Abstract
Background
Eyes with glaucoma are characterized by optic neuropathy with visual field defects in the areas corresponding to the optic disk damage. The exact cause for the glaucomatous optic neuropathy has not been determined. Myopia has been shown to be a risk factor for glaucoma. The purpose of this study was to determine whether a significant correlation existed between the microcirculation of the optic disk and the visual field defects and the retinal nerve fiber layer thickness (RNFLT) in glaucoma patients with myopic optic disks.
Methods
Sixty eyes of 60 patients with myopic disks were studied; 36 eyes with glaucoma (men:women = 19:17) and 24 eyes with no ocular diseases (men:women = 14:10). The mean deviation (MD) determined by the Humphrey field analyzer, and the peripapillary RNFLT determined by the Stratus-OCT were compared between the two groups. The ocular circulation was determined by laser speckle flowgraphy (LSFG), and the mean blur rate (MBR) was compared between the two groups. The correlations between the RNFLT and MBR of the corresponding areas of the optic disk and between MD and MBR of the optic disk in the glaucoma group were determined by simple regression analyses.
Results
The average MBR for the entire optic disk was significantly lower in the glaucoma group than that in the control group. The differences of the MBR for the tissue in the superior, inferior, and temporal quadrants of the optic disk between the two groups were significant. The MBR for the entire optic disk was significantly correlated with the MD (r = 0.58, P = 0.0002) and the average RNFLT (r = 0.53, P = 0.0008). The tissue MBR of the optic disk was significantly correlated with the RNFLT in the superior, inferior, and temporal quadrants.
Conclusions
Our study suggests that there is a causal relationship between the thinner RNFLT that led to the MD and reduction in the microcirculation in the optic nerve head.
Keywords: glaucoma, visual field defects, optic disk, optic neuropathy, myopia, microcirculation, optic nerve head, retinal nerve fiber layer
Introduction
Glaucoma is a multifactorial disease characterized by a degeneration of the axons in the optic nerve and the death of retinal ganglion cells. The death of retinal ganglion cells then leads to defects in the corresponding visual fields. Glaucoma is a progressive disease, and the aim of glaucoma therapy is to stop or slow the progression of the visual field defects and thus maintain good vision over the entire visual field. Controlling the intraocular pressure (IOP) at normal levels is one of the critical factors in preventing the progression of the visual field defects. However among Asians, normal tension glaucoma (NTG) is the major type of glaucoma,1,2 and even with a 30% reduction of the IOP, progression in visual field defects can still occur.
The risk factors for glaucoma are gender, ethnicity, family history, migraine, and myopia.1–6 The Tajimi study,1 an epidemiological study of ocular diseases in Japan, showed that myopia was a risk factor for primary open angle glaucoma (POAG). This study also reported that the prevalence of myopia was higher in Japanese than in individuals from western countries. The Beijing eye study also showed a high prevalence of myopia in individuals living in Beijing.2 The higher prevalence of myopia among Asians is important because patients with higher myopia have a greater risk for glaucoma: eyes with <−3 diopters (D) had an odds ratio [OR]: 2.60, 95% confidence interval [CI]: 1.56–4.35 in the Tajimi study; and eyes with <−6 D of myopia had an OR: 2.28, 95% CI: 0.99–5.25 in the Beijing study.
How myopia and glaucoma are linked has not been determined. However, we recently found that the blood circulation in the optic nerve head was reduced (filling defect) in glaucoma patients with decreased visual acuity.7 In addition, Lam and his coworkers showed that pulsatile ocular blood flow (POBF) was reduced in myopic patients.8 Thus, one common factor in glaucoma and myopia is reduction of the circulation in the optic disk area. These observations motivated us to investigate whether the glaucomatous visual field defects and thinner retinal nerve fiber layer thickness (RNFLT) were significantly correlated with ocular circulation in glaucoma patients with myopic glaucomatous optic disks.
At present, angiography with fluorescein or indocyanine green (ICG) is used to evaluate ocular circulation, and a significant positive correlation exists between the size of the dye filling defect and the degree of visual field loss.9 However, these angiographic methods are invasive, time consuming, and difficult to quantify accurately. These methods are also not used routinely to evaluate the degree of glaucoma. Color Doppler imaging (CDI) and laser Doppler flowmetry (LDF) have been used to quantify the velocity of ocular flow in patients with glaucoma. However, CDI measures the retrobulbar circulation and not the retinal circulation, and LDF examines only a small area of the retina. Thus, these methods for measuring ocular circulation have serious limitations.
Laser speckle flowgraphy (LSFG) is another instrument that has been used to assess intraocular circulation. LSFG is noninvasive and the data can be collected in less than 5.0 seconds.10–17 An examination of the ocular fundus illuminated by laser light shows a speckle pattern that arises from the scatter of the laser irradiation from the tissue. Changes in the velocity of the blood flow blur the speckle pattern, and the changes are called “blurring”. Specialized software for LSFG calculates mean blur rate (MBR) from the light intensity of the speckle pattern on a point-by-point basis. The MBR obtained by the current version of LSFG (LSFG-NAVI, Softcare Ltd, Fukuoka, Japan) is proportional to the velocity of the scatter and is expressed as the product of the square blur rate (SBR). The SBR is the indicator used in the earlier version of the LSFG and is a constant factor. However the current version of LSFG is equipped with a commercial CCD camera, and a clearer color-coded map of MBR can be obtained by a new method of analyzing the speckle pattern.18
The purpose of this study was to determine whether there are significant correlations between the circulation in the optic disk and the degree of RNFL thinning and visual field defects. We focused on glaucoma patients with myopic glaucomatous disks because myopia is a major risk factor for glaucoma. We used LSFG to determine the ocular circulation for each quadrant of the optic disk and also for the average circulation over the entire disk. The mean deviation (MD) of the Humphrey field analyzer (HFA) was used to quantify the degree of visual field defects, and optical coherence tomography (OCT) was used to determine the RNFLT in different quadrants of the optic disks.
Materials and methods
Patients
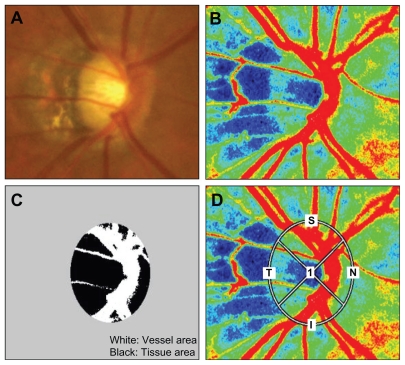
The procedures used in this retrospective study followed the tenets of the Declaration of Helsinki and were approved by the Institutional Review Board of the Tohoku Graduate School of Medicine. Sixty eyes of 60 Japanese adults (>40 years old) who had myopic optic disks were studied. Thirty-six eyes were diagnosed as having glaucoma (men:women = 19:17) and had a glaucomatous change in the optic disk (refractive error >−8 diopters). Twenty-four eyes had no ocular diseases except myopia (men:women = 14:10). A myopic disk was defined by its oval shape and tilting to the temporal side with crescent peripapillary atrophy. A myopic glaucomatous disk was defined as a myopic disk with glaucomatous neuropathy (Figure 1A).19,20 Patients were excluded if they had: glaucoma with other types of optic disk pattern; any other ocular diseases; systemic diseases affecting the visual fields; had intraocular surgery; and had refractive errors (spherical equivalent) <−8 diopters (D). The best-corrected visual acuity (BCVA) was measured with a standard Japanese decimal visual acuity chart and was converted to the logarithm of the minimum angle of resolution (logMAR) units. If both eyes met the inclusion criteria, the eye with the lower BCVA was used in the statistical analyses, and if both eyes had the same visual acuity, one eye was selected randomly for the analyses. The IOP was measured by Goldmann applanation tonometry. The systolic blood pressure (SBP), and diastolic blood pressure (DBP) were recorded before the measurement of LSFG and the ocular perfusion pressure (OPP) was calculated as OPP = 2/3[1/3SBP + 2/3DBP] − IOP.
Figure 1.
(A) A fundus photograph showing a typical myopia-type optic disk. The disk is oval and is tilted to the temporal side with crescent peripapillary atrophy. (B) The color-coded map of the optic nerve head. The colors in this map represent the time averages of MBR over one heartbeat. (C) The disk area was divided into the vessel area and tissue area by LSFG analyzer. The white area represents the vessel area, and the black area represents the tissue area of the optic disk. (D) The elliptical area where MBR was determined by LSFG was set at the outer edge of the myopic optic disk manually. Measurements were taken for the superior, temporal, inferior, and nasal quadrants.
Abbreviations: MBR, mean blur rate; LSFG, Laser speckle flowgraphy.
Diagnosis of glaucoma
Glaucoma was diagnosed by the presence of cupping of the optic disk with visual field defects in the corresponding visual field. The perimetric data selected for the statistical analyses were those collected within 6 months of the LSFG examination. Thirty-six eyes with myopic disks had glaucomatous visual field defects according to the Anderson–Pattela classification: (1) results of the glaucoma hemifield test was outside of the normal limits; (2) there was a cluster of three or more non-edge points at a location typical for glaucoma, all were depressed on the pattern deviation plot at a P < 5% level and one was depressed at a P < 1% level; and (3) corrected pattern standard deviation was significant at the P < 5% level.
Visual field analyses
To evaluate the visual fields, the MD values were obtained by the Swedish interactive threshold algorithm (SITA)-standard strategy of the 30-2 program of the HFA (Carl Zeiss Meditec, Dublin, CA). MDs of only the reliable visual field results (<20% fixation errors, <33% false-positives, and <33% false-negatives) were used.
Laser speckle flowgraphy
To evaluate the microcirculation of the optic nerve head, MBR in the optic disk was determined by LSFG-NAVI (Softcare Ltd, Fukuoka, Japan) as shown in Figure 1B. The measurement of LSFG was carried out after the pupil was dilated with 0.4% tropicamide (Midrin-M, Santen Pharmaceutical Co Ltd, Osaka, Japan). Before the examination with LSFG, the patients rested on a chair for 10 minutes in the dark room, and they were all examined by one experienced investigator. The edge detection of the optic disk in the MBR image was performed manually and the margin of the disk area was saved. The MBR in three areas of the optic disk were calculated: MBRD was the average MBR over the entire optic disk, MBRV was the average over the vessel area, and MBRT was the average of the MBR of the optic disk area minus the vessel area (Figure 1C). These values of the MBR were calculated using the LSFG Analyzer (v 3.0.43.0). Three measurements of the same subject were taken consecutively within a few minutes and MBR in the same area was calculated using the saved area data. The averages were used in the statistical analyses. Similar calculations were made for the superior, temporal, inferior, and nasal quadrants of the optic disk (Figure 1D).
Retinal nerve fiber layer thickness
The RNFLT around the optic disk was determined by Stratus- OCT (Carl Zeiss Meditec, Dublin, CA), and the average RNFLT, and the RNFLT of the superior, inferior, temporal, and nasal quadrants were determined.
Statistical analyses
The relationships between the values of LSFG and the degree of alterations including the MD values and the RNFLT were determined by simple regression analysis. The Mann–Whitney U test was used to compare two independent groups. The chi-square test was used for the categorized data. A P value of <0.05 was considered significant.
Results
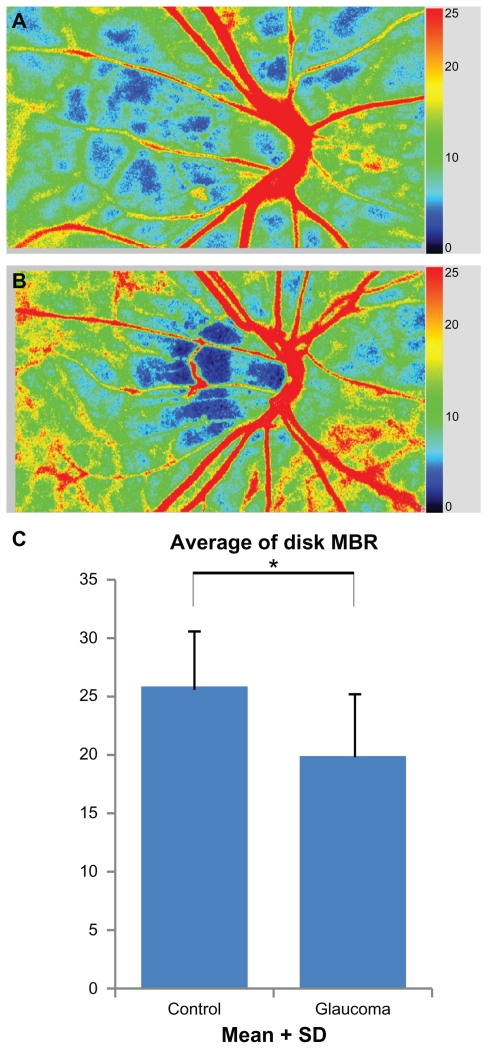
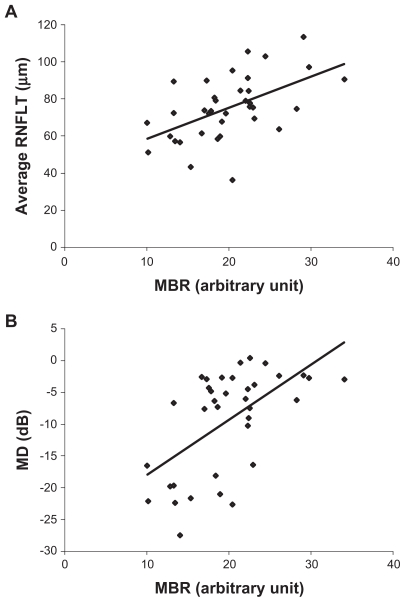
The demographics of the subjects in the control and glaucoma groups are presented in Table 1. The differences in the ages, gender distribution, diastolic blood pressures, and perfusion pressures between the two groups were not significant. The differences in the systolic blood pressure and the refractive errors were also not statistically significant. In the glaucoma group, IOP was significantly lower than that of the controls because they were being treated for glaucoma and their IOP was being controlled. The RNFLT of the superior, temporal, and inferior quadrants in the myopic glaucoma patients were significantly thinner than those in the control group but not in the nasal quadrant (Table 2). The MBR of the optic nerve head in the two groups is shown in Figure 2. A sample color-coded map of the control eye (Figure 2A) measured by LSFG shows warm colors at areas of myopic-type disk and peripapillary choroid, however, a sample of a glaucomatous eye shows cool colors in the same sites (Figure 2B). This means that myopic glaucomatous disks have low MBR for the optic nerve head and choroid. The MBRD in the control group was 25.7 ± 4.9 which was significantly higher than 19.9 ± 5.4 in the glaucoma group (P = 0.0001; Figure 2C). The MBRD is positively correlated with average RNFLT and MD (Figure 3; Y = 1.68X + 41.8, r = 0.532, P < 0.001; Y = 0.867X − 26.6, r = 0.580, P < 0.001, respectively).
Table 1.
Subject demographics
| Control (n = 24) | Glaucoma (n = 36) | P value | |
|---|---|---|---|
| Men:women | 14:10 | 19:17 | 0.67* |
| Age | 54.54 ± 6.83 (40–65) | 53.42 ± 8.52 (40–72) | 0.54** |
| Visual acuity (LogMAR) | −0.05 ± 0.05 (−0.18–1.22) | 0.02 ± 0.24 (−0.08–0) | 0.56** |
| Spherical equivalent (diopter) | −3.07 ± 2.49 | −4.14 ± 2.26 | 0.13** |
| Intraocular pressure (mmHg) | 14.83 ± 3.26 | 13.06 ± 2.26 | 0.02** |
| Systolic blood pressure (mmHg) | 126.29 ± 14.70 | 119.28 ± 15.35 | 0.08** |
| Diastolic blood pressure (mmHg) | 75.79 ± 13.81 | 73.25 ± 11.50 | 0.93** |
| Perfusion pressure (mmHg) | 46.91 ± 9.54 | 46.00 ± 7.60 | 0.70** |
Notes: Mean ± SD (range); Perfusion pressure = 2/3(1/3 systolic blood pressure + 2/3 diastolic blood pressure) – intraocular pressure;
Chi-square test;
Mann-Whitney U test.
Table 2.
Comparison of RNFLT between control and glaucoma groups
| RNFLT | Control (n = 24) | Glaucoma (n = 36) | P value |
|---|---|---|---|
| Average | 97.82 ± 11.84 | 75.20 ± 17.12 | <0.0001 |
| Superior quadrant | 120.29 ± 17.91 | 92.14 ± 29.49 | <0.0001 |
| Temporal quadrant | 79.58 ± 12.65 | 58.75 ± 20.45 | <0.0001 |
| Inferior quadrant | 129.96 ± 22.17 | 88.89 ± 25.19 | <0.0001 |
| Nasal quadrant | 61.38 ± 18.41 | 61.67 ± 14.45 | 0.94 |
Note: Mean ± SD; Student’s t-test.
Abbreviation: RNFLT, retinal nerve fiber layer thickness.
Figure 2.
Comparisons of MBR of the entire optic disk area between control and glaucoma group. The color scale bars in Figure 2A and B show the degree of MBR. The proportions of cool colors in the optic disk, which represent low MBR, are increased in the map of the glaucomatous eye rather than those of the control. (A) A color-coded map of ocular fundus of a control eye. The eyes in control group have myopic disk without glaucomatous visual field loss. (B) A color-coded map of ocular fundus in a glaucomatous eye. (C) Comparisons of MBRD between two groups. There are significant differences in these parameters between control and glaucoma groups.
Notes: *Glaucoma group significantly different from control group (Mann–Whitney U test; P < 0.05)
Abbreviations: MBR, mean blur rate; MBRD, average MBR over the entire optic disk.
Figure 3.
(A) Correlation between the average retinal nerve fiber thickness (RNFLT) and the average MBR over the entire optic disk (MBRD) in myopic glaucoma patients (Y = 1.6767X + 41.844; r = 0.5324, P = 0.0008). (B) Correlation between MD of Humphrey SITA 30-2 program and MBRD in myopic glaucoma patients (Y = 0.8673X − 26.646, r = 0.5797, P = 0.0002).
Abbreviations: MBR, mean blur rate; MBRD, average MBR over the entire optic disk; MD, mean deviation.
Simple regression analyses were performed to determine the correlations between MBR and RNFLT in the glaucomatous eyes (Table 3). In this table, the correlations of MBRV and MBRT with corresponding RNFLT at each quadrant are shown. MBRT is correlated more strongly with RNFLT than MBRV.
Table 3.
Correlation between MBR and RNFLT at the corresponding segment of optic nerve head in glaucoma group
| Measurement | Correlation coefficient | P value |
|---|---|---|
| MBRD | 0.532 | <0.001 |
| MBRV | ||
| Average | 0.240 | 0.159 |
| Superior | 0.211 | 0.217 |
| Temporal | 0.220 | 0.197 |
| Inferior | 0.132 | 0.443 |
| Nasal | 0.194 | 0.257 |
| MBRT | ||
| Average | 0.571 | <0.001 |
| Superior | 0.519 | 0.001 |
| Temporal | 0.364 | 0.029 |
| Inferior | 0.436 | 0.008 |
| Nasal | 0.213 | 0.212 |
Abbreviations: MBRD, MBR at entire optic disk area; MBRV, MBR at vessel area of optic disk; MBRT, MBR at tissue area of optic disk; RNFLT, retinal nerve fiber layer thickness.
Our analyses showed that MBR over the entire optic disk was correlated significantly and positively with average RNFLT (r = 0.532, P < 0.001). MBRT of the superior (r = 0.519, P = 0.001), temporal (r = 0.364, P = 0.029), and inferior quadrants (r = 0.436, P = 0.008) were also significantly and positively correlated with the corresponding RNFLT. These correlations indicate that lower MBR values were found in eyes with thinner RNFLT. However, the correlation between the nasal MBRT and the nasal RNFLT was not significant (r = 0.213, P = 0.212).
Discussion
LSFG has been shown to be a valid and reliable method to measure the blood flow on the optic nerve head and peripapillary choroid.12,21,22 The SBR, which was used as an arbitrary unit in early version of LSFG, was recently demonstrated to be significantly correlated with the blood flow and blood velocity.10 We used the LSFG–NAVI to determine the microcirculation in glaucoma patients with the myopic disk type, and our results showed significant and positive correlations between the circulation on the optic disk and the visual field defects and RNFLT.
The color-coded map provides a very effective way to visually grasp the degree of MBR in ocular fundus (Figure 1A and B). The proportions of warm colors which indicate high MBR are increased in control rather than glaucomatous eyes. By contrast, the proportions of cool colors in the whole optic disk area are increased in glaucomatous eyes. In Figure 1C, a significant difference was found between the control and glaucoma groups in MBRD. The reduction of blood supply to an eye has been said to have an association with the degree of myopic change in past studies.8,23 Furthermore, we showed that there was lower blood flow in the ocular fundus with myopic glaucomatous disks than in myopic disks without glaucomatous change using LSFG.
The tissue MBRs for the superior and inferior quadrants of the glaucoma group were significantly lower than those of the control. These findings suggested that the circulation of myopic glaucomatous disks may decrease in the superior and inferior quadrant, but not the temporal quadrant, in eyes with myopic glaucomatous disk.
The significant correlation between MBRD and the MD agrees with the results of Yaoeda et al who reported that the average SBR at the superior or inferior temporal neuroretinal rim was positively and significantly correlated with the MD in eyes with NTG (r = 0.349, P = 0.020).15 Interestingly, our results showed that glaucoma patients with myopic glaucomatous optic disks had a higher correlation between all disk MBR and MD (r = 0.580, P < 0.001) than that of Yaoeda’s studies (r = 0.349, P = 0.020), which determined the ocular circulation using the earlier version of LSFG. The different degree of association may result from different experimental conditions, including that (1) they used SBR obtained using the earlier version of LSFG with a small area of superior temporal and inferior temporal neuroretinal rim, while we used the MBR of the whole optic nerve head and (2) they examined a range of glaucoma patients, while we examined only glaucoma patients with myopia. These data suggest that MBR of all disk areas obtained by LSFG–NAVI may enable us to assess the status of glaucoma patients with myopic disks.
The RNFLT in our glaucoma patients was significantly thinner than that of the controls in the superior, inferior and temporal quadrant but not in the nasal quadrant (Table 2). The RNFLT was more strongly associated with MBRT than with MBRV (Table 3). The reduced MBR of the tissue in the superior, temporal, and inferior quadrants was significantly and positively correlated with the thinner RNFLT. These findings indicate that lower blood flow to the tissue area of the optic disk, ie, lower MBRT, is correlated with thinner RNFLT at the superior, temporal, and inferior quadrants.
Our findings are in good agreement with earlier studies which showed that patients with higher myopia had thinner RNFLT and decreased ocular circulation. Kim et al24 and Wang et al25 classified myopia patients without glaucoma into three groups according to the refractive error and determined RNFLT by OCT. They reported that eyes with higher myopia had thinner RNFLT than eyes with lower myopia. Dimitrova and colleagues investigated whether the refractive error and the axial length were significantly correlated with the blood flow in the central retinal artery, central retinal vein, and the posterior ciliary arteries measured by CDI.23 They found significant positive correlations between the refractive error and the retrobulbar circulation. Lam et al reported the negative correlation between POBF measured by the OBF tonometer and the axial lengths in young normal subjects (r = −0.57, P < 0.01).8 In another study, they investigated the effect of axial length on POBF in subjects with axial anisometropia and showed that the POBF in the eye with longer axial length was lower than in the contralateral shorter eye (P < 0.001).26 Nicolela at el reported that the more affected eye in glaucomatous patients with asymmetric visual field loss had lower blood velocity in the central retinal artery than the contralateral eye.27 They suggested that low blood velocities might be one of the lateralizing factors in those patients and that this may have a possible role in the pathogenesis of glaucoma. These reports support the hypothesis that myopic eyes tend to have reduced RNFLT and reduced ocular circulation and these changes are risk factors for glaucoma. Our results showed that thinning of RNFL and decreased optic nerve head microcirculation were present in glaucomatous subjects with myopic glaucomatous disks. In addition, RNFLT and myopic disk circulation were significantly correlated.
In conclusion, we found a decrease in microcirculation in glaucoma patients with myopic disks using LSFG-NAVI. The significant correlations between the microcirculation in myopic glaucomatous disks in glaucoma patients and the RNFLT and MD suggest that there is a cause-effect relationship between a reduction of the microcirculation and a reduction in RNFLT that led to MD.
Acknowledgments
The authors thank Professor Duco Hamasaki for editing this manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Suzuki Y, Iwase A, Araie M, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006;113(9):1613–1617. doi: 10.1016/j.ophtha.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 2.Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007;114(2):216–220. doi: 10.1016/j.ophtha.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106(10):2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 4.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53(Suppl 1):S3–S10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115(1):85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131(6):699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 7.Omodaka K, Nakazawa T, Yokoyama Y, Doi H, Fuse N, Nishida K. Correlation between peripapillary macular fiber layer thickness and visual acuity in patients with open-angle glaucoma. Clin Ophthalmol. 2010;4:629–635. doi: 10.2147/opth.s11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam AK, Wong S, Lam CS, To CH. The effect of myopic axial elongation and posture on the pulsatile ocular blood flow in young normal subjects. Optom Vis Sci. 2002;79(5):300–305. doi: 10.1097/00006324-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Arend O, Plange N, Sponsel WE, Remky A. Pathogenetic aspects of the glaucomatous optic neuropathy: fluorescein angiographic findings in patients with primary open angle glaucoma. Brain Res Bull. 2004;62(6):517–524. doi: 10.1016/j.brainresbull.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Nagahara M, Tamaki Y, Tomidokoro A, Araie M. In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Invest Ophthalmol Vis Sci. 2011;52(1):87–92. doi: 10.1167/iovs.09-4422. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama T, Schwartz B, Takamoto T, Azuma I. Evaluation of the circulation in the retina, peripapillary choroid and optic disk in normal-tension glaucoma. Ophthalmic Res. 2000;32(2–3):79–86. doi: 10.1159/000055594. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama T, Utsumi T, Azuma I, Fujii H. Measurement of optic nerve head circulation: comparison of laser speckle and hydrogen clearance methods. Jpn J Ophthalmol. 1996;40(3):339–343. [PubMed] [Google Scholar]
- 13.Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H. Noncontact, two-dimensional measurement of retinal microcirculation using laser speckle phenomenon. Invest Ophthalmol Vis Sci. 1994;35(11):3825–3834. [PubMed] [Google Scholar]
- 14.Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res. 1995;60(4):373–383. doi: 10.1016/s0014-4835(05)80094-6. [DOI] [PubMed] [Google Scholar]
- 15.Yaoeda K, Shirakashi M, Fukushima A, et al. Relationship between optic nerve head microcirculation and visual field loss in glaucoma. Acta Ophthalmol Scand. 2003;81(3):253–259. doi: 10.1034/j.1600-0420.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 16.Yaoeda K, Shirakashi M, Funaki S, Funaki H, Nakatsue T, Abe H. Measurement of microcirculation in the optic nerve head by laser speckle flowgraphy and scanning laser Doppler flowmetry. Am J Ophthalmol. 2000;129(6):734–739. doi: 10.1016/s0002-9394(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 17.Yaoeda K, Shirakashi M, Funaki S, et al. Measurement of microcirculation in optic nerve head by laser speckle flowgraphy in normal volunteers. Am J Ophthalmol. 2000;130(5):606–610. doi: 10.1016/s0002-9394(00)00723-6. [DOI] [PubMed] [Google Scholar]
- 18.Konishi N, Tokimoto Y, Kohra K, Fujii H. New laser speckle flowgraphy system using CCD camera. Opt Rev. 2002;9:163–169. [Google Scholar]
- 19.Broadway DC, Nicolela MT, Drance SM. Optic disk appearances in primary open-angle glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S223–S243. doi: 10.1016/s0039-6257(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 20.Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: clinical correlations. Ophthalmology. 1996;103(4):640–649. doi: 10.1016/s0161-6420(96)30640-4. [DOI] [PubMed] [Google Scholar]
- 21.Isono H, Kishi S, Kimura Y, Hagiwara N, Konishi N, Fujii H. Observation of choroidal circulation using index of erythrocytic velocity. Arch Ophthalmol. 2003;121(2):225–231. doi: 10.1001/archopht.121.2.225. [DOI] [PubMed] [Google Scholar]
- 22.Tamaki Y, Araie M, Tomita K, Nagahara M, Tomidokoro A, Fujii H. Real-time measurement of human optic nerve head and choroid circulation, using the laser speckle phenomenon. Jpn J Ophthalmol. 1997;41(1):49–54. doi: 10.1016/s0021-5155(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 23.Dimitrova G, Tamaki Y, Kato S, Nagahara M. Retrobulbar circulation in myopic patients with or without myopic choroidal neovascularisation. Br J Ophthalmol. 2002;86(7):771–773. doi: 10.1136/bjo.86.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MJ, Lee EJ, Kim TW. Peripapillary retinal nerve fibre layer thickness profile in subjects with myopia measured using the Stratus optical coherence tomography. Br J Ophthalmol. 2010;94(1):115–120. doi: 10.1136/bjo.2009.162206. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Qiu KL, Lu XH, et al. The effect of myopia on retinal nerve fibre layer measurement: a comparative study of spectral-domain optical coherence tomography and scanning laser polarimetry. Br J Ophthalmol. 2011;95(2):255–260. doi: 10.1136/bjo.2009.176768. [DOI] [PubMed] [Google Scholar]
- 26.Lam AK, Chan ST, Chan B, Chan H. The effect of axial length on ocular blood flow assessment in anisometropes. Ophthalmic Physiol Opt. 2003;23(4):315–320. doi: 10.1046/j.1475-1313.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 27.Nicolela MT, Drance SM, Rankin SJ, Buckley AR, Walman BE. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol. 1996;121(5):502–510. doi: 10.1016/s0002-9394(14)75424-8. [DOI] [PubMed] [Google Scholar]