Abstract
According to several studies, some nuclear magnetic resonance (NMR) structures are of lower quality, less reliable and less suitable for structural analysis than high-resolution X-ray crystallographic structures. We present a public database of 2405 refined NMR solution structures [statistical torsion angle potentials (STAP) refinement of the NMR database, http://psb.kobic.re.kr/STAP/refinement] from the Protein Data Bank (PDB). A simulated annealing protocol was employed to obtain refined structures with target potentials, including the newly developed STAP. The refined database was extensively analysed using various quality indicators from several assessment programs to determine the nuclear Overhauser effect (NOE) completeness, Ramachandran appearance, χ1-χ2 rotamer normality, various parameters for protein stability and other indicators. Most quality indicators are improved in our protocol mainly due to the inclusion of the newly developed knowledge-based potentials. This database can be used by the NMR structure community for further development of research and validation tools, structure-related studies and modelling in many fields of research.
INTRODUCTION
Protein structure determination has contributed greatly to our understanding of structural biology by providing the three-dimensional protein structure and the role of the associated conformational dynamics of the protein. Despite the importance of protein structures, three-dimensional protein structure determination has been limited to X-ray crystallography in the solid state (single crystals) or nuclear magnetic resonance (NMR) spectroscopy in solution. Recently, 9049 macromolecular structures obtained by NMR spectroscopy were available in the Protein Data Bank (PDB) (1,2). NMR is uniquely suited to the characterization of protein structure and dynamics in solution, and it is not hampered by the ability or inability of a protein to crystallize. Despite these advantages, NMR protein structures are usually not a first choice for studies of protein structure and function. Because NMR structures can be of lower quality, less reliable and less suitable for structural analysis than crystallography, they are often excluded (3–5).
Because of the uncertainty in NMR structures, numerous refinement protocols and force fields have been developed to improve the quality of NMR structures (3,5–7). Even with the recent advancements in protocols and force fields, the structures obtained can still be of unsatisfactory quality, indicating that there is room for further improvement. Numerous studies have advised that the poor quality of the Ramachandran plot, backbone conformation and/or side-chain packing were caused by the low quality of the NMR structures (8–10). To address these weaknesses, re-refinement and other protocols have been introduced in several projects [DRESS (11), RECOORD (12), etc.]. Re-refinement usually leads to an improvement in structure quality.
The re-refinement in this study is focused on 2405 of the 9049 selected solution NMR structures deposited in the PDB. We developed a new refinement protocol to refine the solution NMR structure by correcting the backbone polypeptide torsion angles. A detailed protocol and target selection are introduced in the next section. We illustrate the clear overall improvement of the structures in our database, statistical torsion angle potentials (STAP) refinement of the NMR database, compared to the currently available refinement databases.
STAP refinement of NMR database
Unlike crystallography, NMR structure determination uses very heterogeneous geometrical information: distances, dihedral angles and orientations (12). Among this geometrical information, we focused on the dihedral angle. Torsion angles are assumed to be a very important factor in the quality of NMR structures (8–10). Its deviation sways the inter-atomic distance and greatly influences the nuclear Overhauser effect (NOE) signals in experiments (13,14). Torsion angle population analysis provides a firm ground for deriving knowledge-based energy functions. STAP was developed with 18 353 high-resolution X-ray crystallographic structures with a resolution below 2.0 Å from the PDB. From that, we removed the structures with redundancies and built two-dimensional histograms as a function of the two backbone torsion angles (φ and ψ) with a grid point for every 15°. By applying a log transformation to these histograms (15), we obtained the two-dimensional knowledge-based potentials on φ/ψ torsion angles for favourable conformational isomerism. We call this potential STAP. STAP gives weight to suitable conformations and lets preferable ones remain. It especially works with the simulated annealing protocol.
The 2405 structures are presented in the STAP refinement of the NMR database. Most of the experimental structures available from the PDB were loaded directly into the automatic pipeline for refinement. Each NOE distance restraint was downloaded from the Biological Magnetic Resonance Bank (BMRB) (16). This restraint set was used to refine the corresponding ensembles deposited in the PDB. Targets that carry contradicting inter-atomic distances to the NOE data were discarded. We selected only the X-PLOR restraint format.
Briefly, the refinement protocol used is as follows: (i) after STAP refining potentials are applied to all of the structures, the implicit solvation model is applied (17) and energy-minimized; (ii) the system is heated from 100 to 500 K using 1000 steps of molecular dynamics; (iii) 2000 steps of molecular dynamics at 500 K are performed; (iv) cooling down to 25 K runs during 4000 steps; and (v) a short minimization with 200 steps is performed. This protocol was executed using CHARMM (ver. C35) (18).
Quality assessment of NMR structure
The programs PROCHECK (ver. 3.5.4) (8) and MolProbity (ver. 2.12) (10) were used to measure the Ramachandran appearance and steric clash score. The program WHAT_CHECK (ver. 8.0) (9) was used to measure the root mean square (RMS) Z-score distribution of several parameters of the protein structure. There are three optimal energy properties for protein structures: Discrete Optimised Protein Energy (DOPE, ver. 9v7) (19), normalized DOPE (nDOPE, ver. 9v7) (20) and dipolar Distance-scaled, Finite-Ideal gas REference (dDFIRE, ver. 1.1) (21). NOE distance violations for all ensemble restraints were calculated with AQUA (ver. 3.2) (22).
Statistical analysis for STAP refinement of the NMR database
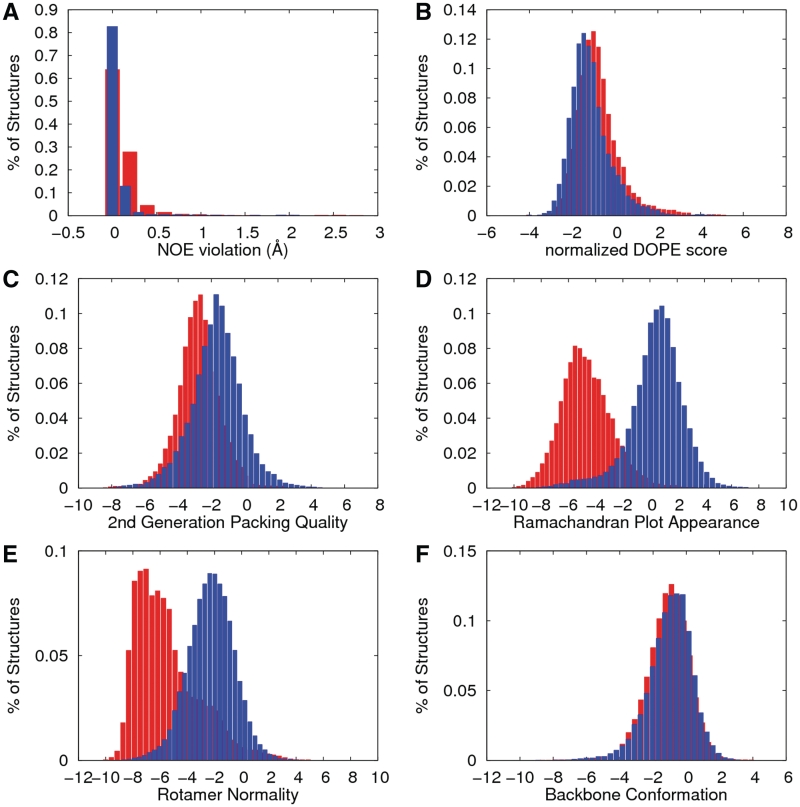
Statistical RMS Z-score distribution, RMS NOE violations and nDOPE scores for the original and refined structure are shown in Figure 1. As we expected, the RMS Z-score for the Ramachandran plot appearance (Figure 1D) showed a great improvement because it shows two separate Gaussian distributions. In addition, the result of the RMS Z-score distribution for the χ1–χ2 rotamer normality (Figure 1E) also appears similar to that of the Ramachandran plot appearance. Other parameters show remarkable improvement with a stable low energy profile for nDOPE and 2nd-generation packing quality. Interestingly, the influence of the torsion angle conformation on the NMR structure may cause RMS NOE violations to provide better quality, indicating that NOE violations directly affect the geometrical conformation of the torsion angle (23,24).
Figure 1.
Distribution of the protein quality assessment of the original (red) and refined structures (blue bars). The figures present the distributions of the 2405 selected NMR structures as follows: (A) the RMS value of the NOE violations, (B) the normalized DOPE score, (C) the 2nd-generation packing quality (all backbone and side chain contacts), (D) the Ramachandran plot appearances, (E) the χ1–χ2 rotamer normality and (F) the backbone conformation. The values were measured by dDFIRE, AQUA and WHAT_CHECK.
The average values of the original and refined parameters pertaining to the structural quality are presented in Table 1. Evaluation of the structural quality indicated that our protocol, STAP, provides improved quality in the polypeptide backbone conformation. Remarkably, the number of RMS NOE violations was closer to zero in the refined structure than in the original structure, which is shown above in the histogram depicted in Figure 1.
Table 1.
Summary of the average quality indicators of the original and refined NMR structures
| Original | Refined (STAP)a | |
|---|---|---|
| Steric clash score | 33.16 ± 39.52 | 1.57 ± 5.90 |
| RMS NOE distance violations | 0.20 ± 0.35 | 0.13 ± 0.24 |
| Optimal protein energy | ||
| DOPE score | −8050.74 ± 4572.84 | −8587.34 ± 4817.02 |
| Normalized DOPE score | −0.62 ± 1.02 | −1.02 ± 0.97 |
| dDFIRE Score | −163.12 ± 89.44 | −179.07 ± 96.77 |
| Ramachandran indicators (%) | ||
| MolProbity | ||
| Favourable | 83.50 ± 10.09 | 95.51 ± 4.83 |
| Allowed | 12.95 ± 7.36 | 3.80 ± 3.92 |
| Disallowed | 3.54 ± 4.04 | 0.69 ± 1.48 |
| PROCHECK | ||
| Favourable | 75.66 ± 12.08 | 89.83 ± 7.45 |
| Allowed | 21.04 ± 9.65 | 8.01 ± 5.80 |
| Generously | 2.35 ± 3.31 | 0.84 ± 1.55 |
| Disallowed | 0.96 ± 1.72 | 1.32 ± 2.01 |
| Structure Z-score distributionb | ||
| 1st-generation packing quality | −3.14 ± 1.68 | −2.63 ± 1.80 |
| 2nd-generation packing quality | −2.59 ± 1.27 | −1.52 ± 1.55 |
| Ramachandran plot appearance | −4.50 ± 1.86 | 0.65 ± 1.95 |
| χ1–χ2 Rotamer normality | −5.25 ± 2.36 | −2.07 ± 1.68 |
| Backbone conformation | −0.93 ± 1.28 | −0.80 ± 1.33 |
| RMS Z-score distributionc | ||
| Bond lengths | 0.48 ± 0.34 | 0.84 ± 0.12 |
| Bond angles | 0.70 ± 0.39 | 1.04 ± 0.16 |
| Ω Angle restraints | 0.36 ± 0.51 | 1.30 ± 0.43 |
| Side-chain planarity | 0.59 ± 0.92 | 0.68 ± 0.45 |
| Improper dihedral distribution | 0.64 ± 0.39 | 0.79 ± 0.25 |
| Inside/outside distribution | 1.05 ± 0.10 | 1.03 ± 0.10 |
aBold font indicates the best scores.
bPositive is better than average.
cRMS Z-score should be close to 1.0.
Comparison with DRESS and RECOORD
From analysis of the STAP data, there are 70 structures present in the two public NMR refined databases (DRESS and RECOORD databases), as shown in Table 2. The refined data of DRESS (refined PDB files) and RECOORD (CNW and CYW PDB files) were loaded directly from their websites to measure the protein structure qualities. The quality improvement by the three protocols (STAP, DRESS and RECOORD) is shown in Table 2. The STAP refinement protocol has a great improvement on the Ramachandran backbone conformation, especially on the percentage of favourable indicators by MolProbity and PROCHECK. The Z-score distributions by WHAT_CHECK indicated that our protocol provides slightly better quality than the other two protocols [steric clash score, nDOPE score, RMS Z-score distributions on the structure (2nd-generation packing quality, χ1–χ2 rotamer normality, Ramachandran plot appearance and backbone conformation) and RMS Z-score distributions (bond angles and inside/outside distribution)]. The result of the optimized energy comparison indicated that the other databases (RECOORD and DRESS) are slightly better than our approach. With the many positive attributes of STAP, it can be confidently concluded that STAP provides comparable performance to justify the geometric consensus for torsion angles and shows comparable or slightly better performance than the two known protocols.
Table 2.
Comparison of the 70 NMR structures that are common among the STAP, DRESS and RECOORD databases
| Original | STAPa | DRESS | RECOORD-CNW | RECOORD-CYW | |
|---|---|---|---|---|---|
| Steric clash score | 68.26 ± 57.86 | 1.03 ± 2.44 | 16.79 ± 9.56 | 16.31 ± 8.62 | 16.56 ± 8.46 |
| Optimal protein energy | |||||
| DOPE score | −6098.15 ± 4586.33 | −6941.80 ± 5130.94 | −6996.31 ± 5244.70 | −7274.30 ± 5902.43 | −7330.00 ± 6150.59 |
| Normalized DOPE score | −0.21 ± 1.17 | −1.00 ± 1.06 | −1.01 ± 1.13 | −0.95 ± 1.11 | −0.96 ± 1.10 |
| dDFIRE score | −125.95 ± 88.72 | −147.52 ± 102.79 | −143.07 ± 101.01 | −146.95 ± 114.19 | −148.66 ± 120.39 |
| Ramachandran indicators (%) | |||||
| MolProbity | |||||
| Favourable | 75.02 ± 12.37 | 94.80 ± 4.51 | 86.38 ± 8.18 | 84.69 ± 9.00 | 83.59 ± 9.35 |
| Allowed | 18.76 ± 8.87 | 4.27 ± 3.64 | 10.71 ± 6.71 | 11.89 ± 7.03 | 12.75 ± 7.58 |
| Disallowed | 6.22 ± 5.25 | 0.92 ± 1.55 | 2.90 ± 2.92 | 3.43 ± 3.34 | 3.66 ± 3.29 |
| PROCHECK | |||||
| Favourable | 67.02 ± 15.14 | 88.45 ± 7.60 | 77.92 ± 11.70 | 75.33 ± 13.02 | 73.73 ± 13.88 |
| Allowed | 27.18 ± 11.95 | 8.73 ± 6.08 | 18.52 ± 9.79 | 20.20 ± 10.73 | 21.48 ± 11.54 |
| Generously | 4.38 ± 4.10 | 1.00 ± 1.59 | 2.06 ± 2.49 | 2.70 ± 3.00 | 2.83 ± 2.99 |
| Disallowed | 1.42 ± 1.93 | 1.82 ± 2.19 | 1.50 ± 2.12 | 1.77 ± 2.26 | 1.97 ± 2.29 |
| Structure Z-score distributionb | |||||
| 1st-generation packing quality | −3.48 ± 1.71 | −2.41 ± 1.73 | −2.14 ± 1.72 | −2.37 ± 1.97 | −2.44 ± 2.03 |
| 2nd-generation packing quality | −2.96 ± 1.19 | −1.28 ± 1.50 | −1.89 ± 1.12 | −2.09 ± 1.27 | −2.08 ± 1.26 |
| Ramachandran Plot Appearance | −5.85 ± 1.92 | 0.98 ± 2.03 | −4.13 ± 1.44 | −4.31 ± 1.60 | −4.43 ± 1.59 |
| χ1–χ2 rotamer normality | −6.35 ± 2.35 | −1.68 ± 1.68 | −2.58 ± 1.59 | −2.50 ± 1.42 | −2.77 ± 1.40 |
| Backbone conformation | −1.67 ± 1.50 | −1.07 ± 1.42 | −1.50 ± 1.40 | −1.64 ± 1.37 | −1.63 ± 1.33 |
| RMS Z-score distributionc | 0.83 ± 0.34 | 0.85 ± 0.06 | 0.84 ± 0.14 | 0.84 ± 0.13 | 0.86 ± 0.13 |
| Bond lengths | 1.06 ± 0.42 | 1.03 ± 0.12 | 0.78 ± 0.12 | 0.78 ± 0.11 | 0.80 ± 0.11 |
| Bond angles | 0.16 ± 0.27 | 1.31 ± 0.37 | 0.75 ± 0.15 | 0.74 ± 0.15 | 0.75 ± 0.14 |
| Ω Angle restraints | 1.71 ± 1.42 | 0.70 ± 0.37 | 0.81 ± 0.18 | 1.27 ± 0.54 | 1.31 ± 0.58 |
| Side-chain planarity | 0.92 ± 0.39 | 0.79 ± 0.17 | 1.01 ± 0.16 | 1.19 ± 0.36 | 1.23 ± 0.38 |
| Improper dihedral distribution | 1.07 ± 0.13 | 1.06 ± 0.12 | 1.07 ± 0.12 | 1.08 ± 0.13 | 1.08 ± 0.13 |
aBold font indicates the best scores.
bPositive is better than average.
cRMS Z-score should be close to 1.
We have discussed the statistical analysis of the original and refined structures and compared our refined structure quality with that of other known NMR refined databases. Based on these facts, we believe that the quality of our 2405 NMR structure database is significantly improved, and this database will be a good start for further development of research and validation tools, structure-related studies and modelling in many fields of research.
Web interface and system
The STAP refinement of the NMR database can be accessed using a web-portal (http://psb.kobic.re.kr/STAP/refinement) that provides a 2405 refined NMR structure database, which runs on the CentOS operation system (ver. 5.2). The web server uses a standard web browser as the graphic user interface to the front-end server based on PHP (ver. 5.1.6)/SQL, the application server based on Apache (ver. 2.2.3) and the MySQL (ver. 5.0.45) back-end database server.
The following five website features are described in detail: (i) the main page, (ii) the general structure information page, (iii) the results page, (iv) the visualization page and (v) the download page. (i) The Front page allows the user to enter the selected PDB ID to access the database. On the main page, there are three different ways to access the database (PDB List, Lucky and manual). The PDB List is somewhat similar to the MS Windows file explorer interface, which is one of the search engines using the tree structure as the user interface, sequentially listing PDB IDs. If a user clicks the ‘PDB List’ button, a tree browser layer will open at the upper left side of the window on the main page. The top-level directory consists of the first letter of each PDB ID. When the user clicks the ‘+’ button placed on the left side of the directory image, the next lower level of the tree node appears. When the user clicks ‘first two letters of ID’, the user can see the list of PDB IDs. The second way is using ‘Lucky’, which is known as the ‘online demonstration’, and uses the pseudo-random number generator algorithms (25) to select a random target. ‘Lucky’ provides visual information with a mouse–rollover–tooltip layer, so that a user can learn our system quickly. Finally, for the manual search, a user types the PDB ID to access the database. For user convenience, the auto-completion function is available. (ii) General information on the target NMR structure (Figure 2) is provided, including information on the protein, experimental information, visualization of the structure using the Jmol viewer (ver. 11.4.RC4) (26) and links to the PDB, BMRB, PubMed (dependent on PDB data; if the PDB data do not include the PubMed information then the PubMed icon will be disabled), and the NCBI Taxonomy Browser. (iii) The Results page shows the calculated results for the structural quality assessment based on the original and refined structures. It sometimes provides information on the evaluation results with DRESS and RECOORD with a link to the target protein sites and an overall summary table for the target structure. (iv) Visualization information on the details of the target structure and Ramachandran plot for protein quality assessment are provided. Both the original and refined structures are displayed (detailed view of Ramachandran indicators, location of steric clash sites, etc.), and the location of the Ramachandran indicator is shown on the plot with KiNG display software (27). (v) A download area is available for various data, for example, the result of the protein quality assessment for the original and refined structures, both PDB files, and the visualization inputs for the KiNG display.
Figure 2.
General information on the target NMR structure: Jmol viewer, link-out to other databases, comparison of original and refinement structures.
FUNDING
Funding for open access charge: A grant from the ‘Korean Research Institute of Bioscience and Biotechnology Research Initiative Program’; and the Korean Ministry of Education, Science and Technology (MEST) under grant numbers 20110002321 and 20110019747, respectively.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
PLSI supercomputing resources of Korea Institute of Sciences and Technology Information supported this work.
REFERENCES
- 1.Rose PW, Beran B, Bi C, Bluhm WF, Dimitropoulos D, Goodsell DS, Prlic A, Quesada M, Quinn GB, Westbrook JD, et al. The RCSB Protein Data Bank: redesigned web site and web services. Nucleic Acids Res. 2011;39:D392–D401. doi: 10.1093/nar/gkq1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penkett CJ, van Ginkel G, Velankar S, Swaminathan J, Ulrich EL, Mading S, Stevens TJ, Fogh RH, Gutmanas A, Kleywegt GJ, et al. Straightforward and complete deposition of NMR data to the PDBe. J. Biomol. NMR. 2010;48:85–92. doi: 10.1007/s10858-010-9439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melnik BS, Garbuzinskii SA, Lobanov M, Galzitskaia OV. The difference between protein structures that are obtained by X-ray analysis and magnetic resonance spectroscopy. Mol. Biol. 2005;39:129–138. [PubMed] [Google Scholar]
- 4.Spronk CA, Linge JP, Hilbers CW, Vuister GW. Improving the quality of protein structures derived by NMR spectroscopy. J. Biomol. NMR. 2002;22:281–289. doi: 10.1023/a:1014971029663. [DOI] [PubMed] [Google Scholar]
- 5.Clore GM, Gronenborn AM. New methods of structure refinement for macromolecular structure determination by NMR. Proc. Natl Acad. Sci. USA. 1998;95:5891–5898. doi: 10.1073/pnas.95.11.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Im W, Brooks CL., III Refinement of NMR structures using implicit solvent and advanced sampling techniques. J. Am. Chem. Soc. 2004;126:16038–16047. doi: 10.1021/ja047624f. [DOI] [PubMed] [Google Scholar]
- 7.Linge JP, Williams MA, Spronk CA, Bonvin AM, Nilges M. Refinement of protein structures in explicit solvent. Proteins. 2003;50:496–506. doi: 10.1002/prot.10299. [DOI] [PubMed] [Google Scholar]
- 8.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 9.Hooft RW, Vriend G, Sander C, Abola EE. Errors in protein structures. Nature. 1996;381:272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]
- 10.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, III, Snoeyink J, Richardson JS, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabuurs SB, Nederveen AJ, Vranken W, Doreleijers JF, Bonvin AM, Vuister GW, Vriend G, Spronk CA. DRESS: a database of REfined solution NMR structures. Proteins. 2004;55:483–486. doi: 10.1002/prot.20118. [DOI] [PubMed] [Google Scholar]
- 12.Nederveen AJ, Doreleijers JF, Vranken W, Miller Z, Spronk CA, Nabuurs SB, Guntert P, Livny M, Markley JL, Nilges M, et al. RECOORD: a recalculated coordinate database of 500+ proteins from the PDB using restraints from the BioMagResBank. Proteins. 2005;59:662–672. doi: 10.1002/prot.20408. [DOI] [PubMed] [Google Scholar]
- 13.Kuszewski J, Gronenborn AM, Clore GM. Improving the quality of NMR and crystallographic protein structures by means of a conformational database potential derived from structure databases. Protein Sci. 1996;5:1067–1080. doi: 10.1002/pro.5560050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein EG, Rice LM, Brunger AT. Torsion-angle molecular dynamics as a new efficient tool for NMR structure calculation. J. Magn. Reson. 1997;124:154–164. doi: 10.1006/jmre.1996.1027. [DOI] [PubMed] [Google Scholar]
- 15.Sippl MJ. Calculation of conformational ensembles from potentials of mean force. An approach to the knowledge-based prediction of local structures in globular proteins. J. Mol. Biol. 1990;213:859–883. doi: 10.1016/s0022-2836(05)80269-4. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, Livny M, Mading S, Maziuk D, Miller Z, et al. BioMagResBank. Nucleic Acids Res. 2008;36:D402–D408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazaridis T, Karplus M. Effective energy function for proteins in solution. Proteins. 1999;35:133–152. doi: 10.1002/(sici)1097-0134(19990501)35:2<133::aid-prot1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Brooks BR, Brooks CL, III, Mackerell AD, Jr, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Kihara D. Estimating quality of template-based protein models by alignment stability. Proteins. 2008;71:1255–1274. doi: 10.1002/prot.21819. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Zhou Y. Specific interactions for ab initio folding of protein terminal regions with secondary structures. Proteins. 2008;72:793–803. doi: 10.1002/prot.21968. [DOI] [PubMed] [Google Scholar]
- 22.Doreleijers JF, Raves ML, Rullmann T, Kaptein R. Completeness of NOEs in protein structure: a statistical analysis of NMR. J. Biomol. NMR. 1999;14:123–132. doi: 10.1023/a:1008335423527. [DOI] [PubMed] [Google Scholar]
- 23.Laskowski RA. Structural quality assurance. Methods Biochem. Anal. 2003;44:273–303. [PubMed] [Google Scholar]
- 24.Shaanan B, Gronenborn AM, Cohen GH, Gilliland GL, Veerapandian B, Davies DR, Clore GM. Combining experimental information from crystal and solution studies: joint X-ray and NMR refinement. Science. 1992;257:961–964. doi: 10.1126/science.1502561. [DOI] [PubMed] [Google Scholar]
- 25.Wichmann BA, Hill ID. Algorithms AS 183: an efficient and portable pseudo-random number generator. Appl. Statist. 1982;31:188–190. [Google Scholar]
- 26.Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem. Mol. Biol. Educ. 2006;34:255–261. doi: 10.1002/bmb.2006.494034042644. [DOI] [PubMed] [Google Scholar]
- 27.Chen VB, Davis IW, Richardson DC. KING (Kinemage, Next Generation): a versatile interactive molecular and scientific visualization program. Protein Sci. 2009;18:2403–2409. doi: 10.1002/pro.250. [DOI] [PMC free article] [PubMed] [Google Scholar]