Abstract
Increased sea-surface temperatures linked to warming climate threaten coral reef ecosystems globally. To better understand how corals and their endosymbiotic dinoflagellates (Symbiodinium spp.) respond to environmental change, tissue biomass and Symbiodinium density of seven coral species were measured on various reefs approximately every four months for up to thirteen years in the Upper Florida Keys, United States (1994–2007), eleven years in the Exuma Cays, Bahamas (1995–2006), and four years in Puerto Morelos, Mexico (2003–2007). For six out of seven coral species, tissue biomass correlated with Symbiodinium density. Within a particular coral species, tissue biomasses and Symbiodinium densities varied regionally according to the following trends: Mexico≥Florida Keys≥Bahamas. Average tissue biomasses and symbiont cell densities were generally higher in shallow habitats (1–4 m) compared to deeper-dwelling conspecifics (12–15 m). Most colonies that were sampled displayed seasonal fluctuations in biomass and endosymbiont density related to annual temperature variations. During the bleaching episodes of 1998 and 2005, five out of seven species that were exposed to unusually high temperatures exhibited significant decreases in symbiotic algae that, in certain cases, preceded further decreases in tissue biomass. Following bleaching, Montastraea spp. colonies with low relative biomass levels died, whereas colonies with higher biomass levels survived. Bleaching- or disease-associated mortality was also observed in Acropora cervicornis colonies; compared to A. palmata, all A. cervicornis colonies experienced low biomass values. Such patterns suggest that Montastraea spp. and possibly other coral species with relatively low biomass experience increased susceptibility to death following bleaching or other stressors than do conspecifics with higher tissue biomass levels.
Introduction
Coral reefs are highly diverse, productive, and economically-important ecosystems found in shallow marine environments throughout the world's tropical and sub-tropical oceans. Currently, populations of reef-building corals are in global decline due to increased sea-surface temperatures associated with climate change [1]. The worldwide degradation of coral reefs has been further exacerbated at local and regional scales by an array of anthropogenic stressors, such as nutrient inputs, overfishing, destructive fishing practices, exotic species introductions, increased sedimentation and pollution [2], [3]. As a result of this ecological crisis, there is an urgent need to understand how acute and chronic stressors affect the physiological integrity of reef-building corals.
Perhaps the greatest of the problems facing reefs today is the warming of sea-surface temperatures that, in conjunction with intense solar radiation, causes widespread coral bleaching and mortality [1]. Bleaching is defined as the loss of endosymbiotic dinoflagellates in the genus Symbiodinium (a genetically diverse group that is commonly known as zooxanthellae) from the coral's tissue, but it may also include loss of pigments by the endosymbiont and/or the host [4]. Coral bleaching is often associated with El Niño Southern Oscillation (ENSO) events and climate change [1]. Because reef-building corals receive energy from their Symbiodinium communities [5], severe bleaching often results in coral colony death through starvation or disease [1], [6], [7]. For example, an intense ENSO event in 1997–1998 led to loss of approximately 16% of coral reefs globally, including corals in the Caribbean Sea [8].
Surveys of mass bleaching events have documented differential mortality among coral colonies and species [6], [9]. In some cases, neighboring colonies of the same coral species were differentially affected by bleaching [10]. The factors underlying differential mortality of corals under seemingly identical conditions of depth and reef position remain poorly understood. It has been proposed that this response is due to a combination of genotypic and physiological variation among coral colonies [11] and/or their endosymbionts [12], [13]. One additional factor affecting post-bleaching mortality is a colony's level of energy reserves, which is correlated with tissue biomass or ash-free dry weight (AFDW).
Coral colony biomass is the product of a complex array of factors. Reef-building corals obtain energy from polytrophic sources, including photosynthetic endosymbionts, heterotrophic feeding, and absorption of dissolved organic matter (DOM), and this energy is allocated to metabolism, reproduction, skeletal growth (i.e., calcification), mucus production, tissue repair and defense, and production of new biomass (e.g., tissue growth and energy reserves) [14]–[16]. A colony's biomass level thus depends upon the equilibrium between various energetic sources vs. expenditures and is subject to dynamic change over time. Such change can be observed in the fluctuations of coral tissue biomass and Symbiodinium population density in response to seasonal variation in temperature and light, with both parameters reaching annual nadir during the hottest times of the year [17], [18]. Tissue biomass and Symbiodinium density are further depleted during mass bleaching events [18], which jeopardize colony survival due to corals' reliance on energy reserves for sustenance during stress. If biomass reserves and energy inputs from autotrophic symbionts, heterotrophic feeding, and absorption are insufficient, then a colony may perish from starvation or due to secondary stressors (e.g., disease).
Based on the above, colony biomass may be an indicator of coral health and resilience to stress. If this hypothesis is correct, biomass differences among colonies and species across a coral reef could explain inter-colony differences in bleaching mortality. Here, we examine this hypothesis and add additional perspectives on coral physiology and the bleaching phenomenon from long-term analysis of tissue biomass and the density of endosymbionts in seven species of reef-building corals. Corals were collected from nine different reefs spanning three geographic regions from a time span of 3–13 years (Fig. 1, Table 1). Our goals were to 1) examine the relationship between tissue biomass and Symbiodinium density, 2) determine how these two parameters vary spatially (i.e., by region, habitat, and depth) and temporally (i.e., season), 3) assess changes in these two parameters following two bleaching events (1998 and 2005), and 4) determine the relationship between coral tissue biomass and colony mortality.
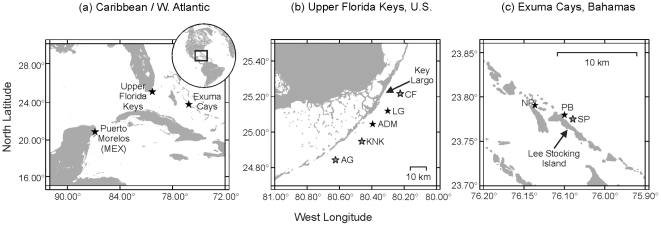
Figure 1. Regional and local maps of collection sites for this study.
Coral collection depth is indicated in panels b and c by star shading; shallow reefs (1–4 m) are indicted by a black star whereas deep reefs (12–15 m) are denoted by a gray star. Reef abbreviations are as follows: Upper Florida Keys, U.S.: CF = Carysfort Reef, LG = Little Grecian Reef, ADM = Admiral Patch Reef, KNK = Conch Reef, AG = Alligator Reef; Exuma Cays, Bahamas: NP = North Norman's Patch Reef, PB = Palmata Beach Reef, SP = South Perry Reef; Puerto Morelos, Mexico: MEX = UNAM. Figure adapted from Thornhill et al. [20].
Table 1. Species of corals sampled at each reef in this study.
| Region | Reef | Latitude | Longitude | Depth | Coral Species | Duration Sampled |
| Exuma Cays, Bahamas | Norman's Pond (NP) | 23.79°N | 76.14°W | 0–4 m | M. annularis | 1995–2006 |
| M. faveolata | 1995–2006 | |||||
| A. cervicornis | 2001–2006 | |||||
| P. astreoides | 2002–2003 | |||||
| S. siderea | 2002–2005 | |||||
| Palmata Beach (PB) | 23.78°N | 76.10°W | 0–4 m | A. cervicornis | 1996–2005 | |
| A. palmata | 1995–2005 | |||||
| South Perry (SP) | 23.77°N | 76.09°W | 12–15 m | M. annularis | 1995–2006 | |
| M. faveolata | 1995–2006 | |||||
| M. franski | 1997–2006 | |||||
| A. cervicornis | 1996–2006 | |||||
| Upper Florida Keys, USA | Little Grecian Reef (LG) | 25.12°N | 80.30°W | 0–4 m | M. annularis | 1999–2007 |
| M. faveolata | 1999–2007 | |||||
| A. cervicornis | 1996–2007 | |||||
| A. palmata | 1996–2007 | |||||
| S. siderea | 2002–2007 | |||||
| Admiral Reef (ADM) | 25.05°N | 80.39°W | 0–4 m | M. annularis | 1995–2007 | |
| M. faveolata | 1994–2007 | |||||
| A. cervicornis | 1996–2007 | |||||
| P. astreoides | 2002–2007 | |||||
| S. siderea | 2002–2007 | |||||
| Carysfort Reef (CF) | 25.22°N | 80.23°W | 12–15 m | M. annularis | 1995–1999 | |
| M. faveolata | 1996–1999 | |||||
| M. franski | 1997–1999 | |||||
| A. cervicornis | 1996–1998 | |||||
| Conch Reef (KNK) | 24.94°N | 80.46°W | 12–15 m | M. annularis | 1999–2001 | |
| M. faveolata | 2000–2002 | |||||
| M. franksi | 1999–2002 | |||||
| Alligator (AG) | 24.84°N | 80.62°W | 12–15 m | M. annularis | 2003–2007 | |
| M. faveolata | 2003–2007 | |||||
| M. franksi | 1997–2007 | |||||
| A. cervicornis | 2003–2005 | |||||
| Puerto Morelos, Mexico | UNAM (MEX) | 20.87°N | 86.85°W | 0–4 m | M. annularis | 2003–2007 |
| M. faveolata | 2003–2007 | |||||
| P. astreoides | 2003–2007 |
Results
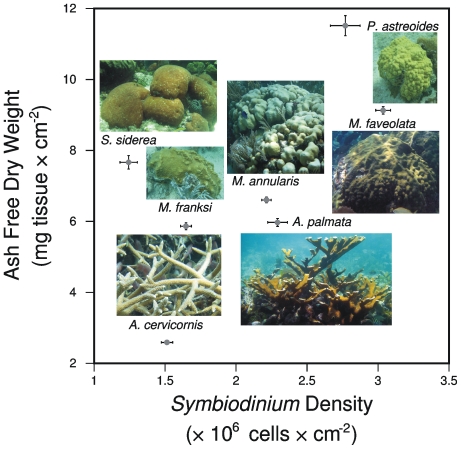
Each coral species exhibited a species-specific mean tissue biomass and symbiont density value (Fig. 2). Porites astreoides had the highest mean coral tissue biomass (11.52±0.28 mg tissue×cm−2; data presented as mean ± SE throughout this manuscript), whereas the lowest biomass was found in Acorpora cervicornis (2.59±0.05 mg tissue×cm−2). The highest and lowest Symbiodinium densities occurred in Montastraea faveolata (3.04±0.05 cells×106×cm−2) and Siderastrea siderea (1.24±0.06 cells×106×cm−2), respectively. Data for the remaining coral species fell between these extremes of ash free dry weight and symbiont density (Fig. 2).
Figure 2. Species-specific values for tissue biomass versus Symbiodinium density from seven species of scleractinian corals.
Biomass was measured by ash-free dry weight in mg tissue×cm−2 whereas Symbiodinium density was quantified as cells×106×cm−2. Data presented as mean values ± standard error. A photograph of each coral species is provided for reference. Photographs are not to scale. Photo credits: D.J. Thornhill, G.C. Chilcoat, and D.W. Kemp.
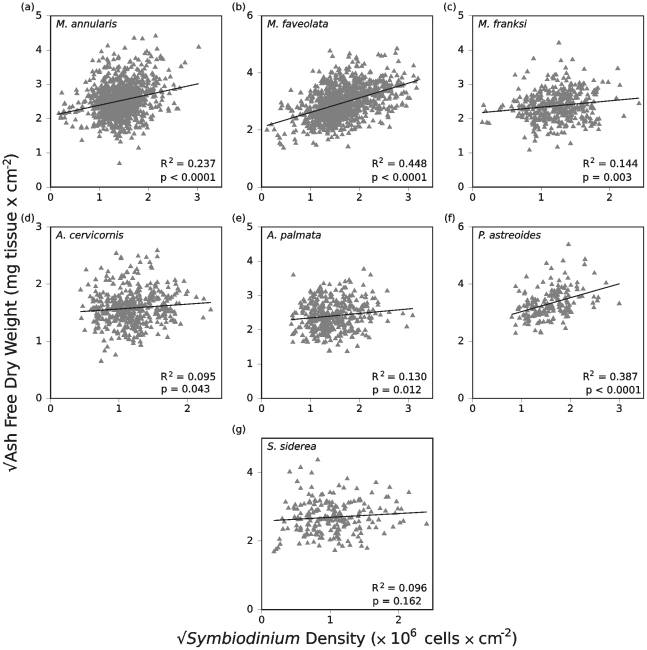
For six of the seven reef-building coral species, tissue biomass and Symbiodinium density were positively and significantly correlated with one another (Fig. 3). The exception was S. siderea, where biomass and symbiont density were not correlated (r = 0.096, p = 0.162, Fig. 3g). Among the remaining six species, the degree of correlation between the two parameters varied considerably (r = 0.095–0.448). The correlation between tissue biomass and Symbiodinium density was strongest for M. faveolata (r = 0.448, p<0.0001) and P. astreoides (r = 0.387, p<0.0001), whereas the relationship was considerably weaker in A. cervicornis (r = 0.095, p = 0.043) relative to the other five coral species (Fig. 3). The remaining three species had correlations of intermediate strength, including A. palmata (r = 0.130, p = 0.012), M. annularis (r = 0.237, p<0.0001), and M. franksi (r = 0.144, p = 0.003).
Figure 3. Scatter plots depicting the relationship between tissue biomass versus Symbiodinium density for seven reef-building corals.
Data have been square root transformed in order to meet normality assumptions and a trend line has been fitted to the transformed dataset for each species. Coral species identity (upper left), degree of correlation (R2 value, lower right), and p value (lower right) are provided for each species.
In all seven coral species, the correlation coefficients suggested that much of the relationship between tissue biomass and Symbiodinium density was left unexplained. In the results that follow we examine other factors that affect this variation by examining how tissue biomass and endosymbiont density vary spatially among regions, depths and reefs, temporally across seasons, following two mass bleaching events, and in relation to coral colony mortality.
Regional and depth patterns
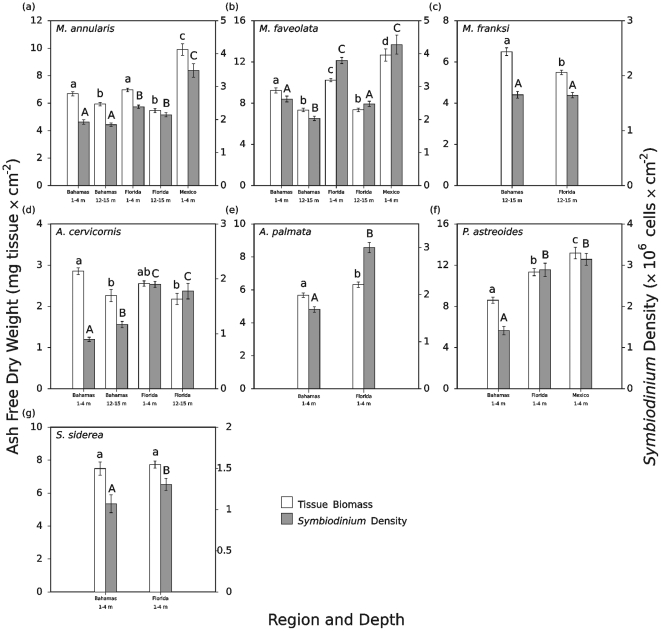
Patterns of coral tissue biomass and symbiont density varied geographically among the Bahamas, the Florida Keys (U.S.), and Mexico for many of the coral species examined (Fig. 4). For example, at shallow depths (1–4 m), corals from the Bahamas often had lower mean tissue biomass and/or Symbiodinium density values than conspecifics from other regions (Fig. 4). This pattern occurred in the endosymbiont cell densities for M. annularis (p values<0.0001), A. cervicornis (p<0.0001), and S. siderea (p = 0.039, two-sample t-test), as well as in both parameters for M. faveolata (p values<0.0001), A. palmata (p values≤0.004, two-sample t-test), and P. astreoides (p values<0.0001) (Fig. 4, unless otherwise noted, regional and depth comparisons and hypotheses testing conducted via two-factor ANOVA and Tukey's honestly-significant-difference tests). By contrast, values for the tissue biomass and Symbiodinium density of shallow water corals in the Florida Keys were either intermediate to those of the Bahamas and Mexico (e.g., M. faveolata tissue biomass p values<0.001; M. annularis symbiont density p values<0.0001; P. astreoides tissue biomass, Florida vs. Mexico p = 0.004, Florida vs. the Bahamas p<0.001), statistically indistinguishable from those of the Bahamas (e.g., M. annularis, tissue biomass p = 0.827; S. siderea tissue biomass two sample t-test p = 0.465), or statistically indistinguishable from those of Mexico (e.g., M. faveolata Symbiodinium density p = 0.554; P. astreoides Symbiodinium density p = 0.258) (Fig. 4). Finally, shallow M. annularis, M. faveolata, and P. astreoides from Mexico had the highest mean tissue biomasses and/or symbiont densities relative to conspecifics from other regions (Fig. 4, p values<0.0001 except M. faveolata Florida vs. Mexico symbiont density where p = 0.554 and P. astreoides Florida vs. Mexico where tissue biomass p = 0.004 and symbiont density p = 0.258). Thus, the regional tissue biomass and Symbiodinium density patterns at shallow depths (1–4 m) can be summarized as follows: Mexico≥Florida≥Bahamas (Fig. 4).
Figure 4. Coral tissue biomass and Symbiodinium density by region and depth within regions.
Coral tissue biomass (left axis and white bars) and Symbiodinium density (right axis and shaded bars) data presented as mean values ± standard error. Statistically distinguishable versus indistinguishable patterns (at α = 0.05) are indicated letters above the histograms for each species (using lower case letters for tissue biomass and upper case letters for Symbiodinium densities). Statistical analyses conducted by either two-factor ANOVAs (for M. annularis, M. faveolata, A. cervicornis, and P. astreoides) or T-tests (for M. franksi, A. palmata, and S. siderea). Coral species identity is indicated (upper left) for each sub-panel.
At depths of 12–15 m, comparisons between regions were only possible in the Bahamas and Florida Keys for four of the species (i.e., M. annularis, M. faveolata, M. franksi, and A. cervicornis). At deep reefs, M. annularis, M. faveolata, and A. cervicornis exhibited mean tissue biomasses that did not differ significantly (p>0.05) between regions (Fig. 4). Montastraea franksi was an exception to this pattern, having higher mean tissue biomass in the Bahamas relative to the Florida Keys (Fig. 4c, two sample t-test p<0.0001). Symbiodinium densities either did not differ significantly between regions (i.e., M. franksi two sample T-test p = 0.923) or were higher in Florida than in the Bahamas (i.e., for M. annularis p = 0.002, M. faveolata p = 0.003, and A. cervicornis p = 0.003; Fig. 4). Therefore, at greater depth, the typical regional tissue biomass patterns were Florida = Bahamas, with the exception of M. franksi. The generalized regional Symbiodinium density patterns were Florida≥Bahamas.
As noted above, within a region and coral species, patterns of coral tissue biomass and symbiont density frequently varied by depth (Fig. 4). As with geographic comparisons, depth-based comparisons were limited by the natural occurrence of the target species and sampling design of this study. Therefore, inter-depth comparisons were only possible for M. annularis, M. faveolata, and A. cervicornis within the Bahamas and Florida Keys. For the Montastraea species, mean tissue biomass was significantly lower in deep-dwelling corals relative to shallow conspecifics in both the Bahamas and Florida (Fig. 4, p values<0.0001). In contrast, shallow and deep A. cervicornis from Florida (p = 0.201), but not the Bahamas (p = 0.001), had mean tissue biomasses that did not differ significantly (Fig. 4).
Within a given region, Symbiodinium densities either did not differ significantly (i.e., for M. annularis [Bahamas p = 0.908, Florida p = 0.145] and Florida A. cervicornis [p = 0.696]) or were lower at depth when compared to their shallow water counterparts (i.e., for M. faveolata p values<0.0001). However, A. cervicornis in the Bahamas was exceptional in that deep-water A. cervicornis exhibited significantly higher (p = 0.007) symbiont densities than did shallow conspecifics (but note the low sample size at deep reefs). In summary, mean AFDW values and symbiont densities could be generalized as shallow (1–4 m)≥deep (12–15 m), with the exception of A. cervicornis endosymbiont densities in the Bahamas.
Seasonal patterns
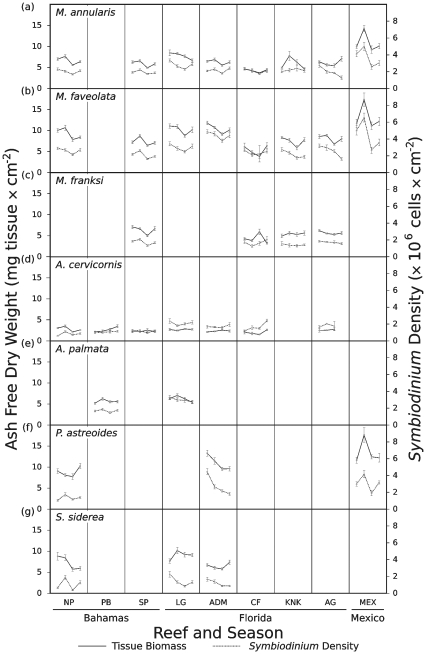
The sampling design of this study also included examination of seasonal fluctuations in tissue biomass and Symbiodinium density (i.e., from winter to spring to summer to fall). Mean seasonal tissue biomass and symbiont density values are presented for each coral species and reef in Fig. 5 and a summary of the statistical analyses performed on these data is provided in Table S1. Both coral tissue biomass and endosymbiont cell density varied seasonally, with the highest values often occurring in the winter or spring and lowest values frequently occurring during the summer or fall (Fig. 5). Notably, significant variation was detected among reefs, among seasons, and among the interaction of reef and season for both physiological parameters in four of the seven coral species sampled, including M. annularis, M. faveolata, P. astreoides (endosymbiont density only), and S. siderea (Table S1). Variation among reefs reflected the differences between regions and depths explored above (Fig. 4), as well as differences between the specific sites sampled (Fig. 5). Variation among seasons was typically due to high tissue biomass and symbiont density in the winter/spring and low values in the summer/fall.
Figure 5. Seasonal variation in coral biomass and Symbiodinium density for seven coral species from nine reefs.
Coral tissue biomass (left axis and solid lines) and Symbiodinium density (right axis and dashed lines) data presented as mean values for a given season ± standard error. Within each line plot, four sample dates are provided, one each for winter (January to March), spring (April to June), summer (July to September) and fall (October to December). Seasonal means are presented chronologically beginning with winter and ending with fall. A blank sub-panel indicates that a particular coral species did not occur at that specific location. Reef abbreviations provided in Fig. 1. Statistical analyses of these data are given in Table S1.
Four coral species deviated from the seasonal patterns described above. Deep-dwelling M. franksi exhibited significant tissue biomass variation among reefs, but not between seasons or in the interaction between reefs and seasons (Table S1, Fig. 5c). The lack of significant seasonality stems from a combination of no seasonal change at certain sites and asynchronous seasonality among other sampled reefs. For M. franski Symbiodinium densities, significant variation occurred seasonally as well as in the interaction between reef and season, but not among reefs alone (Table S1, Fig. 5c). The second exception to the seasonality described above was in both the tissue biomass and Symbiodinium densities of A. cervicornis. Here, statistical analyses revealed significant variation between reefs, but no effect of season or of the interaction between season and reef (Table S1). As illustrated in Fig. 5d, the tissue biomass and symbiont densities of A. cervicornis generally remained constant and low relative to other species throughout the year. For A. palmata, significant variation was detected between reefs for both parameters and significant seasonality was detected in tissue biomass (Table S1). However, there was no significant seasonality in A. palmata Symbiodinium density or in the interaction between reef and season for either parameter (Table S1). This may have resulted from the asynchronous seasonality between reefs for A. palmata Symbiodinium density and the relatively low overall fluctuations in this coral (Fig 5e). Finally, the tissue biomass of P. astreoides varied significantly among reefs and in the interaction between reef and season, but not significantly by season alone (Table S1). The lack of significant seasonal variation in P. astreoides biomass may result from seasonal highs occurring at different times of the year for each of the reefs sampled (i.e., asynchronous seasonality, Fig. 5f). Beyond these three exceptions, tissue biomass and Symbiodinium density fluctuated significantly across seasons for the sampled coral species (Table S1).
The 1998 and 2005 coral bleaching episodes
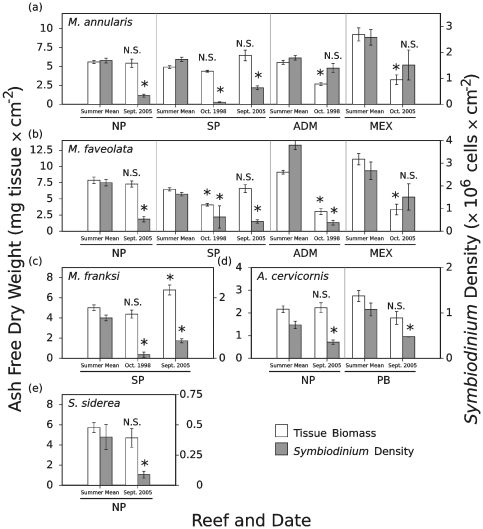
Two major bleaching episodes occurred in the Caribbean Sea and western Atlantic Ocean during the study. In 1998, tropical coral reefs across the world, including reefs of the Florida Keys, Bahamas, and Mexico, experienced a devastating mass bleaching event associated with elevated sea-surface temperatures [1], [8]. Although circumstances prevented the sampling of some Florida reefs during this bleaching event and sampling had not yet begun in Mexico, data were collected for many of the colonies and species under investigation in the Bahamas and for Montastraea spp. at ADM reef in Florida. A second bleaching event occurred in 2005 in the Bahamas and Mexico, but not on the reefs we sampled in Florida [19], [20].
Changes in Symbiodinium density and tissue biomass in relation to the 1998 and 2005 bleaching events are presented in Fig. 6. For M. annularis, M. faveolata, M. franksi, A. cervicornis, and S. siderea from the Bahamas, bleaching was characterized by statistically significant reductions in Symbiodinium density relative to the normal mean summer minima for a given coral species and location (p<0.001 for all comparisons with the exception of S. siderea where p = 0.004; Fig. 6). The remaining two species, A. palmata and P. astreoides, did not experience reductions in their endosymbiont densities. In Florida, M. faveolata at ADM reef experienced significant reductions in Symbiodinium density (p<0.0001) during the bleaching event. However, changes in Symbiodinium density were not significant in M. annularis at ADM reef (p = 0.164). No statistically significant change in Symbiodinium density was detected for M. annularis (p = 0.124) or M. faveolata (p = 0.184; Fig. 6a–b) in Mexico in 2005, likely because the site was sampled approximately two weeks into recovery from bleaching, following a cold front.
Figure 6. Changes in Symbiodinium density and tissue biomass coinciding with bleaching events for five coral species.
Data are presented as mean values of tissue biomass (left axis and white bars) and Symbiodinium density (right axis and shaded bars) ± standard error. Significant deviation from the mean summer tissue biomass and Symbiodinium density, as evaluated using T-tests, indicated above the histograms for each species (N.S. = no significant difference, * = significant deviation from the summer mean at α = 0.05). Reef abbreviations provided in Fig. 1. Note that bleaching impacts are not provided for certain coral species due to either a lack of sampling during the bleaching events (e.g., certain corals from the Florida Keys during the 1998 bleaching) or due to a lack of visible and statistically significant bleaching for that species and location (e.g., A. palmata from all regions in 2005).
In contrast to symbiont densities, tissue biomass values were less often correlated with bleaching in the immediate aftermath of the 1998 and 2005 events (Fig 6). Nevertheless this, visible bleaching was linked in a few cases to reduced tissue biomass, including M. annularis from ADM reef (p<0.0001) in 1998, SP reef in 2005 (p = 0.04), and Mexico in 2005 (p<0.0001), as well as in M. faveolata from ADM reef in 1998 (p<0.0001), SP reef in 1998 (p<0.0001), and Mexico in 2005 (p = 0.002) (Fig. 6).
Coral colony mortality and tissue biomass
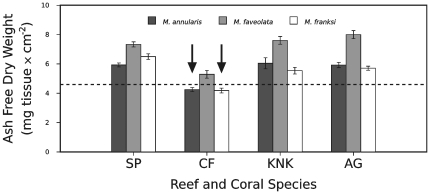
The majority of coral colonies survived throughout the study period, including through the 1998 and 2005 bleaching events. However, colony mortality was observed in M. annularis and M. franksi from CF reef in the Florida Keys in the months following the 1998 bleaching (black arrows, Fig. 7). Montastraea annularis and M. franksi colonies that died at CF reef had consistently lower mean tissue biomass values relative to M. faveolata from CF reef, and relative to conspecific and congeneric corals from other deep (12–15 m) reefs (Figs. 5, 7). The low tissue biomass of M. annularis and M. franksi at CF reef predated the 1998 bleaching event and no sudden dip in biomass levels was observed after the 1998 bleaching. Instead, the Montastraea spp. corals with a low tissue biomass simply did not recover from bleaching and these colonies died within 4 months of the event.
Figure 7. Tissue biomass of Montastraea spp. at 12–15 m depth in relation to colony mortality.
A black arrow indicates that all coral colonies of that species and location died during the course of this study. Data presented as mean values of tissue biomass ± standard error. Coral species identity indicated by bar color (see legend). Reef abbreviations provided in Fig. 1. Dashed line indicates the estimated threshold for mortality susceptibility (see text).
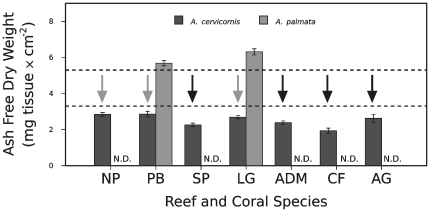
Coral colony death also occurred in 100% of the A. cervicornis colonies sampled from SP, ADM, CF, and AG reefs (black arrows, Fig. 8). At NP, PB, and LG reefs, A. cervicornis colonies experienced reductions in the surface area of living tissue and death of numerous colonies by the conclusion of sampling in 2006–2007 (gray arrows, Fig. 8). Mortality in A. cervicornis was associated with the 1998-bleaching event at CF reef. Because of the high incidence of mortality and tissue decline, conspecific tissue biomass comparisons between healthy and dying corals were not possible for A. cervicornis. However, it is noteworthy that the mean tissue biomass values for A. cervicornis were substantially lower than the mean biomasses of its sister taxon, A. palmata, regardless of the reef, depth, or region sampled (Fig. 8). None of the sampled A. palmata died over the duration of this study.
Figure 8. Tissue biomass of Acropora spp. in relation to colony mortality.
A black arrow indicates that all coral colonies of that species and location died during the course of this study. Gray arrows indicate substantial reductions in live coral tissue for all coral colonies from that location. Data presented as mean values of tissue biomass ± standard error. Coral species is indicated by bar color (see legend). The designation ‘N.D.’ (i.e., no data) denotes the lack of a particular coral species at a specific location. Reef abbreviations provided in Fig. 1. Dashed lines demarcate the statistically significant range of thresholds for mortality susceptibility (see text).
The correlations between colony mortality and low tissue biomass in M. annularis, M. franksi, and potentially A. cervicornis, led to the following hypothesis: low tissue biomass renders coral colonies susceptible to death if a stressful event, such as bleaching or disease, should occur. If correct, the threshold for mortality susceptibility is species or genera specific. Specifically, M. annularis and M. franksi at CF reef that died had mean tissue biomasses of 4.25±0.14 and 4.19±0.16 mg tissue×cm−2, respectively (Fig. 7). By contrast, the mean tissue biomass for A. cervicornis ranged between 1.94±0.15 mg tissue×cm−2 at CF reef to 2.85±0.10 mg tissue×cm−2 at NP reef (Fig. 8).
To mathematically represent this minimum tissue biomass hypothesis, mortality thresholds for Montastraea were calculated (in increments of 0.1 mg tissue×cm−2) between the mean tissue biomass values for M. faveolata at CF reef (which survived with a mean value of 5.30±0.26 mg tissue×cm−2) and those of M. annularis and M. franksi from CF reef (which perished). Consistent with our minimum tissue biomass hypothesis, the value of 4.6 mg tissue×cm−2 was found to be the optimal threshold for Montastraea spp. (Fig. 7; Text S1). Threshold analysis was also conducted for Acropora spp., and a range was determined between the values of 2.9 and 5.6 mg tissue×cm−2. This range covered the maximum tissue biomass observed among dying A. cervicornis (at NP and PB) and the minimum in surviving A. palmata (at PB) respectively. For Acropora spp., values ranging between 3.3 and 5.3 mg tissue×cm−2 represented acceptable thresholds for mortality; the optimal threshold was 4.0 (Fig. 8; Text S1).
Discussion
Examination of coral tissue biomass and Symbiodinium density from seven species of reef-building corals from the Caribbean Sea and western Atlantic Ocean revealed a number of statistically robust patterns. Specifically, coral species displayed a species-specific mean biomass and endosymbiont density, as well as a positive and significant correlation between these two parameters (with the exception of S. siderea). Tissue biomass and Symbiodinium density often varied over both space and time, with a tendency towards the following generalized patterns: Mexico≥Florida≥Bahamas, shallow≥deep, and winter/spring>summer/fall. The 1998 and 2005 bleaching events were characterized by reduced Symbiodinium densities, but not necessarily host tissue biomass, for many coral species. Finally, colonies with relatively low tissue biomass often succumbed to death when exposed to thermal stress and/or disease. In the following discussion, we consider how tissue biomass and symbiont cell density relate to geography, depth, seasonal variation, bleaching history, and differential mortality.
Tissue biomass and Symbiodinium density are positively correlated
Six out of seven reef-building coral species examined here exhibited a correlation between Symbiodinium density and tissue biomass. One possible explanation for such correlations is that endosymbionts are an important component of overall tissue biomass. This possibility has not been explored in the published scientific literature and would make an important topic for future studies. However, data made available to us by S.C. Kempf provide insight into the contributions of Symbiodinium to tissue biomass. Kempf separated host and endosymbiont tissues in five symbiotic cnidarians from the Florida Keys and measured the ash free dry weight of isolated Symbiodinium cells (S.C. Kempf unpublished data). For M. annularis, that analysis resulted in tissue biomass values of 131±4 pg×Symbiodinium cell−1 (S.C. Kempf unpublished data; n = 10 symbiont cell sub-samples; value presented as mean ± SD). Based on the cell densities recovered in our study and the cell biomass values measured by S.C. Kempf, we estimate that Symbiodinium cell biomass accounts for less than 5% of the M. annularis AFDW value. Assuming that a similar biomass of Symbiodinium cells occurs in other reef-building coral species, it appears that symbionts did not appreciably bias the measurement of host AFDW. This conclusion is further supported in many coral species by both the weak relationships between symbiont density and biomass and the immediate decreases in Symbiodinium densities seen during or just after bleaching events with a subsequent loss of tissue biomass delayed until the next sampling point. Nevertheless, it is possible that the relative contributions of Symbiodinium vary among different host and/or endosymbiont species and/or in space and time. Additional study is clearly needed to better document this relationship. Until such data are available, biological inferences about the correlations between symbiont density and overall biomass should be considered with caution.
Resident populations of photosynthetic dinoflagellates support the metabolic needs of most reef-building corals [5], [21], [22]. Photosynthetic material is translocated in excess to host corals, especially during the cooler seasons [18], [23]. Host tissue biomass in corals such as M. faveolata and P. astreoides were greatest when endosymbiont densities were at their highest, despite the fact that Symbiodinium themselves comprise only a marginal portion of measured AFDW (see above). Thus, nutritional (or energetic) mutualisms between corals and symbiotic dinoflagellates likely drive the positive correlations between Symbiodinium densities and host tissue biomasses for most (i.e., six out of seven) species examined here. Presumably, coral species with a weak or uncorrelated biomass to Symbiodinium density relationship, such as A. cervicornis and S. siderea, either obtain a larger proportion of their energetic needs through alternative sources (i.e., heterotrophic feeding and/or absorption), harbor Symbiodinium populations whose net carbon translocation is uncoupled from cell density, allocate more of their energy to other biological requirements (e.g., calcification, mucus production, reproduction, etc.), or some combination of these scenarios.
Additional factors probably contribute to the variation in AFDW and densities of endosymbiotic dinoflagellates. Individuals, populations, and species of coral living under various habitats or associating with different symbiont genotypes many differentially rely on photosynthesis, heterotrophy, and DOM absorption depending on their circumstances [24], [25]. In Hawaii, for instance, the coral Montipora capitata compensates for stress-related reductions in Symbiodinium density through increased feeding, whereas Porites compressa and P. lobata do not [26]. Physiological and morphological variation among Symbiodinium may partially explain the differences in AFDW and symbiont cell density among host species. Certain Symbiodinium lineages presumably transfer less photosynthate to their hosts than do others [27], which may translate into different degrees of correlation between coral biomass and symbiont density among different coral species. Previous work confirms the presence of considerable genetic diversity of Symbiodinium among the colonies and species examined here [19], [20], [28]. More precise information about the physiological contributions of different Symbiodinium lineages is required to determine the degree of mutualism in various host-symbiont combinations.
Tissue biomass and Symbiodinium density vary regionally
The regional differences in tissue biomass and Symbiodinium density encountered here likely relate to latitudinal changes in light and temperature, as well as bleaching history. Tissue biomass and Symbiodinium densities can diminish as irradiance decreases; for instance, across a depth range [18] or with increased shading in underwater caves [29]. It is therefore possible that such differences might be evident in colonies living in high versus low latitudes. Puerto Morelos, Mexico is located at lower latitude than the other regions sampled resulting in more direct photosynthetically active radiation exposure and less seasonal variation. This increased photosynthetically active radiation and seasonal stability may explain the higher tissue biomass in M. annularis, M. faveolata and P. astreoides from Mexico, relative to other regions.
If latitudinal differences in PAR and temperature are responsible for the geographic differences, it is surprising that tissue biomass and endosymbiont cell densities were occasionally lower in the Exuma Cays, Bahamas relative to conspecifics in the Upper Florida Keys, U.S. Although the regions are located at similar latitudes, the Exuma Cays are farther south than the Florida Keys and receive slightly greater light exposure and lower seasonal variation in environmental conditions [30]. Additionally, reefs of the Upper Florida Keys are proximate to large human populations, presumably leading to greater direct anthropogenic impacts (e.g., nutrient additions) relative to the more isolated reefs of Lee Stocking Island. Despite this, corals of the Exuma Cays recently experienced greater extremes of high temperature and bleached more often (e.g., in 2005 [19], [20]) than colonies from the Upper Florida Keys. Greater bleaching-induced stress and the subsequent years of recovery may override latitudinal effects, resulting in reduced tissue biomass and Symbiodinium density in corals from the Bahamas relative to Floridian conspecifics (see discussion of bleaching below).
Tissue biomass and Symbiodinium densities vary with depth
Deeper corals often have lower tissue biomass and Symbiodinium density relative to individuals of the same species in shallow water [18], [31]. Previous studies attributed this pattern to photoacclimation by Symbiodinium populations to attenuating light [18], [29] and this explanation is consistent with our data for M. faveolata. In contrast, M. annularis experienced depth-related differences in tissue biomass, but not Symbiodinium density. Furthermore, A. cervicornis showed either no difference in tissue biomass or Symbiodinium density among depths (in Florida), or an inverse relationship between these two parameters (in the Bahamas). These contrasting results suggest that additional factors contribute to bathymetric tissue biomass and symbiont density patterns. Compared to shallow habitats, deeper reefs are characterized by reduced light intensity, lower average temperature, and marked differences in currents and advective flow from deeper waters. Such physical patterns could drive differences in quantity and quality of zooplankton, detritus, and DOM that may also influence coral biomass. For example, certain corals increase heterotrophic intake with decreasing light intensity and increasing depth [24], [25]. Thus, biomass differences noted here are likely due, in part, to resource acquisition via autotrophy and heterotrophy, as many corals display a greater reliance on heterotrophically derived carbon and nitrogen in deeper waters [32], [33]. While differences in endosymbiont density, carbon translocation, and Symbiodinium genotype (mentioned below) are probable causes of the depth-related patterns in tissue biomass, more detailed comparative analyses are necessary to further understand corals' carbon and nutrient budgets.
Many corals harbor genetically distinct Symbiodinium sp. and some of the species studied here have polymorphic symbioses with at least three different Symbiodinium genotypes. For example, members of the M. annularis species complex harbor internal transcribed spacer region 2 (ITS2) “types” A3 (Mexico), B1 (all three regions), B10 (Florida), B17 (Mexico), C3 (Florida), C7 (Mexico), C12 (Bahamas), and/or D1a (all three regions) and these “types” exhibit zonation by depth and colony position [13], [20], [28]. In shallow, high-light environments, clade A and B “types” dominate the tops of Montastraea spp. colonies; as depth increases, clade C “types” increase in abundance [28], [34]. Additionally, considerable genetic diversity exists within a given Symbiodinium ITS2 “type” [35]. Even when identical Symbiodinium ITS2 genotypes are found in shallow and deep environments, additional genetic, and possibly physiological, differences likely exist [20], [36]. Consequently, differences in endosymbiont populations may further contribute to the depth-associated differences in host tissue biomass observed here.
Seasonality and bleaching in coral tissue biomass and Symbiodinium density
Previous studies demonstrated that symbiont density [17] and tissue biomass fluctuate seasonally, with intra-annual maximums in the winter/spring and minima in the summer/fall [18]. This pattern was further confirmed for the majority of coral species examined. In addition, some summers are characterized by excessive and/or prolonged periods of heating (many of which correspond to strong ENSO cycles) and result in greater declines in tissue biomass, symbiont populations, and photosynthetic capacity, leading to severe coral bleaching [37], [38]. Results also indicate that certain corals, such as A. cervicornis, A. palmata, M. franksi, and P. astreoides, experience minimal seasonal change in biomass and/or symbiont density.
Seasonal changes in temperature and solar insulation play an important role in modulating coral metabolic rate [18]. High temperatures increase metabolic rate, which influences the depletion of lipid reserves, animal biomass, and skeletal growth [39]. Importantly, most Caribbean coral species reproduce by single annual broadcast spawning events in the late summer (i.e., Montastraea, Acropora, and S. siderea), while others brood and release mature larvae several times over the year (i.e., P. astreoides) [40]. Energetic demands of reproduction would further reduce lipids and overall biomass, thus contributing to the typical fall minima in coral tissue. Likewise, the more frequent reproductive effort and thermally tolerant symbioses of P. astreoides [13] may dampen seasonal biomass fluctuation in this species.
We identified bleaching in the form of significant declines in Symbiodinium density in M. annularis, M. faveolata, M. franksi, A. cervicornis, and S. siderea. Whereas bleaching was seen in the Florida Keys and Mexico in 1998, we were unable to sample most of these locations during the peak of the event, with the exception of ADM reef. Similarly, although bleaching was noted in 2005, the lack of symbiont loss in Montastraea spp. from Mexico in our samples is most likely due to the late sampling time (October), after symbiont populations began recovery. Thus, while many corals appeared pale, this may have been due to a substantial reduction in photosynthetic pigments per algal cell as the remaining symbionts were photoacclimating to the higher post-bleaching light field in hospite [41]. Clearly, annual symbiont loss, and greater decline during bleaching, plays a significant role in dictating resource partitioning in corals. Bleaching reduces the proportion of carbon translocated from Symbiodinium spp. to the host coral, and as a result, corals must consume stored energy reserves [42]–[44]. Our data also show that such losses in biomass do not always immediately coincide with losses of Symbiodinium, suggesting a possible time lag [18]. This was particularly apparent in Mexico, where sampling occurred after the 2005 event. For Montastraea spp., symbiont densities were in the process of recovery during sampling, whereas tissue biomass levels were experiencing significant declines.
Low biomass corals are susceptible to death following stress
Seasonal monitoring revealed a correlation between low coral tissue biomass and colony mortality among individuals of M. annularis, M. franksi, and possibly A. cervicornis. This result is supported by Loya et al. [6], who documented higher survivorship among thick-tissue Pacific scleractinians, relative to thinner-tissue species, during the 1998 bleaching event. Similarly, Hoegh-Guldberg [1] proposed that thicker-tissue corals would be more resistant to bleaching due to increased photo-protective shading. These findings suggest the following hypotheses: 1) tissue biomass is indicative of overall coral colony health, 2) tissue biomass is predictive of colony survival probability following periods of stress, such as high-temperature induced bleaching, and 3) each species of coral has a biomass minimum, below which colonies are more susceptible to disease and starvation.
Mortality among Montastraea spp. colonies monitored at deep reef sites correlated with individuals maintaining a low average biomass of approx. 4.6 mg×cm−2 for an extended period and then being subjected to thermal stress. In contrast, colonies of Montastraea spp. with higher biomasses survived (Fig. 7). Similar observations were made for A. cervicornis, which exhibited low tissue biomass throughout the year at all locations sampled (Fig. 5d). At three reefs, A. cervicornis colonies died following either the 1997–1998 El Niño or putative disease incidents. These colonies experienced prolonged periods of low tissue biomass prior to their death (Fig. 8). While surviving A. cervicornis colonies exhibited slightly higher biomass values, all colonies experienced major reductions in the area of living coral tissue on their skeletons and many colonies died over the course of our sampling. Because all A. cervicornis colonies died or shrank substantially in tissue area (i.e., were dying), it was not possible to precisely estimate a minimum tissue threshold for this species. Based on comparisons to A. palmata, we estimated the minimum tissue threshold for A. cervicornis to be between 3.3 and 5.3 mg tissue×cm−2. However, comparisons between Acropora spp. should be interpreted cautiously, as the biomass differences may result primarily from intrinsic properties of each species as opposed to dissimilarity in colony health.
Based on the observations and correlations outlined above, we propose that corals that are unable to replenish their nutrient and energy reserves to a healthy level become metabolically fragile, and are thus increasingly susceptible to dying during episodes of stress when metabolic reserves are most needed. If this hypothesis is correct, tissue biomass represents a potentially useful indicator of the health of different corals. Field survey studies of mass bleaching events have noted differential mortality among coral colonies, species, and reefs [6], [9], [10]. This variability has been partially attributed to genetic and physiological differences among corals or their endosymbionts [11]–[13], however, such explanations do not account for all available data. Evidence presented here suggests that differences in colony biomass also contribute to the mortality patterns in reef-building corals and may in some cases underlie differential survival following bleaching.
Materials and Methods
Study site and collection of corals
This study targeted seven species of corals, including M. annularis, M. faveolata, M. franksi, A. cervicornis, A. palmata, P. astreoides, and S. siderea for seasonal monitoring. Each coral species was sampled based on its local availability from up to three reefs in the Exuma Cays, Bahamas, five reefs in the Upper Florida Keys, United States (U.S.), and one reef in Puerto Morelos, Mexico. Site coordinates, depth, and coral species collected at each site are provided in Table 1 and a map of the collection localities is shown in Fig. 1. Each reef was sampled seasonally, with sample periods designated here as winter (January–March), spring (April–June), summer (July–September), and fall (October–December). Occasionally, circumstances (e.g., storm activity) prohibited the collection of samples during a particular time point, and as a result, collection frequency ranged between two to four samplings per site per year. Collection durations at each site are listed in Table 1.
At each site, six representative colonies of each species were tagged at the beginning of the study to ensure that subsequent collections were from the same coral colony. Due to the difficulty in permanently tagging the branches of A. cervicornis, this coral was sampled from up to six distinct colonies from the same population at every collection period, but not necessarily the same colonies each time. (Note: sample sizes of A. cervicornis at deeper sites were reduced [n = 2–3] due to its rarity at depth.) At each sampling interval, approximately 10-cm2 fragments were removed from each colony using a hammer and chisel or punch core. All colony fragments were taken from non-shaded areas at or near the tops of each colony in order to minimize potential light micro-environmental variability effects across the colony surface. Fragments were placed in pre-labeled plastic bags filled with seawater and transported in an insulated cooler to the laboratory where they were processed immediately.
Determination of coral tissue biomass and Symbiodinium density
Each coral fragment was split into two pieces. The first sub-fragment was processed for coral tissue biomass following a modified protocol based on Johannes and Wiebe [45]. Tissue was removed from the skeleton with a recirculating Waterpik™ using distilled water and then frozen at −20°C. The frozen slurry was then lyophyilized and subsequently baked in a muffle furnace (Fisher Scientific) for at least 4 h at 500°C. Ash-free dry weight was calculated as the difference between dry weight and ash weight following the protocol of Fitt et al. [18]. The second coral sub-fragment was processed to determine Symbiodinium densities. This fragment was ‘waterpiked’ with 0.45 µm-filtered seawater. Algal densities were calculated from replicate (n = 6–10) hemocytometer counts of homogenized tissue. Fragment surface area was determined by the aluminum foil method [46] and tissue biomass and symbiont density were normalized to the surface area of the coral sample. The resulting raw data are publically available at the database DRYAD (http://datadryad.org; doi:10.5061/dryad.gm005fg8).
Statistical analyses
We used Pearson correlations to determine the degree to which tissue biomass and Symbiodinium density were correlated in each of the seven coral species across locations, depths, and collection time points. Assumptions of normality and homogeneity of variance were tested and, because all data lacked normality, each dataset was square root transformed to satisfy normality assumptions. Unless otherwise noted, all subsequent statistical tests were performed on the transformed datasets.
For the following analyses, coral tissue biomass and Symbiodinium density were examined independently of one another using the analytical software JMP version 8 (SAS Institute Inc., Cary, NC, U.S.) and Systat version 13 (Cranes Software International, Bangalore, India). The effects of region, depth, and their interaction on tissue biomass or Symbiodinium density were tested using two-factor analyses of variance (ANOVA) and Tukey's honestly-significant-difference tests (M. annularis, M. faveolata, A. cervicornis, and P. astreoides). When total population size was less than 3 across all sites, T-tests were used instead (M. franksi, A. palmata, and S. siderea). We used separate two-factor ANOVAs to test for effects on coral tissue biomass and Symbiodinium density instead of a single MANOVA because tissue biomass and symbiont density were often correlated (see results), thus violating assumptions of colinearity of variances in dependent variables. For all seven coral species, the effects of location, season, and their interaction on tissue biomass or Symbiodinium density were tested using two-factor ANOVAs. Changes in tissue biomass and Symbiodinium density coincident with bleaching events were examined by comparing parameter values during the bleaching event to the mean summer biomass or symbiont density value for that reef and species, excluding bleaching year data, using two-sample protected T-tests. The mean summer value was used for these comparisons because this is the period in which tissue biomass and Symbiodinium density are typically the lowest each year [18].
To test the hypothesis that low tissue biomass renders coral colonies susceptible to mortality, optimal mortality thresholds were determined within a differential range separating colony outcomes (survival versus mortality). The method (see Text S1) identified the single threshold value in the range between greatest “dying” value and lowest “surviving” value that had the lowest probability of incorrectly classifying a coral as surviving or dying based on its actual measured tissue biomass and known fate (given that the data were normally distributed). Thresholds were tested in increments of 0.1 across the differential range; threshold values were considered to be acceptable if their probability of misclassification was less than 0.05. Thus, mortality threshold analysis resulted in a single optimum value and range of acceptable values for each differential range tested.
Supporting Information
Two-factor ANOVA with interaction terms summary based on the biomass and Symbiodinium densities of seven corals.
(DOCX)
Protocol and programs for mortality threshold analysis, designed and calculated with MATLAB R2007b.
(DOCX)
Acknowledgments
We thank the numerous undergraduates who worked on this project with us as well as the support staffs of the Perry Institute for Marine Science, the Unidad Académica Puerto Morelos UNAM in Mexico, the National Oceanic and Atmospheric Administration (NOAA) Day Boat Program, and the Florida Keys National Marine Sanctuary in Key Largo, Florida. The authors thank J.R. Chabot for his assistance in designing and coding the algorithm for the mortality threshold analysis and S.C. Kempf for providing unpublished data on the contributions of Symbiodinium cells to holobiont tissue biomass. We are also grateful to the handling editor and two anonymous reviewers for providing constructive comments that improved the manuscript. All coral collection was conducted with permits from the appropriate regulatory agency in the countries where collection occurred. In the Florida Keys, U.S., permits were issued by the Florida Keys National Marine Sanctuary or Florida Department of Environmental Protection depending on the year of collection. In the Bahamas, collection was conducted under permits issued by the Bahamas Department of Fisheries to the Perry Institute of Marine Science at Lee Stocking Island. In Mexico, collection was conducted under permits issued by the Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación to the Universidad Nacional Autónoma de México. This is publication #30 from the Key Largo Marine Research Laboratory.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The National Science Foundation (grants 9906976 and 0137007), National Oceanic and Atmospheric Administration, the Office of Naval Research and the Bleaching Group of the Coral Reef Targeted Research and Capacity Building for Management funded this research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshwater Res. 1999;50:839–866. [Google Scholar]
- 2.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 3.Lesser MP. Experimental biology of coral reef ecosystems. J Exp Mar Biol Ecol. 2004;300:217–252. [Google Scholar]
- 4.Fitt WK, Brown BE, Warner ME, Dunne RP. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 2001;20:51–65. [Google Scholar]
- 5.Muscatine L, McCloskey LR, Marian RE. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr. 1981;26:601–611. [Google Scholar]
- 6.Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, et al. Coral bleaching: the winners and the losers. Ecol Lett. 2001;4:122–131. [Google Scholar]
- 7.Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc Natl Acad Sci U S A. 2008;105:10444–10449. doi: 10.1073/pnas.0708049105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson C. Townsville: Australian Institute of Marine Science; 2000. Status of coral reefs of the world: 2000.363 [Google Scholar]
- 9.Glynn PW. Widespread coral mortality and the 1982–83 El Niño warming event. Environ Conserv. 1984;11:133–146. [Google Scholar]
- 10.Fitt WK, Spero HJ, Halas J, White MW, Porter JW. Recovery patterns of the coral Montastrea annularis in the Florida Keys after the 1987 Caribbean “bleaching event.”. Coral Reefs. 1993;12:57–64. [Google Scholar]
- 11.Edmunds PJ. Evidence that reef-wide patterns of coral bleaching may be the result of distribution of bleaching susceptible clones. Mar Biol. 1994;121:137–142. [Google Scholar]
- 12.Rowan R, Knowlton N, Baker A, Jara J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature. 1997;388:265–269. doi: 10.1038/40843. [DOI] [PubMed] [Google Scholar]
- 13.Warner ME, LaJeunesse TC, Robison JD, Thur RM. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol Oceanogr. 2006;51:1887–1897. [Google Scholar]
- 14.Sorokin YI. On the feeding of some scleractinian corals with bacteria and dissolved organic matter. Limnol Oceanogr. 1973;18:380–385. [Google Scholar]
- 15.Sorokin YI. Biomass, metabolic rates and feeding of some common reef zoantharians and octocorals. Aust J Mar Fresh Res. 1991;42:729–741. [Google Scholar]
- 16.Trench RK. Nutritional potentials in Zoanthus sociathus (Coelenterata, Anthozoa). Helgoländ Wiss Meer. 1974;26:174–216. [Google Scholar]
- 17.Fagoonee I, Wilson HB, Hassell MP, Turner JF. The dynamics of zooxanthellae populations: a long-term study in the field. Science. 1999;283:843–845. doi: 10.1126/science.283.5403.843. [DOI] [PubMed] [Google Scholar]
- 18.Fitt WK, McFarland FK, Warner ME, Chilcoat GC. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in corals and in relation to coral bleaching. Limnol Oceanogr. 2000;45:677–685. [Google Scholar]
- 19.Thornhill DJ, Fitt WK, Schmidt GW. Highly stable symbioses among western Atlantic brooding corals. Coral Reefs. 2006;25:515–519. [Google Scholar]
- 20.Thornhill DJ, Xiang Y, Fitt WK, Santos SR. Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. PLoS ONE. 2009;4:e6262. doi: 10.1371/journal.pone.0006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. Light and bioenergetics of a symbiotic coral. Bioscience. 1984;34:705–709. [Google Scholar]
- 22.Edmunds PJ, Davies SP. An energy budget for Porites porites (Scleractinia). Mar Biol. 1986;92:339–347. [Google Scholar]
- 23.Muscatine L, Falkowski PG, Porter JW, Dubinsky Z. Fate of photosynthetic fixed carbon in light-adapted and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc Roy Soc Lond B. 1984;222:181–202. [Google Scholar]
- 24.Anthony K RN, Fabricius KE. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol. 2000;252:221–253. doi: 10.1016/s0022-0981(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 25.Palardy JE, Rodrigues LJ, Grottoli AG. The importance of zooplankton to the daily metabolic carbon requirements of healthy and bleached corals at two depths. J Exp Mar Biol Ecol. 2008;367:180–188. [Google Scholar]
- 26.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- 27.Sachs J, Wilcox T. A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc Roy Soc Lond B. 2006;273:425–429. doi: 10.1098/rspb.2005.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornhill D J, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol. 2006;148:711–722. [Google Scholar]
- 29.Anthony KRN, Hoegh-Guldberg O. Variation in coral photosynthesis, respiration and growth characteristics in contrasting light microhabitats: an analogue to plants in forest gaps and understoreys? Funct Ecol. 2003;17:246–259. [Google Scholar]
- 30.van Woesik R, Shiroma K, Koksal S. Phenotypic variance predicts symbiont population densities in corals: a modeling approach. PLoS ONE. 2010;5:e9185. doi: 10.1371/journal.pone.0009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battey JF, Porter JW. Photoadaptation as a whole organism response in Montastraea annularis. Proc 6th Int Coral Reef Symp. 1988;3:79–87. [Google Scholar]
- 32.Muscatine L, Porter JW, Kaplan IR. Resource partitioning by reef corals as determined from stable isotope composition. I. δ13C of zooxanthellae and animal tissue vs. depth. Mar Biol. 1989;100:185–193. [Google Scholar]
- 33.Muscatine L, Goiran C, Land L, Jaubert J, Cuif JP, et al. Stable isotopes (δ13C and δ15N) of organic matrix from coral skeleton. Proc Natl Acad Sci U S A. 2005;102:1525–1530. doi: 10.1073/pnas.0408921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowan R, Knowlton N. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc Natl Acad Sci U S A. 1995;92:2850–2853. doi: 10.1073/pnas.92.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coffroth MA, Santos SR. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist. 2005;156:19–34. doi: 10.1016/j.protis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Santos SR, Shearer TL, Hannes AR, Coffroth MA. Fine-scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium, Dinophyceae) of the Caribbean. Mol Ecol. 2004;13:459–469. doi: 10.1046/j.1365-294x.2003.02058.x. [DOI] [PubMed] [Google Scholar]
- 37.Warner ME, Fitt WK, Schmidt GW. Damage to photosystem II in symbiotic dinoflagellates; a determinant of coral bleaching. Proc Natl Acad Sci U S A. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warner ME, Chilcoat GC, McFarland FK, Fitt WK. Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Mar Biol. 2002;141:31–38. [Google Scholar]
- 39.Cantin NE, Cohen AL, Karnauskas KB, Tarrant AM, McCorkle DC. Ocean warming slows coral growth in the central Red Sea. Science. 2010;329:322–325. doi: 10.1126/science.1190182. [DOI] [PubMed] [Google Scholar]
- 40.Szmant AM. Reproductive ecology of Caribbean reef corals. Coral Reefs. 1986;5:43–54. [Google Scholar]
- 41.Rodriguez-Roman A, Iglesias-Prieto R. Regulation of photochemical activity in cultured symbiotic dinoflagellates under nitrate limitation and deprivation. Mar Biol. 2005;146:1063–1073. [Google Scholar]
- 42.Porter JW, Fitt WK, Spreo HJ, Rogers CS, White MW. Bleaching in reef corals: physiological and stable isotopic responses. Proc Natl Acad Sci U S A. 1989;86:9342–9346. doi: 10.1073/pnas.86.23.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szmant AM, Gassman NJ. The effects of prolonged “bleaching” on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs. 1990;8:217–224. [Google Scholar]
- 44.Mendes JM, Woodley JD. Effect of the 1995–1996 bleaching event of polyp tissue depth, growth, reproduction and skeletal band formation in Montastraea annularis. . Mar Ecol Prog Ser. 2002;235:93–102. [Google Scholar]
- 45.Johannes RD, Wiebe WJ. Determination of coral tissue biomass and composition. Limnol Oceanogr. 1970;15:822–824. [Google Scholar]
- 46.Marsh JA. Primary productivity of reef-building calcareous red algae. Ecology. 1970;51:255–263. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-factor ANOVA with interaction terms summary based on the biomass and Symbiodinium densities of seven corals.
(DOCX)
Protocol and programs for mortality threshold analysis, designed and calculated with MATLAB R2007b.
(DOCX)