Abstract
The soil bacterium Bacillus thuringiensis is a pathogen of insects and nematodes and is very closely related to, if not the same species as, Bacillus cereus and Bacillus anthracis. The defining characteristic of B. thuringiensis that sets it apart from B. cereus and B. anthracis is the production of crystal (Cry) proteins, which are pore-forming toxins or pore-forming proteins (PFPs). Although it is known that PFPs are important virulence factors since their elimination results in reduced virulence of many pathogenic bacteria, the functions by which PFPs promote virulence are incompletely understood. Here we study the effect of Cry proteins in B. thuringiensis pathogenesis of the nematode Caenorhabditis elegans. We find that whereas B. thuringiensis on its own is not able to infect C. elegans, the addition of the PFP Cry protein, Cry5B, results in a robust lethal infection that consumes the nematode host in 1–2 days, leading to a “Bob” or bag-of-bacteria phenotype. Unlike other infections of C. elegans characterized to date, the infection by B. thuringiensis shows dose-dependency based on bacterial inoculum size and based on PFP concentration. Although the infection process takes 1–2 days, the PFP-instigated infection process is irreversibly established within 15 minutes of initial exposure. Remarkably, treatment of C. elegans with Cry5B PFP is able to instigate many other Bacillus species, including B. anthracis and even “non-pathogenic” Bacillus subtilis, to become lethal and infectious agents to C. elegans. Co-culturing of Cry5B-expressing B. thuringiensis with B. anthracis can result in lethal infection of C. elegans by B. anthracis. Our data demonstrate that one potential property of PFPs is to sensitize the host to bacterial infection and further that C. elegans and probably other roundworms can be common hosts for B. cereus-group bacteria, findings with important ecological and research implications.
Introduction
The Bacillus cereus group of bacteria comprises six species, including three highly related species, B. cereus sensu stricto, B. anthracis and B. thuringiensis, which are sometimes considered a single species [1], [2], [3]. Although closely related, these bacteria are associated with very different diseases. B. anthracis is the causative agent of anthrax [4], B. cereus can cause food poisoning and various opportunistic and nosocomial infections [5], and B. thuringiensis is an invertebrate-specific pathogen [6]. The relationship between these three closely related species of Bacillus in the wild, e.g. how they replicate in the soil environment and what environmental niches they occupy relative to one another, has been the subject of much speculation [7], [8].
The defining characteristic of B. thuringiensis is the production of Crystal (Cry) proteins, a large family of related proteins that kill insects and nematodes [8], [9], [10]. Each B. thuringiensis strain found in the wild can produce one or multiple (typically 2–4) Cry proteins. These Cry proteins are pore-forming proteins (PFPs), and thus are members of the single largest class of bacterial virulence factors [11]. Indeed, roughly 25–30% of all protein toxins made by human bacterial pathogens are PFPs, many with proven roles in disease pathogenesis. Purified Cry proteins alone, even in the absence of B. thuringiensis, produce dose-dependent lethality in invertebrates, and as such can be expressed in transgenic crops to control insect pests.
Here for the first time we characterize in detail the effect of Cry protein PFP administration on the outcome of B. thuringiensis infection in the nematode Caenorhabditis elegans, and uncover an unexpected interaction of Cry proteins with other Bacillus species bacteria during C. elegans infection.
Results
Infectivity of C. elegans by Bacillus species in presence of Cry5B pore-forming protein
In our numerous studies of nematicidal Cry proteins in the absence of B. thuringiensis but in the presence of its standard laboratory food source, Escherichia coli, we have found that Cry proteins produce a dose-dependent mortality in nematodes but do not promote an E. coli infection. Likewise, non-Cry protein-producing B. thuringiensis strains, such as HD1 cry-, do not cause an infection when fed to C. elegans in the absence of Cry proteins (Figure 1A; Table 1; [12]). We therefore investigated what would happen if we fed C. elegans Cry5B, a Cry protein that forms pores in membranes [13], in the presence of B. thuringiensis. Purified Cry5B produces mortality in C. elegans over a 6 day time course at 25°C with a calculated LD50 of ∼8 µg/ml [14]. Under these conditions, E. coli is used as the food source and no infections (internal multiplication of E. coli) are seen; the nematodes die from Cry protein intoxication. Conversely, when we fed Cry5B to C. elegans at 10 µg/ml in the presence of B. thuringiensis, we observed accelerated killing of nematodes in 24–48 h, with the internal structures of the nematodes completely digested by the proliferating bacteria (Figure 1B, Table 1). With B. thuringiensis, the majority of the killing takes place in the first 24 h, although we typically let the assays run for 48 h to ensure maximum killing was reached. Nematodes killed by B. thuringiensis with Cry5B are filled with vegetative B. thuringiensis cells or B. thuringiensis spores (Figure 1B), a phenotype we have named “Bob” for Bag of bacteria, where the undigested cuticle of the nematode represents the bag that contains the bacteria.
Figure 1. Infection of C. elegans by B. thuringiensis and B. anthracis.
Top row: Dissecting microscope view of nematodes cultured under various conditions. Scale bar of all images in top row is 500 µm. Bottom row: Compound microscope view of nematodes cultured under various conditions. For all images in the bottom row, anterior of the worm is top right and scale bar is 50 µm. (A) C. elegans cultured in a well with B. thuringiensis without Cry5B. Top row: None of the six nematodes are infected. All are healthy. The blur associated with some of the worms in the top row is due to their movement in the well. Bottom row: The internal structures of C. elegans fed B. thuringiensis without Cry5B, including the pharynx and intestine, are all intact. (B) C. elegans cultured in a well with B. thuringiensis and Cry5B. Top row: Five of the six worms are completely infected (rigid, lack of internal structures and normal coloration); one is not. Bottom row: Infected animals show complete or near complete digestion of internal structures by the bacteria. Vegetative and sporulated bacteria can be seen in these lethally infected animals. (C) Similar images as in (B) except the bacterium cultured with the nematodes is Bacillus anthracis.
Table 1. Infectivity of Bacillus sp. on C. elegans in the presence/absence of pore-forming Cry5B.
| N2 wild-type animals | ||||
| Without Cry5B PFP | With Cry5B PFP | |||
| Bacillus species | Total number of animals | Number of Bobs | Total number of animals | Number of Bobs |
| Bt HD1 | 107 | 0 | 133 | 102 |
| B. subtilis PY79 | 119 | 0 | 128 | 45 |
| B. subtilis 6051 | 115 | 0 | 122 | 53 |
| B. megaterium | 132 | 0 | 149 | 8 |
Quantitation of infected animals in the presence and absence of Cry5B demonstrates that the PFP is absolutely required for the infection process—in the absence of Cry5B no B. thuringiensis infections are seen whereas in the presence of Cry5B a very high percentage of the nematodes succumb to accelerated lethal infections (Table 1). To confirm that the infections seen are independent of internal hatching of progeny in hermaphroditic C. elegans, we performed similar studies using the C. elegans mutant glp-4(bn2), which are sterile at the restrictive temperature and do not produce progeny although they do have a similar response to Cry5B as wild-type C. elegans [15]. Similar robust Cry5B-mediated infections by B. thuringiensis are found in this genetic background as well (Table 1). In these experiments and other experiments with other Bacillus sp., Cry5B purified from B. thuringiensis was added exogenously so that all the experiments could be directly compared. We have confirmed that B. thuringiensis HD-1 endogenously producing Cry5B also produces a robust number of Bobs (32/33) whereas an isogenic HD-1 strain of B. thuringiensis lacking Cry5B production does not (0/51). Thus Cry5B PFP is an essential virulence factor required for lethal infection of C. elegans by B. thuringiensis.
Cry5B PFP intoxicates and kills C. elegans via binding to glycolipid receptors found in the intestine [16]. To confirm that Cry5B-stimulated B. thuringiensis infections require the established PFP intoxication pathways, we repeated the challenge experiments in glycolipid-receptor deficient bre-4(ye13) C. elegans animals [16], [17]. As predicted, nematodes lacking the receptor for Cry5B are not infected by B. thuringiensis even in the presence of Cry5B (Table 1).
Dependency of the infection process upon PFP dose and bacterial inoculum size
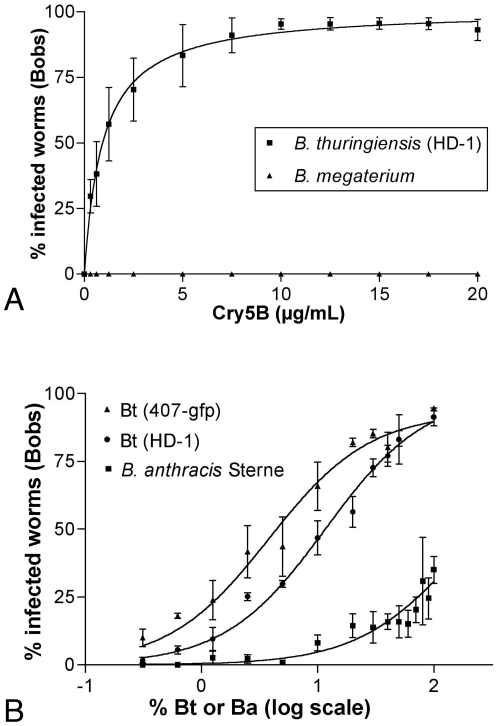
Our experiments demonstrate that infectivity is fully dependent both upon Cry5B sensitization and the presence of a pathogenic bacterium (B. thuringiensis). We next set out to determine the relationship between each of these elements and their dosage. Infection assays were performed with either highly infective B. thuringiensis or weakly infective B. megaterium (as a negative control; see below) at increasing doses of Cry5B (Figure 2A). As demonstrated above, in the absence of Cry5B, no infections were seen with either bacterium (Figure 2A). As the dose of Cry5B increases, the percent of worms infected by B. thuringiensis HD-1 increases until maximum infectivity is reached at ∼10 µg/ml Cry5B (Figure 2A). Thus, B. thuringiensis lethal infection depends upon Cry5B levels in a dose-dependent manner. Because it was the smallest dose that still achieved penetrant infectivity, we utilized 10 µg/mL as our Cry5B dose in all our fixed dose experiments.
Figure 2. Dose-dependency of lethal infections by Bacillus and Cry5B.
(A) Infections increase in a dose-dependent manner with increasing Cry5B. Doses from 0 to 20 µg/ml Cry5B were prepared and standard infection assays with B. thuringiensis HD-1 carried out. No worms were infected at any of the Cry5B concentrations when exposed to the relatively non-pathogenic B. megaterium in the negative control experiment. Error bars indicate standard error. n = 3 trials, with an average of 40 worms per condition per trial. The glp-4(bn2) strain was used to eliminate any concerns of internal hatching of larvae as the source of death. (B) The lethal infection rate induced by Cry5B increases with increasing doses of B. thuringiensis or B. anthracis. Varying volumes of B. thuringiensis or B. anthracis were mixed with volumes of the non-pathogenic B. megaterium to create different ‘doses’ of the pathogen while presenting a constant total bacterial load to the worms. Error bars indicate the SEM. n = 3 trials, with an average of 60 worms per condition per trial. The right-most data point is 100% B. thuringiensis or B. anthracis. The data for 0% B. thuringiensis or B. anthracis were collected but cannot be displayed on this log plot; there were no lethal infections at this dose.
Many key epidemiological processes are inoculum-dependent, e.g., the chance of infection in a given host increases with increasing pathogen inoculum (reviewed in [18]). To determine if this relationship between pathogen and host are true for B. thuringiensis infections in C. elegans, we varied the dose of inoculum by diluting B. thuringiensis with varying amounts of weakly infective B. megaterium. Two different B. thuringiensis strains were used for this study. We found a dose-dependent relationship in which the C. elegans infection rate increases when the proportion of B. thuringiensis in the inoculum is raised (Figure 2B). This well-behaved inoculum-dependent infectivity of B. thuringiensis in Cry5B-sensitized C. elegans is rather unique, as C. elegans–pathogenic bacterial studies generally focus on percent animals killed at different time points at fixed doses of bacteria versus percent animals killed at different bacterial concentrations at a fixed time.
Temporal requirements of the infection process
Our data demonstrate that incubation of B. thuringiensis and Cry5B continually with C. elegans results in a lethal infection within 48 h. We next set out to study the temporal requirements for the infection process—e.g., does B. thuringiensis and Cry5B need to be continually fed to C. elegans during this entire time period or are shorter incubation periods also effective in establishing an infection? To determine when the infection process was established, a B. thuringiensis pulse-chase experiment was designed (Figure 3A, schematic). Young adult C. elegans hermaphrodites were exposed to pulses of the B. thuringiensis and Cry5B PFP for varying lengths of time that ranged from 5 minutes to 8 hours. Following these pulses, the nematodes were passed through a series of five washes containing B. megaterium (no Cry5B) to dilute out both the infectious bacteria and Cry5B. Animals were incubated in the final wash for a total of 48 hours and then scored for Bobs (Figure 3A, graph). Surprisingly, high levels of lethal infections are established in as little as 15 minutes of exposure to the pathogen plus Cry PFP. Indeed, the high levels of infections seen upon exposure to pathogenic conditions for any of the time points between 15 minutes and 8 hours are not statistically different (ANOVA, P>0.05, Tukey's post test).
Figure 3. Temporal aspects of the infection process.
(A) Infections are established upon a short exposure to pathogen. Upper: schematic of experiment. Worms are added to the well on the left containing B. thuringiensis and Cry5B. Following either 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, or 8 h, the worms were then moved through a series of five wells lacking Cry5B protein and containing non-infectious B. megaterium instead of B. thuringiensis. Infection outcomes in the final well were scored 48 h later. Lower: results of the experiment depicted in upper schematic. Data shown represent a total of three independent experiments with ∼30 animals per time point per experiment. Error bars indicate SEM. Only the infection rate for a 5-minute pulse is significantly different from the other data points (ANOVA, p<0.05, Tukey's post test). (B) Cry5B acts early in the infection process as temporal addition of Cry5B protein after, and separate from, pathogen exposure results in a significant drop in infections. Upper: schematic of experiment in which worms are exposed to pathogen first and then to Cry5B. Lower: results in which two sets of experiments were set up simultaneously—a normal 15 minute pulse chase with both Cry5B PFP and pathogen added together (light gray bars; see (A) for set up) and pulse chase in which Cry5B was not added until the end (dark gray bars). Error bars represent SEM. ∼30 worms/condition/trial; 3–4 independent trials per condition. (C) The infection process appears to begin with colonization of the anterior intestine. The anterior intestine of a nematode 3 hr after exposure to B. thuringiensis (407-gfp) under pathogenic conditions. The left panel is a DIC image; the right panel deconvolved fluorescent (FITC) of the same animal. Images taken at 600×. Anterior is down.
To confirm that the infections seen in the pulse-chase experiment were not the result of residual B. thuringiensis and Cry5B in the final well containing B. megaterium, the experiment was set up as above but no worms were placed in the initial B. thuringiensis well. The same volume as pipetted in the above experiments (without nematodes) was then passaged from the initial well through four B. megaterium dilution wells to the final B. megaterium well as above. Nematodes and Cry5B were then added to this final well. No infections were ever detected in four repetitions of this experiment. Thus, the amount of B. thuringiensis transferred from the first to the final well is not sufficient on its own to cause infection and the infections seen in Figure 3 are the result of interactions between the worms and bacteria exposed to one another in the first well.
It is known that the intestinal pores formed by a short pulse of Cry5B on C. elegans can last for up to 1 d [19]. Thus, it is possible that the effects of Cry5B PFP on the infection process occurs late even though the pulse used in the above experiments was early and brief. To address the issue of whether Cry5B PFP is acting late or early, we first exposed the animals to the infectious bacteria, washed these away, and then added Cry5B (Figure 3B, schematic). If Cry5B were only acting late in the process, we rationalized that there should be no difference between adding Cry5B with the bacteria in the very beginning or adding Cry5B after a slight delay of ∼10 min. In fact, we found that adding Cry5B subsequent to the bacteria leads to a significant drop in infection levels. These data indicate that the PFP instigates infection of B. thuringiensis in C. elegans at the earliest stages.
To see if we could detect visual cues of the beginning of the infection process, green-fluorescent protein (GFP)- expressing B. thuringiensis was fed to C. elegans along with Cry5B PFP. The earliest and most common phenotype we could see (∼3–6 h after initiation of the experiment) was the formation of a cluster of vegetative bacteria in the interior lumen of the intestine in many of the nematodes (Figure 3C). These data suggest that, at least by this time-point, the focus point of the infection process is in the anterior intestine, from which it likely spreads.
Cry5B PFP potentiates the infectivity of many Bacillus sp
Our data demonstrate that exposure to the crystal PFP Cry5B allows B. thuringiensis to initiate a lethal infection in C. elegans. We tested that the PFP might also potentiate the infectivity of other Bacillus sp.. First, we tested Bacillus subtilis. B. subtilis is nonpathogenic towards C. elegans, and C. elegans have increased longevity when fed on B. subtilis than when fed on E. coli OP50, the standard laboratory C. elegans food source [20], [21]. We quantitated the ability of B. subtilis (two different strains) to infect C. elegans in the absence and presence of Cry5B PFP. Whereas B. subtilis alone is unable to infect C. elegans, this “nonpathogenic” bacterium is able to infect C. elegans in the presence of Cry5B PFP (Table 1). As with B. thuringiensis, B. subtilis infections occur even in the sterile glp-4(bn2) C. elegans background (Table 1). Thus, internal hatching of progeny is not required for infection by B. subtilis, although some contribution may be reflected by a drop-off in infection rate in glp-4(bn2) animals (Table 1). As with B. thuringiensis, Cry5B intoxication via the glycolipid receptor is required for subsequent infection by B. subtilis, as bre-4(ye13) receptor-less mutants are not infected by B. subtilis in the presence of PFP (Table 1).
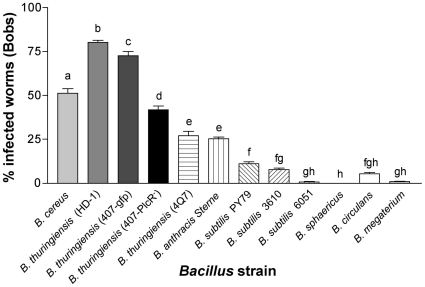
To look closer at the specificity on the Bacillus species, we repeated these Cry5B PFP-mediated infection assays using a broader range of Bacillus sp. for these experiments and the C. elegans strain glp-4(bn2) to avoid any complication in interpretation from internal hatching of larvae (Figure 4). We found that the bacteria from the B. cereus group- B. cereus, B. thuringiensis 407-gfp, B. thuringiensis 4Q7 and B. anthracis Sterne are the most virulent, each infecting >25% of the nematodes (Figure 4). As reported in Table 1, B. subtilis is able to infect at an appreciable level and B. megaterium at a barely detectable level (Figure 4). Interestingly, B. circulans, which is phylogenetically quite divergent from the other Bacillus, is also capable of infection, but B. sphaericus, which is used as an insecticidal bacterium like B. thuringiensis, is not (Figure 4). B. anthracis-infected nematodes look similar to B. thuringiensis-infected nematodes under low and high-magnification microscopy (Figure 1C).
Figure 4. Infection of C. elegans by different Bacillus species.
The percent-infected worms by different Bacillus are shown along with standard error. Means below the same letter were not significantly different at P<0.05; means below different letters are significantly different at P<0.05. Each bar represents the mean for 3–4 independent trials. The total number of animals screened from left to right are: 117, 209, 117, 122, 107, 142, 96, 96, 96, 72, 96, and 216.
It has been previously shown that B. thuringiensis is capable of causing lethal infections in insects and that this infectivity is greatly dependent on the master regulator of virulence, PlcR [22], [23]. In Galleria mellonella larvae, co-ingestion of the B. thuringiensis parental strain with a sublethal concentration of Cry1C toxin caused 70% mortality whereas only 7% mortality was recorded if a ΔplcR mutant strain were used [23]. Interestingly, in Cry5B-sensitized C. elegans, loss of PlcR from B. thuringiensis still produces a robust, albeit reduced, percentage of infected dead animals (Figure 4; 44% of Bobs with B. thuringiensis 407ΔplcR versus 73% with the B. thuringiensis-407-gfp parental strain). Moreover, B. anthracis Sterne, which lacks a functional PlcR [22], is able to produce a level of infection in Cry5B-sensitized C. elegans comparable to one of the B. thuringiensis strain (4Q7) (Figure 4). PlcR is therefore dispensable for infection in C. elegans and much less important for infection in C. elegans than in Galleria. Taken together, Cry5B PFP is able to potentiate the infectivity of many, but not all, Bacillus towards C. elegans, with the tightly knit B. cereus group as the most potentiated.
B. anthracis, which naturally lacks crystal proteins, can infect in the presence of Cry5B-B. thuringiensis
By definition B. thuringiensis is the bacterium that encodes for and produces Cry proteins whereas none of the other Bacillus sp., including the other B. cereus family members, do. We therefore wondered whether there might be any instance in which a Cry protein-minus Bacillus bacterium, such as B. anthracis, might be able to infect C. elegans with the help of B. thuringiensis. This question is important because it is still a mystery how B. anthracis maintains its life cycle in the soil so that it can be periodically transmitted to herbivore hosts [7], [8], [24]. We hypothesized that B. anthracis might be able to infect C. elegans if it was present coincidently with B. thuringiensis—i.e. B. anthracis could cause a super infection when B. thuringiensis was present.
We took a B. thuringiensis strain with a Cry5B plasmid, as might be found in the soil, and sporulated it to express Cry5B. We then mixed these B. thuringiensis spore-crystal lysates with sporulated B. anthracis expressing green-fluorescent protein (GFP). In these experiments, the bacteria were mixed at a ratio of about 16 B. thuringiensis spores for one B. anthracis-GFP spore. After 48 h of incubation at 25°C, we counted the number of Bobs and looked at them under the fluorescence microscope. We found that some of these infected worms were full of the gfp-anthrax (Figure 5; Table 2). Thus, even with an initially much larger load of B. thuringiensis being given to the animals and at a temperature (25°C) where B. thuringiensis might be expected to perform better, on occasion B. anthracis is able to overtake and drive the infection process.
Figure 5. Infection of C. elegans by B. anthracis-gfp in presence of B. thuringiensis expressing Cry5B spores (non-gfp) and B. anthracis-gfp spores.
(A) Transmitted-light view of the worm cuticle filled with vegetative and sporulated Bacillus. (B) View of the same worm using the fluorescence light: rod-shaped bacteria expressing GFP are visible, indicating that B. anthracis is capable of infecting C. elegans in presence of Cry5B expressing-B. thuringiensis. In this animal and all similar animals examined, the vast majority of bacteria inside the nematode cuticle were GFP positive. Scale bar is 50 µm.
Table 2. Infectivity of B. anthracis in the presence of Cry-plus B. thuringiensis.
| Condition | Number animals screened | Number Bobs seen | No. GFP-filled Bobs |
| B. anthracis-gfp | 110 | 0 | 0 |
| B. anthracis-gfp plus Cry5B-Bt | 281 | 72 | 19 |
Discussion
Here for the first time we characterize in detail the infectivity of Bacillus family bacteria in a nematode, C. elegans. Beginning with the invertebrate-specific pathogen, B. thuringiensis, we find that the infection of C. elegans by B. thuringiensis is dependent upon the presence of a PFP crystal protein virulence factor, Cry5B. The Cry5B pore-forming protein either can be added exogenously or can be naturally expressed by B. thuringiensis in order to elicit infection. The B. thuringiensis strains used in this study cannot infect the in the absence of added Cry5B (either exogenous or endogenous). Infectivity is proportionally dependent upon inoculum size and dose of the pore-forming protein. Although the fact that B. thuringiensis (but not other Bacillus species) can infect C. elegans has been seen before [25], [26], the dependence of this infection on crystal protein has not been studied. We furthermore demonstrate that the PFP acts early in the process to instigate infection. The process is likely to be distinct from B. thuringiensis infections characterized in insects since the dependence upon the PlcR virulence regulator, which has been shown to be central for infectivity in an insect [23], is minimal in the nematode system.
Surprisingly we find that many Bacillus species also possess the ability to infect C. elegans in the presence of Cry5B. This list of Bacillus sp. that can infect C. elegans includes B. subtilis, a bacterium considered non-pathogenic to C. elegans and even more benign than the standard laboratory E. coli fed to it [20], [21]. Of all Bacillus sp. tested, B. cereus group bacteria show the highest level of infectivity in the presence of the Cry5B PFP. The relative role of Cry toxin to various Bacillus species has also been investigated in the larvae of the greater wax moth (G. mellonella). In this insect Cry1C toxin induced higher mortality to B. thuringiensis and B. cereus strains and to a much lower level to B. subtilis and B. megaterium and not at all with B. anthracis [23], [27], suggesting that B. anthracis develops better in nematode than in insects. We speculate that infectivity of nematodes is an ancient hallmark of the Bacillus genus and that either many Bacillus sp. have subsequently lost the ability to infect nematodes or in the wild factors other than crystal proteins (e.g., environmental stress, starvation of the nematodes, virulence factors not normally expressed in the laboratory) instigate infection.
This work establishes the Bacillus-Cry5B-C. elegans interaction system as a model for studying specific requirements of the nematode infection process of Bacillus, most notably B. cereus-family bacteria such as B. anthracis, for studying the role of PFPs in promoting bacterial pathogenesis, and for studying host responses to B. cereus family bacteria. The fact that both the bacteria and the host are amenable to genetics makes it possible to investigate the interactions of B. cereus family bacteria and an animal host at a level not previously attained.
This research also begins to address an important question in the B. anthracis field—what are the potential reservoirs of this bacterium in nature? B. anthracis causes periodic, large-scale episodes of infection in ruminants. The question has remained: where does it reside and how does it propagate between episodes? One suggestion is that earthworms are reservoirs for B. anthracis and B. cereus multiplication and persistence [28]. Our data suggest that nematodes are potential targets too, when present with B. thuringiensis, B. anthracis is able to replicate within a nematode. Therefore, nematodes might provide a key, and heretofore elusive, natural reservoir for B. cereus and B. anthracis along with earthworms and could play a specific epidemiological role in anthrax outbreaks in the wild. Furthermore, nematodes may provide a key ecological niche linking the three bacteria in the B. cereus-group and facilitating genetic exchange, such as the many that have already been observed [29], [30] and thus helping shape the common evolution of these bacteria.
Materials and Methods
Bacterial strains
Bacteria were cultured in Brain Heart Infusion (BHI) media (BD, Sparks, MD, USA) at 30°C (37°C for B. anthracis Sterne). For sporulation, B. anthracis was cultured in Difco Sporulation Medium [31]. The Bacillus strains used in this work were: B. cereus (ATCC 14579), B. thuringiensis (HD-1), B. thuringiensis (407-gfp), B. thuringiensis (407-ΔPlcR-gfp), B. thuringiensis (4Q7), B. anthracis Sterne, B. anthracis 7702 harboring pUTE610 (a gift from Theresa Koehler, University of Texas), B. subtilis PY79 (a gift from Richard Losick, Harvard University), B. subtilis 3610, B. subtilis 6051, B. sphaericus (ATCC 4525), B. circulans (ATCC 4513), and B. megaterium (ATCC 14581). B. thuringiensis 407-gfp and B. thuringiensis (407-ΔPlcR-gfp) contain a constitutively expressed Mut1-green fluorescent protein (GFP) carried on a multicopy plasmid (pHT315 papha3::gfp) with an erythromycin selection factor [32].
Nematode strains
C. elegans were maintained using standard techniques [33]. Unless otherwise mentioned, glp-4(bn2) worms were used in all assays to prevent matricidal death due to premature hatching of embryos. At 25°C, this strain lacks germline and does not produce progeny. This strain is often used in C. elegans assays involving bacterial pathogens [15], [34], [35], [36], [37], [38]. We found that many fertile C. elegans hermaphrodites can die from the internal hatching of larvae when exposed to Bacillus spp. under conditions of the infection model. Invariably, these dead worms would subsequently become infected. Under these circumstances, it was not easy to differentiate between worms that had become infected before dying versus worms that had died from internal hatching of larvae before becoming infected. Thus, we use glp-4(bn2) in many of our assays to avoid this ambiguity. We do not believe the use of glp-4(bn2) significantly alters our findings. Similarly robust infections are achieved using wild-type N2 C. elegans as with glp-4(bn2), infected animals look the same under high power magnification regardless of which strain is used, and a completely different sterile strain, fer-1(ba576), shows similar levels of infection to glp-4(bn2) (data not shown). glp-4(bn2) worms were maintained at 15°C and shifted at the appropriate times to 25°C when sterility was desired.
Expression and Purification of Cry5B protein
Purified Cry5B protein was prepared using a modified sucrose gradient [39]. Purified Cry5B protein was precipitated, resuspended in double-distilled H2O, frozen into aliquots using liquid nitrogen, and stored at −80°C until needed. On the day of use, aliquots were thawed at room temperature and centrifuged at 16,000 g to pellet the protein and allow aspiration of the supernatant. Pellets were then solubilized in 20 mM citrate buffer (pH 3.0) to a final concentration of 0.4 mg/ml.
Bacillus infection assays
Unless otherwise specified, infection assays were carried out in 48-well assay plates. The final volume in each assay well was 200 µl. Standard S-medium was used to maintain C. elegans in liquid cultures [33].
Preparation of bacteria
Bacteria were grown overnight in 5 ml liquid BHI in a standard large test tube (25×150 mm) at 250 rpm, 30°C (37°C for B. anthracis). The overnight culture was then centrifuged in 1.5-ml tubes in a microcentrifuge at 16,000 g for 4 min to pellet cells and the supernatant was aspirated. Bacterial cells were resuspended in S-medium for a final OD600 of 0.3+/−0.02. 190 µl of bacteria in S-media was added to the assay well. This methodology allows for relatively easy normalization amongst the different bacterial strains.
For assays with mixing of B. anthracis and Cry5B-expressing B. thuringiensis, B. anthracis-gfp, spores were used since B. thuringiensis produces Cry5B during sporulation. Bacteria were grown in 5 ml liquid Difco Sporulation Medium for 48 hours at 37°C [31]. Cultures were checked using a compound microscope for sproulation and were found to be >99% spores. 0.1 µl of Cry5B spore crystal lysates, containing about 4.0×106 spores were added with 10 µl of B. anthracis-gfp spores (containing about 2.5 105 spores) to 190 µl of S-media in a 48-well plate. glp-4(bn2) animals were prepared as described below.
Addition of Cry5B protein
5 µl of 0.4 mg/ml Cry5B in 20 mM citrate buffer (pH 3.0) was added to appropriate wells for a final well concentration of 10 µg/ml Cry5B. Citrate buffer (pH 3.0) alone was used as a negative control.
Preparation of nematodes
Synchronized populations of glp-4(bn2) nematodes were prepared using standard techniques [39]. Two days prior to setting up the infection assay, a synchronous population of L1s was seeded onto standard 3.5-cm NG plates containing OP50. The worms were incubated at 25°C for 52 h to sterilize the hermaphrodites and allow them to reach the young adult stage. The synchronized nematodes were then washed off the plates with M9 and rinsed three times; twice with M9 and the final time with S-medium. Worms were centrifuged at 500 g for 40 sec to pellet worms for removal of wash supernatants. Worms were then resuspended in S-medium for the desired concentration of 8–12 worms per 5 µl, and 5 µl of this suspension was then added to wells. The assay plates were incubated at 25°C for 48 h, after which they were scored for dead infected worms with a dissecting microscope. Although the majority of nematodes that would end up infected were infected and dead in one day, the assays were allowed to go for two days to be sure the infections had gone to completion. Worms were only counted as infected worms if they were filled with bacteria, which can be detected by dark, rigid, and immobile worms. We have confirmed numerous times by high-resolution microscopy that such worms represent terminally infected worms.
Cry5B dose-response assay
Preparation of bacteria and worms was identical to the described procedure of the C. elegans infection assay. Different doses of the Cry5B PFP were prepared by serial dilution so that the same volume of protein would be added to each well. Each condition was tested in multiple wells (from 4–6 wells).
Bacillus dose-response assay
Bacteria were prepared in S-medium according to the C. elegans infection assay protocol. Different doses of pathogenic Bacillus were prepared by mixing varying amounts of pathogenic Bacillus (i.e. B. thuringiensis HD-1 or B. thuringiensis 407-gfp) with the non-pathogenic B. megaterium, all at the same OD600. The inoculum amount of pathogenic Bacillus was expressed as the percentage of the mixed bacteria by volume. These doses were pipetted into appropriate wells and the rest of the assay was set up per the C. elegans infection assay protocol. Each condition was tested in quadruplicate wells.
Bacillus pulse-chase assay
This pulse-chase assay was designed to test the minimum length of time needed for the bacteria to establish an infection. To allow for ease of manipulation, 24-well plates were used. Each row was dedicated to one condition. The total volume in each assay well was 500 µl. The Bacillus strains, purified Cry5B, and nematodes were prepared in an identical manner to the Bacillus infection assay. The first well of each row was designated the ‘Pulse’ well and contained 475 µl pathogenic Bacillus and 12.5 µl purified Cry5B protein (final concentration 10 µg/ml). The remaining 5 wells of each row were designated the ‘Chase’ wells, each containing 450 µl non-pathogenic B. megaterium. At t0 12.5 µl of glp-4 young adults in S-media (approximately 40 worms) were added to the ‘Pulse’ well and the plate was incubated at 25°C for 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, or 8 h. At the end of the exposure time, the worms were serially transferred through the ‘Chase’ wells in a volume of 50 µl (spending less than 1 min in each intermediate well), such that the final chase well had a total volume of 500 µl. During the chase transfers, the plates were kept tilted at a 60° angle on the bench top to allow worms to more rapidly settle to the bottom and collect them for pipetting to the next well. Plates were then returned to 25°C. At t48 the final chase wells were scored for dead infected worms. Worms that were not transferred in the 50 µl serial washes and thus did not make it into the final ‘Chase’ well were excluded from analysis.
Microscopy
Images of worms in wells were taken with a Nikon Coolpix digital camera on an Olympus dissecting microscope. Images of the infection process were captured using differential interference contrast (DIC) and fluorescence optics on a Zeiss AxioImager A1 microscope using an AxioCam HRm camera and a 63×1.4 NA PlanApochromat lens. Images of colony formation were taken on an Olympus IX70 DeltaVision microscope (Applied Precision, Issaquah, WA) with a Nikon 60×, 1.4 NA PlanApo oil objective lens. Nematodes were mounted on glass slides with 2% agarose pads with 15 mM sodium azide as an anesthetic prior to imaging. Images were recorded and deconvolved using the SoftWorx program (Applied Precision).
Statistics
Statistical analyses were performed with GraphPad Prism (GraphPad Software, San Diego). For multiple-group comparisons, ANOVA was performed followed by Tukey's posttest. Values of p<0.05 were considered statistically significant.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding for this project was provided by National Institutes of Health grants to RVA (NIAID R01 AI056189) and to RVA/VN (NIAID R21 AI065993). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Helgason E, Okstad OA, Caugant DA, Johansen HA, Fouet A, et al. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis–one species on the basis of genetic evidence. Appl Environ Microbiol. 2000;66:2627–2630. doi: 10.1128/aem.66.6.2627-2630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolsto AB, Tourasse NJ, Okstad OA. What sets Bacillus anthracis apart from other Bacillus species? Annual review of microbiology. 2009;63:451–476. doi: 10.1146/annurev.micro.091208.073255. [DOI] [PubMed] [Google Scholar]
- 3.Rasko DA, Altherr MR, Han CS, Ravel J. Genomics of the Bacillus cereus group of organisms. FEMS microbiology reviews. 2005;29:303–329. doi: 10.1016/j.femsre.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Mock M, Fouet A. Anthrax. Annual review of microbiology. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 5.Drobniewski FA. Bacillus cereus and related species. Clinical microbiology reviews. 1993;6:324–338. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and molecular biology reviews : MMBR. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen GB, Hansen BM, Eilenberg J, Mahillon J. The hidden lifestyles of Bacillus cereus and relatives. Environmental microbiology. 2003;5:631–640. doi: 10.1046/j.1462-2920.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 8.Vilas-Boas GT, Peruca AP, Arantes OM. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can J Microbiol. 2007;53:673–687. doi: 10.1139/W07-029. [DOI] [PubMed] [Google Scholar]
- 9.Griffitts JS, Aroian RV. Many roads to resistance: how invertebrates adapt to Bt toxins. BioEssays : news and reviews in molecular, cellular and developmental biology. 2005;27:614–624. doi: 10.1002/bies.20239. [DOI] [PubMed] [Google Scholar]
- 10.de Maagd RA, Bravo A, Crickmore N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends in genetics : TIG. 2001;17:193–199. doi: 10.1016/s0168-9525(01)02237-5. [DOI] [PubMed] [Google Scholar]
- 11.Alouf JE. Molecular features of the cytolytic pore-forming bacterial protein toxins. Folia Microbiol (Praha) 2003;48:5–16. doi: 10.1007/BF02931271. [DOI] [PubMed] [Google Scholar]
- 12.Marroquin LD, Elyassnia D, Griffitts JS, Feitelson JS, Aroian RV. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics. 2000;155:1693–1699. doi: 10.1093/genetics/155.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao CY, Los FC, Huffman DL, Wachi S, Kloft N, et al. Global functional analyses of cellular responses to pore-forming toxins. PLoS pathogens. 2011;7:e1001314. doi: 10.1371/journal.ppat.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Platzer EG, Bellier A, Aroian RV. Discovery of a highly synergistic anthelmintic combination that shows mutual hypersusceptibility. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5955–5960. doi: 10.1073/pnas.0912327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, et al. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc Natl Acad Sci U S A. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffitts JS, Haslam SM, Yang T, Garczynski SF, Mulloy B, et al. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science. 2005;307:922–925. doi: 10.1126/science.1104444. [DOI] [PubMed] [Google Scholar]
- 17.Griffitts JS, Huffman DL, Whitacre JL, Barrows BD, Marroquin LD, et al. Resistance to a bacterial toxin is mediated by removal of a conserved glycosylation pathway required for toxin-host interactions. The Journal of biological chemistry. 2003;278:45594–45602. doi: 10.1074/jbc.M308142200. [DOI] [PubMed] [Google Scholar]
- 18.Regoes RR, Ebert D, Bonhoeffer S. Dose-dependent infection rates of parasites produce the Allee effect in epidemiology. Proc Biol Sci. 2002;269:271–279. doi: 10.1098/rspb.2001.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Los FC, Kao CY, Smitham J, McDonald KL, Ha C, et al. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell host & microbe. 2011;9:147–157. doi: 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garsin DA, Sifri CD, Mylonakis E, Qin X, Singh KV, et al. A simple model host for identifying Gram-positive virulence factors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10892–10897. doi: 10.1073/pnas.191378698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 22.Agaisse H, Gominet M, Okstad OA, Kolsto AB, Lereclus D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Molecular Microbiology. 1999;32:1043–1053. doi: 10.1046/j.1365-2958.1999.01419.x. [DOI] [PubMed] [Google Scholar]
- 23.Salamitou S, Ramisse F, Brehelin M, Bourguet D, Gilois N, et al. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology. 2000;146(Pt 11):2825–2832. doi: 10.1099/00221287-146-11-2825. [DOI] [PubMed] [Google Scholar]
- 24.Blackburn JK, Curtis A, Hadfield TL, O'Shea B, Mitchell MA, et al. Confirmation of Bacillus anthracis from flesh-eating flies collected during a West Texas anthrax season. Journal of wildlife diseases. 2010;46:918–922. doi: 10.7589/0090-3558-46.3.918. [DOI] [PubMed] [Google Scholar]
- 25.Borgonie G, Van Driessche R, Leyns F, Arnaut G, De Waele D, et al. Germination of Bacillus thuringiensis spores in bacteriophagous nematodes (Nematoda: Rhabditida). J Invertebr Pathol. 1995;65:61–67. doi: 10.1006/jipa.1995.1008. [DOI] [PubMed] [Google Scholar]
- 26.Schulenburg H, Muller S. Natural variation in the response of Caenorhabditis elegans towards Bacillus thuringiensis. Parasitology. 2004;128:433–443. doi: 10.1017/s003118200300461x. [DOI] [PubMed] [Google Scholar]
- 27.Fedhila S, Buisson C, Dussurget O, Serror P, Glomski IJ, et al. Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella. Journal of invertebrate pathology. 2010;103:24–29. doi: 10.1016/j.jip.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Schuch R, Pelzek AJ, Kan S, Fischetti VA. Prevalence of Bacillus anthracis-like organisms and bacteriophages in the intestinal tract of the earthworm Eisenia fetida. Applied and environmental microbiology. 2010;76:2286–2294. doi: 10.1128/AEM.02518-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmaster AR, Ravel J, Rasko DA, Chapman GD, Chute MD, et al. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8449–8454. doi: 10.1073/pnas.0402414101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasko DA, Ravel J, Okstad OA, Helgason E, Cer RZ, et al. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic acids research. 2004;32:977–988. doi: 10.1093/nar/gkh258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steichen CT, Kearney JF, Turnbough CL., Jr Non-uniform assembly of the Bacillus anthracis exosporium and a bottle cap model for spore germination and outgrowth. Molecular Microbiology. 2007;64:359–367. doi: 10.1111/j.1365-2958.2007.05658.x. [DOI] [PubMed] [Google Scholar]
- 32.Daou N, Buisson C, Gohar M, Vidic J, Bierne H, et al. IlsA, a unique surface protein of Bacillus cereus required for iron acquisition from heme, hemoglobin and ferritin. PLoS pathogens. 2009;5:e1000675. doi: 10.1371/journal.ppat.1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Boulder: Cold Spring Harbor Laboratory; 1988. pp. 587–606. [Google Scholar]
- 34.Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, et al. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- 36.Moy TI, Ball AR, Anklesaria Z, Casadei G, Lewis K, et al. Identification of novel antimicrobials using a live-animal infection model. Proc Natl Acad Sci U S A. 2006;103:10414–10419. doi: 10.1073/pnas.0604055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapira M, Hamlin BJ, Rong J, Chen K, Ronen M, et al. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci U S A. 2006;103:14086–14091. doi: 10.1073/pnas.0603424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenor JL, McCormick BA, Ausubel FM, Aballay A. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr Biol. 2004;14:1018–1024. doi: 10.1016/j.cub.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 39.Cappello M, Bungiro RD, Harrison LM, Bischof LJ, Griffitts JS, et al. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc Natl Acad Sci U S A. 2006;103:15154–15159. doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]