Abstract
About one-fifth of the genes in the budding yeast are essential for haploid viability and cannot be functionally assessed using standard genetic approaches such as gene deletion. To facilitate genetic analysis of essential genes, we and others have assembled collections of yeast strains expressing temperature-sensitive (ts) alleles of essential genes. To explore the phenotypes caused by essential gene mutation we used a panel of genetically engineered fluorescent markers to explore the morphology of cells in the ts strain collection using high-throughput microscopy. Here, we describe the design and implementation of an online database, PhenoM (Phenomics of yeast Mutants), for storing, retrieving, visualizing and data mining the quantitative single-cell measurements extracted from micrographs of the ts mutant cells. PhenoM allows users to rapidly search and retrieve raw images and their quantified morphological data for genes of interest. The database also provides several data-mining tools, including a PhenoBlast module for phenotypic comparison between mutant strains and a Gene Ontology module for functional enrichment analysis of gene sets showing similar morphological alterations. The current PhenoM version 1.0 contains 78 194 morphological images and 1 909 914 cells covering six subcellular compartments or structures for 775 ts alleles spanning 491 essential genes. PhenoM is freely available at http://phenom.ccbr.utoronto.ca/.
INTRODUCTION
Essential genes are indispensable for the survival of an organism and are typically involved in fundamental biological processes, such as cell wall and membrane biogenesis, ribosome biosynthesis, DNA replication and cytoskeletal functions (1). As one of the most thoroughly characterized model organisms, the budding yeast Saccharomyces cerevisiae is frequently used to study genes involved in conserved biological pathways. In S. cerevisiae, ∼18% of the genes are considered to be essential for growth on rich medium with glucose as the carbon source (1,2). Essential genes are evolutionarily more conserved between yeast and humans than non-essential genes, indicating that a deeper understanding of the functions of yeast essential genes will extend our understanding of human gene function (3). Previous studies have characterized functions of yeast essential genes using a variety of alleles which allow conditional or partial inactivation of essential gene function. Many mutant strains have been constructed for genetic analysis of essential genes in the past several years, including those harbouring tetracycline (tet)-repressible promoter alleles (1), decreased abundance by mRNA perturbation alleles (4) or temperature-sensitive (ts) alleles (5,6). Among these methods, ts alleles are considered the most powerful for genetic analysis as they can perturb specific aspects of essential gene function (5–8).
Temperature-sensitive alleles of essential genes allow cell viability at the permissive temperature since the protein encoded by the ts allele resembles that encoded by the normal or wild-type allele and the mutant cells are often morphologically indistinguishable from the wild-type cells. However, at the restrictive (non-permissive) temperature, the ts gene product is defective and may result in a loss-of-function. Consequently the cells will show growth or morphological defects at the restrictive temperature. We have recently developed a method to quantify cell morphological changes for thousands of mutants in parallel by coupling synthetic genetic analysis with high-content screening (SGA–HCS) (9,10). We used SGA–HCS to study essential yeast genes and recently published a phenotypic profiling of 497 yeast strains carrying ts conditional alleles of essential genes (6).
To facilitate access to these valuable single-cell morphological data including the raw images, we implemented a web server with an integrated database and the capability to allow visualization and analysis of morphological data. PhenoM (Phenomics of Yeast Mutants) is a web-based platform that contains quantitative single-cell measurements and morphological images of yeast cells carrying ts alleles in essential genes generated by HCS technology (10–13) [reviewed in (9)]. Figure 1 shows a detailed record for allele smc4-1, which describes the basic information of the gene and phenotypic images. We have developed a user-friendly system with a fast and accurate search engine embedded with two data mining tools, PhenoDev and PhenoBlast. PhenoDev is a set of tools for calculating phenotypic differences between two mutant strains or between a mutant and a wild-type strain grown at the same or different temperatures. These tools can be used to identify genes that, if compromised, can give rise to a specific abnormal phenotype of interest; conversely, for a specific ts allele, these tools can also quickly identify the most significant phenotypic changes associated with the temperature shift. In contrast, the other tool, PhenoBlast, serves to quantify the morphological similarity between multiple ts alleles. This tool can be best used to discover other ts alleles which cause abnormal phenotypes similar to a ts allele of interest. Our system also provides tools for Gene Ontology (GO) enrichment analysis to find common GO annotation(s) among the top-ranked mutants identified by PhenoDev or PhenoBlast.
Figure 1.
An example of the allele detail interface based on allele smc4-1. The interface includes basic information of the allele and phenotypic images of the allele in different subcellular compartments or structures.
Currently, PhenoM version 1.0 holds 78 194 morphological images of 1 909 914 cells, covering six yeast subcellular compartments or structures, including actin filaments, DNA damage foci, mitochondria, nucleus, plasma membrane and mitotic spindle. These data were assembled by studying strains carrying 775 different ts alleles spanning 491 essential genes. To our knowledge, PhenoM is the first database of phenotypes caused by mutation of yeast essential genes that contains and analyses quantitative data derived from single-cell morphological images. PhenoM should be a valuable resource for the yeast and genomics communities since the large volume of data and the efficient analysis tools will facilitate the study of essential genes function. Images are also available in a downloadable format to facilitate future re-analysis using more sophisticated software developed by us or by other computational experts.
DATA SOURCE
The PhenoM database contains morphological images and quantitative single-cell measurements of yeast strains carrying 775 different ts alleles associated with 491 essential genes, covering the following six subcellular compartments or structures: actin filaments, DNA damage foci, mitochondria, nucleus, plasma membrane and mitotic spindle. As described in our previous report, MATα query strains carrying different fluorescent markers were mated to the ts allele array and MATα haploid ts strains expressing different Green Fluorescent Protein (GFP) and/or Red Fluorescent Protein (RFP) fusion proteins were isolated using SGA technology (6,10,14). The following cellular markers were used: a plasma membrane marker (Psr1p-GFP), a reporter of DNA damage (Ddc2p-GFP), a nuclear marker (Mad1p-Nuclear localization Signal-RFP), a mitotic spindle reporter (GFP-Tub1), a mitochondrial marker (OM45p-GFP) and an actin reporter (Sac6p-GFP). Morphological images were generated by using the SGA-HCS protocol (9,15) and quantitative measurements of a particular morphological feature were extracted at the single-cell level. For every reporter, several independent images were captured at different temperatures and these are referred to as ‘sites’ in the database. The quantitative measurements of each cell were categorized by several factors such as the temperature at which the yeast strain was grown, the cell cycle stage of the cell and the subcellular compartment or structure from which a feature was extracted. Since yeast cells have a relatively simple ellipsoidal shape, the dimensions of the cells can serve as a proxy to determine cell cycle phase (16). In PhenoM, we categorized cells into one of four phases of the cell cycle according to the ratio of daughter cell area to mother cell area: (i) unbudded cell; (ii) small budded cell; (iii) medium budded cell; and (iv) large budded cell (detailed information is provided on PhenoM website). Gene annotations were downloaded from SGD (17), while GO annotations were downloaded from the GO website (18) and were customized according to the gene set within the database.
DATA MINING
The current PhenoM database contains data for 775 ts mutants covering 491 yeast essential genes. For each mutant strain, up to 865 morphological parameters were catalogued in the database, corresponding to different temperatures, cellular compartments and cell cycle stages. For example, the following parameters describing the morphology of the spindle were quantified and stored in the database: area, perimeter, length and orientation. To aid biologists in extracting biologically useful information, we designed and implemented two data mining tools, PhenoDev and PhenoBlast, which summarize the phenotypic deviations of genes among isogenic cell populations and quantify the morphological similarities among different mutants, respectively.
Phenotypic deviation: PhenoDev
PhenoDev is a collection of analytical tools that can help users evaluate phenotypic deviations for a given morphological parameter in response to condition changes. It consists of three modules: PhenoTempDev, PhenoMutaDev and PhenoCycleDev. We provide a detailed user manual and examples for using these tools on the PhenoM website; the tools have user-friendly interfaces that have been extensively tested and optimized.
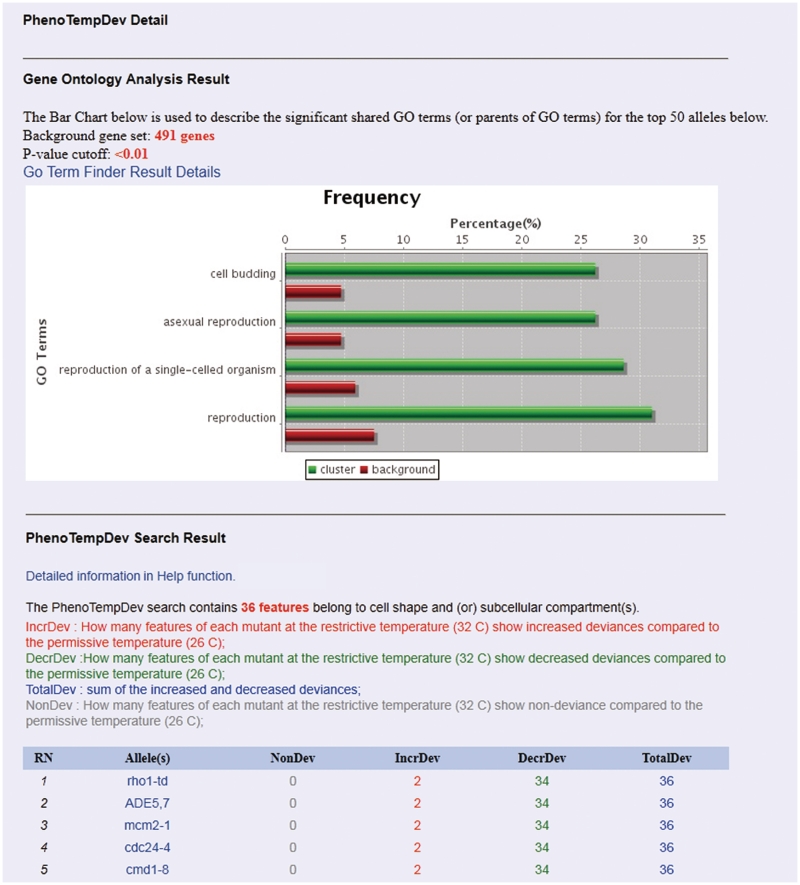
The phenotypic temperature deviation (PhenoTempDev) module can help users identify ts strains that have significant changes in a specific morphological parameter when grown at the permissive or restrictive temperature (see Figure 2 for an example). For example, if a researcher is interested in asking whether a ts allele affects ‘spindle length’, he or she can use PhenoTempDev to extract two sets of measurements for ‘spindle length’ across the two cell populations. A statistical test then allows ranking of the ts alleles according to the significance of the difference between the tested strains. In essence, this tool can help researchers identify genes that are implicated in a particular yeast morphological trait. Phenotypic mutation deviation (PhenoMutaDev) is intended to quantify the phenotypic deviation between the wild-type strain and a ts mutant strain for a selected set of morphological parameters at the same temperature. In contrast to PhenoTempDev, which detects morphological changes caused by temperature shift, PhenoMutaDev can identify mutants that have a significantly different phenotype than wild-type cells.
Figure 2.
An example of the ‘PhenoTempDev Detail’ interface. The top five alleles were presented in the figure.
Finally, phenotypic cell cycle deviation (PhenoCycleDev) allows users to examine the phenotypic changes for a single mutant throughout the cell cycle. As described above, we categorized the cell cycle into four phases based on daughter bud and mother cell ratios. Our system queries three contiguous cell cycle transitions: (i) from unbudded to small budded cells (G1-S phase); (ii) from small budded to medium budded cells (S-G2); and (iii) from medium budded to large budded cells (G2-M). For each cell cycle transition, measurements of morphological parameters are extracted and compared. The program then ranks all the mutants according to changes in morphological measurements associated with the three transitions. With this tool, for any essential mutant, users can readily determine which phenotypic features are affected during a particular cell cycle phase transition.
PhenoBlast
The rationale for using morphological data to study gene function stems from our recent observation that genes that are annotated to have similar functions tend to give rise to similar morphological defects upon perturbation (6). The huge volume of morphology data generated from high-content screens and catalogued in the PhenoM database offers a unique and useful resource to search for alleles that show similar morphological phenotypes and thus have similar cellular functions.
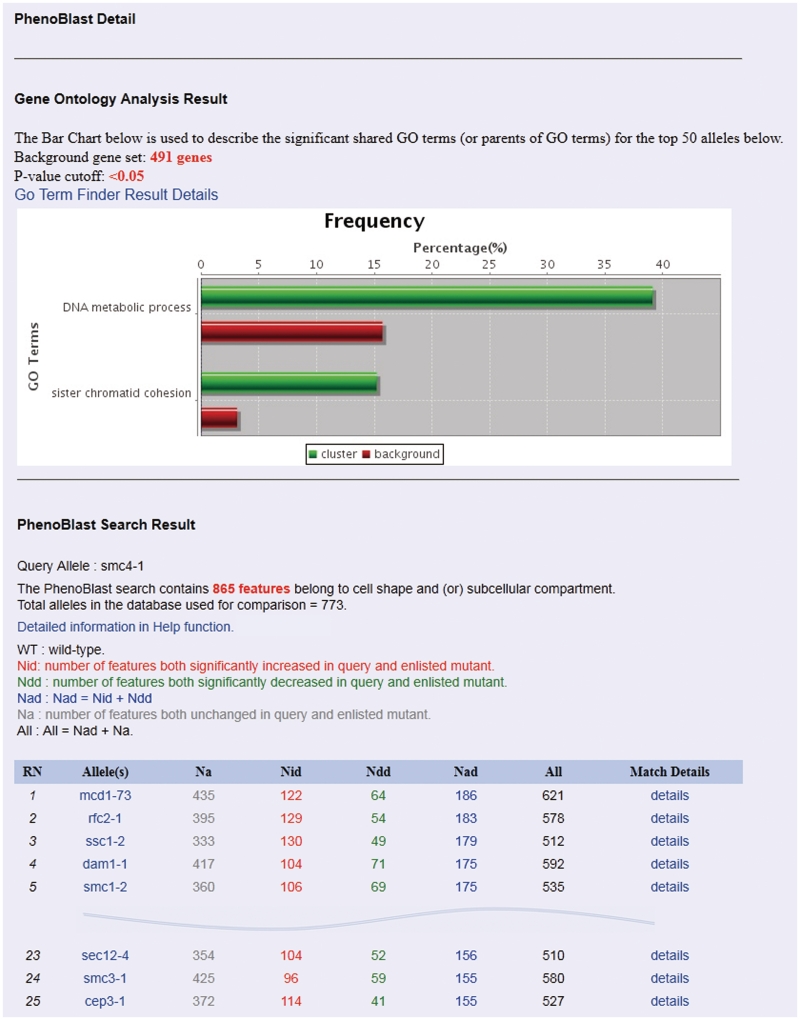
In contrast to searching databases for genes that have sequence similarities, searching for morphological similarities between any pair of yeast strains is computationally and algorithmically more challenging. In an earlier study on phenotypic changes in Caenorhabditis elegans in RNAi interference experiments, Gunsalus and colleagues developed a software tool called PhenoBlast, in an analogy to BLAST, where gene pairs are scored based on the similarities in morphological phenotypes caused by knockdown of gene expression by RNAi. With this tool, 47 phenotypic parameters were used to assess morphological phenotypes in Caenorhabditis elegans (19). We adopted PhenoBlast for comparing yeast morphology data. In our algorithm, we do not directly compare the morphology between two different mutant strains. Instead, for each mutant strain, the morphological measurements of the population of mutant cells were first compared against the population of wild-type cells. We then used Wilcoxon rank-sum test to determine whether a particular morphological parameter (e.g. spindle length) is significantly increased (labelled as ‘+1’), unchanged (labelled as ‘0’) or significantly decreased (labelled as ‘−1’) in the mutant strain. In this way, we obtain a vector of 865 values consisting of ‘+1’, ‘0’ or ‘−1’ for each mutant strain, which describes the overall morphological change in comparison to wild-type cells. We can then quantify the morphological similarities between any two mutant strains by calculating the similarities between their corresponding vectors (for detailed information, please see the PhenoM server). The algorithm described above has been implemented and optimized in PhenoBlast. Users can query a single mutant of interest and see other mutants with similar phenotypic profiles in real time. Figure 3 shows an example of a PhenoBlast comparison which used default settings to query the smc4-1 strain. The search identified the mcd1-73 strain, which contains a ts allele in the MCD1 gene as the top hit—the smc4-1 and mcd1-73 strains shared similarity in 621 morphological parameters [122 parameters significantly increased, 435 unchanged and 64 significantly decreased (Figure 4)].
Figure 3.
An example of the ‘Search’ interface of PhenoBlast.
Figure 4.
An example of the ‘PhenoBlast Detail’ interface. The interface includes GO analysis result section and the ‘Search Result’ section. Smc4-1 is used as the query allele and the top 5 and 23–25 alleles were presented in the figure.
Search
The search engine of the system provides two options, Quick Search and Morphology Search. In Quick Search, users can directly query any mutant of interest from the database by specifying its gene name, open reading frame (ORF) name, or aliases. A partial-word-match method is also incorporated in the Quick Search. On the other hand, if users want to find mutant strains that share morphological similarity with a pre-specified gene, a search by morphology profiles can be performed. The Morphology Search utility is very flexible as it allows users to conduct specific advanced searches including: (i) searching for mutants in a certain cell cycle phase; (ii) searching for mutants grown at a certain temperature; (iii) searching for mutants with a number of objects in a single cell in a particular compartment; (iv) searching for mutants in a certain range of a particular cell shape parameter. All of these options are unified in the Morphology Search.
GO enrichment analysis
Depending on the users’ objective, running PhenoDev or PhenoBlast automatically generates a list of mutants showing phenotypic abnormality. We also provide a module for GO enrichment analysis to test whether genes associated with some phenotypic defects are enriched for known functions or pathways. This functionality is particularly useful when gauging the biological significance of some highly ranked genes whose mutation causes extreme phenotypic defects. To implement GO analysis, we compared several tools such as GoMiner (20), GOEAST (21) and GO::TermFinder (22). We chose to use the GO::TermFinder package (v0.86) for PhenoM since it uses a modular design and thus was easy to integrate into our system.
DATABASE IMPLEMENTATION AND USER INTERFACE
Database implementation
We designed and implemented PhenoM using Struts-Spring-Hibernate, a lightweight enterprise-level developmental framework (23,24). MySQL (v5.0.77) was used as the underlying relational database to store the large volume of quantitative measurements and the links to the raw morphological images in the file system. At the front end, Apache Server 2.0 is deployed to handle user requests and to forward requests for Java servlets and Java Server Pages to Apache Tomcat 6.0. To provide a user-friendly interface, JavaScript and AJAX (Asynchronous Javascript and XML) technology were adopted to program the client-side functionality and Apache TilesTM was used to create reusable interface components. For online graphical visualization and image manipulation, ImageJ (v1.44) was incorporated to assist users with morphological image processing via Java applets, and the Java open source package JFreeChart (http://www.jfree.org/jfreechart/) was used to plot bar charts. All source code development was performed in Eclipse v3.4.2 (http://www.eclipse.org).
User interface
The PhenoM server provides the following primary web interfaces, which allow users to perform focused query and analysis tasks.
The ‘Allele detail interface’ presents detailed information for a mutant strain. It consists of three parts:
Basic information and cross links to other databases such as SGD (17) or BioGRID (25);
Phenotypic images and statistical information for each of the six subcellular compartments or structures. Several functional links are also provided, such as the option to download an original micrograph in TIFF format, viewing an image through ImageJ, and also a link to the dataset for a particular image. For ease of analysis, all images are annotated by names that correspond to the mutant name, the reporter imaged, the temperature at which the images were captured and the site number (which indicates the set of independent images used to produce a particular image for a mutant) and
The PhenoBlast results list, containing up to fifty other mutants that share similar morphological profiles (Figures 1 and 4).
PhenoDev: this is a series of tools to measure phenotypic deviations of strains carrying different ts alleles under varying conditions. We take PhenoTempDev as an example to navigate the interfaces of this function (Figure 2). Multiple options, such as morphological features in a certain compartment, bud size and others are provided and can be customized. An analysis with the default parameters will lead to the PhenoTempDev results interface (Figure 2), which includes two sections: (i) the results of GO analysis and (ii) the deviation details of the top 50 mutants listed in descending order.
PhenoBlast: this is a comparison tool for phenotypic similarity and can be accessed either from the home interface of PhenoM or from the navigation bar. To execute PhenoBlast, both a query mutant and the relevant morphological features must be specified. Upon typing characters in the query allele box, the system will automatically suggest a list of the most similar mutant names. To facilitate a customized search, the list of 865 morphological features is divided into three parts: (i) 386 features from phenotypic mutation deviation; (ii) 193 features from phenotypic temperature deviation; and (iii) 286 features from phenotypic cell cycle deviation (detailed information is provided on the PhenoM server). At least one feature must be chosen to conduct the analysis. The PhenoBlast results interface will be displayed following the execution of a successful query and contains two parts: (i) the GO analysis results and (ii) the details of 50 other mutants that have similar phenotypes. Figures 3 and 4 shows an example of navigating through PhenoBlast (Figures 3 and 4). The SMC4 gene encodes a member of a ubiquitous family of chromosome-associated ATPases (26). The Smc4 protein is a subunit of the condensin complex which is required for chromosome condensation and dynamics (27,28). To search for mutants sharing similar morphological profiles with the smc4-1 mutant strain, we can input smc4-1 in the query allele box in the Search interface of PhenoBlast (Figure 3) and then press the button ‘Run PhenoBlast’ using default parameters. The system automatically leads to the Detail interface of PhenoBlast. In the search result section, the first, fifth and twenty fourth ranked mutants, mcd1-73, smc1-2 and smc3-1, respectively, all carry mutant alleles in genes encoding condensin subunits and the list of mutants overall is enriched in both DNA metabolic process (P = 0.01218) and sister chromatid cohesion (P = 0.04455) (Figure 4). The results from PhenoBlast are thus consistent with known biology (6,29–31), and we believe that it will become a useful tool for suggesting new biological gene associations for the yeast research community.
Download: PhenoM allows users to download either data for the full set of all mutants or from a single selected mutant in tab-delimited format.
AVAILABILITY AND FUTURE DIRECTION
PhenoM can be freely accessed at http://phenom.ccbr.utoronto.ca/. The future development of PhenoM will include the following aspects: (i) PhenoM will include phenotypic information for additional yeast strains carrying mutant alleles of non-essential or essential genes as such data become available from our groups or elsewhere; (ii) data for more subcellular compartments or structures will be incorporated into the database to help biologists uncover the association between cell morphology and gene function; and (iii) more data mining and statistical analysis tools will be developed, especially tools that can integrate other types of data such as protein complexes or protein interactions.
Our database ultimately aims to provide a centralized platform for the analysis of gene function by using quantitative measurements and morphological images from yeast to mammalian cells. To accomplish this goal, PhenoM will provide a submission system to collect data from other image-based HCS experiments in the future.
FUNDING
A Fudan University - University of Toronto Exchange Scholarship (in part to K.J.); a postdoctoral fellowship from the Best Foundation (to F.J.V.); the Canadian Institutes of Health Research grants (GMX-201237 to B.A. and C.B.) and (GMX-211012 to B.A., C.B. and Z.Z.). Infrastructure for automated imaging in the Boone and Andrews Lab is funded through the Canadian Foundation for Innovation (LEF-21475) and the Ontario Research Fund. Funding for open access charge: the Canadian Institutes of Health Research (GMX-201237) and (GMX-211012).
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We would like to thank Xiaoli Wu for helping design the style of the interfaces and Dr Yufang Zheng from Fudan University for critical readings of an early version of the article. We also wish to thank Dr Judice L.Y. Koh for her helpful suggestions.
REFERENCES
- 1.Mnaimneh S, Davierwala AP, Haynes J, Moffat J, Peng WT, Zhang W, Yang X, Pootoolal J, Chua G, Lopez A, et al. Exploration of essential gene functions via titratable promoter alleles. Cell. 2004;118:31–44. doi: 10.1016/j.cell.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TR. Yeast and drug discovery. Funct. Integr. Genomics. 2002;2:199–211. doi: 10.1007/s10142-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 4.Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, Ihmels J, Andrews B, Boone C, Greenblatt JF, et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Aroya S, Coombes C, Kwok T, O'Donnell KA, Boeke JD, Hieter P. Toward a comprehensive temperature-sensitive mutant repository of the essential genes of Saccharomyces cerevisiae. Mol. Cell. 2008;30:248–258. doi: 10.1016/j.molcel.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Vizeacoumar FJ, Bahr S, Li J, Warringer J, Vizeacoumar FS, Min R, Vandersluis B, Bellay J, Devit M, et al. Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat. Biotechnol. 2011;29:361–367. doi: 10.1038/nbt.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartwell LH, Culotti J, Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc. Natl Acad. Sci. USA. 1970;66:352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simchen G. Cell cycle mutants. Ann. Rev. Genet. 1978;12:161–191. doi: 10.1146/annurev.ge.12.120178.001113. [DOI] [PubMed] [Google Scholar]
- 9.Vizeacoumar FJ, Chong Y, Boone C, Andrews BJ. A picture is worth a thousand words: genomics to phenomics in the yeast Saccharomyces cerevisiae. FEBS Lett. 2009;583:1656–1661. doi: 10.1016/j.febslet.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 10.Vizeacoumar FJ, van Dyk NS, Vizeacoumar F, Cheung V, Li J, Sydorskyy Y, Case N, Li Z, Datti A, Nislow C, et al. Integrating high-throughput genetic interaction mapping and high-content screening to explore yeast spindle morphogenesis. J. Cell. Biol. 2010;188:69–81. doi: 10.1083/jcb.200909013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakal C, Aach J, Church G, Perrimon N. Quantitative morphological signatures define local signaling networks regulating cell morphology. Science. 2007;316:1753–1756. doi: 10.1126/science.1140324. [DOI] [PubMed] [Google Scholar]
- 12.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Perlman ZE, Slack MD, Feng Y, Mitchison TJ, Wu LF, Altschuler SJ. Multidimensional drug profiling by automated microscopy. Science. 2004;306:1194–1198. doi: 10.1126/science.1100709. [DOI] [PubMed] [Google Scholar]
- 14.Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 15.Baryshnikova A, Costanzo M, Dixon S, Vizeacoumar FJ, Myers CL, Andrews B, Boone C. Synthetic genetic array (SGA) analysis in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Methods Enzymol. 2010;470:145–179. doi: 10.1016/S0076-6879(10)70007-0. [DOI] [PubMed] [Google Scholar]
- 16.Saito TL, Ohtani M, Sawai H, Sano F, Saka A, Watanabe D, Yukawa M, Ohya Y, Morishita S. SCMD: Saccharomyces cerevisiae Morphological Database. Nucleic Acids Res. 2004;32:D319–D322. doi: 10.1093/nar/gkh113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong EL, Balakrishnan R, Dong Q, Christie KR, Park J, Binkley G, Costanzo MC, Dwight SS, Engel SR, Fisk DG, et al. Gene Ontology annotations at SGD: new data sources and annotation methods. Nucleic Acids Res. 2008;36:D577–D581. doi: 10.1093/nar/gkm909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. The Gene Ontology Consortium Gene ontology: tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunsalus KC, Yueh WC, MacMenamin P, Piano F. RNAiDB and PhenoBlast: web tools for genome-wide phenotypic mapping projects. Nucleic Acids Res. 2004;32:D406–D410. doi: 10.1093/nar/gkh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Narasimhan S, Kane DW, Reinhold WC, Lababidi S, et al. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Q, Wang XJ. GOEAST: a web-based software toolkit for Gene Ontology enrichment analysis. Nucleic Acids Res. 2008;36:W358–W363. doi: 10.1093/nar/gkn276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Cherry JM, Sherlock G. GO::TermFinder–open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun LV, Jin K, Liu Y, Yang W, Xie X, Ye L, Wang L, Zhu L, Ding S, Su Y, et al. PBmice: an integrated database system of piggyBac (PB) insertional mutations and their characterizations in mice. Nucleic Acids Res. 2008;36:D729–D734. doi: 10.1093/nar/gkm790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Jin K, Xie X, Li D, Yang J, Wang L, Gu N, Zhong Y, Sun LV. Development of a database system for mapping insertional mutations onto the mouse genome with large-scale experimental data. BMC Genomics. 2009;10(Suppl. 3):S7. doi: 10.1186/1471-2164-10-S3-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stark C, Breitkreutz BJ, Chatr-Aryamontri A, Boucher L, Oughtred R, Livstone MS, Nixon J, Van Auken K, Wang X, Shi X, et al. The BioGRID Interaction Database: 2011 update. Nucleic Acids Res. 2011;39:D698–D704. doi: 10.1093/nar/gkq1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koshland D, Strunnikov A. Mitotic chromosome condensation. Ann. Rev. Cell Dev. Biol. 1996;12:305–333. doi: 10.1146/annurev.cellbio.12.1.305. [DOI] [PubMed] [Google Scholar]
- 27.Hirano T. SMC-mediated chromosome mechanics: a conserved scheme from bacteria to vertebrates? Genes Dev. 1999;13:11–19. doi: 10.1101/gad.13.1.11. [DOI] [PubMed] [Google Scholar]
- 28.Strunnikov AV, Jessberger R. Structural maintenance of chromosomes (SMC) proteins: conserved molecular properties for multiple biological functions. Eur. J. Biochem./FEBS. 1999;263:6–13. doi: 10.1046/j.1432-1327.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 29.Guacci V, Koshland D, Strunnikov A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 1997;91:47–57. doi: 10.1016/s0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein F, Mahr P, Galova M, Buonomo SB, Michaelis C, Nairz K, Nasmyth K. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell. 1999;98:91–103. doi: 10.1016/S0092-8674(00)80609-1. [DOI] [PubMed] [Google Scholar]
- 31.Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]