motion pattern overlap, whereas the ectoderm moved downward at a slightly lower speed (Fig. 8A,B,A′,B′, compare also with Fig. 3A–C). The ectodermal cell sheet thus resembled a ‘sleeve’ being pushed up relative to the mesoglea and endoderm (Fig. 8B′). In the vicinity of the budding region and in the outgrowing bud itself, mesoglea and the overlying cells of both layers moved at different rates (see below). In the lower peduncle, both epithelial layers and the adjacent mesoglea were displaced as a composite structure until they reached the aboral end of the animal (data not shown). In the tentacles, only ectodermal cells and the associated mesoglea were examined. Both tissue elements moved to the tip of the tentacle at identical rates (Fig. 8C,D), which contrasts with the situation at the tentacle base (see below). The tissue displacement velocities of epithelial cells and mesoglea observed at different body column positions and in the tentacles are in good agreement with previous measurements on Hyda vulgaris (Otto and Campbell, 1977b) and are about 30% slower than similar measurements on Hydra littoralis (Campbell, 1967b) and Hydra viridissima (Campbell, 1973).
Fig. 8.
Epithelial cell and mesoglea dynamics in the body column and tentacles. Representative examples of labeled tissue displacement in individual animals. (A,B) A graft labeled for collagen-1 (green), ectodermal epithelial cells (bright yellow) and endodermal epithelial cells (orange) in the upper body column on day 1 and day 8. (A′,B′) Magnified views. (A″,B″) Schematic views. The perspective of A is about 180° rotated from B, as indicated by the position of cell cluster 2 in A″ and B″. (C,D) Labeled patch of collagen-1 (green) and ectodermal epithelial cells (yellow) in a tentacle on day 0 and day 4. Cells and ECM retain a 100% overlap. (E) Results of mesoglea and cell sheet movement illustrated schematically based on observations in multiple animals (n=10 in head and tentacle base, n=10 in tentacle, n=30 in the upper body column, n=50 in budding area and bud, n=15 in the lower peduncle region). Regions where epithelial cells and mesoglea move together (blue) are shown. In the upper body column, the ectodermal layer moves slightly slower than the endodermal layer and the mesoglea (asterisk). The stationary region for ectodermal epithelial cells is located at a body column position of about 13–18% (yellow double-headed arrow) and for endodermal epithelial cells at a body column position of about 5–10% (orange double-headed arrow). Scale bars: 1 mm (B), 500 μm (B,D).
Mesoglea and epithelial cells move at different rates during tentacle and bud formation
Epithelial cells of the tentacle originate in the body column (Fig. 3A–C) (Campbell, 1967b; Hobmayer et al., 1990), whereas the new tentacle mesoglea is made at the base of the tentacle (Fig. 4A,B). Consequently, when cells moved into the tentacles, they appeared to leave the stationary mesoglea in the head region and moved onto newly forming mesoglea at the tentacle base (Fig. 9A,B). Once in the tentacle, ectodermal cells were displaced together with their underlying mesoglea (Fig. 8C,D). A very similar situation could also be observed during budding. Numerous cells of both epithelial layers moved from the parent body column into developing buds (Otto and Campbell, 1977a), whereas the amount of recruited mesoglea remained relatively small (Fig. 9C–E, Fig. 4D,E; supplementary material Fig. S3B–E). The area of recruited cells had a diameter approximately 3–4 times larger than the area of recruited ECM (Fig. 9F, n=5).
Fig. 9.
Epithelial cell and mesoglea dynamics at the tentacle base and during budding. (A) Head region of an animal with labeled mesoglea (green) extending from the head region into the tentacle and a group of labeled ectodermal epithelial cells (yellow, circled in red) between two tentacles at day 0. (A′) Schematic. (B) On day 2, the group of epithelial cells (circled in red) have moved from the stationary head region into the tentacle. Note the gap with strongly reduced green fluorescence at the proximal end of the tentacle (arrowheads) indicating mesoglea expansion. (B′) Schematic. (C–E) A graft labeled for collagen-1 (green) and epithelial cells (yellow) in the body column over the course of three consecutive days before (C) and during (D,E) budding. Cells move relative to their underlying substrate into the newly developing animal (D,E). Note that only a small part of the labeled, parental mesoglea is recruited into the bud (arrowheads). (F) Fate map of epithelial cells and ECM of the parent body column recruited into a bud. The schematic shows the area of epithelial cells recruited to the bud (yellow) compared with the area of ECM recruited to the bud (green). The fate map was constructed based on an idealized body column diameter (Otto and Campbell, 1977a). Scale bars: 500 μm.
Discussion
The new experimental approach of labeling ECM in living systems (Davidson et al., 2008; Larsen et al., 2006; Ohashi et al., 1999; Sivakumar et al., 2006) provides a promising tool to assess the role of the ECM during tissue growth and morphogenesis. At the whole organism level, such an analysis has so far only been done on the vertebrate avian embryo (Czirok et al., 2004; Zamir et al., 2006; Zamir et al., 2005; Zamir et al., 2008). These studies revealed that the ECM is unexpectedly dynamic, and contributes significantly to overall tissue movements during gastrulation and neurulation. Here, we have investigated the behaviour of the basement membrane system in a simple invertebrate animal, the cnidarian Hydra. The epithelium of Hydra is one of the earliest examples of true epithelial organisation in metazoan evolution (Tyler, 2003). The surprisingly dynamic nature of Hydra ECM observed in our experiments – together with similar observations in the distantly related vertebrate embryo – suggests that ECM in the tissues of all multicellular animals has a more dynamic nature than so far assumed.
The role of mesoglea during Hydra tissue movements
Although the mesoglea appears to be a stable structure and can be isolated intact free of cells from Hydra, our experiments in vivo with patches of labeled mesoglea indicate that it is in fact a dynamic structure that undergoes constant remodeling (Fig. 10A). Mesoglea is produced in the upper body column and displaced to the basal disk, where it is degraded. Similarly, mesoglea is produced at the tentacle base and displaced to the tip of the tentacle, where it is degraded. This pattern of ECM flow from central body regions to the periphery of the animal is similar to the flow of epithelial cells, and overlaps with it to a significant degree (Fig. 8E). Thus, not only epithelial sheets but also the intervening mesoglea is subjected to a constant centrifugal tissue renewal. These observations reject the assumption that the general nature of Hydra tissue movements represents active epithelial migration relative to a stationary mesoglea (Burnett and Hausman, 1969; Shostak and Globus, 1966; Shostak et al., 1965). Rather, they support the view of Campbell (Campbell, 1973; Campbell, 1974) that the apparent ‘movement’ of epithelial cells relative to the morphology of the animal can be understood as continuous ‘outward’ expansion of the whole tissue.
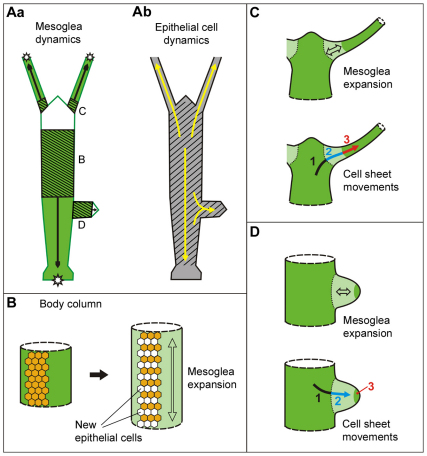
Fig. 10.
Fate map of Hydra mesoglea and a model of cell–matrix interactions during growth and morphogenesis. (A) Mesoglea (a) and epithelial cell (b) dynamics. Black arrows in a indicate the direction of mesoglea movements; shaded areas indicate mesoglea stretching and growth; white areas indicate stationary mesoglea in the head and bud tip; stars mark sites of mesoglea degradation. Yellow arrows in b indicate the direction of epithelial cell movements; shaded area marks the area of epithelial cell proliferation (Campbell, 1967a; Holstein et al., 1991). (B) Mesoglea growth in the body column is associated with cell proliferation. (C) Mesoglea stretching (top) and cell movements (bottom) during steady state tentacle evagination. Light green represents the area of mesoglea expansion (double-headed arrow); dark green represents the region of stable mesoglea; arrow indicates the direction of cell movement. The relationship between cells and substrate changes during these morphogenetic movements: cells move over stable mesoglea (1); cells move over expanding mesoglea (2); cells moved together with mesoglea (3). (D) Expansion of the mesoglea (top) and cell movements (bottom) during bud evagination. Labeling analogous to that in C.
Despite these general similarities in the dynamic behaviour of epithelial cells and the mesoglea, there is not a simple relationship between cells and matrix. At least five different situations can be distinguished: (1) Mesoglea in the head region is very stable and appears to change neither in size nor in position. The overlying epithelial cells, however, undergo continuous proliferation and slow movement toward the base of tentacles and the tip of the hypostome (Campbell, 1967b; Dubel, 1989). (2) Mesoglea in the upper gastric region grows in length along the oral aboral axis essentially at the same rate as the overlying epithelial sheet expands due to cell proliferation (Fig. 8A,B). (3) Mesoglea at the base of tentacles expands extensively to accommodate new epithelial cells, which flow in from the head region (Fig. 9A,B). (4) Similarly, mesoglea of the evaginating bud is subjected to extensive stretching and thinning to accommodate large numbers of epithelial cells, which flow in from the parent body column (Fig. 9C–E). (5) In the distal tentacles, labeled mesoglea patches remain constant in size and move together with overlying epithelial cells distally along the tentacle (Fig. 8C,D). Similarly, patches of stained mesoglea in the peduncle change little in size while moving down the body column together with overlying epithelial cells.
These apparently diverse behaviours of cells and matrix in different body parts are consistent with the following principles. Epithelial sheets can change shape easily by rearranging individual cells within the epithelial sheet, as can be observed during their movement into buds and tentacles (Clarkson and Wolpert, 1967; Philipp et al., 2009). Furthermore, epithelia are able to detach from the mesoglea in situations where they move over its surface. By comparison, the mesoglea is rigid and requires biochemical remodeling to change shape, including both softening to permit stretching of existing networks and subsequent new synthesis to restore a mature mesoglea structure. Most interestingly, and in contrast to epithelial cells, the mesoglea is made separately in every body part (Fig. 10Aa) and these ‘compartments’ of mesoglea do not mix (except for the bud mesoglea, which is derived from a small patch of parental mesoglea and subsequently strengthened by newly synthesized mesoglea components). These results suggest the need for local patterning signals to achieve this body-part-specific mesoglea fate. Because mesoglea is made by the overlying epithelial sheet, this implies that the epithelial cells in different regions of the animal secrete a different repertoire of modifying enzymes and different amounts of new mesoglea components according to the local need.
Mechanisms of mesoglea morphogenesis in different body regions
In view of the mechanical stability of mesoglea (Day and Lenhoff, 1981; Hausman and Burnett, 1969; Wainwright et al., 1976), it is clear that changing mesoglea shape, as occurs during bud formation, tentacle formation and more slowly during body column expansion, requires alterations to the network of constituent macromolecules. This might involve proteolytic remodeling of the mesoglea. Results shown in Fig. 7 demonstrate that enzymes that degrade laminin display different activities in different body parts of the animal, which is consistent with the level of mesoglea shape change observed during in vivo tracking experiments. Lysates from evaginating buds for instance, where high rates of mesoglea shape change occur, display much higher rates of laminin degradation compared with head lysates, where the mesoglea is comparably stable. Matrix metalloproteinases (MMPs) have been shown to cleave ECM components in vertebrates (Page-McCaw et al., 2007) and two such MMPs have been described in Hydra. HMMP is expressed in the distal end of tentacles and the basal disc (Leontovich et al., 2000; Shimizu et al., 2008). It has been shown to cleave collagen in vitro and might be involved in the degradation of mesoglea at the tip of the tentacles and the basal disc. MMP-A3 (Munder et al., 2010) is expressed in a narrow region at the base of late bud stages and might function in the separation of buds from parent animals. In addition to these MMPs, there are at least five more MMPs of unknown function present in the genome of Hydra (Chapman et al., 2010) (data not shown). Local activity of these metalloproteinases during bud evagination, at the base of tentacles and in the upper body column could, in principle, loosen the stable mesoglea structure and permit shape changes.
Although enzymatic remodeling of mesoglea by matrix modifying enzymes such as MMPs might help to make existing ECM scaffolds more susceptible to shape change by ‘softening’ rigid networks, the shape change itself must be brought about by physical forces created by the morphogenetic activities of overlying cells. Mesoglea growth in the upper body column is associated with epithelial expansion (Fig. 8), which happens as a result of cell proliferation (Otto and Campbell, 1977b). Changing the feeding rate affects both cell proliferation (Holstein et al., 1991; Otto and Campbell, 1977b) and mesoglea displacement rates, suggesting that mesoglea growth in this region is related to epithelial cell proliferation. In the simplest case, cell proliferation and the resulting increase in epithelial area could directly generate the physical force necessary to stretch the underlying mesoglea (Fig. 10B). Recent observations based on clones of proliferating GFP-expressing epithelial cells in the body column (Wittlieb et al., 2006) (R.A., unpublished results) show that the clones are aligned along the oral–aboral axis of the animal, supporting the hypothesis of cell proliferation as the driving force for mesoglea expansion along that axis. It remains unclear, however, why the mesoglea in other body regions, where epithelia proliferate and expand, such as the head or the lower gastric region, does not change in size. A lower level of proteolytic remodeling and the resulting higher mechanical stability of mesoglea could account for this phenomenon.
By contrast, mesoglea expansion at the base of tentacles and in the evaginating bud is based on a different mechanism. Both tentacle and bud outgrowth are associated with rapid epithelial movements, which are independent of cell proliferation (Clarkson and Wolpert, 1967; Diehl and Burnett, 1965; Otto and Campbell, 1977b). These movements appear to be driven by lateral intercalation of individual epithelial cells (Philipp et al., 2009) (supplementary material Fig. S3), a cell behaviour that has been shown to generate physical forces necessary for morphogenesis in other systems (Bertet et al., 2004; Rauzi et al., 2008). The movements are also accompanied by radical changes in the relationship of epithelial sheets to their underlying substrate. In the head region, epithelial cells migrate over the stationary mesoglea until they reach the border between head and tentacle tissue (Fig. 10C, 1). Distal to this border, they step onto an expanding mesoglea (Fig. 10C, 2). Even further distally along the tentacle, cells stably attach to unchanging mesoglea and both are displaced together towards the tentacle tip (Fig. 10C, 3). Cell-matrix interactions during bud evagination are very similar (Fig. 10D, 1–3). We hypothesize that physical forces generated during epithelial cell movements induce the observed local mesoglea shape change (Fig. 10C,D, top). It remains unclear, however, how forces transmitted from migrating epithelial sheets result only in a very localized shape change of the mesoglea at the tentacle base (Fig. 10C, 2) and in the evaginating bud after passing the parent–bud border (Fig. 10D, 2). Again, local proteolytic remodeling might make the mesoglea in these areas more susceptible to shape change.
Whereas mesoglea expansion in the tentacle base and the evaginating bud are both associated with epithelial cell movements, the rate at which cells enter tentacles and buds differs significantly. Tentacle tissue grows out by 10–12% of its length per day, which corresponds to roughly 100 epithelial cells (Otto and Campbell, 1977a). By contrast, bud area multiplies four to five times in size during the first 24 hours and more than 4000 cells are recruited from the parental body column in this time interval (Graf and Gierer, 1980; Otto and Campbell, 1977a). In both young buds and tentacle bases, high levels of laminin and collagen expression reflect the need for new ECM material (Zhang et al., 2007; Zhang et al., 2002) (supplementary material Fig. S2). In the evaginating bud, however, the rate of expansion is so great that ECM synthesis cannot keep up and for 24 hours, young buds show reduced levels of collagen staining (Fig. 5, Fig. 6A,B; supplementary material Fig. S1). By contrast, no such thinning of ECM was observed at the tentacle base, probably because of the slower rate of mesoglea expansion. Nevertheless de novo synthesis is not essential for rapid evagination, because inhibition of collagen biosynthesis with DP did not inhibit the initial phase of bud evagination. This suggests that remodeling of the existing ECM network, but not new ECM synthesis, is critical for early bud evagination.
Materials and Methods
Animal culture
Hydra vulgaris (strain Basel) was used in all experiments. The animals were cultured at 18°C in mass culture dishes of 1000–2000 individuals per dish containing 2 liters of M-solution (Lenhoff, 1983) and were fed with freshly hatched Artemia salina larvae. During tracking experiments, individual animals were kept in single wells containing 2 ml culture medium and were fed either daily or every other day with 10–15 Artemia salina larvae.
In vivo mesoglea labeling
Monoclonal antibodies against Hydra collagen-1 (mAb39) and laminin (mAb52) (Sarras et al., 1991a; Sarras et al., 1993) were labeled with Alexa Fluor 488 and Alexa Fluor 546 Monoclonal Antibody Labeling Kits (Molecular Probes) following the manufacturer's instructions. Fluorescently tagged antibodies were loaded into pulled glass needles at a concentration of 1 mg/ml and injected directly into the mesoglea with a PLI-90 pressure injector (Harvard Apparatus). Single injections of 10–50 nl volumes resulted in labeling of 10–20% of the entire mesoglea of an animal. Multiple injections allowed us to label the entire animal.
In vivo labeling of epithelial cells
Ectodermal epithelial cells were labeled with Fluoresbrite polychromatic 1.0 micron microspheres (Polysciences, Warrington, PA) either by incubation for 12 hours in a 0.026% solution (Technau and Holstein, 1992) or by direct injection of a 0.13% solution together with the fluorescently tagged antibodies. Endodermal epithelial cells were labeled with Fluoresbrite polychromatic microspheres by injection of a 2.6% solution into the gastric cavity or by injection of 20 mg/ml Rhodamine Dextran (Sigma). These two labeling methods resulted in uniform labeling of the endodermal cell layer. Alternatively, labeling of small clusters of endodermal cells with CM-DiI (Molecular Probes) was carried out according to the method described previously (Kishimoto et al., 1996).
Tissue grafting
Grafting experiments were performed 24 hours after mesoglea and cell labeling and were carried out according to the procedure described earlier (Teragawa and Bode, 1990).
Imaging of living animals
Living animals were relaxed in 2% urethane in culture medium for 2–5 minutes before observation under a Nikon SMZ 1500 stereomicroscope equipped a filter-set for 450–490 nm excitation and 515 nm long pass emission. The long-pass emission filter allowed us to observe double- and triple-labeled specimens with the Alexa Fluor 488 in green emission and Fluoresbrite polychromatic microspheres and DiI in yellow–orange emission by using only blue excitation. The stereomicroscope was equipped with an EXFO X-cite series 120 fluorescent light source. Images were taken with an Olympus DP70 digital camera.
Confocal microscopy
Immunofluorescent staining with monoclonal antibodies mAb39 against collagen-1 and JK-2 against collagen-4 was performed as described earlier (Shimizu et al., 2008). Animals labeled in vivo by injection of fluorescently tagged antibodies were relaxed in 2% Urethane for 2 minutes and fixed in 4% paraformaldehyde for 30 minutes at room temperature. After three washes in PBS, they were mounted in vectashield (Vector Laboratories) and observed under a Zeiss 510 LSM or a Nikon eclipse 50i microscope.
Mesoglea isolation
Isolation of mesoglea of single buds was performed as described for whole animals (Day and Lenhoff, 1981).
Morphometry and measurements of fluorescence intensity
Morphometric measurements and measurements of the fluorescence intensity were performed on digital images using ImageJ software. For measurements of fluorescence intensities of collagens in buds, average grey values along a central line were compared with average grey values along a central line in the upper body column (Fig. 5D).
ECM degradation essay
ECM turnover was monitored on protein levels by assaying the proteolytic stability of laminin in isolated mesoglea samples incubated with different tissue lysates. For each set of experiments, the mesogleas of 15 animals were extracted and solubilized in 30 μl PBS. Cell lysates from different body parts of Hydra (15–25 animals) were generated by passing the tissue through a syringe needle several times on ice. The lysate was cleared by centrifugation and the protein concentration of each supernatant was adjusted to 1 mg/ml by adding PBS. The cell lysates were immediately mixed with the mesoglea samples in a 1:1 ratio and incubated at room temperature. Samples were taken at different time points, mixed with sample buffer and boiled. Laminin stability was assayed by western blotting using monoclonal Hydra laminin antibody (mAB 52) at 1:1000 and secondary anti-mouse HRP at 1:10,000. Mouse anti-α-tubulin was applied at 1:2000, secondary anti-mouse HRP at 1:10,000. The mesoglea samples had to be preincubated in the respective tissue lysates for 1 hour to release the laminin from the ECM network. After preincubation, the levels of laminin were approximately equal in all samples (time point=0).
In situ hybridization
Experiments were carried out using probes to Hydra collagen-1 and collagen-4 (Deutzmann et al., 2000; Fowler et al., 2000). The procedure for in situ hybridization is the same as described previously (Zhang et al., 2007).
Drug treatment with 2,2′-dipyridyl
Treatment was performed by soaking the animals in 0.05 mM or 0.1 mM DP (Sigma) and 0.1% DMSO in culture medium at 1 ml solution per individual. All controls were incubated in 0.1% DMSO in culture medium. Inhibitor treatment was performed in the dark and solutions were changed routinely every 24 hours.
Acknowledgements
The authors would like to thank Dale R. Abrahamson for providing the Hydra mesoglea specific antibodies and Stefanie Pontasch for critical reading and helpful comments on the manuscript. This work was initiated and carried out mainly in the lab of X.Z. by R.A. when R.A. was a visiting PhD candidate.
Footnotes
Funding
Funding for this work was provided by a program project grant from the National Institutes of Health NIDDK [grant number 5 P01 DK065123] to D.R.A. and M.P.S. and by an award from the G. Harold and Lila Y. Mathers Charitable Foundation to C.D.L. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.087239/-/DC1
References
- Benazeraf B., Francois P., Baker R. E., Denans N., Little C. D., Pourquie O. (2010). A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466, 248-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C., Sulak L., Lecuit T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667-671 [DOI] [PubMed] [Google Scholar]
- Bosch T. C., David C. N. (1984). Growth regulation in Hydra: relationship between epithelial cell cycle length and growth rate. Dev. Biol. 104, 161-171 [DOI] [PubMed] [Google Scholar]
- Burnett A. L., Hausman R. E. (1969). Mesoglea of Hydra. 2. Possible Role in Morphogenesis. J. Exp. Zool. 171, 15-24 [DOI] [PubMed] [Google Scholar]
- Campbell R. D. (1967a). Tissue dynamics of steady state growth in Hydra littoralis. I. Patterns of cell division. Dev. Biol. 15, 487-502 [DOI] [PubMed] [Google Scholar]
- Campbell R. D. (1967b). Tissue dynamics of steady state growth in Hydra littoralis. II. Patterns of tissue movement. J. Morphol. 121, 19-28 [DOI] [PubMed] [Google Scholar]
- Campbell R. D. (1973). Vital marking of single cells in developing tissues: India ink injection to trace tissue movements in hydra. J. Cell Sci. 13, 651-661 [DOI] [PubMed] [Google Scholar]
- Campbell R. D. (1974). Cell movements in Hydra. Amer. Zool. 14, 523-535 [Google Scholar]
- Chapman J. A., Kirkness E. F., Simakov O., Hampson S. E., Mitros T., Weinmaier T., Rattei T., Balasubramanian P. G., Borman J., Busam D., et al. (2010). The dynamic genome of Hydra. Nature 464, 592-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson S. G., Wolpert L. (1967). Bud morphogenesis in hydra. Nature 214, 780-783 [DOI] [PubMed] [Google Scholar]
- Czirok A., Rongish B. J., Little C. D. (2004). Extracellular matrix dynamics during vertebrate axis formation. Dev. Biol. 268, 111-122 [DOI] [PubMed] [Google Scholar]
- Davidson L. A., Dzamba B. D., Keller R., Desimone D. W. (2008). Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev. Dyn. 237, 2684-2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. M., Lenhoff H. M. (1981). Hydra mesoglea: a model for investigating epithelial cell-basement membrane interactions. Science 211, 291-294 [DOI] [PubMed] [Google Scholar]
- Deutzmann R., Fowler S., Zhang X., Boone K., Dexter S., Boot-Handford R. P., Rachel R., Sarras M. P., Jr (2000). Molecular, biochemical and functional analysis of a novel and developmentally important fibrillar collagen (Hcol-I) in hydra. Development 127, 4669-4680 [DOI] [PubMed] [Google Scholar]
- Diehl F. A., Burnett A. L. (1965). The role of interstitial cells in the maintenance of Hydra. II. Budding. J. Exp. Zool. 158, 283-295 [DOI] [PubMed] [Google Scholar]
- Dubel S. (1989). Cell differentiation in the head of Hydra. Differentiation 41, 99-109 [Google Scholar]
- Epp L., Smid I., Tardent P. (1986). Synthesis of the Mesoglea by Ectoderm and Endoderm in Reassembled Hydra. J. Morphol. 189, 271-279 [DOI] [PubMed] [Google Scholar]
- Fowler S. J., Jose S., Zhang X., Deutzmann R., Sarras M. P., Jr, Boot-Handford R. P. (2000). Characterization of hydra type IV collagen. Type IV collagen is essential for head regeneration and its expression is up-regulated upon exposure to glucose. J. Biol. Chem. 275, 39589-39599 [DOI] [PubMed] [Google Scholar]
- Graf L., Gierer A. (1980). Size, shape and orientation of cells in budding hydra and regulation of regeneration in cell aggregates. Dev. Genes Evol. 188, 141-151 [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Burnett A. L. (1969). The mesoglea of Hydra. I. Physical and histochemical properties. J. Exp. Zool. 171, 7-14 [Google Scholar]
- Hausman R. E., Burnett A. L. (1971). Mesoglea of Hydra. IV. Qualitative radioautographic study of protein component. J. Exp. Zool. 177, 435-446 [Google Scholar]
- Hobmayer E., Holstein T. W., David C. N. (1990). Tentacle morphogenesis in hydra. II. Formation of a complex between a sensory nerve cell and a battery cell. Development 109, 897-904 [Google Scholar]
- Holstein T. W., Hobmayer E., David C. N. (1991). Pattern of epithelial cell cycling in hydra. Dev. Biol. 148, 602-611 [DOI] [PubMed] [Google Scholar]
- Ingber D. E. (2006). Mechanical control of tissue morphogenesis during embryological development. Int. J. Dev. Biol. 50, 255-266 [DOI] [PubMed] [Google Scholar]
- Kawano H., Li H. P., Sango K., Kawamura K., Raisman G. (2005). Inhibition of collagen synthesis overrides the age-related failure of regeneration of nigrostriatal dopaminergic axons. J. Neurosci. Res. 80, 191-202 [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Murate M., Sugiyama T. (1996). Hydra regeneration from recombined ectodermal and endodermal tissue. I. Epibolic ectodermal spreading is driven by cell intercalation. J. Cell Sci. 109, 763-772 [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllyla R., Pihlajaniemi T. (1989). Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 3, 1609-1617 [PubMed] [Google Scholar]
- Larsen M., Wei C., Yamada K. M. (2006). Cell and fibronectin dynamics during branching morphogenesis. J. Cell Sci. 119, 3376-3384 [DOI] [PubMed] [Google Scholar]
- Lenhoff H. M. (1983). Culturing large numbers of Hydra. In Hydra: Research Methods (ed. Lenhoff H.M.), pp. 53-62 New York: Plenum Press; [Google Scholar]
- Leontovich A. A., Zhang J., Shimokawa K., Nagase H., Sarras M. P., Jr (2000). A novel hydra matrix metalloproteinase (HMMP) functions in extracellular matrix degradation, morphogenesis and the maintenance of differentiated cells in the foot process. Development 127, 907-920 [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., Yasumasu I. (1983). Inhibition of archenteron formation by the inhibitors of prolyl hydroxylase in sea urchin embryos. Cell Differ. 12, 225-231 [DOI] [PubMed] [Google Scholar]
- Munder S., Kasbauer T., Prexl A., Aufschnaiter R., Zhang X., Towb P., Bottger A. (2010). Notch signalling defines critical boundary during budding in Hydra. Dev. Biol. 344, 331-345 [DOI] [PubMed] [Google Scholar]
- Ohashi T., Kiehart D. P., Erickson H. P. (1999). Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc. Natl. Acad. Sci. USA 96, 2153-2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto J. J., Campbell R. D. (1977a). Budding in Hydra attenuata: bud stages and fate map. J. Exp. Zool. 200, 417-428 [DOI] [PubMed] [Google Scholar]
- Otto J. J., Campbell R. D. (1977b). Tissue economics of hydra: regulation of cell cycle, animal size and development by controlled feeding rates. J. Cell Sci. 28, 117-132 [DOI] [PubMed] [Google Scholar]
- Page-McCaw A., Ewald A. J., Werb Z. (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp I., Aufschnaiter R., Ozbek S., Pontasch S., Jenewein M., Watanabe H., Rentzsch F., Holstein T. W., Hobmayer B. (2009). Wnt/beta-catenin and noncanonical Wnt signaling interact in tissue evagination in the simple eumetazoan Hydra. Proc. Natl. Acad. Sci. USA 106, 4290-4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzi M., Verant P., Lecuit T., Lenne P. F. (2008). Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401-1410 [DOI] [PubMed] [Google Scholar]
- Rongish B. J., Drake C. J., Argraves W. S., Little C. D. (1998). Identification of the developmental marker, JB3-antigen, as fibrillin-2 and its de novo organization into embryonic microfibrous arrays. Dev. Dyn. 212, 461-471 [DOI] [PubMed] [Google Scholar]
- Sarras M. P., Jr, Deutzmann R. (2001). Hydra and Niccolo Paganini (1782-1840)--two peas in a pod? The molecular basis of extracellular matrix structure in the invertebrate, Hydra. Bioessays 23, 716-724 [DOI] [PubMed] [Google Scholar]
- Sarras M. P., Jr, Madden M. E., Zhang X. M., Gunwar S., Huff J. K., Hudson B. G. (1991a). Extracellular matrix (mesoglea) of Hydra vulgaris. I. Isolation and characterization. Dev. Biol. 148, 481-494 [DOI] [PubMed] [Google Scholar]
- Sarras M. P., Jr, Meador D., Zhang X. M. (1991b). Extracellular matrix (mesoglea) of Hydra vulgaris. II. Influence of collagen and proteoglycan components on head regeneration. Dev. Biol. 148, 495-500 [DOI] [PubMed] [Google Scholar]
- Sarras M. P., Jr, Zhang X., Huff J. K., Accavitti M. A., St John P. L., Abrahamson D. R. (1993). Extracellular matrix (mesoglea) of Hydra vulgaris III. Formation and function during morphogenesis of hydra cell aggregates. Dev. Biol. 157, 383-398 [DOI] [PubMed] [Google Scholar]
- Shimizu H., Aufschnaiter R., Li L., Sarras M. P., Jr, Borza D. B., Abrahamson D. R., Sado Y., Zhang X. (2008). The extracellular matrix of hydra is a porous sheet and contains type IV collagen. Zoology (Jena) 111, 410-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak S., Globus M. (1966). Migration of epithelio-muscular cells in Hydra. Nature 210, 218-219 [DOI] [PubMed] [Google Scholar]
- Shostak S., Kankel D. R. (1967). Morphogenetic movements during budding in Hydra. Dev. Biol. 15, 451-463 [DOI] [PubMed] [Google Scholar]
- Shostak S., Patel N. G., Burnett A. L. (1965). The role of mesoglea in mass cell movement in Hydra. Dev. Biol. 12, 434-450 [DOI] [PubMed] [Google Scholar]
- Sivakumar P., Czirok A., Rongish B. J., Divakara V. P., Wang Y. P., Dallas S. L. (2006). New insights into extracellular matrix assembly and reorganization from dynamic imaging of extracellular matrix proteins in living osteoblasts. J. Cell Sci. 119, 1350-1360 [DOI] [PubMed] [Google Scholar]
- Sudhop S., Coulier F., Bieller A., Vogt A., Hotz T., Hassel M. (2004). Signalling by the FGFR-like tyrosine kinase, Kringelchen, is essential for bud detachment in Hydra vulgaris. Development 131, 4001-4011 [DOI] [PubMed] [Google Scholar]
- Technau U., Holstein T. W. (1992). Cell sorting during the regeneration of Hydra from reaggregated cells. Dev. Biol. 151, 117-127 [DOI] [PubMed] [Google Scholar]
- Teragawa C. K., Bode H. R. (1990). Spatial and temporal patterns of interstitial cell migration in Hydra vulgaris. Dev. Biol. 138, 63-81 [DOI] [PubMed] [Google Scholar]
- Tyler S. (2003). Epithelium–The primary building block for metazoan complexity. Integr. Comp. Biol. 43, 55-63 [DOI] [PubMed] [Google Scholar]
- Wainwright S. A., Biggs W. D., Currey J. D., Gosline J. M. (1976). Support in Organisms. In Mechanical Design in Organisms, pp. 287-344 New York: Halsted Press, John Wiley and Sons; [Google Scholar]
- Wittlieb J., Khalturin K., Lohmann J. U., Anton-Erxleben F., Bosch T. C. (2006). Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc. Natl. Acad. Sci. USA 103, 6208-6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E. A., Czirok A., Rongish B. J., Little C. D. (2005). A digital image-based method for computational tissue fate mapping during early avian morphogenesis. Ann. Biomed. Eng. 33, 854-865 [DOI] [PubMed] [Google Scholar]
- Zamir E. A., Czirok A., Cui C., Little C. D., Rongish B. J. (2006). Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc. Natl. Acad. Sci. USA 103, 19806-19811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E. A., Rongish B. J., Little C. D. (2008). The ECM moves during primitive streak formation–computation of ECM versus cellular motion. PLoS Biol. 6, e247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Fei K., Agbas A., Yan L., Zhang J., O'Reilly B., Deutzmann R., Sarras M. P., Jr (2002). Structure and function of an early divergent form of laminin in hydra: a structurally conserved ECM component that is essential for epithelial morphogenesis. Dev. Genes. Evol. 212, 159-172 [DOI] [PubMed] [Google Scholar]
- Zhang X., Boot-Handford R. P., Huxley-Jones J., Forse L. N., Mould A. P., Robertson D. L., Lili, Athiyal M., Sarras M. P., Jr (2007). The collagens of hydra provide insight into the evolution of metazoan extracellular matrices. J. Biol. Chem. 282, 6792-6802 [DOI] [PubMed] [Google Scholar]