Abstract
Reversible interactions of glycoconjugates on leukocytes with P- and E-selectin on endothelial cells mediate tethering and rolling of leukocytes in inflamed vascular beds, the first step in their recruitment to sites of injury. Although selectin ligands on hematopoietic precursors have been identified, here we review evidence that PSGL-1, CD44, and ESL-1 on mature leukocytes are physiologic glycoprotein ligands for endothelial selectins. Each ligand has specialized adhesive functions during tethering and rolling. Furthermore, PSGL-1 and CD44 induce signals that activate the β2 integrin LFA-1 and promote slow rolling, whereas ESL-1 induces signals that activate the β2 integrin Mac-1 in adherent neutrophils. We also review evidence for glycolipids, CD43, L-selectin, and other glycoconjugates as potential physiologic ligands for endothelial selectins on neutrophils or lymphocytes. Although the physiologic characterization of these ligands has been obtained in mice, we also note reported similarities and differences with human selectin ligands.
Introduction
The selectins mediate adhesion of hematopoietic cells to vascular surfaces and to each other.1 These interactions are important for host defense, hematopoiesis, immune cell surveillance, hemostasis, and inflammation. Each of the 3 selectins is a type I transmembrane protein with an N-terminal C-type lectin domain, an epidermal growth factor-like domain, a series of consensus repeats, a transmembrane domain, and a short cytoplasmic tail. L-selectin is constitutively expressed on most leukocytes. P-selectin is rapidly mobilized from secretory granules to the plasma membranes of platelets and endothelial cells on stimulation. E-selectin expression on endothelial cells is regulated at the transcriptional level by inflammatory mediators, such as tumor necrosis factor-α.
The rolling cell adhesion mediated by selectins is a dynamic process that requires rapid formation and breakage of bonds under flow.2 Rolling enables cells to receive signals that activate integrins, another class of adhesion receptors, which cause the cells to roll slower and to arrest.3 Here we discuss how leukocytes, particularly neutrophils, interact with endothelial selectins during inflammation. We review evidence that surprisingly few neutrophil glycoproteins are physiologic selectin ligands, defined by their ability to mediate rolling adhesion. Rolling can be studied with flow chambers in vitro or ex vivo and in transparent tissues or by epifluorescence in vivo.
The selectins are Ca2+-dependent lectins. The minimal glycan determinant for selectin binding is sialyl Lewis x (sLex; NeuAcα2,3Galβ1,4[Fucα1,3]GlcNAcβ1-R).1 The fucose moiety of sLex expressed on selectin ligands forms critical interactions with the Ca2+-coordination site on the lectin domain of selectins.2 Leukocytes from mice lacking the 2 α1,3-fucosyltransferases that add fucose to form sLex on hematopoietic cells cannot roll on P- and E-selectin.4 Because sLex can potentially cap N- and O-glycans on many proteins and also glycans on lipids, neutrophils might display many selectin ligands. However, α1,3-fucosylation occurs at limited sites on some proteins on human myeloid cells,5 and there is very little α1,3-fucosylation on murine myeloid cells.6 A subset of these glycoproteins might cluster sLex-capped glycans to increase avidity. As described in the next section, P-selectin binds with higher affinity to an N-terminal region of P-selectin glycoprotein ligand-1 (PSGL-1) through cooperative interactions with sulfated tyrosines and other amino acids and with an adjacent sLex-capped O-glycan. However, the affinity or avidity of a glycoprotein for a selectin in solution may not predict physiologic relevance. During rolling, selectins interact with their ligands under 2-dimensional conditions where force regulates off-rates.2 Rolling of a selectin-expressing cell on an isolated glycoprotein does not prove the latter's function in the context of the complex topography of the leukocyte surface (Figure 1A). Factors, such as the number of molecules per cell, molecular length, dimerization or oligomerization, clustering in lipid rafts or microvilli, or cytoskeletal anchorage, may be crucial determinants of function.2 Therefore, the physiologic roles of P- and E-selectin ligands require confirmation in primary leukocytes.
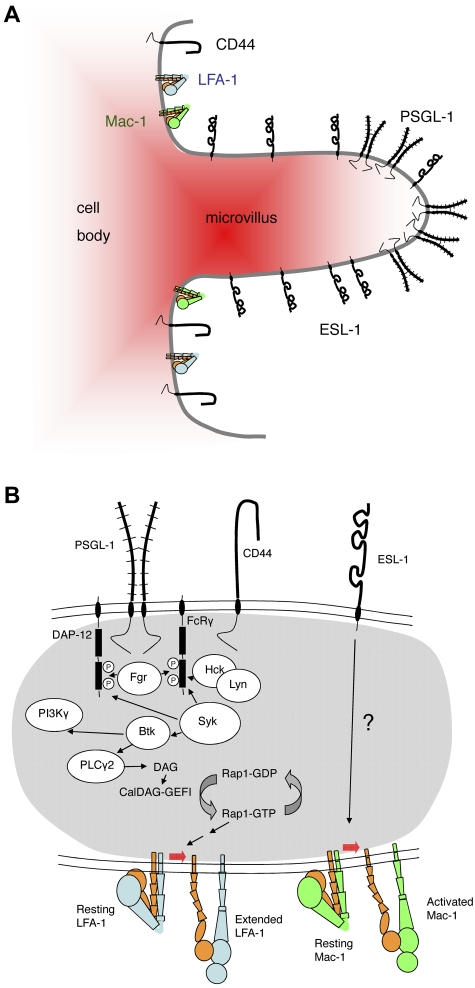
Figure 1.
Topography and signaling pathways triggered by selectin ligands in neutrophils. (A) Topography of selectin ligands on neutrophils. Based on biochemical and electron microscopic evidence, PSGL-1 is thought to be concentrated in lipid rafts on the tips of microvilli.13 Electron microscopy places (some of) ESL-1 on microvilli, but not necessarily the tips,69 whereas CD44 is concentrated in the valleys between microvilli.86 LFA-1 and Mac-1 are thought to be mostly on the cell body. (B) Signaling pathways of selectin ligands in neutrophils. Engagement of PSGL-1 by P-selectin or E-selectin or engagement of CD44 by E-selectin induces activation of the SFKs Fgr, Hck, and Lyn.40,82 The activated SFKs phosphorylate the ITAM domains of DAP-12 and FcRγ, enabling them to recruit spleen tyrosine kinase Syk.82 Knocking out Fgr or knocking out both Hck and Lyn blocks this signaling pathway.40,82 Direct physical association between PSGL-1, CD44, and the SFKs has not been demonstrated. Syk activity is needed to activate Bruton tyrosine kinase (Btk), which leads to phospholipase C-γ2 (PLCγ2) activation, providing diacylglycerol (DAG) for the activation of CalDAG-GEFI, an exchange factor for the small G protein Rap-1.84 Rap-1 drives LFA-1 extension through other signaling intermediates (not shown). Engagement of ESL-1 by E-selectin has been shown to activate Mac-1,85 but the signaling pathway is unknown.
Each approach to identify physiologic selectin ligands on a leukocyte has strengths and limitations. Gene knockout or gene silencing (mRNA knockdown) in mice allows assessment of ligand activity under physiologic conditions, but loss of a glycoprotein might impair rolling through an indirect effect on cellular function rather than by eliminating a key selectin ligand. Definitive identification of a glycoprotein as a selectin ligand should ideally meet several criteria, including: (1) the capacity to support rolling of selectin-bearing cells or beads on isolated ligand; (2) gene depletion or silencing must impair selectin-mediated functions on intact cells in vitro and in vivo (eg, rolling or signaling); and (3) monoclonal antibodies (mAbs) against a specific glycoprotein must also impair selectin-mediated functions. To date, only mAbs to the unique N-terminal P-selectin-binding region of PSGL-1 fulfill the third requirement.1,2 Importantly, mAbs to glycoproteins that reproducibly block binding to E-selectin have not been described. This has significantly hindered testing of physiologic functions of candidate E-selectin ligands on primary human leukocytes, where gene knockout and gene silencing methods are less feasible. In mice, as we discuss in “Additional ligands for E-selectin,” the functional redundancy of E-selectin ligands has required simultaneous deletion of more than one glycoprotein to unmask their functions. Even when all 3 of the aforementioned conditions are met, the ability of mAb or knockout/knockdown approaches to abrogate leukocyte rolling on a selectin may not definitively identify all glycoprotein ligands. Other glycoproteins might be necessary but not sufficient without the targeted glycoproteins.
Despite these challenges, significant progress has been made in identifying physiologic selectin ligands on leukocytes that mediate not only rolling but also signaling, thus enabling integrins to stabilize interactions with endothelial cells and other blood cells. These advances offer the opportunity to identify new physiologic contributions of selectins and their ligands to homeostasis and disease. Here we review the evidence that 3 glycoproteins act as physiologic ligands for P- and/or E-selectin on mouse neutrophils, describe the specialized roles of each ligand for neutrophil recruitment during inflammation, discuss limitations of current data and some controversies, note other leukocyte glycoproteins and glycolipids that might be physiologic ligands, and suggest avenues for future research. The contributions of selectin ligands on hematopoietic precursors to in vivo trafficking are less well characterized and will not be discussed here.
Ligands for endothelial selectins
PSGL-1
PSGL-1 is a major selectin ligand on leukocytes. PSGL-1 binds to P-selectin,7 E-selectin,8,9 and L-selectin10 under flow conditions. It is the predominant physiologic ligand for P-selectin and L-selectin on leukocytes, and it cooperates with additional ligands to mediate leukocyte rolling on E-selectin. In addition to mediating leukocyte tethering and rolling, it transduces signals into rolling leukocytes and into leukocytes decorated with platelets.11
PSGL-1 is a type I membrane protein that is preferentially located in lipid rafts12 on the tips of microvilli13 (Figure 1A). It is expressed as a disulfide-linked homodimer; each subunit consists of an extracellular, transmembrane, and cytoplasmic domain14,15 (Figure 1B). The extracellular domain of PSGL-1 is rich in prolines, serines, and threonines, most of which are located in a series of decameric repeats (14-16 in humans and 15 in mice).16,17 Posttranslational modifications of PSGL-1 are important for optimal selectin binding.15 Protein O-glycosylation is initiated by a polypeptide N-acetylgalactosamine transferase (ppGalNAcT) that adds GalNAc to serine and threonine residues. Leukocytes from mice lacking the glycosyltransferase ppGalNAcT-1 roll poorly on P- and E-selectin in vitro.18 To bind to P-selectin, PSGL-1 requires an α2,3-sialylated and α1,3-fucosylated core 2 O-glycan attached to a specific N-terminal threonine.15 Three core 2 β1,6-N-acetylglucosaminyltransferase enzymes (C2GnT) transfer GlcNAc to the Galβ1,3GalNAc core 1 structure.19 Studies with knockout mice have established the contribution of C2GnT1 to leukocyte interactions with all 3 selectins.20–24 C2GnT1 is required for the trafficking of neutrophils and activated T cells to sites of inflammation.20,22 Sulfation of tyrosine residues near the N-terminus optimizes the binding of PSGL-1 to P-selectin.15,25 To bind to E-selectin, PSGL-1 requires core 2 α1,3-fucosylated and α2,3-sialylated O-glycans but not tyrosine sulfation.15,26 Interactions of chemokines with the N-terminal domain of PSGL-1 may enhance recruitment of specific leukocyte subsets into inflamed or secondary lymphoid tissues.27,28 Interestingly, PSGL-1 glycosylation, which promotes selectin binding, negatively affects chemokine binding.28
The sequences of the transmembrane and cytoplasmic domains of PSGL-1 are highly conserved. In the endoplasmic reticulum, cooperative interactions between transmembrane domains and between cytoplasmic domains facilitate the formation of PSGL-1 dimers.29,30 Each noncovalent dimer is then stabilized by a single juxtamembrane disulfide bond. An export signal in the cytoplasmic domain promotes transfer of PSGL-1 from the endoplasmic reticulum to the Golgi apparatus, where O-glycans are added en route to the cell surface.30 The cytoplasmic tail of PSGL-1 consists of 67 amino acids in mice and 69 amino acids in humans and may interact with different proteins.11 In vitro, the cytoplasmic domain binds to ezrin/radixin/moesin (ERM) proteins, which in turn interact with actin filaments.31,32 Because both ERM proteins and PSGL-1 move to the uropod on polarization, the PSGL-1-ERM interaction might play a role in later steps of the leukocyte adhesion cascade, such as intravascular crawling or transendothelial migration. Nef-associated factor 1 (Naf-1) forms a constitutive complex with the juxtamembrane region of the cytoplasmic tail of PSGL-1.33 The PSGL-1 tail also binds to selectin ligand interactor cytoplasmic-1 (human ortholog of the mouse sorting nexin 20), which binds phosphoinositides and targets PSGL-1 to endosomes in transfected cells. However, selectin ligand interactor cytoplasmic-1 does not participate in PSGL-1-mediated leukocyte adhesion and signaling in vivo.34
mAbs to the N-terminal region of human or murine PSGL-1 block P- and L-selectin binding and abolish leukocyte rolling on L-selectin and P-selectin in vivo.7,10,35,36 L- and P- selectin bind to the same or closely overlapping sites near the N-terminus of PSGL-1, whereas E-selectin appears to bind to at least one more site.15 In vivo, PSGL-1-deficient leukocytes have markedly impaired tethering to and rolling on P-selectin.37 They tether less well to E-selectin, but those that tether roll with normal velocities.8,38–40 These observations confirmed that PSGL-1 is the predominant ligand for P-selectin. They also demonstrated that PSGL-1, albeit an important E-selectin ligand, must cooperate with other physiologic ligands for E-selectin, as we discuss in the following 2 sections. In the absence of an inflammatory or infectious challenge, the phenotype of PSGL-1-deficient (Selplg−/−) mice is remarkably mild.8,37
CD44
CD44 is a class I transmembrane glycoprotein that is expressed on most vertebrate cells, including hematopoietic stem cells, monocytes, neutrophils, lymphocytes, and endothelial cells. CD44 is involved in many cellular processes, including growth, survival, differentiation, and motility. A glycoform of CD44 isolated from human, but not murine, hematopoietic progenitors binds to L- and E-selectin in vitro. This glycoform has been termed hematopoietic cell E-/L-selectin ligand.41,42 Whether hematopoietic cell E-/L-selectin ligand functions as a selectin ligand on intact primary cells in vitro or in vivo has not been established. CD44 on neutrophils and some lymphocytes is a physiologic E-selectin ligand, suggesting cell-specific posttranslational modifications of CD44.39,43 The binding activity of neutrophil-derived CD44 requires its decoration by sialylated, α1,3-fucosylated, N-linked glycans.39 Altered glycosylation of CD44 proteins may account for pathologic conditions. For example, CD44 is hypofucosylated in neutrophils from patients with leukocyte adhesion deficiency type II syndrome.39
CD44, although encoded by a single gene, has more than 40 isoforms.44 The heterogeneity results from posttranslational modifications, such as sulfation and glycosylation as well as alternative splicing. Cells can simultaneously express multiple CD44 isoforms. The expression profile of the isoforms is dependent on the type of tissue and differentiation stage.45,46 The “standard” form of CD44 is composed of an extracellular amino-terminal globular protein domain, a stem structure, a transmembrane region, and a cytoplasmic tail. Hematopoietic cells express this standard form (Figure 1B), but glycosylation of the standard form varies as cells differentiate.
The N-terminal globular domain of CD44 has motifs that function as docking sites for several components of the extracellular matrix (eg, hyaluronan, collagen, laminin, fibronectin, and glycosaminoglycans).47,48 Binding of hyaluronan by CD44 is tightly regulated by posttranslational modifications.49–51 Physiologic stimuli can alter these modifications, resulting in the induction of hyaluronan binding.50,52
The stem structure (46 amino acids) links the amino-terminal globular domain to the transmembrane domain, which consists of 23 hydrophobic amino acids and a cysteine residue. The transmembrane domain may be responsible for the association of CD44 proteins with lipid rafts.53 Although the cytoplasmic tail of CD44 has no intrinsic catalytic activity, it interacts with several intracellular signaling molecules, including Src family kinases (SFKs), Rho GTPase, Rho kinase, and protein kinase C.54 It is not known whether these interactions are direct or indirect or whether they have functional impact in leukocytes.
CD44-deficient mice develop normally but have altered immune responses.55 CD44 has hyaluronan-dependent and -independent functions. Intravital microscopy has documented CD44-dependent rolling of T-cell subsets on hyaluronan in vivo.56–58 In vivo experiments also demonstrated that CD44 and hyaluronan are required for T-cell recruitment into the inflamed peritoneal cavity.59 CD44 and hyaluronan may enhance neutrophil recruitment to sites of inflammation.60–62 However, neutrophils do not tether to and roll on hyaluronan,61,62 and this agrees with the normal recruitment of neutrophils to the inflamed peritoneal cavity of Cd44−/− mice.39 In contrast, sequestration of neutrophils within liver sinusoids has been shown to be CD44- and hyaluronan-dependent.62 CD44-deficient neutrophils show reduced adhesion to the inflamed endothelium and subsequently increased rolling flux60,63 and increased rolling velocities,39 suggesting that CD44 is important for adhesion and/or sequestration.
T-lymphocytes require CD44 for integrin α4β1-mediated firm adhesion to the endothelium; this function requires the cytoplasmic tail of CD44.64,65 CD44-dependent rolling of T-helper (Th1) and Th2 CD4 lymphocytes has been observed in a mouse model of tumor necrosis factor-α–induced inflammation.56 CD44 extracted from Th1 lymphocytes binds to soluble E-selectin in vitro and cooperates with PSGL-1 in vivo by controlling rolling velocities and promoting firm arrest.43 Competitive recruitment assays demonstrated that T cells lacking both CD44 and PSGL-1 have more severe defects in migration to inflamed sites than T cells lacking only PSGL-1.43
ESL-1
Deleting PSGL-1 and CD44 in murine neutrophils strongly reduces but does not eliminate rolling on E-selectin in vitro or in vivo.39 A third key glycoprotein ligand for E-selectin on murine neutrophils is E-selectin ligand-1 (ESL-1). ESL-1 (also called MGF-160 or CFR-1, encoded by the gene Glg1) is a type I transmembrane protein. It consists of 1148 amino acids with 16 conserved cysteine-rich repeats and 5 potential N-glycosylation sites in the extracellular domain, a 21-residue transmembrane domain, and a short 13-residue cytoplasmic tail66,67 (Figure 1B). Although ESL-1 primarily localizes in the Golgi apparatus, a minor portion of ESL-1 is also exported to the plasma membrane, perhaps because of differential processing of the C-terminal domain.68,69 ESL-1 is expressed in many cell types,70 but selectin-binding activity has only been demonstrated in myeloid cells and human metastatic prostate cancer cells.71–73 Available antibodies to ESL-1 do not detect the protein on leukocyte surfaces by flow cytometry. Biotinylation strategies have demonstrated surface expression of ESL-1 on murine neutrophils69 and lymphocytes (A.H., unpublished data, January 2007), but not on human neutrophils and lymphocytes (D. Vestweber, oral communication, Max-Planck Institute for Molecular Medicine, Münster, Germany, August 2011). Although we focus here on its role as a selectin ligand, ESL-1 may exert pleiotropic effects. It functions as a receptor for several members of the fibroblast growth factor family,70,74 and it modulates intracellular processing and secretion of TGF-β.75 Consequently, mice deficient in ESL-1 show growth retardation and skeletal dysplasia.70,75
The first evidence that ESL-1 could bind E-selectin was obtained by applying myeloid cell lysates to E-selectin affinity columns.73 ESL-1 requires appropriate modifications of N-glycans to bind to E-selectin,67,72,73 whereas O-glycosylation of the glycoprotein has not been described. As with all other selectin ligands, α1,3 fucosylation of ESL-1 is required for interactions with E-selectin. Neutrophils appear to use fucosyltransferase IV to modify ESL-1 and fucosyltransferase VII to modify PSGL-1.76 Although biochemical and cell-based interactions of ESL-1 with E-selectin were documented many years ago,67 a physiologic role for ESL-1 in mediating rolling of murine leukocytes on E-selectin under flow was only recently documented.
Knockdown of ESL-1 by a short hairpin RNA strategy demonstrated that binding of a recombinant soluble form of E-selectin is mildly reduced when ESL-1 alone is absent but is abrogated when both PSGL-1 and ESL-1 are absent.38 Intravital studies of leukocyte rolling in venules of inflamed tissues further showed that leukocyte tethering requires the combined presence of PSGL-1 and ESL-1, where PSGL-1 interacts with both P- and E-selectin and ESL-1 interacts only with E-selectin.
Selectin ligand-mediated signaling
Binding of selectins to their ligands on leukocytes induces the activation of different signaling pathways (Figure 1B). Neutrophils rolling on P-selectin partially activate integrin αLβ2, also known as lymphocyte-associated antigen-1 (LFA-1), which slows their rolling velocities by enhancing transient LFA-1 binding to intercellular cell adhesion molecule-1 (ICAM-1). E- or P-selectin binding induces LFA-1 extension in a Syk-dependent manner.77 The cytoplasmic tail of PSGL-1 is required for LFA-1 activation and slower rolling on ICAM-1.78 However, deleting the cytoplasmic tail of PSGL-1 does not change the rolling of neutrophils on P-selectin or its localization in microvilli, lipid rafts, and uropods.78
In transfected cells, PSGL-1 interacts with the p85 subunit of PI3K in the presence of Naf-1.33 Under nonflow conditions, stimulation of human neutrophils with a soluble P-selectin-Fc chimeric protein induces SFK-dependent phosphorylation of Naf-1, which recruits the phosphoinositide-3-OH kinase p85-p110δ (PI3Kδ) heterodimer and leads to leukocyte integrin activation.33 These conditions may occur as leukocytes adhere to activated platelets, which express P-selectin at high densities, but are less likely to occur as leukocytes roll on P-selectin expressed on activated endothelial cells. Indeed, murine neutrophils lacking PI3Kδ exhibit normal LFA-1-dependent slow rolling on P-selectin and ICAM-1.40
Three studies using flow chambers have shown that PSGL-1 participates in E-selectin–mediated slow rolling of murine neutrophils.40,78,79 In one study using unfractionated murine blood, PSGL-1-deficient neutrophils did not roll slower on E-selectin and ICAM-1 than on E-selectin alone. In contrast, CD44-deficient neutrophils rolled much slower on E-selectin and ICAM-1 than on E-selectin alone, although they rolled slightly faster than wild-type neutrophils.79 In another study using isolated murine leukocytes, reduced rolling velocity was abolished only in neutrophils that lacked both PSGL-1 and CD44.40 In flow chamber experiments using whole human blood, blocking the N-terminal P-selectin–binding site on PSGL-1 with monovalent Fab fragments of mAb PL1 was sufficient to prevent slow rolling on E-selectin and ICAM-1, suggesting that PSGL-1 engagement is necessary for effective signaling in human neutrophils.77 This surprising result implies that the N-terminal region of PSGL-1 must engage E-selectin to trigger signaling because PSGL-1 has more than one binding site for E-selectin and PL1 does not completely block binding of PSGL-1 to E-selectin.80 Four in vivo studies failed to find a rolling velocity difference between murine wild-type and PSGL-1–deficient neutrophils.10,38–40 However, in these experiments, rolling behavior of knockout and wild-type cells could not be compared in the same microvessels. Natural variations in wall shear stress, vessel diameter, and flow velocity may introduce some experimental noise that can make small differences difficult to detect in vivo.79 On the other hand, intravenous injection of a blocking mAb to β2 integrins significantly increased rolling velocities in wild-type mice81 or in mice lacking PSGL-1 or CD44,40 suggesting that either PSGL-1 or CD44 is sufficient to trigger slow rolling in vivo. At present, technical differences probably account for these apparent discrepancies, and the relative roles of PSGL-1 and CD44 in E-selectin–triggered signaling require further study. To date, no direct physical interaction of PSGL-1 or CD44 with downstream signaling molecules has been demonstrated.
E-selectin ligand engagement on neutrophils induces signals that partially activate LFA-1, which mediates slow rolling on ICAM-1.77,79,82 E-selectin–mediated signaling requires intact lipid rafts on neutrophils40 and an intact cytoplasmic domain of PSGL-1.40 E-selectin binding induces the phosphorylation of the SFKs Fgr, Hck, and Lyn40,82 and of the ITAM-containing adaptor proteins DAP12 and FcRγ, which subsequently interact with the tyrosine kinase Syk.82 PSGL-140,77,79 or CD4440 and the activation of Syk79 are required for E-selectin–mediated slow rolling (Figure 1B). DAP12 and Syk phosphorylation is absent in neutrophils from Fgr−/− mice and Lyn−/−/Hck−/− mice after E-selectin engagement.40,82 Likewise, elimination of both ITAM-containing adaptor proteins, DAP12 and FcRγ, abolishes Syk phosphorylation and slow rolling.40,82 The Tec family kinase Bruton tyrosine kinase acts downstream of Syk40,83 and regulates 2 pathways: one requires phospholipase C-γ2; the other may require PI3K-γ,83 although another study did not observe this requirement.40 Because the rolling velocity defect in PI3Kγ-deficient neutrophils is small, it may fall below the limit of detection in some assays. The small GTPase Rap1 is activated after E-selectin engagement, and blocking Rap1a in Pik3cg−/− mice by a dominant-negative TAT-fusion mutant completely abolishes E-selectin–mediated slow rolling.84 CalDAG-GEFI (gene name Rasgrp2) and p38 MAPK are key signaling intermediates between phospholipase C-γ2 and Rap1a. Interestingly, the extension of LFA-1 induced by E-selectin binding is only partially dependent on CalDAG-GEFI, whereas chemokine-triggered LFA-1 activation is completely defective in Rasgrp2−/− mice.84
ESL-1 also appears to contribute to the pro-adhesive action of E-selectin38,85 and probably cooperates with CD44 for this function as suggested by the observation that combined deficiency in both ligands results in elevated rolling flux fractions at the expense of reduced firm adhesion.38 These experiments also showed that slow leukocyte rolling requires ESL-1 to an extent similar to that described for CD44.38,39 Although there is evidence that signaling is defective when ESL-1 is not expressed,85 the precise function of ESL-1 in controlling slow rolling remains unknown. The rolling phenotype of ESL-1–deficient (as opposed to knocked-down, where silencing may be not fully specific) leukocytes has not been described. An interesting particularity of ESL-1 is its role in maintaining steady rolling kinetics by allowing continuous contact with the inflamed endothelium.38 Overall, these studies suggest that ESL-1 is a versatile ligand, capable of cooperating with PSGL-1 to mediate tethering on E-selectin while also contributing to steady rolling on endothelial cells once the leukocyte has tethered. Why ESL-1 is endowed with these functional properties is not well understood. One possibility is that its topologic distribution underlies this versatility: it is homogeneously expressed on the microvilli surface,69 whereas PSGL-1 expression is mostly restricted to the tips of these structures13 and CD44 is expressed in the planar cell body86 (Figure 1A).
Besides controlling rolling velocities, E-selectin ligand engagement also induces redistribution or “capping” of adhesion molecules on the cell surface of neutrophils.38,87 Interestingly, this effect appears to be exclusively mediated by CD44 and involves activation of p38 MAPK.38,87 Why receptor translation along the membrane is controlled by CD44 but not PSGL-1 is unknown. There is also evidence that engagement of PSGL-1 by P-selectin or E-selectin can trigger proliferative and differentiation signals in hematopoietic cells, including hematopoietic progenitors88 and dendritic cells,89 with functional consequences during inflammation.90 The signaling pathways controlling these processes have not been described.
Little is known about the possible pathophysiologic roles of ESL-1, but a potential contribution to vascular occlusion in sickle cell disease has been recently described.85 In a murine model of sickle cell disease after challenge with tumor necrosis factor-α, interactions between sickle-shaped erythrocytes and neutrophils generate intravascular cell aggregates that trigger the vaso-occlusive episodes characteristic of patients with this disease.85,91 In this murine model, ESL-1 transduces signals that activate integrin αMβ2 (also known as Mac-1) on neutrophils (Figure 1B), thus favoring interactions with circulating erythrocytes and promoting vaso-occlusion.85 PSGL-1 and CD44 do not contribute significantly to integrin activation in this model, which suggests ligand-specific signaling pathways. Indirect evidence with chemical inhibitors in vivo suggested that SFKs, but not p38 MAPK or Syk, are required for integrin activation downstream of ESL-1.85 Thus, although ESL-1 shares the SFK signaling pathway with PSGL-1 and CD44 to modulate integrin activation,33,40,82 it appears to have its own activating functions. One possible explanation is that each ligand has a temporally restricted signaling function: PSGL-1 and CD44 predominating during early (ie, tethering and rolling) phases of recruitment and ESL-1 at later stages (ie, during the crawling phase), but this hypothesis needs to be experimentally tested. How ESL-1 initiates signaling events on leukocytes also requires further study. Because ESL-1 is important for the processing and signaling of various growth factors,70,75 caution is needed to discriminate between effects that may be purely selectin-triggered and those related to other physiologic inputs.
Additional ligands for E-selectin
E-selectin can bind to multiple glycoconjugates. Loss of PSGL-1, CD44, and ESL-1 in murine neutrophils virtually eliminates rolling on E-selectin, suggesting that these 3 glycoproteins compose all physiologically relevant ligands for E-selectin on these cells.38 However, loss of core 1-derived O-glycans in murine neutrophils also virtually eliminates rolling on E-selectin, even though CD44 and ESL-1 from these cells (which require N-glycans to bind to E-selectin) still bind to E-selectin in biochemical assays.26 One possible explanation for the discrepant results is that neutrophils express at least one more glycoprotein ligand for E-selectin that requires specific O-glycosylation. In the absence of this putative ligand, the N-glycans on CD44 and ESL-1 are insufficient to support rolling. In the absence of PSGL-1, CD44, and ESL-1, the O-glycans on this putative ligand are also insufficient to support rolling. Another possibility is that loss of core 1 O-glycans indirectly impairs the functions of CD44 and ESL-1 by altering their cell-surface distributions or by other mechanisms. A third possibility is that loss of the other biologic functions of ESL-1 affects neutrophil properties that indirectly impair rolling on E-selectin.
There is experimental evidence that a different combination of glycoproteins, including PSGL-1, CD44, and CD43, functions in murine inflammatory T cells.43,92–94 Furthermore, human neutrophils may use L-selectin and glycolipids to mediate E-selectin binding95,96 (Table 1). In vitro, E-selectin binds to human CD66/carcinoembryonic antigen,97 integrin Mac-1,98 podocalyxin-like protein, or melanoma cell adhesion molecule,99 which may be relevant in specific cellular contexts. Thus, the identification of the full repertoire of ligands for E-selectin is still a matter of debate and active research. We briefly summarize additional glycoconjugates on hematopoietic cells with stronger evidence as bona fide ligands for E-selectin.
Table 1.
Comparison of mouse and human leukocyte ligands for endothelial selectins
| Mouse |
Human |
|||
|---|---|---|---|---|
| Function | Evidence | Function | Evidence | |
| PSGL-1 (PMN and T cells) | Tethering to and rolling on P- and E- selectin | Antibody blocking, and knockout mice, flow chamber and IVM | Tethering to and rolling on P-selectin | Antibody blocking, flow chamber |
| Signaling, β2 integrin activation for slow rolling on P- and E-selectin | Flow chamber and IVM | Signaling, integrin activation for slow rolling on E-selectin | Antibody blocking, flow chamber | |
| CD44 (PMN and T cells) | Cooperates with PSGL-1 for rolling on E-selectin | IVM, flow chamber | Contributes to binding to fluid-phase E-selectin | Flow cytometry |
| Signaling for β2 integrin activation and slow rolling on E-selectin | Knockout mice, flow chamber, IVM | |||
| Signaling for receptor clustering on E-selectin | Knockout mice, IVM | |||
| Cooperates with PSGL-1 for leukocyte migration during inflammation | Inflammatory models in knockout mice | |||
| ESL-1 (PMN) | Present on the surface of neutrophils and Th1 lymphocytes | Surface biotinylation and Western blotting | Not detected on the surface of human leukocytes | Surface biotinylation and Western blotting |
| Binds to E-selectin | E-selectin affinity columns | Unknown contribution to E-selectin binding | ||
| Antibody blocking on myeloid cell line | ||||
| Cooperates with PSGL-1 for tethering to E-selectin Cooperates with CD44 for slow rolling on E-selectin Allows steady rolling on E-selectin Signaling for β2 integrin activation |
shRNA silencing and IVM | |||
| CD43 (T cells) | Cooperates with PSGL-1 for binding to E-selectin | Flow cytometry and static adhesion in knockout mice | Supports binding and rolling of E-selectin-expressing cells | In vitro binding and blot-rolling assays |
| Cooperates with PSGL-1 for Th1 cell migration during inflammation | Skin inflammation model in knockout mice | |||
| L-selectin (PMN) | Does not bind to P- or E-selectin | E-selectin affinity columns and antibody blocking | Binding to E-selectin | E-selectin affinity columns |
| Binding to PSGL-1 mediates secondary tethers | Flow chamber and IVM in knockout mice | Mediates rolling on E-selectin | Flow chamber and antibody blocking | |
| Glycolipids (PMN) | Unknown contribution to selectin binding | Ligands for P- and E-selectin are protease-sensitive | Mediate rolling of E-selectin expressing cells | Flow chamber and use of inhibitors of glycosphingolipid biosynthesis |
| Other differences (PMN and T cells) | Ligands for P- and E-selectin are protease sensitive | Ligands for E-selectin are protease insensitive | ||
| Antibodies to sLex and Lex do not bind murine neutrophils | Antibodies to sLex and Lex strongly bind to human neutrophils | |||
| PSGL-1, CD43, CD44 and ESL-1 cooperate for tethering, rolling and migration to inflamed sites | Knockout and shRNA silencing using IVM, and inflammation models | Unknown repertoire of E-selectin ligands; in vitro evidence exists for PSGL-1, CD44, L-selectin, and glycolipids on neutrophils; evidence for PSGL-1 and CD43 on T cells | ||
Listed are glycoconjugates with strong evidence as P- or E-selectin ligands in at least some assays. The leukocyte subset (neutrophils, PMN; or T lymphocytes) for which the function of each putative ligand has been best studied is indicated in parentheses.
IVM indicates intravital microscopy; sLex, sialyl Lewis x structure; and Lex, Lewis x structure.
CD43
The high level of expression on leukocytes, extensive glycosylation, and molecular length of CD43 (also known as leukosialin) were proposed to favor both pro-adhesive or anti-adhesive roles.100,101 Biochemical and in vitro cellular studies as well as in vivo data support a role for CD43 as an E-selectin ligand on human and murine inflammatory T cells (Th1 type)93,102 but not on neutrophils.26,103 Delayed-type hypersensitivity models have been used to demonstrate the physiologic contribution of CD43 to skin inflammation during T cell–dependent responses. In all cases, ablation of PSGL-1 was required to unmask a role for CD43.92,94 Like PSGL-1, CD43 localizes to microvilli,13,104 but it is not known whether both receptors cooperate in mediating T-cell tethering. These findings, together with the observation that CD43 expression on murine neutrophils is not sufficient to support tethering, rolling, or recruitment in the absence of PSGL-1, CD44, and ESL-1,38 and the differential contributions of CD44 on murine T cells and neutrophils,43 are consistent with the proposal that lymphoid and myeloid cells use a different repertoire of selectin ligands.105
L-selectin
This selectin is exclusively expressed on leukocytes, where it directs the migration of naive and central memory T cells to lymph nodes through recognition of glycoproteins expressed on high endothelial venules.106 L-selectin–deficient mice exhibit impaired leukocyte recruitment to sites of inflammation,107 which may reflect the inability of L-selectin–deficient neutrophils to bind to adherent neutrophils and neutrophil fragments.10 Furthermore, L-selectin is expressed on the tips of microvilli and, on human neutrophils, is decorated with N-glycans capped with sLex.95,108 L-selectin from human (but not mouse) neutrophils binds to E-selectin affinity columns and supports the rolling of E-selectin–transfected cells96 (Table 1). Antibodies that recognize the lectin domain of human L-selectin partially inhibit in vitro neutrophil rolling on E-selectin,95,96 but this was later explained by inhibition of secondary neutrophil-neutrophil tethering.109–111 In vivo, the reduced recruitment of leukocytes in L-selectin–deficient mice appears to be the result of loss of secondary tethers between circulating leukocytes and those already attached to the endothelium, which are mediated by interactions between L-selectin and PSGL-1.10
Glycolipids
E-selectin binds to sialylated and fucosylated lactosylceramides extracted from human neutrophils,112 and immobilized lipids modified with sLex or sLea mediate tethering and rolling of E-selectin–expressing cells under flow.113 These early findings, performed mostly using human samples, conflict with the recent description that a limited array of glycoproteins accounts for the full repertoire of E-selectin ligand activity on mouse neutrophils.38 Given reported differences in the structure and function of selectin ligands between mouse and human neutrophils,6,96 it is conceivable that glycolipids play a more prominent role as E-selectin ligands in human neutrophils (Table 1). In agreement with this, sialylated glycosphingolipids containing several terminal repeats of N-acetyl-lactosamine with 2 or 3 fucose residues have been purified from human neutrophils. These glycosphingolipids support tethering and rolling of E-selectin–expressing cells at densities similar to those found on intact cells.114 Inhibition of glycosphingolipid synthesis on neutrophils partially abrogates E-selectin binding,114 although this finding could be explained by indirect effects through membrane stiffening.115 It has been proposed that the high density of glycolipids on the cell membrane compensates for their reduced accessibility compared with extended glycoproteins presented on microvilli.113 Thus, glycolipid-mediated interactions may be particularly important during the slow rolling phase, when the cell's body is in close proximity to the endothelial membrane. Notwithstanding these observations, the physiologic relevance of glycolipids for tethering and rolling of human neutrophils or other leukocyte subsets on E-selectin awaits definitive confirmation.
Future directions
More than 2 decades after the initial description of selectins, the complete repertoire of physiologic ligands that interact with endothelial selectins remains to be elucidated. The precise nature of all ligands, their contribution to leukocyte rolling and signaling, and the possible interspecies differences (Table 1) remain to be identified. At the same time, because the majority of research on selectin ligands has focused on myeloid cell lines and neutrophils, it will be important to establish whether the same repertoire of ligands functions in other leukocyte subsets, including inflammatory T cells, hematopoietic progenitors, or leukemic cells. Differences in the use of ligands among these cell types could be exploited to interfere with the extravasation of damaging subsets (eg, self-reactive lymphocytes, pro-atherogenic monocytes, or leukemic clones) without compromising homeostatic host defense.
From a mechanistic standpoint, signaling initiated by engagement of various selectin ligands is now well established. However, the complete sequence of events leading from selectin engagement of PSGL-1 and CD44 at the cell surface to integrin activation needs to be fully characterized (Figure 1B). A number of signaling intermediaries have been identified, but the potential contributions of Ca2+, diacylglycerol, protein kinase C, or PI3Kγ remain to be defined. It will also be important to define the exact signaling mechanisms by which ESL-1 contributes to leukocyte recruitment. The continuous advances in this field are rapidly reshaping our perception of selectin ligands as specialized signal transducers in immune cells; this perception should open new therapeutic avenues for the treatment of vascular and immune disorders.
Acknowledgments
The authors thank Dr A. Urzainqui for helpful comments on the manuscript.
This work was supported by the Interdisciplinary Clinical Research Center (IZKF, Münster, Germany) and the German Research Foundation (A.Z.), the National Institutes of Health (K.L. and R.P.M.), a Ramón y Cajal fellowship, the Spanish Ministry of Science and Innovation, and the FP7-People-IRG Program (A.H.). The Centro Nacional de Investigaciones Cardiovasculares is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
Authorship
Contribution: All authors wrote and edited the manuscript and designed the figures.
Conflict-of-interest disclosure: R.P.M. has interest in Selexys, a company that is developing inhibitors of selectins and selectin ligands. The remaining authors declare no competing financial interests.
Correspondence: Andrés Hidalgo, Department of Epidemiology, Atherothrombosis and Imaging, Centro Nacional de Investigaciones Cardiovasculares, Melchor Fernandez Almagro 3, Madrid 28039, Spain; e-mail: ahidalgo@cnic.es.
References
- 1.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79(1):181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 2.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Homeister JW, Thall AD, Petryniak B, et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15(1):115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem. 1996;271(31):18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 6.Kobzdej MM, Leppanen A, Ramachandran V, Cummings RD, McEver RP. Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells. Blood. 2002;100(13):4485–4494. doi: 10.1182/blood-2002-06-1799. [DOI] [PubMed] [Google Scholar]
- 7.Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86(12):4417–4421. [PubMed] [Google Scholar]
- 8.Xia L, Sperandio M, Yago T, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109(7):939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. P-selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J Exp Med. 2000;192(11):1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperandio M, Smith ML, Forlow SB, et al. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197(10):1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarbock A, Muller H, Kuwano Y, Ley K. PSGL-1-dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86(5):1119–1124. doi: 10.1189/jlb.0209117. [DOI] [PubMed] [Google Scholar]
- 12.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 13.Moore KL, Patel KD, Bruehl RE, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128(4):661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEver RP. P-selectin glycoprotein ligand-1 (PSGL-1). In: Ley K, editor. Adhesion Molecules: Function and Inhibition. Basel, Switzerland: Birkhauser Verlag; 2007. pp. 3–26. [Google Scholar]
- 15.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100(11 suppl):S97–S103. [PubMed] [Google Scholar]
- 16.Afshar-Kharghan V, Diz-Kucukkaya R, Ludwig EH, Marian AJ, Lopez JA. Human polymorphism of P-selectin glycoprotein ligand 1 attributable to variable numbers of tandem decameric repeats in the mucinlike region. Blood. 2001;97(10):3306–3307. doi: 10.1182/blood.v97.10.3306. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Galipeau J, Kozak CA, Furie BC, Furie B. Mouse P-selectin glycoprotein ligand-1: molecular cloning, chromosomal localization, and expression of a functional P-selectin receptor. Blood. 1996;87(10):4176–4186. [PubMed] [Google Scholar]
- 18.Tenno M, Ohtsubo K, Hagen FK, et al. Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Mol Cell Biol. 2007;27(24):8783–8796. doi: 10.1128/MCB.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schachter H, Brockhausen I. The biosynthesis of branched O-glycans. Symp Soc Exp Biol. 1989;43:1–26. [PubMed] [Google Scholar]
- 20.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9(6):881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 21.Smith MJ, Smith BR, Lawrence MB, Snapp KR. Functional analysis of the combined role of the O-linked branching enzyme core 2 beta1-6-N-glucosaminyltransferase and dimerization of P-selectin glycoprotein ligand-1 in rolling on P-selectin. J Biol Chem. 2004;279(21):21984–21991. doi: 10.1074/jbc.M402731200. [DOI] [PubMed] [Google Scholar]
- 22.Snapp KR, Heitzig CE, Ellies LG, Marth JD, Kansas GS. Differential requirements for the O-linked branching enzyme core 2 beta1-6-N-glucosaminyltransferase in biosynthesis of ligands for E-selectin and P-selectin. Blood. 2001;97(12):3806–3811. doi: 10.1182/blood.v97.12.3806. [DOI] [PubMed] [Google Scholar]
- 23.Sperandio M, Thatte A, Foy D, Ellies LG, Marth JD, Ley K. Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood. 2001;97(12):3812–3819. doi: 10.1182/blood.v97.12.3812. [DOI] [PubMed] [Google Scholar]
- 24.Xia L, Ramachandran V, McDaniel JM, Nguyen KN, Cummings RD, McEver RP. N-terminal residues in murine P-selectin glycoprotein ligand-1 required for binding to murine P-selectin. Blood. 2003;101(2):552–559. doi: 10.1182/blood-2001-11-0036. [DOI] [PubMed] [Google Scholar]
- 25.Westmuckett AD, Thacker KM, Moore KL. Tyrosine sulfation of native mouse Psgl-1 is required for optimal leukocyte rolling on p-selectin in vivo. PLoS One. 2011;6(5):e20406. doi: 10.1371/journal.pone.0020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yago T, Fu J, McDaniel JM, Miner JJ, McEver RP, Xia L. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc Natl Acad Sci U S A. 2010;107(20):9204–9209. doi: 10.1073/pnas.1003110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirata T, Furukawa Y, Yang BG, et al. Human P-selectin glycoprotein ligand-1 (PSGL-1) interacts with the skin-associated chemokine CCL27 via sulfated tyrosines at the PSGL-1 amino terminus. J Biol Chem. 2004;279(50):51775–51782. doi: 10.1074/jbc.M409868200. [DOI] [PubMed] [Google Scholar]
- 28.Veerman KM, Williams MJ, Uchimura K, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8(5):532–539. doi: 10.1038/ni1456. [DOI] [PubMed] [Google Scholar]
- 29.Epperson TK, Patel KD, McEver RP, Cummings RD. Noncovalent association of P-selectin glycoprotein ligand-1 and minimal determinants for binding to P-selectin. J Biol Chem. 2000;275(11):7839–7853. doi: 10.1074/jbc.275.11.7839. [DOI] [PubMed] [Google Scholar]
- 30.Miner JJ, Shao B, Wang Y, et al. Cytoplasmic domain of P-selectin glycoprotein ligand-1 facilitates dimerization and export from the endoplasmic reticulum. J Biol Chem. 2011;286(11):9577–9586. doi: 10.1074/jbc.M110.208777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso-Lebrero JL, Serrador JM, Dominguez-Jimenez C, et al. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood. 2000;95(7):2413–2419. [PubMed] [Google Scholar]
- 32.Urzainqui A, Serrador JM, Viedma F, et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17(4):401–412. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 33.Wang HB, Wang JT, Zhang L, et al. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007;8(8):882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- 34.Schaff UY, Shih HH, Lorenz M, et al. SLIC-1/sorting nexin 20: a novel sorting nexin that directs subcellular distribution of PSGL-1. Eur J Immunol. 2008;38(2):550–564. doi: 10.1002/eji.200737777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borges E, Eytner R, Moll T, et al. The P-selectin glycoprotein ligand-1 is important for recruitment of neutrophils into inflamed mouse peritoneum. Blood. 1997;90(5):1934–1942. [PubMed] [Google Scholar]
- 36.Borges E, Tietz W, Steegmaier M, et al. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185(3):573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Hirata T, Croce K, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190(12):1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26(4):477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201(8):1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yago T, Shao B, Miner JJ, et al. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood. 2010;116(3):485–494. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merzaban JS, Burdick MM, Gadhoum SZ, et al. Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells. Blood. 2011;118(7):1774–1783. doi: 10.1182/blood-2010-11-320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Invest Dermatol. 2004;122(5):1061–1069. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 43.Nacher M, Blazquez AB, Shao B, et al. Physiological contribution of CD44 as a ligand for E-selectin during inflammatory T-cell recruitment. Am J Pathol. 2011;178(5):2437–2446. doi: 10.1016/j.ajpath.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz P, Schwarzler C, Gunthert U. CD44 isoforms during differentiation and development. Bioessays. 1995;17(1):17–24. doi: 10.1002/bies.950170106. [DOI] [PubMed] [Google Scholar]
- 47.Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6(5):726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 48.Sleeman JP, Kondo K, Moll J, Ponta H, Herrlich P. Variant exons v6 and v7 together expand the repertoire of glycosaminoglycans bound by CD44. J Biol Chem. 1997;272(50):31837–31844. doi: 10.1074/jbc.272.50.31837. [DOI] [PubMed] [Google Scholar]
- 49.Katoh S, Miyagi T, Taniguchi H, et al. Cutting edge: an inducible sialidase regulates the hyaluronic acid binding ability of CD44-bearing human monocytes. J Immunol. 1999;162(9):5058–5061. [PubMed] [Google Scholar]
- 50.Maiti A, Maki G, Johnson P. TNF-alpha induction of CD44-mediated leukocyte adhesion by sulfation. Science. 1998;282(5390):941–943. doi: 10.1126/science.282.5390.941. [DOI] [PubMed] [Google Scholar]
- 51.Skelton TP, Zeng C, Nocks A, Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol. 1998;140(2):431–446. doi: 10.1083/jcb.140.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levesque MC, Haynes BF. Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNF-alpha and is inhibited by IL-4 and IL-13. J Immunol. 1997;159(12):6184–6194. [PubMed] [Google Scholar]
- 53.Perschl A, Lesley J, English N, Hyman R, Trowbridge IS. Transmembrane domain of CD44 is required for its detergent insolubility in fibroblasts. J Cell Sci. 1995;108(3):1033–1041. doi: 10.1242/jcs.108.3.1033. [DOI] [PubMed] [Google Scholar]
- 54.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 55.Schmits R, Filmus J, Gerwin N, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90(6):2217–2233. [PubMed] [Google Scholar]
- 56.Bonder CS, Clark SR, Norman MU, Johnson P, Kubes P. Use of CD44 by CD4+ Th1 and Th2 lymphocytes to roll and adhere. Blood. 2006;107(12):4798–4806. doi: 10.1182/blood-2005-09-3581. [DOI] [PubMed] [Google Scholar]
- 57.DeGrendele HC, Estess P, Picker LF, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med. 1996;183(3):1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278(5338):672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 59.DeGrendele HC, Kosfiszer M, Estess P, Siegelman MH. CD44 activation and associated primary adhesion is inducible via T cell receptor stimulation. J Immunol. 1997;159(6):2549–2553. [PubMed] [Google Scholar]
- 60.Hutas G, Bajnok E, Gal I, Finnegan A, Glant TT, Mikecz K. CD44-specific antibody treatment and CD44 deficiency exert distinct effects on leukocyte recruitment in experimental arthritis. Blood. 2008;112(13):4999–5006. doi: 10.1182/blood-2008-04-150383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan AI, Kerfoot SM, Heit B, et al. Role of CD44 and hyaluronan in neutrophil recruitment. J Immunol. 2004;173(12):7594–7601. doi: 10.4049/jimmunol.173.12.7594. [DOI] [PubMed] [Google Scholar]
- 62.McDonald B, McAvoy EF, Lam F, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med. 2008;205(4):915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szanto S, Gal I, Gonda A, Glant TT, Mikecz K. Expression of L-selectin, but not CD44, is required for early neutrophil extravasation in antigen-induced arthritis. J Immunol. 2004;172(11):6723–6734. doi: 10.4049/jimmunol.172.11.6723. [DOI] [PubMed] [Google Scholar]
- 64.Siegelman MH, Stanescu D, Estess P. The CD44-initiated pathway of T-cell extravasation uses VLA-4 but not LFA-1 for firm adhesion. J Clin Invest. 2000;105(5):683–691. doi: 10.1172/JCI8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest: CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20(4):455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- 66.Gonatas JO, Mourelatos A, Stieber A, Lane WS, Brosius J, Gonatas NK. MG-160, a membrane sialoglycoprotein of the medial cisternae of the rat Golgi apparatus, binds basic fibroblast growth factor and exhibits a high level of sequence identity to a chicken fibroblast growth factor receptor. J Cell Sci. 1995;108(2):457–467. doi: 10.1242/jcs.108.2.457. [DOI] [PubMed] [Google Scholar]
- 67.Steegmaier M, Levinovitz A, Isenmann S, et al. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373(6515):615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- 68.Gonatas JO, Chen YJ, Stieber A, Mourelatos Z, Gonatas NK. Truncations of the C-terminal cytoplasmic domain of MG160, a medial Golgi sialoglycoprotein, result in its partial transport to the plasma membrane and filopodia. J Cell Sci. 1998;111(2):249–260. doi: 10.1242/jcs.111.2.249. [DOI] [PubMed] [Google Scholar]
- 69.Steegmaier M, Borges E, Berger J, Schwarz H, Vestweber D. The E-selectin-ligand ESL-1 is located in the Golgi as well as on microvilli on the cell surface. J Cell Sci. 1997;110(6):687–694. doi: 10.1242/jcs.110.6.687. [DOI] [PubMed] [Google Scholar]
- 70.Miyaoka Y, Tanaka M, Imamura T, Takada S, Miyajima A. A novel regulatory mechanism for Fgf18 signaling involving cysteine-rich FGF receptor (Cfr) and delta-like protein (Dlk). Development. 2010;137(1):159–167. doi: 10.1242/dev.041574. [DOI] [PubMed] [Google Scholar]
- 71.Dimitroff CJ, Descheny L, Trujillo N, et al. Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res. 2005;65(13):5750–5760. doi: 10.1158/0008-5472.CAN-04-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lenter M, Levinovitz A, Isenmann S, Vestweber D. Monospecific and common glycoprotein ligands for E- and P-selectin on myeloid cells. J Cell Biol. 1994;125(2):471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levinovitz A, Muhlhoff J, Isenmann S, Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol. 1993;121(2):449–459. doi: 10.1083/jcb.121.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Z, Zuber ME, Burrus LW, Olwin BB. Identification and characterization of a fibroblast growth factor (FGF) binding domain in the cysteine-rich FGF receptor. J Biol Chem. 1997;272(8):5167–5174. doi: 10.1074/jbc.272.8.5167. [DOI] [PubMed] [Google Scholar]
- 75.Yang T, Mendoza-Londono R, Lu H, et al. E-selectin ligand-1 regulates growth plate homeostasis in mice by inhibiting the intracellular processing and secretion of mature TGF-beta. J Clin Invest. 2010;120(7):2474–2485. doi: 10.1172/JCI42150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang MC, Zollner O, Moll T, et al. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275(40):31353–31360. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- 77.Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood. 2010;116(4):617–624. doi: 10.1182/blood-2010-01-266122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miner JJ, Xia L, Yago T, et al. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112(5):2035–2045. doi: 10.1182/blood-2008-04-149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26(6):773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel KD, Moore KL, Nollert MU, McEver RP. Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest. 1995;96(4):1887–1896. doi: 10.1172/JCI118234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99(1):336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- 82.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205(10):2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mueller H, Stadtmann A, Van Aken H, et al. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115(15):3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stadtmann A, Brinkhaus L, Mueller H, et al. Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur J Immunol. 2011;41(7):2074–2085. doi: 10.1002/eji.201041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82(6):989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 87.Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils. J Immunol. 2004;172(12):7780–7790. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- 88.Eto T, Winkler I, Purton LE, Levesque JP. Contrasting effects of P-selectin and E-selectin on the differentiation of murine hematopoietic progenitor cells. Exp Hematol. 2005;33(2):232–242. doi: 10.1016/j.exphem.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 89.Urzainqui A, Martinez G, del Hoyo A, et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol. 2007;179(11):7457–7465. doi: 10.4049/jimmunol.179.11.7457. [DOI] [PubMed] [Google Scholar]
- 90.Nunez-Andrade N, Lamana A, Sancho D, et al. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J Pathol. 2011;224(2):212–221. doi: 10.1002/path.2850. [DOI] [PubMed] [Google Scholar]
- 91.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A. 2002;99(5):3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alcaide P, King SL, Dimitroff CJ, Lim YC, Fuhlbrigge RC, Luscinskas FW. The 130-kDa glycoform of CD43 functions as an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J Invest Dermatol. 2007;127(8):1964–1972. doi: 10.1038/sj.jid.5700805. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto M, Atarashi K, Umemoto E, et al. CD43 functions as a ligand for E-selectin on activated T cells. J Immunol. 2005;175(12):8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 94.Matsumoto M, Shigeta A, Furukawa Y, Tanaka T, Miyasaka M, Hirata T. CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin. J Immunol. 2007;178(4):2499–2506. doi: 10.4049/jimmunol.178.4.2499. [DOI] [PubMed] [Google Scholar]
- 95.Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 96.Zollner O, Lenter MC, Blanks JE, et al. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol. 1997;136(3):707–716. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuijpers TW, Hoogerwerf M, van der Laan LJ, et al. CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol. 1992;118(2):457–466. doi: 10.1083/jcb.118.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crutchfield KL, Shinde VR, Patil CJ, et al. CD11b/CD18-coated microspheres attach to E-selectin under flow. J Leukoc Biol. 2000;67(2):196–205. doi: 10.1002/jlb.67.2.196. [DOI] [PubMed] [Google Scholar]
- 99.Barthel SR, Wiese GK, Cho J, et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci U S A. 2009;106(46):19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stockton BM, Cheng G, Manjunath N, Ardman B, von Andrian UH. Negative regulation of T cell homing by CD43. Immunity. 1998;8(3):373–381. doi: 10.1016/s1074-7613(00)80542-7. [DOI] [PubMed] [Google Scholar]
- 101.Woodman RC, Johnston B, Hickey MJ, et al. The functional paradox of CD43 in leukocyte recruitment: a study using CD43-deficient mice. J Exp Med. 1998;188(11):2181–2186. doi: 10.1084/jem.188.11.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107(4):1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carlow DA, Ziltener HJ. CD43 deficiency has no impact in competitive in vivo assays of neutrophil or activated T cell recruitment efficiency. J Immunol. 2006;177(9):6450–6459. doi: 10.4049/jimmunol.177.9.6450. [DOI] [PubMed] [Google Scholar]
- 104.Yonemura S, Nagafuchi A, Sato N, Tsukita S. Concentration of an integral membrane protein, CD43 (leukosialin, sialophorin), in the cleavage furrow through the interaction of its cytoplasmic domain with actin-based cytoskeletons. J Cell Biol. 1993;120(2):437–449. doi: 10.1083/jcb.120.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994;91(16):7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 107.Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181(6):2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stein JV, Cheng G, Stockton BM, Fors BP, Butcher EC, von Andrian UH. L-selectin-mediated leukocyte adhesion in vivo: microvillous distribution determines tethering efficiency, but not rolling velocity. J Exp Med. 1999;189(1):37–50. doi: 10.1084/jem.189.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alon R, Fuhlbrigge RC, Finger EB, Springer TA. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J Cell Biol. 1996;135(3):849–865. doi: 10.1083/jcb.135.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guyer DA, Moore KL, Lynam EB, et al. P-selectin glycoprotein ligand-1 (PSGL-1) is a ligand for L-selectin in neutrophil aggregation. Blood. 1996;88(7):2415–2421. [PubMed] [Google Scholar]
- 111.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1: a mechanism that amplifies initial leukocyte accumulation of P-selectin in vitro. J Clin Invest. 1996;98(5):1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tiemeyer M, Swiedler SJ, Ishihara M, et al. Carbohydrate ligands for endothelial-leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88(4):1138–1142. doi: 10.1073/pnas.88.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alon R, Feizi T, Yuen CT, Fuhlbrigge RC, Springer TA. Glycolipid ligands for selectins support leukocyte tethering and rolling under physiologic flow conditions. J Immunol. 1995;154(10):5356–5366. [PubMed] [Google Scholar]
- 114.Nimrichter L, Burdick MM, Aoki K, et al. E-selectin receptors on human leukocytes. Blood. 2008;112(9):3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yago T, Leppanen A, Qiu H, et al. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J Cell Biol. 2002;158(4):787–799. doi: 10.1083/jcb.200204041. [DOI] [PMC free article] [PubMed] [Google Scholar]