Abstract
Gliomas, the most frequent primitive CNS tumors, have been suggested to originate from astrocytes or from neural progenitors/stem cells. However, the precise identity of the cells at the origin of gliomas remains a matter of debate because no pre-neoplastic state has been yet identified. TGFα, an EGF family member, is frequently over-expressed in the early stages of glioma progression. We previously demonstrated that prolonged exposure of astrocytes to TGFα is sufficient to trigger their reversion to a neural progenitor-like state. To determine whether TGFα de-differentiating effects are associated with cancerous transforming effects, we grafted intra-cerebrally de-differentiated astrocytes. We show that these cells had the same cytogenomic profile as astrocytes, survived in vivo and did not give birth to tumors. When astrocytes de-differentiated with TGFα were submitted to oncogenic stress using gamma irradiation, they acquired cancerous properties: they were immortalized, showed cytogenomic abnormalities, and formed high-grade glioma-like tumors after brain grafting. In contrast, irradiation did not modify the lifespan of astrocytes cultivated in serum-free medium. Addition of TGFα after irradiation did not promote their transformation but decreased their lifespan. These results demonstrate that reversion of mature astrocytes to an embryonic state without genomic manipulation is sufficient to sensitize them to oncogenic stress.
Keywords: Animals; Astrocytes; drug effects; metabolism; radiation effects; Brain Neoplasms; chemically induced; physiopathology; Cell Dedifferentiation; drug effects; physiology; radiation effects; Cell Transformation, Neoplastic; chemically induced; metabolism; radiation effects; Cells, Cultured; Culture Media, Serum-Free; pharmacology; Gamma Rays; adverse effects; Glioma; chemically induced; physiopathology; Mice; Mice, Inbred C57BL; Mice, Nude; Stem Cell Transplantation; Stem Cells; drug effects; metabolism; radiation effects; Stress, Physiological; physiology; radiation effects; Transforming Growth Factor alpha; metabolism; pharmacology
Keywords: EGF, transdifferentiation, metaplasia, radial glia, erbB
Gliomas are highly heterogeneous primitive tumors of the central nervous system and result in poor outcome despite new alkylating and anti-angiogenic treatments. The variability in clinical evolution of patients bearing tumors of similar histopathological appearances and the invasive nature of the tumors impair the development of curative treatments.
The cell at the origin of glioma remains a matter of speculation since no pre-neoplastic state has been identified 1. Morphological and immunohistological similarities of glioma cells to macroglial cells of the CNS have led to the initial proposal that they derive from astrocytes or oligodendrocytes. Conversely, the coexistence within the same tumor of cells of different phenotypes, and the isolation of tumor stem cells from gliomas 2, 3 favor neural progenitors or neural stem cells as the cell of origin of gliomas. The frequent development of gliomas in the vicinity of the sub-ventricular zone, which contains adult neural stem cells, as well as the migratory properties of these cells 4, further support this hypothesis. Nevertheless, experimental development of gliomas in juvenile transgenic mice shows that cancerous transformation can be achieved with retroviral introduction of oncogenes in both progenitor/stem cells expressing nestin and differentiated cells expressing GFAP, a common astrocyte marker, although the transformation occurs with higher efficacy in immature cells 5. A possible reconciliation between these opposite sets of data may be that gliomas result not only from alterations in the regulation of cell proliferation and survival, but also from alterations in the differentiation state of mature cells as proposed for other cancers 6. We previously showed that prolonged exposure of differentiated astrocytes to Transforming Growth Factor (TGFα), a member of the Epidermal Growth factor (EGF) family 7, results in their progressive and functional reversion, first to neural progenitor-like cells akin to radial glia, and then to cells that exhibit properties of neural stem cells 8. TGFα and its receptor erbB1 (or epidermal growth factor receptor, EGFR) are involved in the control of both gliogenesis and glioma development. They constitute the signaling module most frequently deregulated in gliomas. TGFα over-expression is found in about 80% of gliomas, and is observed from the initial steps of their development 9, whereas the over-expression of erbB1 appears in 20–40% of gliomas in later phases 10. Over-expression of TGFα and/or its receptor are, by themselves, insufficient to achieve cancerous transformation of astrocytes in transgenic mice 11, 12.
In the context of regenerative medicine, the ability to revert somatic cells to an embryonic state without nucleic acid delivery of reprogramming factors 13, 14 and with a single modification of their environment 8 is of great interest. This interest is reinforced by the recent report that a subset of reactive astrocytes isolated from the brains of stab wound-injured mice may reacquire, at least in vitro, neural stem cell properties 15, and by the known over-expression of TGFα by reactive astrocytes (reviewed in 9). In light of the deregulation of TGFα expression in the early stages of glioma development, it is important to determine whether reversion of astrocytes to a neural progenitor state predisposes the cells to cancerous transformation. To evaluate this possibility, we used a classical mutagenic treatment, irradiation, to determine whether astrocytes de-differentiated into progenitor-like cells are more sensitive to cancerous transformation than their mature counterparts. Our results show that mature astrocytes, having regressed to a neural progenitor-like stage in response to a single change in their extra-cellular environment, can be the cell of origin of gliomas.
Materials and methods
Animals
C57Bl6/J and Nude mice (Charles River, France) were housed in an air-conditioned room with free access to water and food. NOD/SCID mice (Charles River, France) were maintained in microisolator cages in pathogen-free conditions.
Cell culture and irradiation
Cultures of mouse astrocytes were prepared from cortices of 1-to-2-day-old C57Bl6/J mice as previously described 16. Briefly, cultures were established in defined MEM:F12 medium containing 10% fetal calf serum (Biowest, Nuaillé, France). Medium was changed every 3 days following washes with ice-cold phosphate-buffered saline (PBS, pH 7.4). When confluence was reached (8–10 DIV), cultures were shaken overnight (250 r.p.m.), trypsinised and seeded on poly-L-ornithine-coated glass slides (50,000 cell/cm2). The cells were further cultured for 4–6 days with washes with ice-cold PBS preceding each medium renewal, until an 80–90% confluent cell layer had formed. The cultures were then transferred to serum-free medium for 3 days, prior being cultivated for 7 days in defined medium supplemented or unsupplemented with TGFα (50ng/ml, AbCys, France). GFAP-immunoreactive astrocytes accounted for 91–96% of the cells in all cultures types. The remaining 4–9% of cells were CD11b receptor-immunoreactive microglia. TGFα levels present in these astrocyte culture lysates were determined using an anti-human TGFα sandwich ELISA (R&D systems, France) following the manufacturer’s instructions. Assays of erbB1 levels and activation were performed as previously described 8 with the following antibodies: anti-erbB1 sc-03-G from Santa-Cruz (TEBU, France) for immuno-precipitation, anti-erbB1 and anti-phosphotyrosine antibody 4G10 from UpstateBiotechnologies (Lake Placid, NY) for immuno-blotting. Expression of TACE, the enzyme that controls TGFα release from the cells 17, was verified using Western blot analysis with an anti-TACE antibody from Santa-Cruz (1:250, sc-6416, TEBU, France), as previously described 18.
Astrocytes cultivated with or without TGFα were irradiated with 5 Gy of γ-radiation using a 137Cs irradiator (IBL 637, Institut Curie, Paris, France) at a dose rate of 1.57 Gy/min (Fig. 1a). Controls corresponded to sham-irradiated cultures. The cells were maintained at 37°C throughout the experime ntal procedure, except during irradiation, which was performed at 20°C in ambient a ir. Two hours after irradiation, the cells were washed with ice-cold PBS, and further cultured in their original medium with changes of media every 3 days. In addition, astrocytes cultivated with or without TGFα were treated with 1 μM Tyrphostin AG1478 (La Jolla, CA) between minus 2, and plus 2 hours of the irradiation time-point. AG1478 is a specific inhibitor of erbB1 tyrosine kinase activity 19, known to inhibit TGFα effects on astrocytes 8, 20.
Figure 1.
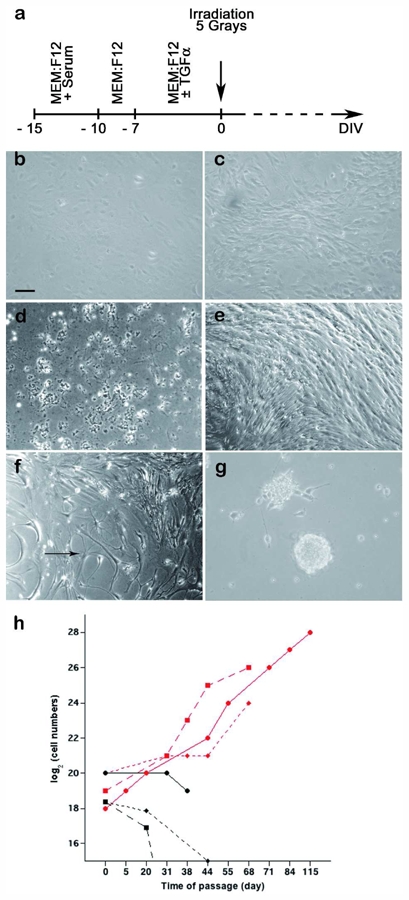
Response to irradiation of astrocytes and astrocytes de-differentiated into progenitor-like cells by TGFα. a. Schematic representation of the experimental protocol. See text for details. b. Mouse astrocytes have a flat, polygonal shape in serum-free medium. c. After 7 days in the presence of TGFα, astrocytes acquire a bipolar shape. d. Irradiation does not affect the survival of astrocyte cultures maintained in serum-free medium. They die within 3 weeks post-irradiation, as do their sham-irradiated counterparts. e. Six weeks post-irradiation, TGFα-treated cultures contain densely packed bipolar cells. f. Giant stellar shaped cells (arrow) co-exist with bipolar cells in irradiated TGFα-treated cultures (6 weeks post-irradiation). g. Three months post-irradiation, cells growing as free-floating cellular spheres appeared in irradiated TGFα-treated cultures. Scale bar = 40 μm in a–g. h. Growth during serial passage of sham-irradiated TGFα-treated astrocyte cultures (black points) and irradiated TGFα-treated astrocyte cultures (red points). Serially passaged irradiated TGFα-treated astrocytes exhibited immortal growth while non-irradiated TGFα-treated astrocytes underwent proliferation arrest. Illustration of 3 independent experiments.
Changes in morphology or growth pattern were verified by visual inspection prior each medium renewal. After 40 DIV, medium was switched to a 1:1 mixture of DMEM and F12 nutrient (Invitrogen, France), containing 0.6% glucose, 2 mM glutamine, 13 mM sodium bicarbonate, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer, 5 IU/ml penicillin and 5 μg/ml streptomycin, and B27, N-2 and G5 supplements (10 μl/ml each, Invitrogen) (NS34 medium, 8). This medium was renewed once per week. Experiments were repeated three times in an independent manner.
Proliferation and survival assays
Seventy days post-irradiation, cultures were trypsinised and counted. Cell viability was determined by Trypan Blue exclusion. To construct growth curves, cultures were submitted to serial passages when reaching 70–90% confluence.
Array CGH
The samples were homogenized, and genomic DNA was extracted according to Qiagen protocols with modifications. Restriction of DNA, quality control of restricted DNA by microanalysis, pooling, and clean-up, were performed following instructions from Agilent. The samples were hybridized in single versus a standard DNA (male or female, Promega) with Mouse Genome CGH 4x44K Agilent micro arrays (G4426B). The scanning and image analysis were performed with Agilent technology using default settings. Raw data .txt files from the Agilent Feature Extraction software 9.5 were then imported for analysis into CGH Analytics 3.4.40. Aberrations were obtained with the ADM2 algorithm and filtering options of a minimum of 5 probes and abs (log 2 ratio) >0.25. Sexual chromosomes were excluded from the analysis because male and female mice were indifferently used to establish the astrocyte cultures.
The microarray data analyzed in this paper have been submitted to the Array Express data repository at the European Bioinformatics Institute (http://www.ebi.ac.uk/arrayexpress/) under the accession number E-TABM-706.
Anchorage-independent Growth assays
The cells (10,000) were uniformly suspended in 1.5 ml NS34 containing 66% methylcellulose medium (M3434; Stem Cell Technologies, France). The suspension was plated in 35 mm dishes. The plates were incubated at 37°C in a 5% CO2 humidified atmosphere after microscopic evaluation to ascertain that no cellular clusters have been seeded. The cells were cultured for 7 days prior to the evaluation of colony formation.
Subcutaneous and intracerebral grafting
Cell suspensions in PBS were prepared prior to grafting from cultures treated with trypsin. Bilateral subcutaneous injections of either 5.106 or 5.105-cells/200μl were performed into the flanks of 6-week-old NOD/SCID mice (n=10). Mice were screened twice weekly for tumor formation. Mice were sacrificed under anesthesia one month post-graft. The tumors were excised and fixed by immersion in paraformaldehyde for 24 hours prior to being embedded into paraffin. Stereotaxic injections of 5.105 cells/5 μl into the striatum of 6-week-old Nude mice (n=8) were performed using the following stereotaxic coordinates (antero-posterior = 0 from the bregma, lateral = 2.5 mm right, ventral = 3.8 mm deep from the dura 21. Body-weights were monitored once a week, and mice were sacrificed 18–25 days post-graft with a transcardial paraformaldehyde perfusion as previously described 22. Tissues were frozen in a 30% sucrose cryoprotective medium after a 24 hr incubation period.
Immunolabelling
Ten μm-thick sections were prepared from paraffin-embedded subcutaneous tumors. Cryostat sections (20 μm thickness) of the brains were cut in the frontal plane. Immunohistochemistry was performed on free-floating sections. Immunocytochemical and immunohistochemical procedures were performed as previously described 23 using the following primary antibodies: polyclonal rabbit or monoclonal anti-GFAP (1:500, Dako or 1:500 ICN, France), polyclonal rabbit anti-BLBP antibody (1:1000), monoclonal anti-nestin (1:200, Chemicon, France), polyclonal rabbit anti-NG2 (1:2000, Tebu, France), polyclonal rabbit anti-TGFα (1:200, RGG-8040, Peninsula Laboratories, San Carlos, CA). Immunofluorescent labelings were viewed using an Axioplan 2 fluorescence microscope, and images were captured using the ApoTome (Zeiss), AxioCam MRm digital camera and AxioVision 4.2 software. Other immunolabelings images were acquired on a Digital still camera (DXM 1200, Nikon, USA) using the Lucia software (Laboratory Imaging, Ltd). The images were prepared for printing using Adobe Photoshop software (Adobe Systems, San Jose, CA).
Results
Irradiation immortalizes astrocytes de-differentiated into progenitor-like cells
Astrocytes maintained for a week in serum-free medium exhibit a flat, polygonal morphology (Fig. 1b), while TGFα-treated astrocytes acquire, after 7 days of treatment, a bipolar morphology (Fig. 1c). Irradiation did not modify the morphology or the life span of astrocytes cultured in serum-free medium. These cultures died within 3 weeks post-irradiation, as did their sister sham-irradiated counterparts (Fig. 1d). In contrast, TGFα-treated cultures survived beyond 3 weeks regardless of whether they had been irradiated (Fig. 1e).
Non-irradiated TGFα-treated astrocytes presented the previously described radial glia-like bipolar morphology 8, with a thin layer of cytoplasm surrounding a fusiform nucleus, and two long thin processes extending in opposite directions (Fig. 1c). This morphology was maintained throughout the survival time of the cultures. On the contrary, irradiated TGFα-treated astrocytes exhibited progressive morphological changes. Forty days post-irradiation, irradiated TGFα-treated cultures contained densely packed bipolar cells (Fig. 1e) that coexisted with giant stellar-shaped cells (Fig. 1f) and small rounded cells that covered large areas of the culture dish. Development of large masses of round, bright cells at the top of the cellular layer was observed in irradiated TGFα-treated astrocyte cultures maintained for 3 months after irradiation in TGFα-supplemented defined medium. Masses were collected from three independent cultures, and subsequently dissociated and further cultured. The cells kept growing in suspension, forming cellular spheres that occasionally attached to the culture flask (Fig. 1g). Eventually, all irradiated TGFα-treated cultures were composed, for the most part, of cells that formed floating spheres. In addition to these morphological changes, irradiated TGFα-treated cells exhibited a higher growth rate than sham-irradiated sister cultures (compare Fig. 1e with 1c). Unlike their sham-irradiated counterparts, irradiated TGFα-treated cells could be trypsinised and successfully re-seeded (Fig. 1h). These data show that irradiation immortalized astrocytes reverted to progenitor-like cells upon TGFα action.
Both ligand-independent and TGFα-dependent erbB1 activations have been shown to underlie the radioresistance of tumoral epithelial cells through enhanced survival and DNA repair 24–27. ErbB1 expression has been previously characterized and shown to be functional in our mouse cortical astrocyte cultures (Fig. 2a, and 20. These cells express TGFα, as detected by ELISA at a concentration of 5 pg TGFα/μg protein in our astrocyte cultures, and as observed using immunocytochemistry (Fig. 2c). They also express TACE, the metalloprotease required for TGFα release, as seen by immunoblotting (Fig. 2b). To evaluate whether irradiation survival of astrocytes depended on the mobilization of the endogenous TGFα-erbB1 pair, we repeated the irradiation procedure on cultures treated or untreated with TGFα and supplemented with 1 μM AG1478, a specific inhibitor of erbB1 tyrosine kinase activity19, for 4 hours that included the irradiation time-point. Longer erbB1 inhibition was not attempted, as we previously observed that TGFα-treated astrocytes underwent apoptosis upon prolonged growth factor signaling inhibition 20.
Figure 2.
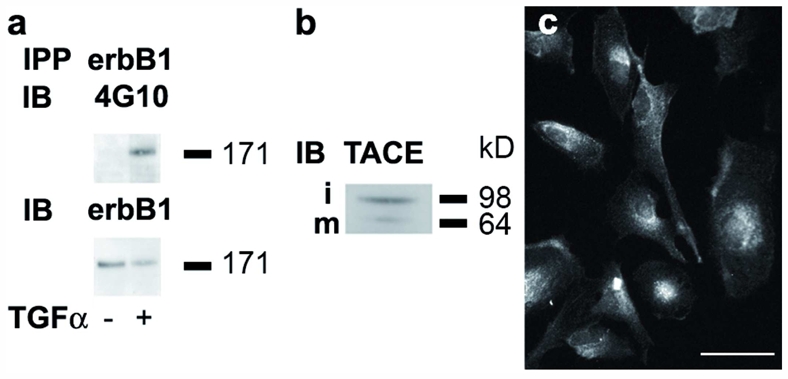
a. ErbB1 is expressed and activated in response to TGFα in mouse astrocyte cultures. Phosphorylated erbB1 was analyzed by Western blot in mouse astrocyte cultures under control conditions and following 5 min TGFα treatment. IPP: immuno-precipitation. IB: immuno-blotting. 4G10: monoclonal anti-phosphotyrosine antibody. b. TACE expression in mouse astrocyte cultured in serum-free medium. i: immature form of TACE; m: mature form of TACE. c. Immunofluorescent detection of TGFα in mouse astrocytes cultured in serum-free medium. Bar= 50 μm.
Blockade of erbB1 signaling had no effect on the survival of control or TGFα-treated astrocytes up to 40 days post-irradiation, the longest time point surveyed. To verify whether TGFα promoted DNA double-strand break repair or survival, we added the growth factor to control cultures within 30 minutes after their irradiation. Surprisingly, all cells died within 6 days, demonstrating that TGFα potentiates irradiation-triggered cellular stress.
Taken together, these results indicate that radioresistance of astrocytes, irrespective of whether they have been previously treated with TGFα, is independent from the erbB1-signalling pathway.
Tumoral transformation of irradiated TGFα–treated astrocytes
In contrast with nuclei of sham-irradiated cells that possessed regular contours, nuclei of irradiated TGFα-treated cells exhibited atypia with irregular contours and segmentation (not shown). This observation raised the possibility that irradiation not only resulted in the immortalization of astrocytes de-differentiated into progenitor-like cells by TGFα, but also in their cancerous transformation. To evaluate this hypothesis, we first assayed their ability to grow in an anchorage-independent manner, a common landmark of cells’ cancerous transformation 28. Cells were seeded in culture medium containing 60% methylcellulose, and the formation of colonies was monitored. Numerous large colonies were observed in cultures of irradiated TGFα–treated astrocytes (Fig. 3a), whereas only rare and small colonies <50 μm in diameter were formed by sham-irradiated TGFα-treated cultures (Fig. 3a, insert), as expected for neural progenitors 29. To assess in vivo tumorigenicity, we performed heterotopic and homotopic grafts. We first performed subcutaneous injections of irradiated TGFα–treated cells, growing either anchored to the culture flask or as floating spheres, into the flanks of 6-week-old NOD-SCID mice. Within 2 weeks, tumors became visible in all animals at the sites of injection. After the death of two mice, all the remaining animals were sacrificed 4 weeks post-injection. Dissection showed that all animals had developed tumors that ranged in size from 10–25 mm (Fig. 3b). Most of the tumors had developed beyond the subcutaneous space, and some grew within the peritoneal cavity. Histological analysis showed that the tumors were formed of bundles of spindle-shaped cells, and contained areas of pseudopalissading necrosis (Fig. 3c). They were highly destructive of the host tissues, as illustrated for the dermis (Fig. 3c), the cartilages (Fig. 3d), and the muscles (Fig. 3e). Their histological aspect evoked either a glioblastoma or a gliosarcoma. No difference was noted between tumors generated from anchored or floating cells (not shown). Immunohistological detection of Ki67 (Fig. 3f) revealed a 33% index of proliferation.
Figure 3.
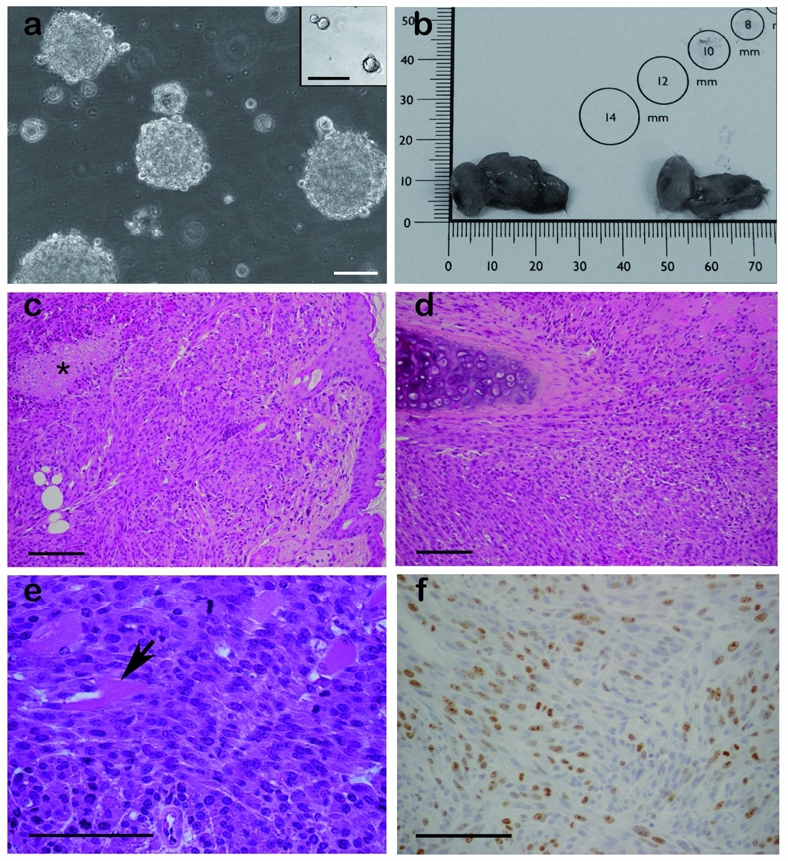
a. Irradiated TGFα-treated astrocyte cultures form large and numerous colonies in semi-solid medium, while their sham-irradiated counterparts form only rare and small colonies (insert). Scale bar = 100 μm. b–f. Subcutaneous tumors derived from irradiated TGFα-treated astrocytes. b. Illustration of the macroscopic appearance of the subcutaneous tumors. c–d. Hematoxylin-eosin staining illustrating the spindle-cell morphology of the tumor cells, and their destruction of the host dermis (c), the cartilages (d), and the muscles (e). Star in c marks an area of necrosis. Arrow in e points to a muscle fiber. f. The tumors contained numerous Ki67-immunoreactive cells. Scale bar = 100 μm in c, d, e and f.
Homotopic grafts were achieved using stereotaxic injections of irradiated TGFα–treated cells growing in the form of floating spheres into the striatum of 6-week-old Nude mice. Striatal grafts of neural stem-like cells and progenitor-like cells derived from TGFα-treated astrocytes were used as controls. Recipients of grafts of neural-stem like cells and progenitor-like cells survived up to 6 weeks post-grafting without displaying any clinical sign. Upon histological examination, cellular grafts were found under the form of small and well-delineated cellular aggregates that remained localized along the injection track (Fig. 4a). In contrast, mice grafted with irradiated TGFα-treated cells displayed reductions in their body weight, and behavioral alterations from around 2 weeks post-graft. All animals were sacrificed at 3–4 weeks post-graft and had developed tumors. Histological analysis showed vast bundles of spindle-shaped cells within the striatum, forming a densely packed cellular tumor (Fig. 4b–d), and patches of spindle-shaped cells could be observed throughout the ipsi-lateral brain, from the thalamus (Fig. 4b) to the olfactory bulb (not shown). Immunochemical analysis was used to determine whether the grafted cells maintained a similar molecular expression profile in vivo as in vitro. We previously reported that astrocytes de-differentiated into progenitor-like cells express markers of neural stem cells or progenitor cells (BLBP, nestin), and half of them express the astroglial marker GFAP 8. In addition, we observed here that neither control nor TGFα–treated astrocytes express NG2, a glycoprotein recently identified in oligodendrocyte precursors 30 (data not shown). Most irradiated TGFα–treated cells maintained GFAP expression in vitro (Fig. 5a). GFAP-immunoreactive cells were observed within the core of the CNS tumors (Fig. 5b and 6a) although the pattern of GFAP-immunostaining showed the presence of reactive astrocytes around the tumor mass (Fig. 5b). Only a fraction of the irradiated TGFα–treated astrocytes retained in vitro expression of BLBP and nestin (Fig. 5c and e), and patches of BLBP- and nestin-immunoreactive cells were observed within the centre of the tumors (Fig. 5d and f). Interestingly, we found that a subset of irradiated TGFα–treated cells had acquired NG2 expression in vitro (Fig. 5g), and some NG2-immunoreactive cells were observed within the brain tumor core (Fig. 5h). Finally, no GFAP-immunoreactive tumor cells were observed in subcutaneous tumors (Supplementary Fig. 1b). Conversely, no cells expressing the neuronal marker synaptophysin were observed in CNS tumors (Supplementary Fig. 1d) while numerous synaptophysin-immunoreactive cells were observed within the subcutaneous tumors (Supplementary Fig. 1c). These data indicate that the environment influences the molecular markers of the tumor cells, without affecting their tumorigenicity.
Figure 4.
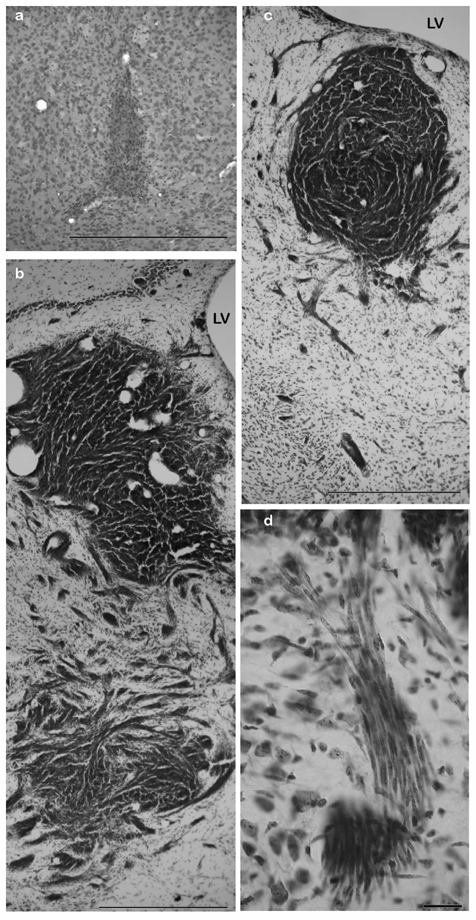
Intra-cerebral tumors derived from irradiated TGFα-treated astrocytes. Cresyl violet staining. a. Striatal grafts of neural stem-like cells derived from TGFα-treated astrocytes do not form tumors. b–c. Examples of tumors derived from irradiated TGFα-treated astrocytes. The tumor cells were spindle-shaped and invaded the host well beyond the transplantation point. LV: lateral ventricle. Scale bar = 500 μm. d. High power view of tumoral cells invading the host tissue at the edge of the tumor. Scale bar = 60 μm.
Figure 5.
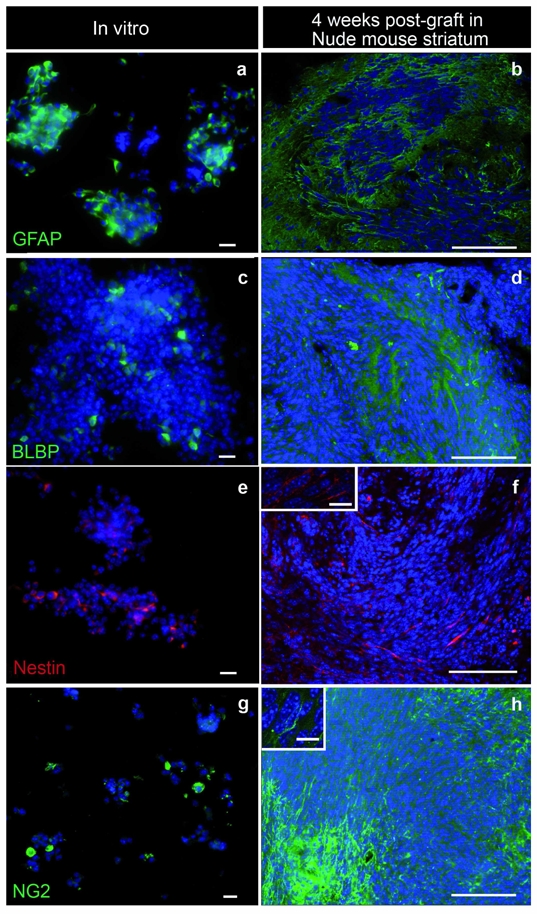
Immunohistochemical characterization of irradiated TGFα-treated astrocytes in vitro and after intra-cerebral grafting. a. Most astrocytes de-differentiated into progenitor-like cells upon TGFα action and transformed by irradiation express the astroglial marker GFAP. b. Patches of GFAP-immunoreactive cells were observed within the core of the CNS tumors. c and e. Part of irradiated TGFα–treated astrocytes express in vitro BLBP and nestin, markers of neural stem cells or progenitor cells. d and f. Patches of BLBP and nestin-immunoreactive cells were observed within the tumor masse. g. A subset of irradiated TGFα–treated cells express NG2, a glycoprotein labeling a population of macroglial cells and of oligodendrocyte progenitors. h. NG2-immunoreactive cells were observed within the brain tumor core. Scale bars = 20 μm in a, c, e, and g; 100 μm in b, d, f and h; 10 μm in the insets of f and h.
Figure 6.
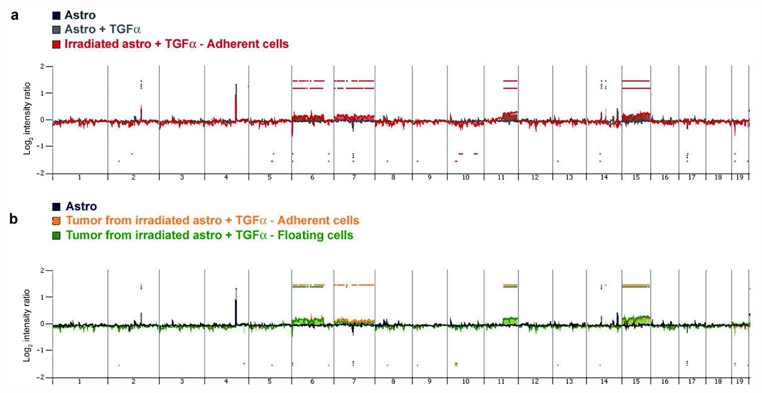
a. Profile of gains and losses of irradiated TGFα-treated astrocytes growing in the form of adherent cells (red), compared with their non-irradiated sister cells (grey), and non-irradiated astrocytes maintained in serum-free medium (black). Shown are log 2 ratios for each chromosome. Non-irradiated astrocytes exhibited the same profile irrespective of exposure to TGFα. Irradiated TGFα-treated astrocytes exhibited gains in chromosomes 6, 7, 11 and 15. b. Profile of gains and losses of subcutaneous tumors derived from irradiated TGFα-treated astrocytes growing in the form of adherent cells (yellow) or floating cells (green).
Taken together, these results demonstrate that irradiation triggered cancerous transformation of astrocytes if they had regressed to a progenitor-like status upon TGFα action.
Cytogenomic abnormalities of transformed TGFα-treated astrocytes
Analysis of the karyotypes at 2 months post-irradiation revealed a hyperploidy in 50% of irradiated TGFα-treated astrocytes (data not shown). A high-resolution oligonucleotide CGH array was used to characterize the cytogenomic alterations of these cells in vitro and in vivo, compared with their non-irradiated sister cells. This analysis was performed on the cells used for subcutaneous grafting, i.e., at 9 months post-irradiation. The detailed results of the analysis are provided in supplementary Table 1. The profile of gains and losses for astrocytes maintained in serum-free medium were highly similar to those for TGFα-treated astrocytes. Both cell types exhibited polymorphisms compared with the control DNA sample provided by the manufacturer, most notably in chromosome 4qD1 (Fig. 6a, supplementary Table 1). A search for genomic imbalances appearing de novo in transformation was performed through the comparison of array CGH patterns of the samples. The profiles of irradiated TGFα-treated astrocytes growing either in the form of adherent (Fig. 6a) or floating cells (not shown) were very similar (Table 1), and differed from non-irradiated cultures most prominently through gains in chromosomes 6qA-E, 7qA-F, 11qB-E and 15qA-F (Fig. 6a and Table 1). Irradiated TGFα-treated astrocytes that grew as floating cells lacked the chromosome 15 and 7qF1-F4 alterations identified in irradiated TGFα-treated astrocytes growing as adherent cells.
Table 1.
Chromosomal copy number alterations. Red and green areas indicate DNA gains and losses, respectively. Aberrations were obtained with ADM2 algorithm and filtering options of a minimum of five probes and log2 (ratio) > 0.25. Genomic coordinates according to the mm9 build (July 2007) and values in log2 (ratio).
| Genomic coordonates (mm9) | chromosome | band | ASTRO | ASTRO + TGFα | IRRADIATED ASTRO + TGFα ADHERENT CELLS | IRRADIATED ASTRO + TGFα FLOATING CELLS | TUMOR 1 FROM IRRADIATED ASTRO + TGFα ADHERENT CELLS | TUMOR 2 FROM IRRADIATED ASTRO + TGFα ADHERENT CELLS | TUMOR 3 FROM IRRADIATED ASTRO + TGFα ADHERENT CELLS | TUMOR 4 FROM IRRADIATED ASTRO + TGFα ADHERENT CELLS | TUMOR 5 FROM IRRADIATED ASTRO + TGFα ADHERENT CELLS | TUMOR 1 FROM IRRADIATED ASTRO + TGFα FLOATING CELLS | TUMOR 2 FROM IRRADIATED ASTRO + TGFα FLOATING CELLS | TUMOR 3 FROM IRRADIATED ASTRO + TGFα FLOATING CELLS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| chr2:81139123-101306651 | 2 | 2qD-E1 | 0 | 0 | −0.28 | 0 | − 0.25 | − 0.26 | 0 | 0 | 0 | − 0.27 | 0 | 0 |
| chr2:115846031-123819240 | 2 | 2qE5 | 0.84 | 0.86 | 1.04 | 0.92 | 0.78 | 0.87 | 0.83 | 0.82 | 0.78 | 0.69 | 0.78 | 0.84 |
| chr3:83835375-93096260 | 3 | 3qF1 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr4:109852334-116510051 | 4 | 4qD1 | 2.65 | 2.63 | 2.38 | 2.42 | 2.12 | 2.44 | 0 | 0 | 2.25 | 2.19 | 2.46 | 0 |
| chr4:132678793-140287612 | 4 | 4qD3 | 0 | 0 | 0 | 0 | −0.54 | −0.47 | −0.58 | 0 | −0.49 | −0.43 | −0.49 | −0.55 |
| chr4:140287612-146469778 | 4 | 4qE1 | 0 | 0 | 0 | 0.48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr4:146469778-155029701 | 4 | 4qE2 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr5:81773799-91394246 | 5 | 5qE1 | 0 | 0 | −0.26 | 0 | −0.25 | −0.28 | 0 | 0 | −0.24 | 0 | 0 | 0 |
| chr6:0-16613965 | 6 | 6qA1 | 0 | 0 | −1.02 | −1.03 | −0.77 | −0.85 | −0.79 | −0.84 | −0.81 | −0.61 | −0.77 | −0.85 |
| chr6:16613965-116786401 | 6 | 6qA2-E3 | 0 | 0 | 0.29 | 0.43 | 0.33 | 0.36 | 0.37 | 0.38 | 0.36 | 0.29 | 0.35 | 0.39 |
| chr6:125093383-139264118 | 6 | 6qF3-G1 | 0 | 0 | −0.27 | −0.27 | −0.31 | −0.31 | −0.25 | 0 | −0.25 | −0.24 | 0 | 0 |
| chr7:0-15170811 | 7 | 7qA1 | 0 | 0 | 0.3 | 0.3 | 0.26 | 0.24 | 0.34 | 0.3 | 0.28 | 0 | 0.22 | 0 |
| chr7:15170811-37421334 | 7 | 7qA2-B2 | 0 | 0 | 0.36 | 0.45 | 0.26 | 0.31 | 0.34 | 0.3 | 0.28 | 0 | 0.28 | 0.25 |
| chr7:37421334-47535208 | 7 | 7qB3 | 0 | 0 | 0.43 | 0.45 | 0.33 | 0.31 | 0.34 | 0.36 | 0.34 | 0 | 0.28 | 0.25 |
| chr7:47535208-54109226 | 7 | 7qB4 | 0 | 0 | 0.3 | 0.3 | 0.26 | 0 | 0.34 | 0 | 0 | 0 | 0 | 0 |
| chr7:54109226-60683244 | 7 | 7qB5 | 0 | 0 | 0.3 | 0.3 | 0 | 0 | 0.34 | 0 | 0 | 0 | 0 | 0 |
| chr7:60683244-71302812 | 7 | 7qC | −0.72 | 0.3 | −0.68 | −0.4 | 0.26 | −0.42 | 0.34 | −0.36 | −0.4 | −0.69 | −0.45 | −0.5 |
| chr7:71302812-90013479 | 7 | 7qD1-D3 | 0 | 0 | 0.3 | 0.22 | 0.26 | 0.24 | 0.34 | 0.3 | 0.28 | 0 | 0 | 0 |
| chr7:90013479-102150128 | 7 | 7qE1-E2 | 0 | 0 | 0.3 | 0.22 | 0.26 | 0 | 0.34 | 0.3 | 0.28 | 0 | 0 | 0 |
| chr7:102150128-111252615 | 7 | 7qE3 | 0 | 0 | 0.3 | 0.22 | 0.26 | 0.24 | 0.34 | 0.3 | 0.28 | 0 | 0.22 | 0 |
| chr7:111252615-145134094 | 7 | 7qF1-F5 | 0 | 0 | 0.3 | 0 | 0.26 | 0 | 0.34 | 0.3 | 0.28 | 0 | 0 | 0 |
| chr9:14343360-24059831 | 9 | 9qA2-A3 | 0 | 0 | 0 | 0 | −0.32 | −0.33 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr10:23539770-41194597 | 10 | 10qA4-B1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.27 | −0.27 | 0 |
| chr10:41194597-63753544 | 10 | 10qB2-B4 | 0 | 0 | −0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr10:95630316-111323496 | 10 | 10qC3-D1 | 0 | 0 | −0.28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr11:62758837-121798632 | 11 | 11qB3-E2 | 0 | 0 | 0.52 | 0.5 | 0.42 | 0.45 | 0.43 | 0.44 | 0.43 | 0.37 | 0.42 | 0.45 |
| chr14:43144646-51575209 | 14 | 14qC1 | 0 | 0 | −0.24 | 0 | −0.31 | −0.32 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr14:51575209-54550702 | 14 | 14qC2 | 0.62 | 0.63 | 0.56 | 0.55 | 0.53 | 0.51 | 0.55 | 0.55 | 0.57 | 0.5 | 0.56 | 0.58 |
| chr14:59509857-68436336 | 14 | 14qD1 | 0 | 0.83 | 0.36 | 0.44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| chr15:0-66107593 | 15 | 15qA1-D1 | 0 | 0 | 0.35 | 0 | 0.31 | 0.35 | 0.39 | 0.43 | 0.36 | 0.31 | 0.43 | 0.52 |
| chr15:66107593-103492577 | 15 | 15qD2-F3 | 0 | 0 | 0.43 | 0 | 0.39 | 0.44 | 0.39 | 0.43 | 0.42 | 0.39 | 0.49 | 0.59 |
| chr17:21829683-31434744 | 17 | 17qA3.3 | −0.5 | −0.54 | −0.56 | −0.51 | −0.53 | −0.49 | −0.53 | −0.49 | −0.53 | −0.49 | −0.53 | −0.56 |
| chr19:0-16650124 | 19 | 19qA | 0 | 0 | −0.99 | −0.99 | −0.67 | −0.7 | −0.76 | −0.81 | −0.73 | 0.3 | −0.67 | −0.78 |
| chr19:38173456-61321190 | 19 | 19qC3-D3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.21 | 0 | 0 |
The array CGH profiles of the different subcutaneous tumors derived from irradiated TGFα-treated astrocytes were overall comparable, and similar to the profile of their cells of origin. One marked exception was the segmental chromosomal copy number alteration (CNA) on chromosome 15; absent in irradiated TGFα-treated astrocytes growing as floating cells, it was acquired in all tumors derived from these cells (n=3). In addition, one tumor derived from irradiated TGFα-treated astrocytes growing as floating cells lacked CNAs on chromosome 7, whereas 2 other tumors exhibited CNAs only on chromosome 7qA1-B3 (Table 1). The profiles of tumors generated from TGFα-treated astrocytes growing as adherent cells exhibited a remarkable degree of similarity (Table 1). The most notable variation was seen in the 7qF1-F5 CNAs, present in irradiated TGFα-treated astrocytes growing as adherent cells and in all but one tumor generated from these cells (n=5).
These results show that irradiation-induced cancerous transformation of TGFα-treated astrocytes was accompanied by the acquisition of several CNAs, among which those located on chromosomes 6, 7 and 11 were conserved among all irradiated cells in vitro, and all tumors in vivo. Interestingly, gains in chromosome 6 and 11 segments encompassed the loci of the TGFα gene and the erbB2 proto-oncogene, an orphan receptor of the erbB1 family, respectively. No segmental alterations were observed in gene loci encoding other members of the EGF and erbB families, erbB1 included. Additional molecular and pharmacologic studies are required to determine which molecular pathways are instrumental in radiation-induced neoplastic transformation of these cells, and in the maintenance of their tumorigenicity.
Discussion
Our results demonstrate that, unlike mature astrocytes, those that revert to neural progenitor-like cells upon TGFα action can be submitted to cancerous transformation.
TGFα has been reported to induce cancerous transformation of cultured fibroblasts 31, renal epithelial cells 32, and mammary epithelial cells 33. Transgenic mice over-expressing TGFα develop, late in their life, malignant tumors in the liver and the mammary glands 7, 34–36. However, sustained activation of the erbB1 signaling module is insufficient to trigger astrocyte neoplasia, unless astrocytes bear mutations in other oncogenes or tumor suppressor genes. For instance, primary cultures of astrocytes derived from P53−/− or P16/P19−/− mice, are immortal but not tumorigenic, but may progress to malignant transformation upon treatment with EGF, the structural and functional analogue of TGFα 12, 37, 38. In agreement with these results, we observed no sign of tumoral transformation of astrocytes either in vitro 8 or in vivo after intra-cerebral grafts (Fig. 4a), despite several months of continuous exposure to TGFα. In addition, we observed that TGFα-treated astrocytes are able to engage into a neuronal differentiation pathway in vivo (data not shown) as they do in vitro 8. Finally, the genomic profiles of astrocytes maintained in serum-free medium are the same as those of astrocytes treated with TGFα.
Our results show that astrocytes survive irradiation regardless of their exposure to TGFα. Several studies demonstrated the participation of erbB1 activation in the cellular defense system against ionizing radiation, and clinical results indicate that erbB1 inhibition sensitizes different solid human tumors, including gliomas, to radiation 39, 40. ErbB1 activation may be achieved within minutes via radiation-induced reactive oxygen/nitrogen species that induce phosphorylation of erbB1 41–43, and within 2 hours via increased proteolytic cleavage of the TGFα precursor 26, resulting in the mobilization of intra-cellular pathways that promote cell survival and DNA repair 44, 45. Although we ascertained that the astrocytes used in the present study express all the molecular components required for a functional endogenous TGFα-erbB1 signaling module, their radioresistance appears independent from erbB1 activity. Indeed, survival of irradiation is unaltered by the blockade of erbB1 tyrosine kinase activity within 4 hours of irradiation.
Although astrocytes resisted radiation, they demonstrated limited growth potential and entered into a senescence-like state 2–3 weeks post-irradiation, like their sham-irradiated counterparts. Addition of TGFα to control astrocytes after their irradiation did not promote their survival or cancerous transformation, but, on the contrary, induced their death. In contrast, irradiation of astrocytes reverted to progenitor-like cells upon TGFα action resulted in their immortalization and cancerous transformation. Sham-irradiated TGFα-treated cultures survived much longer in their original dish than untreated astrocytes, as previously reported 8, but they did not sustain successive passages, indicating that TGFα treatment is insufficient to trigger astrocyte immortalization.
Cancerous transformation was ascertained by the cells’ ability to form colonies in methylcellulose medium, their karyotype anomalies, and, most importantly, by the formation of subcutaneous tumors in NOD-SCID mice and high-grade glioma-like tumors in Nude mouse CNS. In both graft paradigms, the histological aspect of the tumors evoked either a glioblastoma or a gliosarcoma. Immunological characterization of the transformed cells in vitro and in brain grafts revealed, overall, a very immature phenotype, except for GFAP expression that was, at least in vitro, observed in most cells. Only a fraction of the cancerously transformed cells retained neural progenitor markers like BLBP and nestin, which were expressed in the vast majority of non-irradiated TGFα-treated astrocytes 8. In contrast, some had acquired the expression of NG2, a proteoglycan frequently associated with cancerous cells in glioma 46, and involved in chemoresistance 47 and cell motility 48. The high similarity of the genomic profiles of different subcutaneous tumors and the profile of their cells of origin, associated with their rapid development within 2–3 weeks in both graft paradigms, shows that the cells’ tumorigenicity is independent of secondary alterations selected in vivo. In addition, the lack of genomic anomalies in TGFα-treated astrocytes prior to their irradiation shows that TGFα does not predispose the cells to cancerous transformation through the acquisition of an aneuploid state, a phenomenon recently shown to occur in a transient manner during normal differentiation of neural stem cells of the mouse sub-ventricular zone 49.
Conclusion
Our results show that regression of astrocytes to a progenitor-like stage sensitizes the cells to oncogenic events. Immediate exposure of irradiated-astrocytes to TGFα kills them instead of promoting their cancerous transformation. This further shows that the change in the differentiation status of the astrocytes is a prerequisite condition to their cancerous transformation. Whether this sensitization is of the same magnitude for “artificial” and “natural” progenitors remains to be determined. It is indeed possible to envisage that de-differentiated cells possess particular chromatin states and/or inappropriate DNA repair and apoptosis pathways. The present work indicates that alteration of the differentiation state of mature cells could be at the core of glioma development, although it does not allow eliminating neural stem or progenitor cells as parallel cells of origin of these primitive brain tumors.
In any case, our results suggest that the frequent TGFα over-expression observed at the early stages of glioma development testifies to a participation of this growth factor in the earliest pathological events that result in glioma induction.
Supplementary Material
Acknowledgments
This research was supported by ARC (grants 3500, 3972, 3131), the Fondation pour la Recherche Médicale, and the Association pour la Recherche dans les Tumeurs Cérébrales (study fellowships to CD), CAPES-COFECUB (CAPES study fellowship to GPF), and Inserm.
We are grateful to Dr. Heintz for his generous gift of BLBP antibodies, Sophie Daviel and the Curie Institute for providing access to gamma irradiation facilities, and Dr Dutrillaux for helpful discussion. This research was supported by ARC (grants 3500, 3972, 3131), the Fondation pour la Recherche Médicale, and the Association pour la Recherche dans les Tumeurs Cérébrales (study fellowships to CD), CAPES-COFECUB (CAPES study fellowship to GPF), and Inserm.
Footnotes
Authors’ contribution
Dufour C: collection and/or assembly of data, data analysis and interpretation, manuscript writing
Cadusseau J: collection and/or assembly of data, data analysis and interpretation
Varlet P: data analysis and interpretation
Surena AL: collection and/or assembly of data, data analysis and interpretation
de Faria GP: collection and/or assembly of data,
Dias-Morais A: collection and/or assembly of data
Auger N: collection and/or assembly of data, data analysis and interpretation
Léonard N: collection and/or assembly of data
Daudigeos E: collection and/or assembly of data
Dantas-Barbosa C: collection and/or assembly of data
Grill J: financial support, data analysis and interpretation
Lazar V: collection and/or assembly of data
Dessen P: data analysis and interpretation
Vassal G: financial support, administrative support
Prevot V: collection and/or assembly of data, data analysis and interpretation
Sharif A: collection and/or assembly of data, data analysis and interpretation
Chneiweiss H: financial support, manuscript writing, conception and design
Junier MP: conception and design, manuscript writing, collection and/or assembly of data, data analysis and interpretation
References
- 1.Collins VP. Brain tumours: classification and genes. J Neurol Neurosurg Psychiatry. 2004 Jun;75(Suppl 2):ii2–11. doi: 10.1136/jnnp.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004 Sep 20;23(43):7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 3.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004 Oct 1;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 4.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005 Aug 25;353(8):811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 5.Shih AH, Holland EC. Developmental neurobiology and the origin of brain tumors. J Neurooncol. 2004 Nov;70(2):125–136. doi: 10.1007/s11060-004-2746-3. [DOI] [PubMed] [Google Scholar]
- 6.Harris H. Tumour suppression: putting on the brakes. Nature. 2004 Jan 15;427(6971):201. doi: 10.1038/427201a. [DOI] [PubMed] [Google Scholar]
- 7.Lee DC, Fenton SE, Berkowitz EA, Hissong MA. Transforming growth factor alpha: expression, regulation, and biological activities. Pharmacol Rev. 1995 Mar;47(1):51–85. [PubMed] [Google Scholar]
- 8.Sharif A, Legendre P, Prevot V, et al. Transforming growth factor alpha promotes sequential conversion of mature astrocytes into neural progenitors and stem cells. Oncogene. 2007 Apr 26;26(19):2695–2706. doi: 10.1038/sj.onc.1210071. [DOI] [PubMed] [Google Scholar]
- 9.Junier MP. What role(s) for TGFalpha in the central nervous system? Prog Neurobiol. 2000 Dec;62(5):443–473. doi: 10.1016/s0301-0082(00)00017-4. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- 11.Rabchevsky AG, Weinitz JM, Coulpier M, Fages C, Tinel M, Junier MP. A role for transforming growth factor alpha as an inducer of astrogliosis. J Neurosci. 1998 Dec 15;18(24):10541–10552. doi: 10.1523/JNEUROSCI.18-24-10541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002 Apr;1(3):269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008 Dec 4;3(6):595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Buffo A, Rite I, Tripathi P, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevot V, Lomniczi A, Corfas G, Ojeda SR. erbB-1 and erbB-4 receptors act in concert to facilitate female sexual development and mature reproductive function. Endocrinology. 2005 Mar;146(3):1465–1472. doi: 10.1210/en.2004-1146. [DOI] [PubMed] [Google Scholar]
- 17.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005 Jan;6(1):32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 18.Surena AL, de Faria GP, Studler JM, et al. DLG1/SAP97 modulates transforming growth factor alpha bioavailability. Biochim Biophys Acta. 2009 Feb;1793(2):264–272. doi: 10.1016/j.bbamcr.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- 20.Sharif A, Prevot V, Renault-Mihara F, et al. Transforming growth factor alpha acts as a gliatrophin for mouse and human astrocytes. Oncogene. 2006 Jul 6;25(29):4076–4085. doi: 10.1038/sj.onc.1209443. [DOI] [PubMed] [Google Scholar]
- 21.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, USA: Academic Press; 1997. [Google Scholar]
- 22.Boillee S, Cadusseau J, Coulpier M, Grannec G, Junier MP. Transforming growth factor alpha: a promoter of motoneuron survival of potential biological relevance. J Neurosci. 2001 Sep 15;21(18):7079–7088. doi: 10.1523/JNEUROSCI.21-18-07079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharif A, Renault F, Beuvon F, et al. The expression of PEA-15 (phosphoprotein enriched in astrocytes of 15 kDa) defines subpopulations of astrocytes and neurons throughout the adult mouse brain. Neuroscience. 2004;126(2):263–275. doi: 10.1016/j.neuroscience.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003 Sep;28(9):509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 25.Contessa JN, Reardon DB, Todd D, et al. The inducible expression of dominant-negative epidermal growth factor receptor-CD533 results in radiosensitization of human mammary carcinoma cells. Clin Cancer Res. 1999 Feb;5(2):405–411. [PubMed] [Google Scholar]
- 26.Dent P, Reardon DB, Park JS, et al. Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999 Aug;10(8):2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reardon DB, Contessa JN, Mikkelsen RB, et al. Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene. 1999 Aug 19;18(33):4756–4766. doi: 10.1038/sj.onc.1202849. [DOI] [PubMed] [Google Scholar]
- 28.Cooper GM, Okenquist S, Silverman L. Transforming activity of DNA of chemically transformed and normal cells. Nature. 1980 Apr 3;284(5755):418–421. doi: 10.1038/284418a0. [DOI] [PubMed] [Google Scholar]
- 29.Louis SA, Rietze RL, Deleyrolle L, et al. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008 Apr;26(4):988–996. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- 30.Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005 Dec;207(6):707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todaro GJ, Fryling C, De Larco JE. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe S, Lazar E, Sporn MB. Transformation of normal rat kidney (NRK) cells by an infectious retrovirus carrying a synthetic rat type alpha transforming growth factor gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1258–1262. doi: 10.1073/pnas.84.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shankar V, Ciardiello F, Kim N, et al. Transformation of an established mouse mammary epithelial cell line following transfection with a human transforming growth factor alpha cDNA. Mol Carcinog. 1989;2(1):1–11. doi: 10.1002/mc.2940020102. [DOI] [PubMed] [Google Scholar]
- 34.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990 Jun 15;61(6):1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 35.Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 36.Matsui Y, Halter SA, Holt JT, Hogan BL, Coffey RJ. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990 Jun 15;61(6):1147–1155. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- 37.Yahanda AM, Bruner JM, Donehower LA, Morrison RS. Astrocytes derived from p53-deficient mice provide a multistep in vitro model for development of malignant gliomas. Mol Cell Biol. 1995 Aug;15(8):4249–4259. doi: 10.1128/mcb.15.8.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogler O, Nagane M, Gillis J, Huang HJ, Cavenee WK. Malignant transformation of p53-deficient astrocytes is modulated by environmental cues in vitro. Cell Growth Differ. 1999 Feb;10(2):73–86. [PubMed] [Google Scholar]
- 39.Baumann M, Krause M, Dikomey E, et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol. 2007 Jun;83(3):238–248. doi: 10.1016/j.radonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Geoerger B, Gaspar N, Opolon P, et al. EGFR tyrosine kinase inhibition radiosensitizes and induces apoptosis in malignant glioma and childhood ependymoma xenografts. Int J Cancer. 2008 Jul 1;123(1):209–216. doi: 10.1002/ijc.23488. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Ullrich RK, Contessa JN, Dent P, et al. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat Oncol Investig. 1999;7(6):321–330. doi: 10.1002/(SICI)1520-6823(1999)7:6<321::AID-ROI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 42.Dent P, Yacoub A, Contessa J, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003 Mar;159(3):283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Sturla LM, Amorino G, Alexander MS, Mikkelsen RB, Valerie K, Schmidt-Ullrichr RK. Requirement of Tyr-992 and Tyr-1173 in phosphorylation of the epidermal growth factor receptor by ionizing radiation and modulation by SHP2. J Biol Chem. 2005 Apr 15;280(15):14597–14604. doi: 10.1074/jbc.M413287200. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt MH, Furnari FB, Cavenee WK, Bogler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc Natl Acad Sci U S A. 2003 May 27;100(11):6505–6510. doi: 10.1073/pnas.1031790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szumiel I. Epidermal growth factor receptor and DNA double strand break repair: the cell’s self-defence. Cell Signal. 2006 Oct;18(10):1537–1548. doi: 10.1016/j.cellsig.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Shoshan Y, Nishiyama A, Chang A, et al. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10361–10366. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chekenya M, Krakstad C, Svendsen A, et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008 Sep 4;27(39):5182–5194. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burg MA, Nishiyama A, Stallcup WB. A central segment of the NG2 proteoglycan is critical for the ability of glioma cells to bind and migrate toward type VI collagen. Exp Cell Res. 1997 Aug 25;235(1):254–264. doi: 10.1006/excr.1997.3674. [DOI] [PubMed] [Google Scholar]
- 49.Walton NM, Snyder GE, Park D, Kobeissy F, Scheffler B, Steindler DA. Gliotypic Neural Stem Cells Transiently Adopt Tumorigenic Properties During Normal Differentiation. Stem Cells. 2008 Nov 6; doi: 10.1634/stemcells.2008-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


