Abstract
Genes underlying important phenotypic differences between Plasmodium species, the causative agents of malaria, are frequently found in only a subset of species and cluster at dynamically evolving subtelomeric regions of chromosomes. We hypothesized that chromosome-internal regions of Plasmodium genomes harbour additional species subset-specific genes that underlie differences in human pathogenicity, human-to-human transmissibility, and human virulence. We combined sequence similarity searches with synteny block analyses to identify species subset-specific genes in chromosome-internal regions of six published Plasmodium genomes, including Plasmodium falciparum, Plasmodium vivax, Plasmodium knowlesi, Plasmodium yoelii, Plasmodium berghei, and Plasmodium chabaudi. To improve comparative analysis, we first revised incorrectly annotated gene models using homology-based gene finders and examined putative subset-specific genes within syntenic contexts. Confirmed subset-specific genes were then analyzed for their role in biological pathways and examined for molecular functions using publicly available databases. We identified 16 genes that are well conserved in the three primate parasites but not found in rodent parasites, including three key enzymes of the thiamine (vitamin B1) biosynthesis pathway. Thirteen genes were found to be present in both human parasites but absent in the monkey parasite P. knowlesi, including genes specifically upregulated in sporozoites or gametocytes that could be linked to parasite transmission success between humans. Furthermore, we propose 15 chromosome-internal P. falciparum-specific genes as new candidate genes underlying increased human virulence and detected a currently uncharacterized cluster of P. vivax-specific genes on chromosome 6 likely involved in erythrocyte invasion. In conclusion, Plasmodium species harbour many chromosome-internal differences in the form of protein-coding genes, some of which are potentially linked to human disease and thus promising leads for future laboratory research.
Author Summary
With more than 250 million infections and over a million deaths each year, malaria remains one of the most devastating infectious diseases worldwide. With the availability of complete genome sequences of both human and non-human Plasmodium parasites, the causative agents of malaria, it is now possible to use comparative genomics as a tool to look for genes that are present in some but not all Plasmodium species. Such species subset-specific genes possibly underlie important phenotypic differences between malaria parasites and could provide important clues for the development of new strategies to prevent and treat malaria in humans. In this study, we performed a comprehensive computational comparison of the published genomes of six Plasmodium species, including two human (P. falciparum and P. vivax), one monkey (P. knowlesi), and three rodent malaria parasites (P. berghei, P. yoelii, and P. chabaudi). This comparison revealed many species subset-specific genes that are potentially linked to human pathogenicity, human-to-human transmissibility, and human virulence. These genes can now be examined further by targeted experimental analyses to test predicted phenotypic associations and to elucidate gene function.
Introduction
Malaria remains a serious health threat. Every year, more than 250 million people worldwide suffer from malaria and over one million people die as a consequence of the disease, mostly children in Africa under the age of five [1]. Malaria is an infectious disease caused by single-celled intracellular eukaryotic parasites of the genus Plasmodium that are transmitted by mosquitoes. Four species, Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae and Plasmodium ovale, are traditionally recognized as human parasites. Other Plasmodium species are important model parasites in malaria research, including the primate malaria model Plasmodium knowlesi, which parasitizes macaque monkeys in the wild, as well as the three rodent malaria parasites Plasmodium berghei, Plasmodium yoelii, and Plasmodium chabaudi, which are natural parasites of thicket rats in central Africa.
Plasmodium species that naturally infect humans, monkeys, and rodents differ in their ability to cause human disease. Firstly, laboratory experiments have shown that parasites of thicket rats are infectious to various other species of rodents but not primates [2], [3], suggesting that rodent parasites lack essential features required to parasitize primates, including humans. Secondly, the macaque monkey parasite P. knowlesi differs from the four human parasites in that it is not endemic in larger parts of the human population despite its known ability to infect also humans under natural conditions [4]. Recent epidemiological and entomological data suggest that human P. knowlesi malaria is an ancient zoonosis acquired from forest-dwelling macaque monkeys [5]. It is likely that P. knowlesi malaria fails to spread in human settlements and beyond because of the known inability of P. knowlesi to develop in domestic species of Anopheles [6], [7]. However, concerns have been raised that with increased exposure of humans to P. knowlesi the parasite might eventually become epidemic in humans [4], [5]. Thirdly, human malaria parasites differ greatly in human virulence. P. falciparum, which accounts for up to 90% of annual infections worldwide [1], is the most virulent species and is responsible for almost all malarial deaths [8]. P. vivax, the major cause of human malaria outside Africa, rarely kills, although cases of lethal P. vivax malaria have been reported [9]. The more benign nature of P. vivax malaria in humans is commonly attributed to the inability of P. vivax-infected red blood cells to adhere to vascular endothelium and the preference of P. vivax to infect reticulocytes (immature red blood cells), which naturally limits parasitaemia because reticulocytes account for only 1–2% of erythrocytes [10], [11]. Finally, P. vivax and P. ovale, but not P. falciparum and P. malariae, can stay dormant in the liver as hypnozoites, which can cause relapses months or even years after the primary infection in the blood has been cleared [12]. Relapses are thought to be an evolutionary adaptation of the parasite to ensure transmission in more temperate climate zones where mosquitoes are not available throughout the year [11].
Recent genome sequencing of the two human malaria parasites P. falciparum [13] and P. vivax [14], the macaque parasite P. knowlesi [15], and the three rodent parasites P. yoelii [16], P. berghei [17], and P. chabaudi [17] provides an opportunity to identify the genetic basis of the aforementioned important phenotypic differences by means of comparative genomics. An important insight that has been gleaned from early comparative genomics analyses of Plasmodium genomes is that genes mediating parasite-host interactions are frequently restricted to a single Plasmodium species (species-specific) or restricted to a subset of Plasmodium species (species subset-specific). Perhaps the best studied and clinically most relevant example is P. falciparum erythrocyte membrane protein 1 (PfEMP1), whose different isoforms are encoded by about 60 members of the P. falciparum-specific var gene family [13], [18]. PfEMP1 proteins are expressed at the surface of infected red blood cells (iRBC) where they mediate adhesion to both uninfected erythrocytes and host endothelial cells. This causes a great deal of the severe clinical pathologies of P. falciparum malaria. PfEMP1 is therefore considered the prime virulence factor of P. falciparum malaria. Other important species- or species subset-specific gene families have been linked to host immune evasion, including the var and rif/stevor gene families in P. falciparum, vir in P. vivax, SICAvar and kir in P. knowlesi, and the cir/bir/yir family in rodent malaria parasites (reviewed in [19]). Erythrocyte invasion is another critical molecular process at the parasite-host interface facilitated by species subset-specific gene family members, including duffy-binding like (DBL) and reticulocyte-binding-like (RBL) gene family members [14] as well as serine repeat antigens (SERA) and merozoite surface proteins (MSPs), some of which are now leading targets in vaccine development (reviewed in [20], [21]). Comparative genomic studies also have shown that species- or species subset-specific genes in Plasmodium genomes are preferentially located at dynamically evolving subtelomeric regions of chromosomes that are completely devoid of synteny [14], [16], [22], [23]. In contrast, non-subtelomeric or chromosome ‘core’ regions (referred to as chromosome-internal regions in the following) were found to be highly syntenic and to contain comparably few gene differences between species. Nevertheless, important species- and subset-specific genes have been described in chromosome-internal regions as well, including members of the aforementioned var, MSP, and SERA gene families in P. falciparum [13], [16], [23] as well as MSP and RAD genes in P. vivax [14], the latter of which has been associated with P. vivax selectivity for young erythrocytes and/or immune evasion [24]. The P. knowlesi genome is particularly rich in chromosome-internal species- and subset-specific genes, which have been identified as surface antigens of the SICAvar and kir gene families, respectively [15].
The fact that parasite genes mediating parasite-host interactions are frequently restricted to a single or a subset of Plasmodium species suggests that the search for species subset-specific genes is a promising strategy to identify new candidate genes underlying host-specific adaptations of Plasmodium species, in particular adaptations to human hosts and anthropophilic mosquito vectors. Identification and characterization of such genes may hold the key for important insights into molecular processes contributing to human disease. For example, parasite-encoded molecular factors that can explain why P. falciparum, P. vivax, and P. knowlesi but not rodent malaria parasites are infectious to humans are currently unknown. The identification of such pathogenicity factors could lead to new strategies to treat malaria in humans. Similarly, genes allowing P. falciparum and P. vivax but not P. knowlesi to complete their life cycle in anthropophilic mosquito vectors have not been identified, although an understanding of the genetic basis of this difference in human transmission success could help to prevent future host switches from monkey to human and pave the way for new transmission blocking strategies. Regarding human virulence it is likely that P. falciparum contains additional virulence genes that await functional characterization. The identification of new virulence genes would enhance our understanding of virulence mechanisms and could lead to new therapeutic interventions to treat severe malaria in humans. Finally, an understanding of the molecular mechanism underlying P. vivax hypnozoite formation is currently entirely missing but urgently needed, because hypnozoites cannot be killed by most available antimalarial drugs and complicate malaria eradication efforts [11].
We hypothesize that chromosome-internal regions of Plasmodium genomes harbour currently unappreciated species differences in the form of protein-coding genes that contribute to human pathogenicity, human-mosquito-human transmissibility, and human virulence. Other than subtelomeric regions, which contain mostly large and readily identifiable species-specific gene families [17], gene differences in chromosome-internal regions are currently largely unexplored [22], [23]. The goal of this study was therefore to systematically identify and characterize species subset-specific genes in chromosome-internal regions of Plasmodium genomes. Although not all subset-specific genes are expected to be functional due to stochastic processes of gene birth-and-death, a recent study in Drosophila has shown that a significant fraction of genes that differ between species do have important phenotypic effects [25]. We focused on four specific comparisons. First, to identify genes possibly linked to human pathogenicity, we determined genes well conserved in the genomes of P. falciparum, P. vivax, and P. knowlesi but absent in rodent malaria parasites, P. chabaudi, P. berghei, and P. yoelii. Second, we identified genes possibly crucial for parasite transmission success between humans by looking for genes present in P. vivax and P. falciparum but absent in the macaque monkey parasite P. knowlesi. Third, we identified genes that possibly contribute to severe human malaria by looking for P. falciparum-specific genes comparing P. falciparum with its less virulent relative P. vivax. Finally, we identified genes that potentially define unique features of P. vivax malaria by looking for genes present in P. vivax but absent in other sequenced Plasmodium genomes. Each of these comparisons resulted in the identification of several species subset-specific genes, most of which with unknown function. We propose these genes as attractive starting points for follow-up experimental analyses to test predicted phenotypic associations and to further elucidate their functions.
Results
Gene model improvement in P. vivax and P. knowlesi
Six published Plasmodium genomes sequenced to high coverage (ranging from 4 to 14.5-fold coverage) were selected for comparison, including the two clinically most important human parasites (P. falciparum and P. vivax), one monkey parasite (P. knowlesi), and three rodent parasites (P. chabaudi, P. berghei, and P. yoelii) (Figure S1). Preliminary examination of P. vivax and P. knowlesi gene models (Text S1) indicated that many gene models in these two genomes are apparently missing or mispredicted. To facilitate comparative analysis, we therefore started our analysis by improving current P. vivax and P. knowlesi gene models with homology-based gene prediction programs, using validated P. falciparum gene models as queries (see Materials and Methods). In total, we identified 53 and 19 new protein-coding genes and revised 165 and 116 existing gene models in P. vivax and P. knowlesi, respectively, including 31 split or merged genes (Table S1 and Table S2). Figure S2 shows four typical examples of improved gene models, including a novel gene, a split gene that was merged, a merged gene that was split, and an elongated gene model.
Rodent pathogens likely defective in thiamine (vitamin B1) biosynthesis
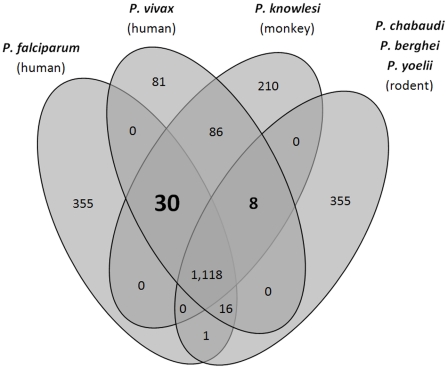
Comparative analyses of the complete proteomes of P. falciparum, P. yoelii, P. berghei and P. chabaudi, and the improved proteomes of P. vivax and P. knowlesi using BLASTP and genBlastG (see Materials and Methods) identified 30 proteins well conserved in the three primate parasites (percent identity (PID) of global protein sequence alignment ≥40) that are putatively absent in all three rodent parasites (global PID≤15) (Figure 1). Examination of their identifiable syntenic genomic regions provided additional support for the absence of 16 of these putatively absent genes, i.e. no orthologous genes were found at the corresponding genomic positions in the rodent parasite genomes.
Figure 1. Proteome comparison reveals 30 proteins conserved in primate malaria parasites but absent in rodent malaria parasites.
Genes were considered specific to a group of parasites if conserved in all in-group species (global protein sequence PID> = 40) but not in any of the out-group species (PID< = 15). In particular, primate-parasite specific genes are genes conserved in the three primate parasite proteomes but not in any of the three rodent parasite proteomes. The Venn diagram shows numbers of species subset-specific genes identified for all possible species combinations. Putative primate parasite-specific genes (30) are shown in bold. Note that gene numbers do not add up to species totals because genes with PIDs between 15% and 40% are not included.
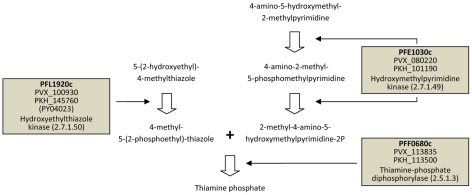
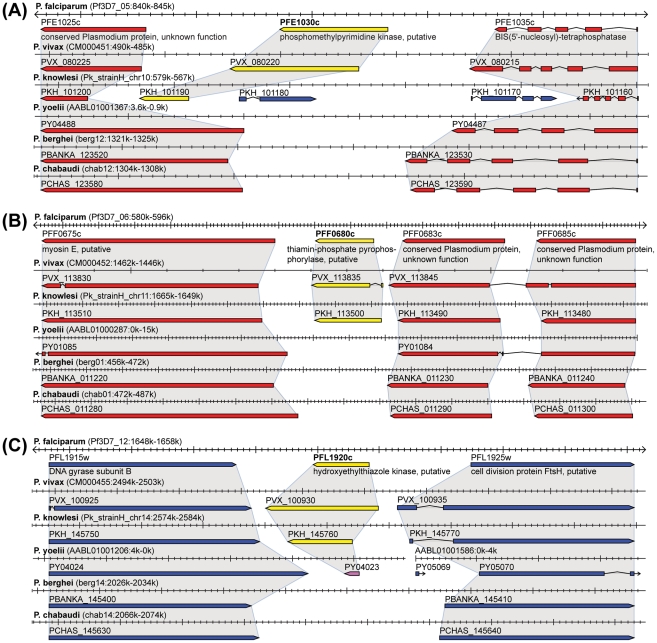
Table 1 shows P. falciparum orthologs of these 16 putative primate parasite-specific genes together with their degree of conservation in P. vivax, functional annotations, and expression profiles (see Table S9 for a list of the same genes but including graphical expression profiles). Among them are three key metabolic enzymes of the thiamine (vitamin B1) biosynthesis pathway: PFL1920c (hydroxyethylthiazole kinase, EC 2.7.1.50), PFE1030c (hydroxylmethylpyrimidine kinase, EC 2.7.1.49), and PFF0680c (thiamine-phosphate diphosphorylase, EC 2.5.1.3). Together, these three genes catalyze essential steps in the de novo synthesis of vitamin B1 (Figure 2). With one exception in P. yoelii, which appears to be a gene relic that should be annotated as a pseudogene, orthologs of all three P. falciparum genes are absent from three independent syntenic positions in all three sequenced rodent pathogen genomes (Figure 3). This data strongly suggests that primate but not rodent malaria parasites are capable of synthesizing vitamin B1 de novo.
Table 1. Genes conserved in primate parasites but absent in rodent parasites.
| PfGene (PvPID/OG) | Product | RNA-seq expr. (IDC) | Protein expr. | GO biological process | Additional information |
| MAL13P1.214 (64/VIRI) | Phosphoethanolamine N-methyltransferase | Min: 0 hMax: 30 h | MZ;GC;SC;SZ;RU;TZ | Phosphatidylcholine biosynth. pr. | methionine and polyamine metabolism |
| MAL8P1.202 (53/ALVE) | apicoplast phosphate-idic acid phosphatase | Min: 15 hMax: 30 h | - | - | dolichol metabolism; SP |
| PF14_0565(44/----) | unknown function | Min: 10 hMax: 35 h | MZ | - | WD40 repeat-like |
| PF14_0036(69/ALVE) | acid phosphatase, putative | Min: 10 hMax: 25 h | MZ;GC;SC;TZ;RU | - | hydrolase activity |
| PF11_0186(65/----) | unknown function | Min: 10 hMax: 35 h | GC;SZ | - | - |
| PF14_0662(50/ALVE) | nucleoside transporter, putative | Min: 15 hMax: 30 h | - | - | nucleoside transmembrane transporter |
| PFF0680c(40/FIRM) | thiamin-phosphate pyrophosphorylase | Min: 15 hMax: 35 h | MZ | thiamine bio-synthetic process | - |
| PFE1030c(67/PROT) | phosphomethylpyrimidine kinase, putative | Min: 20 hMax: 35 h | SC;SZ;RU | thiamine bio-synthetic process | - |
| PFL1920c(76/FIRM) | hydroxyethylthiazole kinase, putative | Min: 15 hMax: 30 h | MZ;GC;TZ;SC;RU | thiamine biosynthetic process | - |
| MAL7P1.339 (46/ALVE) | Ca++ chelating serine protease, putative | Min: 20 hMax: 30 h | - | - | SCP/Tpx-1/Ag5/PR-1/Sc7 family; SP |
| PFI0405w(46/----) | unknown function | Min: 20 hMax: 35 h | - | 1 TM; AP | |
| MAL8P1.111 (42/ALVE) | JmjC domain containing protein | Min: 20 hMax: 35 h | GC | - | [Histone H3]-lysine-36 demethylase |
| PFL2255w(69/ALVE) | unknown function | Min: 25 hMax: 35 h | - | ubiquitin cycle | - |
| PFL1840w(66/ALVE) | unknown function | Min: 30 hMax: 40 h | GC;SZ | - | SP; 4 TM; COPI associated |
| PFL0305c(85/ALVE) | IMP-specific 5′-nucleotidase, putative | Min: -Max: - | GC; oocyst SZ | nucleotide metabolic process | magnesium ion binding; phosphatase activity |
| PFI1220w(47/ALVE) | unknown function | Min: -Max: - | - | - | acyl-CoA N-acyltransfer-ase; upregul. in GC/SZ |
Genes conserved in P. falciparum, P. vivax, and P. knowlesi but absent in P. berghei, P. chabaudi, and P. yoelii as determined by genome-wide genBlastG searches and examination of syntenic genomic regions. Min and Max RNA-seq expression according to scaled expression values from the intraerythrocytic developmental cycle (IDC) as reported by [58]. A table with graphical RNA-seq expression profiles for these genes is provided in Table S9. Abbreviations: Pf: P. falciparum; Pv: P. vivax; PvPID: global protein sequence identity with P. vivax ortholog; OG: closest OrthoMCL DB species out-group with predicted ortholog of this gene; VIRI: Viridiplantae; ALVE: Alveolates; FIRM: Firmicutes; PROT: Proteobacteria; GO: gene ontology; SZ: sporozoites; (el)GC: (early/late) gametocytes; TZ: trophozoite; MZ: merozoites; SC: schizont; RU: rupture; AP: targeted to apicoplast; TM: predicted transmembrane domain; SP: predicted signal peptide.
Figure 2. Rodent malaria parasites likely deficient in de novo synthesis of thiamine (vitamin B1).
The diagram illustrates catalytic steps of the thiamine biosynthesis pathway in P. falciparum, with 4-amino-5-hydroxymethyl-2-methylpyrimidine and 5-(2-hydroxyethyl)-4-methylthiazole as start products and thiamine phosphate as the end product. The three enzymes predicted to be absent in rodent malaria parasites catalyze subsequent reactions in this pathway, suggesting that rodent malaria parasites are deficient in de novo synthesis of vitamin B1. Gene identifiers correspond to P. falciparum genes (bold) and their P. vivax and P. knowlesi orthologs (below). PFL1920c has a predicted but severely truncated ortholog in P. yoelii (PY04023). Figure based on pathway shown in the Malaria Parasite Metabolic Pathways (MPMP) database [26].
Figure 3. Syntenic orthologs of thiamine biosynthesis genes present in primate but not rodent parasites.
Each panel shows one of the three P. falciparum thiamine biosynthesis gene on top (gene identifier in bold) and syntenic genomic regions in P. vivax, P. knowlesi, P. yoelii, P. berghei, and P. chabaudi below. Shaded areas indicate orthology. Thiamine biosynthesis genes displayed in yellow. Flanking genes and their orthologs on forward and reverse strand are shown in blue and red, respectively. The three P. falciparum genes are located on three different chromosomes and in all three cases syntenic orthologs are present in primate but not rodent parasite genomes. The syntenic P. yoelii ortholog of PFL1920c (PY04023, Panel C) is an exception but appears to be a truncated gene relic that should be annotated as pseudogene. Orthologs of PFF0683c and PFF0685c (Panel B) are merged into single genes in P. vivax and P. yoelii and should be split. Images adapted from PlasmoDB 8.0.
Besides the three thiamine biosynthesis enzymes, Table 1 reveals additional enzyme-coding genes conserved in primate but absent in rodent malaria parasites. This includes an acid phosphatase (PF14_0036) involved in riboflavin (vitamin B2) metabolism [26], [27], a highly conserved putative IMP-specific 5′-nucleotidase (PFL0305c) that is involved in purine metabolism, an apicoplast phosphatidic acid phosphatase (MAL8P1.202) catalyzing the production of diacylglycerol as part of the dolichol metabolism [26], as well as two enzymes previously described as absent in rodent malaria parasites, including phosphoethanolamine N-methyltransferase (MAL13P1.214) that plays a role in phospholipid metabolism [28], and Jumonji domain containing protein (MAL8P1.111) serving as one of two functionally distinct P. falciparum histone lysine demethylases [29]. Other functionally annotated proteins conserved in primate malaria parasites but absent in rodent malaria parasites include a putative nucleoside transporter (PF14_0662), a putative acyl-CoA N-acyltransferase (PFI1220w) specifically upregulated in gametocytes and sporozoites [30], and a putative Ca++ chelating serine protease (MAL7P1.339). Taken together, the three primate parasites infectious to humans maintain a limited but conserved subset of genes that is absent in rodent malaria parasites, pointing towards new candidate pathogenicity genes required for parasitizing primate hosts, including humans.
Synteny analysis reveals numerous gene differences in chromosome-internal regions
Further comparisons of the genomes of the three primate parasites P. falciparum, P. vivax, and P. knowlesi were performed using whole-genome synteny analysis. Synteny blocks were detected with OrthoCluster [31] based on our improved gene sets and orthology relationships predicted by Inparanoid (see Materials and Methods). Because our goal was to identify parasite-specific genes in syntenic chromosome-internal regions, we focused on the detection of imperfect synteny blocks (i.e. synteny blocks allowing for minor interruptions) and non-nested synteny blocks (i.e. synteny blocks not contained within larger synteny blocks due to one-to-many orthologous relationships) [32]. Note that in the context of pairwise synteny analysis we refer to genes without predicted ortholog in the other species as parasite-specific and not species-specific, because some of these genes might have predicted orthologs in other species.
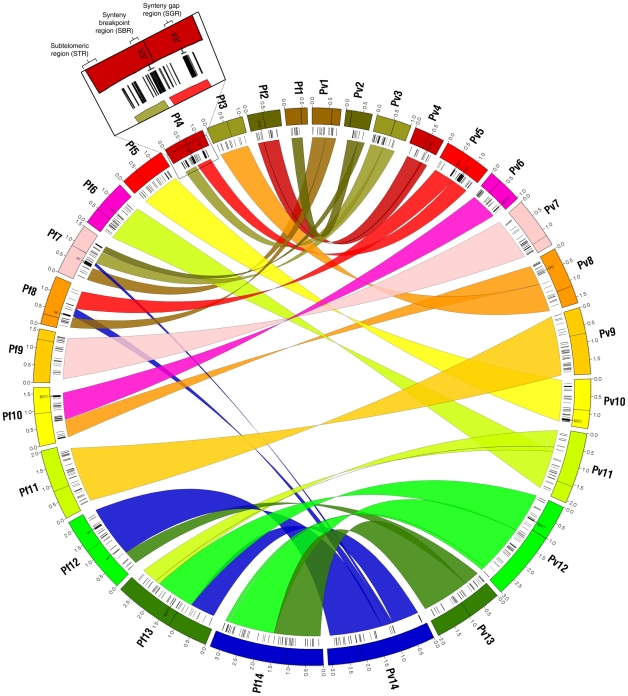
Between the two human parasites P. falciparum and P. vivax (Figure 4 and Table S3), we identified 28 non-nested imperfect synteny blocks with a median size of 144.5 genes (563.7 kb) that collectively cover 90% of protein-coding genes or 85% of the nuclear genome sequence. Between P. vivax and P. knowlesi (Figure 5 and Table S3), OrthoCluster identified 16 non-nested imperfect synteny blocks of median size 300 genes or 1,376 kb (average of both genomes), each of them essentially spanning complete chromosomes with two exceptions on P. vivax chromosomes 3 and 4 (Text S1). OrthoCluster output files listing all detected synteny blocks and their genes are provided in Dataset S2 and Dataset S3. A more detailed discussion of synteny block analysis results can be found in the Supporting Information (Text S1).
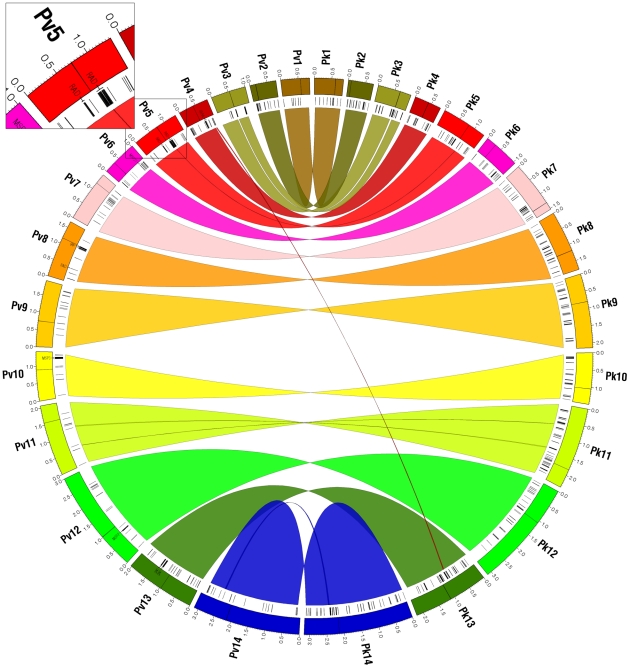
Figure 4. P. falciparum and P. vivax share extensive synteny with hundreds of putative parasite-specific genes in chromosome-internal regions.
Outer segments depict the 14 nuclear chromosomes of P. falciparum (left semicircle, counter-clockwise) and P. vivax (right semicircle, clockwise). Each chromosome is assigned a different color. Ribbons indicate the 29 identified imperfect synteny blocks (28 non-nested and 1 nested) colored according to connected P. vivax chromosomes. Black tick marks underneath chromosomes indicate putative parasite-specific genes located at synteny gap regions (SGR) and synteny breakpoint regions (SBR) (see inset). Parasite-specific genes in subtelomeric regions (STRs) not shown. Text labels within chromosomes indicate parasite-specific genes mentioned in the text, including the newly identified putative MSP3 gene cluster on P. vivax chromosome 6. Black lines within chromosomes indicate putative centromeres. In both species, chromosome-internal regions contain hundreds of putative parasite-specific genes (388 in both species). Image created with Circos [56].
Figure 5. P. vivax and P. knowlesi share almost perfect 1-to-1 chromosomal synteny but also harbor hundreds of putative parasite-specific genes in chromosome-internal regions.
Outer segments depict the 14 nuclear chromosomes of P. vivax (left semicircle, counter-clockwise) and P. knowlesi (right semicircle, clockwise). Ribbons represent the 20 identified imperfect synteny blocks (both nested and non-nested) colored according to connected P. vivax chromosomes. Black tick marks underneath chromosomes indicate putative P. vivax-specific genes (281) and P. knowlesi-specific genes (364) located at SGRs and SBRs. Parasite-specific genes in subtelomeric regions (STRs) not shown. Text labels within chromosomes indicate parasite-specific genes mentioned in the text. The inset shows the largest identified SGR in P. vivax containing 26 RAD genes. Black lines within chromosomes indicate putative centromeres. Excluding subtelomeres, imperfect synteny blocks span complete chromosomes with only two exceptions (P. vivax chromosomes 3 and 4), but also contain many putative parasite-specific genes, particularly in P. knowlesi. Image created with Circos [56].
To better characterize parasite-specific genes revealed by synteny block analysis, we define three different types of non-syntenic regions (inset Figure 4 and Figure S3). A subtelomeric region (STR) is defined as the genomic region from the most distal gene on a chromosome arm to the first syntenic gene that is part of an imperfect synteny block (there are two such STRs on each chromosome). A synteny breakpoint region (SBR) is defined as a genomic region between imperfect synteny blocks. A synteny gap region (SGR) is defined as the genomic region that interrupts perfect synteny within imperfect synteny blocks due to the presence of one or more consecutive non-syntenic genes (defined here either as parasite-specific genes that do not have a predicted ortholog in the compared species or as genes that do have a predicted ortholog in the compared species but not syntenic).
Detected imperfect synteny blocks allowed us to examine chromosome-internal parasite-specific genes within their syntenic contexts (Text S1). In total, syntenic examination confirmed 117 of 388 (30%) P. falciparum-specific genes and 173 of 388 (45%) P. vivax-specific genes in chromosome-internal regions between P. falciparum and P. vivax (Figure 6). Between P. vivax and P. knowlesi, 139 of 281 (49%) P. vivax-specific genes and 222 of 364 (61%) P. knowlesi-specific genes were confirmed (Figure 7). Thus, depending on the comparison, SGRs and SBRs were found to contain 16–58% of the total number of parasite-specific genes in each species (Text S1 and Table S6), representing a considerable amount of the total parasite-specific gene content in each species. The gene content of these SGRs and SBRs is discussed in the following sections.
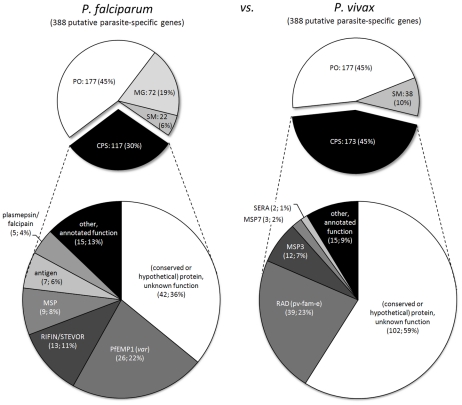
Figure 6. P. falciparum-specific genes in chromosome-internal regions enriched with virulence genes.
Putative parasite-specific genes identified at SGRs and SBRs between P. falciparum (388 genes) and P. vivax (388 genes) were examined within their syntenic context (upper two diagrams). Differences considered as non-reliable were excluded from further analysis, including positional orthologs (PO), potential missing genes (MG), and potential split or merged genes (SM). Confirmed parasite-specific (CPS) genes were examined for annotated functions (lower two diagrams). P. falciparum-specific genes (lower left diagram) were found to be enriched (FDR-adjusted p-value<0.05) for known virulence factors with associated GO biological processes pathogenesis (GO:0009405), adhesion to host (GO:0044406), cell adhesion (GO:0007155), and defense response (GO:0006952), suggesting potential virulence-associated functions for genes currently not implicated in human virulence.
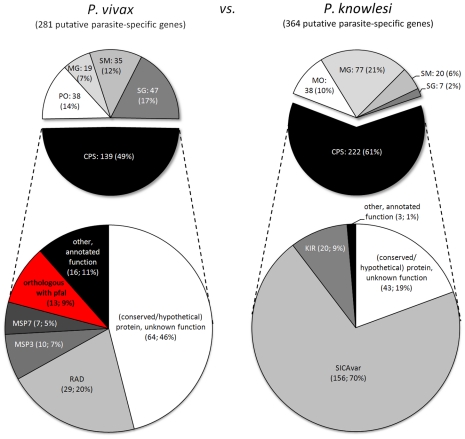
Figure 7. Human parasites P. falciparum and P. vivax share 13 syntenic orthologs that are absent in the monkey parasite P. knowlesi.
Putative chromosome-internal parasite-specific genes between the human parasite P. vivax (281 genes) and the closely related macaque monkey parasite P. knowlesi (364 genes) were examined within their syntenic context. Slices in white and shades of gray in the upper two diagrams show excluded questionable parasite-specific genes, including differences due to positional orthologs (PO), potential missing genes (MG), sequence gaps (SG), and potential split/merged genes (SM). Black slices represent confirmed parasite-specific (CPS) genes examined for their function. Of the 139 confirmed P. vivax-specific genes, 13 genes (9%, shown in red and in Table 2) have a syntenic ortholog in P. falciparum and thus represent genes present in both human parasites but absent in P. knowlesi.
Human parasites share genes absent in P. knowlesi that are specifically up-regulated in gametocytes or sporozoites
Although P. vivax and P. knowlesi are phylogenetically much more closely related to each other than P. vivax is to P. falciparum (Figure S1 and [33]), we identified 13 genes that are syntenic orthologs between P. vivax and P. falciparum but absent from syntenic regions in P. knowlesi (Figure 7, red slice). Indeed, orthologs of those genes were not found anywhere in the P. knowlesi genome, even after screening P. knowlesi genomic sequences with genBlastG to account for unannotated genes (see Materials and Methods).
Table 2 shows P. falciparum orthologs of these 13 genes together with their degree of conservation in P. vivax and gene expression data (see Table S10 for a list of the same genes but including graphical expression profiles). Three genes (PFA0380w, PF14_0236, and PF10_0185) show no or only weak expression in the intraerythrocytic developmental cycle (IDC) and were found to be specifically upregulated in gametocytes or sporozoites [30], which is consistent with the possibility that they may play a role in parasite development within the mosquito host and hence transmission success between humans. One of these three genes (PFA0380w) is annotated as putative serine/threonine kinase. NCBI BLASTP search with this gene revealed that it is much closer related to its P. vivax ortholog (PVX_081395; E = 9e-66; PID = 54%) than to any P. falciparum paralog (best hit PF13_0258 (TKL3); E = 0.008; PID = 48%), suggesting that the presence of this gene is of functional importance.
Table 2. Genes shared between P. falciparum and P. vivax but absent in P. knowlesi.
| PfGene (PvPID/OG) | Product | RNA-seq. expr. (IDC) | Protein expr. | Additional information |
| PFI1405c(44/RODE) | unknown function | Min: 15 hMax: 25 h | TZ; iRBCm | C-terminal TM (InterProScan); antigenic variation (PlasmoDraft) |
| MAL8P1.126(35/ALVE) | serine protease, putative | Min: 10 hMax: 35 h | oocyst SZ | Deg2 chloroplast peptidase (MEROPS); sole member of clan PA/family S1; SP |
| PF14_0454(23/RODE) | unknown function | Min: 10 hMax: 35 h | SZ;TZ; (el)GC | calponin-like actin-binding; winged-helix DNA binding; defense response (PlasmoDraft) |
| PF11_0460(19/RODE) | unknown function | Min: 20 hMax: 35 h | GC; MZ; eStages | upregulated in GC |
| PFL0170w(19/RODE) | transporter, putative | Min: 20 hMax: 35 h | (l)GC; TZ | 12 TM; MFS general substrate transporter |
| PF11_0361-a(55/RODE) | unknown function | Min: 20 hMax: 35 h | - | 6 TM; PQ-loop repeat; SP |
| PF11_0134(52/RODE) | unknown function | Min: 20 hMax: 35 h | - | TM; DUF1704 member (conserved in many species) |
| MAL13P1.107(34/----) | unknown function | Min: 20 hMax: 35 h | SZ; (l)GC | SP and GPI-anchor; similarity with neighboring rhoptry protein 2 (PF13_0116) |
| PFL0360c(8/----) | unknown function | Min: 25 hMax: 35 h | - | 3 TM; ZF; divergent Pv positional ortholog; similar to serine protease (PlasmoDB) |
| PF14_0236(12/----) | unknown function | Min: 25 hMax: 35 h | SZ; MZ | ZF; divergent Pv positional ortholog; antigenic variation (PlasmoDraft); upregul. in GC |
| PFI1216w(52/RODE) | telomeric repeat binding factor 1 | Min: -Max: - | - | homeodomain-like; SANT, DNA binding; MYB-like; EST support |
| PFA0380w(10/RODE) | serine/threonine kinase, putative | Min: -Max: - | SZ | N-terminal TM; divergent Pv positional ortholog; EST support; upregulated in SZ |
| PF10_0185(28/RODE) | unknown function | Min: -Max: - | - | EST support; upregulated in GC |
Shown are all P. falciparum genes that were found to have a syntenic ortholog in the second human parasite P. vivax but no identifiable ortholog (neither syntenic nor non-syntenic) in the monkey parasite P. knowlesi. Four of these genes (MAL8P1.126, MAL13P1.107, PFL0360c, and PF14_0236) also lack an identifiable ortholog in the three rodent parasite genomes and are thus potentially unique to human Plasmodium parasites. Genes ordered and grouped by similarity of their IDC expression profile (see legend Table 1). A table with graphical RNA-seq expression profiles for these genes is provided in Table S10. Abbreviations: Pf: P. falciparum; Pv: P. vivax; PvPID: global protein sequence identity with P. vivax ortholog; OG: closest OrthoMCL DB species out-group with predicted ortholog of this gene; ALVE: Alveolates; RODE: rodent malaria parasites; iRBCm: infected red blood cell membrane (PIESPs)/Schizont; SZ: sporozoites; (el)GC: (early/late) gametocytes; TZ: trophozoite; MZ: merozoites; SC: schizont; ZF: zinc finger domain; TM: predicted transmembrane domain; SP: predicted signal peptide.
Four genes in Table 2 (MAL13P1.107, MAL8P1.126, PF14_0236, and PFL0360c) lack orthologs also in rodent malaria parasites and are thus potentially unique to human malaria parasites. Three of these genes show only weak expression in the intraerythrocytic developmental cycle (IDC) and two protein products were detected in sporozoites, again pointing towards a possible role of these genes in parasite development within the mosquito host. MAL13P1.107 shows sequence similarity (BLASTP PID 30, E = 2e-33) with the neighboring gene rhoptry protein 2 [34], suggesting a function during host cell invasion. MAL8P1.126 is annotated as Deg2 chloroplast peptidases and is the sole P. falciparum member of clan PA [26]. NCBI BLASTP and TBLASTN searches revealed that MAL8P1.126 is conserved in other Apicomplexa species but not non-human malaria parasites, suggesting functional gene loss in non-human malaria parasites. It should be emphasized that a syntenic gene of MAL8P1.126 annotated as DegP-like serine protease 1 precursor is present in P. knowlesi (PKH_011050) but much shorter (409 aa) with very low sequence similarity to MAL8P1.126 (global PID 8; BLASTP e = 6e-7). Thus PKH_011050 is probably a non-functional pseudogene. The remaining two putative human malaria parasite-specific genes (PF14_0236 and PFL0360c) have no annotated function. Both contain a predicted zinc finger domain and PF14_0236 is predicted to be involved in antigenic variation [35] and PFL0360c shows similarity to a serine protease [36].
We found indications that P. knowlesi could lack a functional copy of telomeric repeat binding factor 1 (TRF1). Running GeneWise with TRF1 of P. falciparum (PFI1216w) against the syntenic region in P. knowlesi reveals only residual protein sequence similarity (24% global PID), which is well below the degree of conservation found with P. vivax (52% PID) and probably indicative of recent gene inactivation in P. knowlesi. Both BLASTP and TBLASTN searches using PFI1216w as query against the complete P. knowlesi genome revealed the syntenic region of PFI1216w as best hit. Although almost certainly not linked to parasite transmission success, the potential absence of a fully functional copy of TRF1 in P. knowlesi is interesting, because it could offer an explanation for the presence of hundreds of variant surface antigens and telomeric repeats in chromosome-internal regions of the P. knowlesi genome (see Discussion).
Functions of the remaining genes shared by P. falciparum and P. vivax but absent in P. knowlesi (Table 2) remain largely unknown. Notably, the 13 identified genes are statistically significantly enriched (p = 0.0159) for genes whose expression is induced during the trophozoite stage (20 h post infection) and peaks during the schizont stage (36 h post infection). It remains to be determined if these genes are therefore also functionally related.
Chromosome-internal P. falciparum-specific genes enriched with virulence genes
Looking at parasite-specific genes identified between the highly virulent parasite P. falciparum and the less virulent human parasite P. vivax, our analysis recovers many known human virulence genes in P. falciparum (Figure 6, bottom left). The largest fraction (26 genes, 22%) of the 117 chromosome-internal P. falciparum-specific genes is annotated as chromosome-internal members of the var gene family, the prime virulence factors of P. falciparum [18]. GO term enrichment analysis with Ontologizer [37] reveals that the 117 P. falciparum-specific genes are statistically significantly enriched for GO biological processes pathogenesis (GO:0009405; FDR-adjusted p-value = 9e-5) and adhesion to host (GO:0044406; p = 0.02), mostly because of the presence of these 26 var genes (Table S7). The two second largest subgroups of P. falciparum-specific genes are also involved in important pathogenic processes, including nine MSPs and 13 members of the rif/stevor gene family. Enriched GO terms associated with these genes include cell adhesion (GO:0007155; p = 2e-25) and defense response (GO:0006952; p = 3e-6). More generally, we find P. falciparum-specific genes enriched for GO subcellular locations membrane (GO:0016020; p = 4e-10) and host intracellular part (GO:0033646; p = 8e-4), indicating enrichment for proteins functional at the parasite-host interface.
To identify novel candidate genes potentially linked to severe P. falciparum malaria, we removed known virulence genes and retained genes that contain features commonly associated with human virulence genes, including PEXEL motifs, signal peptides, or transmembrane domains. In addition, we retained P. falciparum-specific genes predicted to have virulence-associated functions based on gene co-expression or protein interaction data with known virulence genes (‘guilt-by-association’ principle) [35], [38].
Among the resulting 15 genes (Table 3 and Table S11) we found two genes with annotated functions, including a putative apyrase (PF14_0297) and a putative sugar transporter (PFE1455w) (see Discussion). The remaining 13 genes are of unknown function. Four genes have predicted human virulence-associated functions based on gene co-expression or protein interaction data [35], [38], including evasion of host defense (PF07_0107), antigenic variation (PFA0360c), biological adhesion (PF10_0350), and immune response (PF10_0044). PlasmoDB annotates another two genes with GO terms cell adhesion (PF13_0071) and immune response (MAL8P1.97), respectively. Eleven genes carry a predicted signal peptide or transmembrane domain and thus potentially function at the parasite-host interface. One of them (PF07_0107) carries an additional PEXEL motif and is thus a predicted erythrocyte surface or exported protein. Looking at RNA-seq expression data for genes with unknown function, all but two genes (MAL8P1.97 and PF10_0044) have associated expression evidence during the intraerythrocytic developmental cycle (IDC). Two genes (PF07_0107 and PF13_0194) are constitutively expressed at high levels, one gene peaks at the trophozoite stage (PF10_0350), seven genes peak at the late trophozoite/early schizont stage, and one gene (PFF0335c) peaks during schizont development. Two genes (PF10_0357 and PF10_0342) appear maximally expressed during the schizont-ring stage transition and co-localize with the MSP3 gene cluster on P. falciparum chromosome 10, suggesting a function in erythrocyte invasion.
Table 3. P. falciparum genes absent in P. vivax with possible role in human virulence.
| PfGene (OG) | Product | RNA-seq expr. (IDC) | Protein expr. | Additional information |
| PF07_0107(----) | exported protein, unknown function | Min: 40 hMax: 25 h | - | 2 TM domains; evasion of host defense (PlasmoDraft, 80%); PX |
| PF13_0194(----) | probable protein, unknown function | Min: 0 hMax: 35 h | TZ | chr13 MSP7-like gene cluster; SP; 1 TM domain; similar to MSP7-like |
| PF10_0350(----) | probable protein, unknown function | Min: 15 hMax: 25 h | - | chr10 MSP gene cluster; SP and GPI anchor; biological adhesion (PlasmoDraft, 66%) |
| PF13_0071(----) | probable protein, unknown function | Min: 15 hMax: 30 h | TZ;SC;RU;SZ | cell adhesion, phosphate transport (PlasmoDB) |
| PF13_0192(----) | conserved, unknown function | Min: 20 hMax: 30 h | TZ;SC;RU;iRBCm | chr13 MSP7-like gene cluster; 2 TM domains; NTP activity (PlasmoDraft) |
| MAL13P1.106(----) | probable protein, unknown function | Min: 15 hMax: 30 h | (el)GC | SP; 1 TM domain; upregulated in GC |
| PF14_0708(PLASM)¥ | probable protein, unknown function | Min: 20 hMax: 35 h | eGC;MZ | 2 TM domains; upregulated in GC; predicted paralog in P. falciparum (MAL8P1.95) |
| PF14_0297(ALVE) | apyrase, putative | Min: 20 hMax: 30 h | - | predicted signal anchor and 2 TM domains; converts ATP to AMP; purine metabolism |
| PFE1455w(PROT) | sugar transporter, putative | Min: 20 hMax: 35 h | - | 12 TM domains; glycoside-pentoside-hexuronide:cation symporter (GPH) family |
| PFA0360c(----) | conserved, unknown function | Min: 25 hMax: 30 h | - | SP; 1 TM domain; antigenic variation (OPI); upregulated in GC & SZ |
| PFF0335c(----) | probable protein, unknown function | Min: 25 hMax: 35 h | eGC;TZ;MZ;eSC;RU | membrane protein from schizonts; possible rhoptry/surface protein; SP |
| PF10_0357(----) | probable protein, unknown function | Min: 20 hMax: 0 h | - | chr10 MSP gene cluster; conserved RESA domain (CCD: PTZ00341) |
| PF10_0342(----) | probable protein, unknown function | Min: 30 hMax: 40 h | - | chr10 MSP gene cluster; SP |
| MAL8P1.97(ALVE) | hypothetical protein | Min: -Max: - | oocyst SZ | 12 TM domains; immune response, MDR trans-porter domain (PlasmoDB); homolog in T. parva |
| PF10_0044(----) | hypothetical protein | Min: -Max: - | - | WD40 repeat-like; full EST support; immune response (PlasmoDraft, 80%); upregulated in SZ |
Selected subset of identified chromosome-internal genes present in the highly virulent human parasite P. falciparum but absent in the less virulent human parasite P. vivax. Potential virulence-associated function of these genes is predicted based on characteristics shared with known virulence factors, including the presence of a PEXEL motif, signal peptides, and transmembrane domains, or is predicted based on co-expression and protein interaction data [35], [38]. Genes ordered and grouped by similarity of their IDC expression profile (see legend Table 1). A table with graphical RNA-seq expression profiles for these genes is provided in Table S11. Abbreviations: OG: closest OrthoMCL DB species out-group with predicted ortholog of this gene; PLASM: Plasmodium; ALVE: Alveolates; PROT: Proteobacteria; SZ: sporozoites; (el)GC: (early/late) gametocytes; TZ: trophozoite; MZ: merozoites; SC: schizont; iRBCm: infected red blood cell membrane (PIESPs)/Schizont; RU: erythrocyte rupture; OPI: ontology-based pattern identification; TM: predicted transmembrane; SP: predicted signal peptide; PX: PEXEL export motif. ¥ PF14_0708 has a predicted OrthoMCL DB ortholog in P. vivax (PVX_123110), but is present as extra copy in P. falciparum.
Uncharacterized gene cluster on P. vivax chromosome 6 possibly involved in erythrocyte invasion
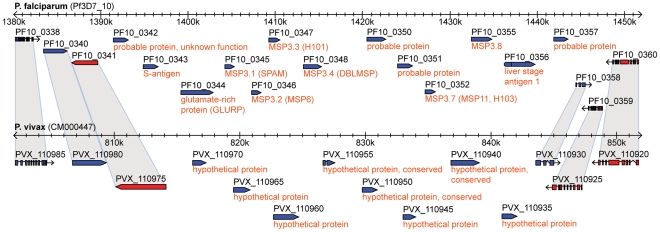
Of the 173 identified P. vivax-specific genes compared to P. falciparum (Figure 6, bottom right), the largest group of genes with named gene products contains members of the previously mentioned RAD gene family (39 genes, 23%), followed by MSP3 genes (12, 7%), and MSP7 genes (3, 2%). Among genes with unannotated function (102 genes, 59%), we identified an interesting and currently uncharacterized P. vivax gene cluster of hypothetical proteins likely involved in erythrocyte invasion (Figure 8). This gene cluster is found on P. vivax chromosome 6 (position 815,000 to 842,000) and contains eight single-exon genes located on the same strand. The syntenic genomic region in P. falciparum maps to the MSP3 gene cluster on chromosome 10 (position 1,390,000 to 1,444,000), which harbors 13 P. falciparum-specific single-exon genes also located on the same strand. The P. falciparum gene cluster consists of several known antigens and genes involved in erythrocyte invasion, including six members of the MSP3 gene family (including MSP6), the glutamate-rich protein (GLURP) as well as the S-antigen (PF10_0343) and liver stage antigen 1 (PF10_0356). All but one of these genes (PF10_0343) have no predicted ortholog in P. vivax. It is possible that the P. vivax genes in the syntenic gene cluster on chromosome 6 have a similar function as the P. falciparum-specific genes on chromosome 10, which makes them prime candidate genes involved in erythrocyte invasion and interesting targets for further functional characterization. Three other lines of evidence support this conclusion. First, for all but one (PVX_110955) of these eight P. vivax genes, top P. falciparum BLASTP hits fall into the syntenic P. falciparum gene cluster (E-value≤0.05; PID≥28%). Second, all eight P. vivax genes carry a predicted signal peptide and are thus likely exported proteins. Third, four P. vivax genes (PVX_110945, PVX_088845, PVX_099900, and PVX_089440) peak in expression during the schizont-ring stage transition, which is typical for invasion-related proteins [24].
Figure 8. Uncharacterized genes on P. vivax chromosome 6 possibly involved in erythrocyte invasion.
The upper part of the figure shows a genomic region on P. falciparum chromosome 10 with a cluster of 13 P. falciparum-specific genes, including the S-antigen, liver stage antigen 1 (LSA1), and five members of the MSP3 gene family, including MSP6. The lower part of the figure shows the syntenic genomic region on P. vivax chromosome 6 containing a cluster of eight P. vivax hypothetical proteins. Shaded segments indicate orthology. Genes on the forward strand are shown in blue, genes on the reverse strand shown in red. Figure adapted from PlasmoDB 8.0.
We identified only few chromosome-internal P. vivax-specific genes absent in both P. falciparum and P. knowlesi that could explain unique biological features of P. vivax malaria, in particular the formation of hypnozoites. After excluding questionable open reading frames, only six candidate genes remained (see Materials and Methods). One gene (PVX_099470) has an annotated function and is one of 25 WD domain, G-beta repeat domain containing proteins in P. vivax, all of which occur chromosome-internally. WD-repeat proteins are a large family of proteins found in all eukaryotes and are implicated in a variety of functions, ranging from signal transduction and transcription regulation to cell cycle control and apoptosis. Using PVX_099470 as query, GeneWise predicts a severely truncated syntenic pseudogene in P. knowlesi with high identity (56% PID), suggesting recent gene inactivation in P. knowlesi. The other five genes have unknown functions. Four genes (PVX_089770, PVX_097730, PVX_110945, and PVX_082710) localize to chromosome-internal RAD, MSP3 (chromosome 10), MSP3 (chromosome 6, putative), and MSP7 gene clusters, respectively, and are thus possibly functionally related to these gene families. The remaining gene (PVX_003710) is a 154 aa single-exon gene with EST expression evidence but of unknown function.
Discussion
In this study, we compared the genomes of six Plasmodium species and proposed several chromosome-internal genes as new candidate genes underlying medically important phenotypic differences, including human pathogenicity, human-mosquito-human transmissibility, and human virulence. Previous studies have shown that important molecular processes at the parasite-host interface, including cytoadherence [13], [18], immune evasion [19], and erythrocyte invasion [20], are typically mediated by species- or species subset-specific genes and that these genes cluster at subtelomeric regions of chromosomes. We hypothesized that human parasites harbor additional human virulence- and pathogenicity genes in chromosome-internal regions. Although we expect parasite virulence and pathogenicity to be primarily the result of gene gain or retention, another possibility not further explored here is that some virulence and pathogenicity is the consequence of adaptive gene loss, as observed in bacteria [39].
We identified 16 genes that are well conserved in the three primate parasites causing human disease but are not found in rodent parasites. Some of these genes could be determinants of primate (and thus human) pathogenicity (Table 1). Most of these 16 genes (9 genes) have predicted OrthoMCL DB orthologs in other Alveolate species (Table 1), suggesting that gene loss in rodent malaria parasites caused these species differences. Multiple lines of evidence suggest that these 16 genes are indeed absent in rodent malaria parasite genomes. First, none of these genes has a predicted Inparanoid or OrthoMCL DB ortholog (neither syntenic nor non-syntenic) in any of the three closely related rodent malaria parasite genomes. Second, screening complete genomic sequences (including the nearly complete chromosome-level assemblies of P. chabaudi and P. berghei available at PlasmoDB 7.1) with genBlastG did not identify these genes in any of the three rodent malaria parasite genomes. If these genes were present in rodent malaria genomes but mis- or unannotated, then we would expect genBlastG to find them, because all 16 P. falciparum genes are well conserved in P. vivax and P. knowlesi, which have a similar phylogenetic distance to P. falciparum as the three rodent parasites. Finally, syntenic genomic regions as determined by flanking orthologs are present in latest chromosome-level assemblies of P. berghei and P. chabaudi and are assembled without gaps, making it less likely that these genes are absent in rodent parasite genomes due to incomplete genome sequences or assemblies. In some cases as shown in Figure 3, we even find evidence of residual sequence similarity in syntenic regions, which is best explained by (recent) gene loss in rodent malaria parasites. Thus our bioinformatics analysis strongly suggests that these 16 genes are not present in rodent malaria parasite genomes, but ultimate proof will require experimental studies.
Among the 16 putative primate parasite-specific genes were several metabolic enzymes, including three key enzymes of the thiamine (vitamin B1) biosynthesis pathway. This pathway has been proposed as attractive antimalarial drug target because of its absence in human hosts [40], [41]. Our analysis suggests that primate but not rodent malaria parasites synthesize thiamine de novo and that rodent malaria parasites depend entirely on thiamine uptake from vertebrate and invertebrate hosts. Indeed, studies have shown that rodent malaria parasites have greatly impaired eryrthrocytic multiplication rates if thiamine is deliberately eliminated from the host [6]. Why particularly primate parasites engage in thiamine biosynthesis is an interesting question. One possibility is that, in primate hosts, thiamine salvage provides the parasite with only insufficient amounts of this essential coenzyme. A more speculative alternative is that thiamine production of the parasite provides the host with this essential enzyme during times when it is only insufficiently available in the host's diet. Interestingly, for all three P. falciparum thiamine enzymes, top BLASTP hits outside Plasmodium are found in bacteria, including Clostridium spp. as top hits in two of three cases (data not shown). In Clostridium ljungdahlii, the three enzymes are located next to each other on the same strand and thus form a potential operon (Figure S5), compatible with the possibility that in the common ancestor of Plasmodium parasites thiamine biosynthesis was horizontally acquired from bacteria (probably from the mitochondrial or apicoplast genome) and subsequently lost in the common ancestor of rodent malaria parasites. Gene loss in rodent malaria parasites (vs. gene gain in primate malaria parasites) as the likely cause for this species-specific difference is supported by residual sequence similarity found in syntenic genomic regions of rodent parasite genomes (Figure 3C).
Comparing the two human parasites P. falciparum and P. vivax with the monkey parasite P. knowlesi, we identified 13 P. vivax genes that have a syntenic ortholog in P. falciparum but no predicted ortholog (neither syntenic nor non-syntenic) in P. knowlesi. The presence of such genes was unexpected because phylogenetically P. vivax is much more closely related to P. knowlesi than to P. falciparum (Figure S1 and [33]). Unlike P. falciparum and P. vivax, P. knowlesi malaria in humans is not endemic in larger parts of the human population and is geographically restricted to forested areas in Malaysian Borneo and peninsular Malaysia [4], [42]. This is most likely due to P. knowlesi's known inability to develop in Anopheles species that preferentially feed on humans [6]. We therefore hypothesize that these 13 genes shared by P. falciparum and P. vivax but absent in P. knowlesi may include genes that permit the entry and survival of parasites in anthropophilic human vectors. Consistent with this possibility, three of the 13 genes (PF14_0236, PFA0380w, and PF10_0185) show only weak expression during the IDC and are specifically up-regulated in sporozoites or gametocytes. Notably, four of the identified 13 genes lack orthologs also in rodent malaria parasites and thus could mediate functions specifically required to parasitize humans. The remaining nine genes have predicted orthologs in rodent malaria parasites and thus likely represent cases of gene loss in P. knowlesi. Further experimental characterization of these 13 genes is required to confirm a potential role in human transmission success. Eventually, these studies may lead to the development of new transmission blocking strategies or to new ideas how future host switches from monkey to human can be prevented. If the ambitious goal of malaria eradication is to be taken seriously [43], a better understanding of molecular factors contributing to the parasite's ability to complete its life cycle in anthropophilic insect vectors is indispensable.
Comparing the highly virulent human parasite P. falciparum with the less virulent human parasite P. vivax, we identified 117 chromosome-internal P. falciparum-specific genes, many of which have known virulence-associated functions (Figure 6). Subtracting genes with known virulence-associated functions, we identified a subset of 15 genes that we proposed as novel candidate genes potentially linked to severe human malaria (Table 3). Because most of these 15 genes are of unknown function and lack also identifiable orthologs in other species [44], experimental analysis in P. falciparum will be required to elucidate their function and to confirm an association with human virulence. The two genes with annotated functions warrant further discussion. The first gene (PF14_0297) is annotated as apyrase, which is a membrane-bound enzyme converting ATP to AMP. Apyrases are involved in purine metabolism [26] and, in mosquitoes, are expressed in salivary glands to inhibit blood clotting [45]. The presence of apyrase in P. falciparum but not in any other Plasmodium parasite (best NCBI BLASTP hit was found in the human apicomplexan parasite Toxoplasma gondii) points towards an increased requirement of this enzymatic function in P. falciparum. Apyrase has been proposed as possible target for antimicrobial therapies [46], but our finding suggests that its use as antimalarial drug target would be limited to P. falciparum malaria. The second gene with annotated function (PFE1455w) is a putative Na+- or H+-driven sugar symporter of the GPH family [47] and one of currently six genes annotated with sugar transmembrane transporter activity in P. falciparum (GeneDB; GO:0051119). NCBI BLASTP and TBLASTN searches reveal homologs of PFE1455w in T. gondii (TGME49_026020) and Neospora caninum (NCLIV_046810) but not in any other Plasmodium species. Host-derived sugars are an essential nutrient of malaria parasites for intraerythrocytic development [48]. In the absence of gluconeogenesis in malaria parasites [13] additional sugar transporters in the membrane of infected erythrocytes likely allow for more efficient glucose uptake from the blood and thus for faster parasite growth, which can be seen as an adaption towards increased virulence of P. falciparum.
Several biological features distinguish P. vivax from other sequenced Plasmodium species, including preference for reticulocytes and its ability to develop dormant hypnozoite forms in the liver that can cause relapses months or even years after primary infection. We hypothesized that genes present in P. vivax but absent in the other sequenced Plasmodium species are candidate genes underlying reticulocyte invasion and hypnozoite formation. We identified a currently uncharacterized chromosome-internal gene cluster on P. vivax chromosome 6 containing several P. vivax-specific genes putatively involved in erythrocyte invasion (Figure 8). Based on synteny, this gene cluster likely encodes for MSPs, including the currently missing P. vivax ortholog of P. falciparum MSP6 [14]. We expect that further characterization of this gene cluster will result in new insights into P. vivax-specific adaptations of erythrocyte invasion. Experimental analyses will be required to test this bioinformatics prediction. In contrast, our search for P. vivax-specific genes potentially linked to hypnozoite formation was largely unsuccessful, suggesting that hypnozoite formation has its roots in regulatory differences and is not primarily associated with protein-coding genes that are unique to P. vivax.
One peculiarity of the P. knowlesi genome is that it has hundreds surface antigens spread all over its genome [15], which we noticed also in our analysis (Figure 7, lower right). How P. knowlesi mobilized its once subtelomeric surface antigens and inserted them into chromosome-internal regions is an intriguing question, especially because this must have happened rather recently after the divergence from the common ancestor with P. vivax and because transposable elements that could have mediated rapid gene dispersal have not been identified in Plasmodium genomes [13]. Our finding that P. knowlesi might lack a fully functional copy of TRF1 could provide a possible explanation for this phenomenon. In mammalian cells, telomeric repeat binding factors play a pivotal role in protection and maintenance of telomeres [49]. Partial or complete loss of function of telomere repeat binding factor 1 in P. knowlesi could cause telomere instability, resulting in frequent DNA breakage events near telomeres whose subsequent repair causes broken subtelomeric fragments to be randomly inserted into the P. knowlesi genome. Such a mechanism would also explain why P. knowlesi harbors telomeric repeat sequences in chromosome-internal regions [15]. Further experimental work can now test this hypothesis and, if confirmed, investigate the important question if the loss-of-function allele of PFI1216w is a fixed wild type allele in the P. knowlesi population or a recently introduced mutation, perhaps only present in the sequenced laboratory strain of P. knowlesi.
Materials and Methods
Genome sequences and gene models
Published chromosome-level assemblies for P. falciparum, P. vivax, and P. knowlesi were downloaded from PlasmoDB version 7.0 [36] (http://plasmodb.org). P. chabaudi and P. berghei chromosome-level assemblies were available but unpublished. Therefore, older contig-level assemblies available at PlasmoDB (version 5.5) were used for genome-wide comparisons. The P. yoelii contig-level assembly was also downloaded from PlasmoDB 5.5. Annotated gene models (GFF3 format) were downloaded from PlasmoDB 7.0 (P. falciparum, P. vivax, and P. knowlesi) and PlasmoDB 5.5 (P. yoelii, P. chabaudi, and P. berghei). If a gene had multiple isoforms, only longest isoforms ( = longest protein sequence) were kept.
Homology-based gene model improvement of P. vivax and P. knowlesi
Missing or incorrectly annotated gene models cause overestimates of genetic differences and, important for this study, false specific-specific genes. We therefore repaired the more obvious defects in P. vivax and P. knowlesi gene model annotations before genome comparisons. Using two homology-based gene predictors, including our own program genBlastG [50] and the widely used and well established program GeneWise [51], an automated pipeline for genome-wide gene model improvement was implemented. Briefly, protein sequences of all protein-coding P. falciparum genes (5,317 genes, excluding pseudogenes and shorter isoforms) were used as query to run both genBlastG and GeneWise against P. vivax and P. knowlesi genomes. To ensure the quality of predicted gene models, only predictions that encoded for protein sequences with high global sequence identity (PID> = 60) with the query gene were kept. If multiple predictions overlapped by more than 5% of their coding exons, only the prediction with the highest PID was kept (filtration step). We use global PID as a measure of sequence conservation because it better captures global similarity between two proteins as compared to for example the BLAST E-value, which measures local sequence similarity and is more prone to various biases, including sequence composition. In a subsequent merging step, predicted and existing gene models were merged into a hybrid gene set, retaining predictions that (a) did not overlap with existing gene models or (b) showed a PID improvement of at least 5% over overlapping existing gene models. As in the filtration step, existing and predicted gene modes were considered as overlapping if more than 5% of their coding exons overlapped. The hybrid gene set served as basis for all subsequent comparisons. A summary of improved gene models, including novel functional annotations predicted with InterProScan [52], can be found in Table S1 (P. vivax) and Table S2 (P. knowlesi). Novel and improved gene models for both P. vivax and P. knowlesi are provided in Dataset S1 (GFF3 format).
Identification of primate parasite-specific genes
Because chromosome-level assemblies for rodent malaria parasites that became available with PlasmoDB 7.0 have not yet been published, we used older, contig-level assemblies (PlasmoDB version 5.5) and a synteny-independent, BLAST-based approach for the initial genome-wide screening for species subset-specific genes. Briefly, complete proteomes of P. falciparum (5,317 proteins), P. vivax (5,156), P. knowlesi (5,143), and P. yoelii (7,802) were used as query to run both NCBI BLASTP (version 2.2.21) [53] and genBlastG (version 1.28) [50] against the other five proteomes and genomes, respectively (including P. berghei and P. chabaudi). Top hits of both BLASTP and genBlastG were used to compute global PIDs with the query protein using ClustalW (version 1.83; BLOSUM62; default parameters) [54]. If the best BLASTP hit was different from the predicted Inparanoid ortholog then the global PID was also computed between query and Inparanoid ortholog. A query protein was considered as conserved in another genome if the maximum of these three PIDs was ≥40 and as absent if the maximum PID was ≤15. In particular, P. falciparum genes were considered primate parasite-specific if conserved in P. vivax and P. knowlesi but not in P. berghei, P. chabaudi, and P. yoelii. The rather conservative margin between high and low PID (25 percent points) was chosen to exclude insignificant PID differences due to fluctuating protein sequence conservation levels or imperfect gene models. Summarized results of this first initial screening are shown in Figure 1. The complete gene list is provided in Table S8. In a second step, P. falciparum orthologs of the 30 putative primate parasite-specific genes were inspected using the newer chromosome-level assemblies of P. chabaudi and P. berghei available at PlasmoDB 7.1. We only kept P. falciparum genes for which (a) genBlastG failed to annotate a gene with a minimum global PID of 15 in the entire genome and (b) no gene was present at the expected syntenic region as defined by the position of flanking syntenic orthologs. Sixteen out of the initial 30 genes fulfilled these two criteria and are shown in Table 1.
The two-step process of first screening for putative primate parasite-specific genes against PlasmoDB 5.5 versions of rodent malaria parasite genomes and gene models then verifying the absence of candidate genes in PlasmoDB 7.1 was chosen because, in agreement with pre-publication data use policies, we restricted all genome-scale comparisons to officially published Plasmodium genomes. Furthermore, we made no efforts to improve rodent parasite gene models based on the now obsolete PlasmoDB 5.5 genome assemblies, because greatly improved P. chabaudi and P. berghei gene models became available with PlasmoDB 7.0. It should be emphasized, however, that using the older PlasmoDB 5.5 rodent parasite genome sequences and gene models in the initial screening step did not affect our final results, because all final candidate genes in Table 1 have been verified to be also absent in PlasmoDB 7.1.
Orthology prediction and synteny block detection
We used OrthoCluster [31] (executable from Dec 17, 2007, downloaded from http://genome.sfu.ca/cgi-bin/orthoclusterdb/download), a program recently developed in our lab, for the gene-based identification of synteny blocks. As input OrthoCluster was provided with genome coordinates of protein-coding genes as well as with gene orthology relationships predicted by Inparanoid (version 4) [55]. Synteny blocks (both perfect and imperfect) were required to have at least two pairs of orthologous genes, irrespective of genomic distance but constrained by the amount of allowed intervening genes. For perfect synteny blocks, we did not allow for any interruptions. For imperfect synteny blocks, we allowed for ≤40% out-map mismatches (i.e. genes without predicted orthologs in the other genome) and ≤10% in-map mismatches (i.e. genes with, but non-syntenic orthologs in the other genome). These two thresholds were chosen after observing that further increasing the percentages did not result in larger imperfect synteny blocks (Figure S4). OrthoCluster was further run with the -rs parameter, which instructs OrthoCluster to report all genes not perfectly preserved in order and strandedness as mismatches. This allowed us to localize all genes for which synteny was not perfectly conserved and to examine the nature of those differences in detail. Synteny analysis was performed only on the 14 nuclear chromosomes excluding mitochondrial and apicoplast genomes. Imperfect synteny blocks were visualized using Circos (version 0.52) [56]. Orthology prediction and synteny analysis was performed using our homology-improved gene models.
Syntenic examination of chromosome-internal parasite-specific genes
To separate questionable differences from likely true genetic differences, we examined all parasite-specific genes in SGRs and SBRs. SGRs were examined in an automated manner using custom Perl scripts. The few SBRs were trickier to deal with due to ambiguous mapping locations in the other genome and were thus examined manually. Briefly, automated BLASTP and GeneWise homology searches were combined with manual visual inspections of non-syntenic regions in a genome browser. Non-random BLASTP sequence similarity (E≤1e-4 and PID≥20) between two ‘syntenic’ parasite-specific genes of which both are flanked by syntenic orthologs was interpreted as evidence of likely orthologous genes missed by Inparanoid. We refer to these genes as positional orthologs. Furthermore, non-random GeneWise alignments (bitscore ≥40) generated in syntenic regions using the putative parasite-specific gene as query are indicative of potentially split/merged genes or missing genes, depending on whether or not alignments overlap with existing gene models. Albeit due to limited sequence similarity GeneWise gene models produced in this step are not entirely reliable, we kept their protein translations for downstream proteomics analyses. In addition, we provide these tentative GeneWise gene models for further inspection (Dataset S4, GFF3 format). Gene structures (gene length, location, number of exons) were also visually examined to validate putative orthologs. We further excluded putative parasite-specific genes for which sequence gaps were present in syntenic regions of the respective other genome, because in this case one cannot reliably exclude the possibility that a syntenic ortholog is present but currently missing from the assembly.
Identification of human parasite-specific genes
Genes shared by P. falciparum and P. vivax but absent in P. knowlesi (Figure 7 and Table 2) were identified by taking all confirmed P. vivax-specific genes absent in P. knowlesi (Figure 7) and then excluding all genes without predicted ortholog in P. falciparum, considering both Inparanoid orthologs and positional orthologs recovered from Figure 6. In addition, we queried OrthoMCL DB [44] for the presence of orthologs of putative human parasite-specific genes in other species than P. falciparum and P. vivax. As a last step, we ran genBlastG with remaining P. falciparum genes against the entire P. knowlesi genome and retained only those candidates that either (a) did not produce a gene model with at least 15% PID and 80% query coverage or (b) produced such a gene model but it overlapped with a P. knowlesi gene that had a different known ortholog in P. falciparum. The resulting gene list is shown in Table 2. Additional information shown in Table 2 is a compilation of data obtained from searching online databases with P. falciparum gene names and sequences, including PlasmoDB 7.1 [36], GeneDB [57], InterPro [52], and NCBI nucleotide and protein archives.
Identification of P. falciparum-specific genes
Starting with all genes in Figure 6 (bottom left diagram) categorized as ‘other, annotated function’ (15 genes) and ‘(conserved or hypothetical) protein, unknown function’ (42 genes), we excluded genes representing chromosome-internal members of previously described (subtelomeric) gene families (7 genes), genes part of gene families with known members in P. vivax (6 genes), genes without expression evidence or questionable open reading frames (9 genes), and genes where visual re-examination revealed the presence of a potential positional ortholog in P. vivax that did not meet our similarity threshold for automatic detection (BLASTP E-value<1e-04; PID> = 20). Of the remaining genes we only retained those with potential virulence-associated functions as predicted by (a) the presence of a PEXEL motif, a signal peptide, or a transmembrane domain, or (b) PlasmoDraft [35] or OPI [38]. We further queried OrthoMCL DB [44] for predicted orthologs in other species. One gene (PF14_0708) was found to have a predicted ortholog in P. vivax, but was nevertheless retained in the final list because this gene is present as an extra copy in P. falciparum (i.e. two genes in P. falciparum and one in P. vivax). As a last step, we ran genBlastG with remaining P. falciparum genes against the entire P. vivax genome and retained only those candidates that either (a) did not produce a gene model with at least 15% PID and 80% query coverage or (b) produced such a gene model but it overlapped with a P. vivax gene that had a different known ortholog in P. falciparum. The resulting gene list is shown in Table 3. The complete, unfiltered list of all 117 identified chromosome-internal P. falciparum -specific genes is provided in Table S4. As before, the additional information shown in Table 3 is a compilation of data obtained from searching online databases with P. falciparum gene names and sequences, including PlasmoDB 7.1, GeneDB, InterPro, and NCBI nucleotide and protein archives. Sequence-independent predictions of virulence-associated functions were obtained from.
Identification of genes unique to P. vivax
To identify genes exclusively present in P. vivax, we overlapped the two P. vivax-specific gene sets of Figure 6 and Figure 7, which resulted in 81 P. vivax-specific genes absent in both P. falciparum and P. knowlesi. We then excluded genes with named gene products (RAD, MSP7, MSP3, SERA), which resulted in 38 chromosome-internal P. vivax-specific genes encoding for hypothetical proteins of unknown function (Table S5). Because median length of encoded protein sequences was short (116 aa), we suspected many false-positive gene predictions among those genes. We therefore further excluded all genes with one or more of the following characteristics: short open reading frames (<100 aa); EST evidence conflicting with the current gene model; coding sequence consisting mostly of low complexity regions or repeat sequences; and the presence of an overlapping gene on opposite strand. Excluding low-confidence ORFs and two other hypothetical genes located near subtelomeric regions resulted in five genes with a likely genuine ORF in chromosome-internal regions.
Supporting Information
Improved P. vivax and P. knowlesi gene models in GFF3 format.
(TXT)
Detected imperfect synteny blocks between P. falciparum and P. vivax . OrthoCluster ‘.cluster’ output file showing both syntenic and non-syntenic genes of identified imperfect synteny blocks.
(TXT)
Detected imperfect synteny blocks between P. vivax and P. knowlesi . OrthoCluster ‘.cluster’ output file showing both syntenic and non-syntenic genes of identified imperfect synteny blocks.
(TXT)
Additional revised P. vivax and P. knowlesi gene models in GFF3 format. This file contains GeneWise gene models produced during syntenic examination of putative parasite-specific genes using genes from the syntenic genomic region of P. falciparum genes as query. Some predictions are based on low sequence similarity (down to ∼15% global protein sequence PID) and should therefore be considered preliminary.
(TXT)
Phylogeny (A) and selected genome features (B) of the six Plasmodium genomes compared in this study. The phylogenetic tree was computed with proml/PHYLIP v3.68 based on concatenated protein sequences from 50 randomly chosen well conserved nuclear 1-to-1 orthologs (see Text S1). Out-group species Babesia bovis not shown. Branch lengths drawn to scale. Scale bar represents 0.025 amino acid substitutions per site, and numbers at branch points represent bootstrap values from 1,000 iterations. Abbreviations: n/a … not available or not applicable; n/d … not determined; CDS: coding sequence; EST: expressed sequence tag. ¥ Includes mitochondrial and apicoplast genome. †Excluding 447 contigs likely representing DNA contamination from host species Saimiri boliviensis boliviensis. ‡Inferred from presence of consensus telomere tandem repeat sequence GGGTT(T/C)A at chromosome ends. § Only longest isoforms.
(DOC)
Examples of improved gene models in P. vivax and P. knowlesi . Panel A shows a newly identified 60S ribosomal protein (L39) in P. knowlesi (PKH_113715), which has 86% global protein sequence identity (PID) and 98% coverage with its P. falciparum ortholog PFF0573c. Panel B shows two P. vivax split genes (PVX_090855 and PVX_090860) merged into a single gene (PVX_090860). The revised gene model is supported by EST evidence and, after improvement, recognized as CPW-WPC domain containing protein by InterProScan. Panel C shows a merged gene in P. knowlesi (PKH_132400) annotated as dynein-associated protein that was split into two genes (PKH_132400a and PKH_132400b), one of which is subsequently recognized as membrane occupation and recognition nexus (MORN)-motif containing protein. Panel D shows the replacement of a truncated hypothetical protein in P. vivax (PVX_088280) with a longer gene model, facilitating its recognition as putative acetyltransferase. Improved gene models shown in yellow. Existing PlasmoDB 7.1 gene models shown in blue (forward strand) or red (reverse strand). The complete set of improved gene models is provided in Dataset S1 (GFF format).
(DOC)
Different types of non-syntenic regions. Subtelomeric regions (STR) range from the first gene on a chromosome arm to the first syntenic gene (two STRs on each chromosome). Synteny breakpoint regions (SBR) are defined as genomic regions between imperfect synteny blocks, which may or may not contain genes. Synteny gap regions (SGR) are defined as genomic regions that interrupt synteny within imperfect synteny blocks due to the presence of one or more parasite-specific genes or non-syntenic orthologs (seven SGRs shown). Shown are two real imperfect synteny blocks identified between P. falciparum (chromosome 4) and P. vivax. The syntenic architecture depicted here (large STRs, few SBRs, many SGRs) is typical for all investigated Plasmodium chromosomes.
(TIF)
Influence of OrthoCluster -ip and -op parameter on the number of identified imperfect synteny blocks between P. falciparum and P. vivax . The graph shows the number of identified imperfect synteny blocks between P. falciparum and P. vivax as a function of allowed in-map mismatches (-ip parameter) and out-map mismatches (-op parameter). After allowing for 10% in-map mismatches and 40% out-map mismatches the number of identified synteny blocks reaches a plateau and does not decrease further. Similar results were observed between P. vivax and P. knowlesi (data not shown).
(XLS)
Gene organization of thiamine biosynthesis genes in Clostridium ljungdahlii suggesting an operonic gene structure. Protein sequences of the three thiamine biosynthesis genes of P. falciparum (PFL1920c, PFE1030c, and PFF0680c) were used as NCBI BLASTP queries to search for homologous proteins (nr database). For all three genes top hits outside Plasmodium were found in bacteria, with top hits in Clostridium ljundahlii in two of three cases. In Clostridium ljundahlii the three enzymes are located next to each other and on the same strand, suggesting that they could form an operon in this species. Screenshot adapted from the NCBI Entrez database (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene&cmd=retrieve&list_uids=9446921).
(DOC)
List of improved gene models in P. vivax . The list includes new and replaced gene models together with characteristics of query and target genes (length, number of exons), EST support, genomic location, method of prediction, sequence conservation levels, query coverage, and newly predicted domains after improvement.
(XLS)
List of improved gene models in P. knowlesi . The list includes new and replaced gene models together with characteristics of query and target genes (length, number of exons), genomic location, method of prediction, sequence conservation levels, query coverage, and newly predicted domains after improvement.
(XLS)
Gene orthology and synteny between P. falciparum and P. vivax as well as between P. vivax and P. knowlesi . Ortholog groups and synteny blocks were identified with Inparanoid4 and OrthoCluster, respectively. Only chromosomal contigs considered for synteny analysis. Orthology prediction included genes from all contigs, chromosomal and non-chromosomal. ‡ Number of protein-coding genes after gene model improvement and excluding annotated pseudogenes and shorter isoforms. § Average percent identity of global protein sequence alignments of all one-to-one orthologs computed with ClustalW. Abbreviations: bp … base pairs; chr … chromosome totals, excluding non-chromosomal contigs.
(DOC)
Complete list of identified parasite-specific genes between P. falciparum and P. vivax . The list includes both subtelomeric and chromosome-internal parasite-specific genes. Chromosome-internal parasite-specific genes are listed together with non-syntenic regions they fall into (SGR or SBR).
(XLS)
Complete list of identified parasite-specific genes between P. vivax and P. knowlesi . The list includes both subtelomeric and chromosome-internal parasite-specific genes. Chromosome-internal parasite-specific genes are listed together with non-syntenic regions they fall into (SGR or SBR). Note that numbers of questionable parasite-specific genes in this table are lower than in Figure 7 because questionable parasite-specific identified in the previous comparison with P. falciparum were excluded for this table.
(XLS)
Number of parasite-specific genes found in non-syntenic regions before (top) and after (bottom) excluding questionable differences. Questionable cases of parasite-specific genes and non-syntenic orthologs are detected and removed through a combination of both automatic and manual examination of SGRs and SBRs (see Materials and Methods). ‡ Includes 374 genes (P. vivax) and 75 genes (P. knowlesi) currently located on non-chromosomal contigs.
(DOC)
Enriched GO terms in P. falciparum -specific genes. P. falciparum-specific genes identified in comparison with P. vivax were subjected to GO term enrichment analysis using Ontologizer. GO terms statistically significantly enriched after correcting for multiple testing (P-value<0.05, Benjamini-Hochberg correction) are highlighted in red.
(XLS)
List of species subset-specific genes as identified by BLAST and genBlastG. Genes in this table underlie the Venn diagram of Figure 1. They are listed together with functional annotations and sequence conservation levels as determined with BLASTP and genBlastG.
(XLS)
Genes conserved in primate parasites but absent in rodent parasites. Same genes as in Table 1 but including graphical RNA-seq expression profiles.
(PDF)
Genes shared between P. falciparum and P. vivax but absent in P. knowlesi . Same genes as in Table 2 but including graphical RNA-seq expression profiles.
(PDF)
P. falciparum genes absent in P. vivax with possible role in human virulence. Same genes as in Table 3 but including graphical RNA-seq expression profiles.
(PDF)
Supplementary information. Additional information about Plasmodium genome features and species phylogeny, homology-based gene model improvement, synteny block analysis, syntenic examination of chromosome-internal parasite-specific genes, compositional bias of Plasmodium genomes, and enriched GO terms of P. falciparum-specific genes.
(DOC)
Acknowledgments
We are grateful to Dr. David Baillie and Dr. Fiona Brinkman for valuable advice and discussions. Special thanks go to Ismael A. Vergara for many fruitful discussions and for providing Perl scripts and OrthoCluster support. We also thank Bora Uyar for providing gene model improvement Perl scripts and Shih-Chieh Chu for genBlastG support. Eric Chen helped working on the P. knowlesi comparison. We further acknowledge Dr. Carl Lowenberger and Spencer Myrtle for discussions and critical proofreading of the manuscript. We thank Duncan Napier, Martin Siegert, and Ata Roudgar for IT support and acknowledge Compute Canada for providing computational resources in form of the WestGrid grid computing facility.
Footnotes
The authors have declared that no competing interests exist.
This project is supported by a Discovery Grant from Natural Sciences and Engineering Research Council (NSERC) of Canada to NC, and the Simon Fraser University Community Trust Endowment Fund (CTEF) through the BCID Project. NC is a Michael Smith Foundation for Health Research (MSFHR) Scholar and a Canadian Institutes of Health Research (CIHR) New Investigator. CF was supported by Simon Fraser University, B.C. Pacific Century, Weyerhaeuser, and Sulzer Pumps graduate scholarships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. World Malaria Report 2008. World Health Organization; 2008. [Google Scholar]
- 2.Cox FEG. Studies on the host parasite relationships of Haemosporidia. 1964. [Ph. D. Thesis]: University of London.
- 3.Killick-Kendrick R, Peters W. Rodent Malaria. London: Academic Press Inc. Ltd; 1978. 62 [Google Scholar]
- 4.Cox-Singh J, Singh B. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 2008;24:406–410. doi: 10.1016/j.pt.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KS, Divis PC, Zakaria SK, Matusop A, Julin RA, et al. Plasmodium knowlesi: Reservoir Hosts and Tracking the Emergence in Humans and Macaques. PLoS Pathog. 2011;7:e1002015. doi: 10.1371/journal.ppat.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnham P. Malaria Parasites and Other Haemosporidia. Oxford: Blackwell Scientific publications; 1966. 94 [Google Scholar]
- 7.Kantele A, Jokiranta TS. Review of Cases With the Emerging Fifth Human Malaria Parasite, Plasmodium knowlesi. Clin Infect Dis. 2011;52:1356–1362. doi: 10.1093/cid/cir180. [DOI] [PubMed] [Google Scholar]
- 8.Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: Severe malaria. Crit Care. 2003;7:315–323. doi: 10.1186/cc2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, et al. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 10.Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 1992;69:1213–1226. doi: 10.1016/0092-8674(92)90642-p. [DOI] [PubMed] [Google Scholar]
- 11.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 12.Krotoski WA. The hypnozoite and malarial relapse. Prog Clin Parasitol. 1989;1:1–19. [PubMed] [Google Scholar]
- 13.Gardner MJ, Hall N, Fung E, White O, Berriman M, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pain A, Bohme U, Berry AE, Mungall K, Finn RD, et al. The genome of the simian and human malaria parasite Plasmodium knowlesi. Nature. 2008;455:799–803. doi: 10.1038/nature07306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlton JM, Angiuoli SV, Suh BB, Kooij TW, Pertea M, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002;419:512–519. doi: 10.1038/nature01099. [DOI] [PubMed] [Google Scholar]
- 17.Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 18.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham D, Lawton J, Jarra W, Preiser P, Langhorne J. The pir multigene family of Plasmodium: antigenic variation and beyond. Mol Biochem Parasitol. 2010;170:65–73. doi: 10.1016/j.molbiopara.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87:377–390. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 22.Debarry JD, Kissinger JC. Jumbled Genomes: Missing Apicomplexan Synteny. Mol Biol Evol. 2011;28:2855–2871. doi: 10.1093/molbev/msr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kooij TW, Carlton JM, Bidwell SL, Hall N, Ramesar J, et al. A Plasmodium whole-genome synteny map: indels and synteny breakpoints as foci for species-specific genes. PLoS Pathog. 2005;1:e44. doi: 10.1371/journal.ppat.0010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, et al. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci U S A. 2008;105:16290–16295. doi: 10.1073/pnas.0807404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Zhang YE, Long M. New genes in Drosophila quickly become essential. Science. 2010;330:1682–1685. doi: 10.1126/science.1196380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginsburg H. Progress in in silico functional genomics: the malaria Metabolic Pathways database. Trends Parasitol. 2006;22:238–240. doi: 10.1016/j.pt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Wilkes JM, Doerig C. The protein-phosphatome of the human malaria parasite Plasmodium falciparum. BMC Genomics. 2008;9:412. doi: 10.1186/1471-2164-9-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dechamps S, Maynadier M, Wein S, Gannoun-Zaki L, Marechal E, et al. Rodent and nonrodent malaria parasites differ in their phospholipid metabolic pathways. J Lipid Res. 2010;51:81–96. doi: 10.1194/jlr.M900166-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui L, Fan Q, Cui L, Miao J. Histone lysine methyltransferases and demethylases in Plasmodium falciparum. Int J Parasitol. 2008;38:1083–1097. doi: 10.1016/j.ijpara.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 31.Zeng X, Pei J, Vergara IA, Nesbitt M, Wang K, et al. OrthoCluster: a new tool for mining synteny blocks and applications in comparative genomics. 2008. In: 11th International Conference on Extending Technology (EDBT); 25–30 March 2008; Nantes, France.
- 32.Ng MP, Vergara IA, Frech C, Chen Q, Zeng X, et al. OrthoClusterDB: an online platform for synteny blocks. BMC Bioinformatics. 2009;10:192. doi: 10.1186/1471-2105-10-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol. 2008;47:261–273. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Tufet-Bayona M, Janse CJ, Khan SM, Waters AP, Sinden RE, et al. Localisation and timing of expression of putative Plasmodium berghei rhoptry proteins in merozoites and sporozoites. Mol Biochem Parasitol. 2009;166:22–31. doi: 10.1016/j.molbiopara.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Brehelin L, Dufayard JF, Gascuel O. PlasmoDraft: a database of Plasmodium falciparum gene function predictions based on postgenomic data. BMC Bioinformatics. 2008;9:440. doi: 10.1186/1471-2105-9-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer S, Grossmann S, Vingron M, Robinson PN. Ontologizer 2.0–a multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics. 2008;24:1650–1651. doi: 10.1093/bioinformatics/btn250. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Young JA, Santrosyan A, Chen K, Yan SF, et al. In silico gene function prediction using ontology-based pattern identification. Bioinformatics. 2005;21:1237–1245. doi: 10.1093/bioinformatics/bti111. http://chemlims.com/OPI/MServlet.ChemInfo. [DOI] [PubMed] [Google Scholar]
- 39.Sokurenko EV, Hasty DL, Dykhuizen DE. Pathoadaptive mutations: gene loss and variation in bacterial pathogens. Trends Microbiol. 1999;7:191–195. doi: 10.1016/s0966-842x(99)01493-6. [DOI] [PubMed] [Google Scholar]
- 40.Du Q, Wang H, Xie J. Thiamin (vitamin B1) biosynthesis and regulation: a rich source of antimicrobial drug targets? Int J Biol Sci. 2011;7:41–52. doi: 10.7150/ijbs.7.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller IB, Hyde JE, Wrenger C. Vitamin B metabolism in Plasmodium falciparum as a source of drug targets. Trends Parasitol. 2010;26:35–43. doi: 10.1016/j.pt.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 43.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, et al. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen F, Mackey AJ, Stoeckert CJ, Jr, Roos DS. OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic Acids Res. 2006;34:D363–368. doi: 10.1093/nar/gkj123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Champagne DE, Smartt CT, Ribeiro JM, James AA. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc Natl Acad Sci U S A. 1995;92:694–698. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansom FM, Robson SC, Hartland EL. Possible effects of microbial ecto-nucleoside triphosphate diphosphohydrolases on host-pathogen interactions. Microbiol Mol Biol Rev. 2008;72:765–781. doi: 10.1128/MMBR.00013-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin RE, Henry RI, Abbey JL, Clements JD, Kirk K. The ‘permeome’ of the malaria parasite: an overview of the membrane transport proteins of Plasmodium falciparum. Genome Biol. 2005;6:R26. doi: 10.1186/gb-2005-6-3-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuster FL. Cultivation of plasmodium spp. Clin Microbiol Rev. 2002;15:355–364. doi: 10.1128/CMR.15.3.355-364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ancelin K, Brunori M, Bauwens S, Koering CE, Brun C, et al. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol Cell Biol. 2002;22:3474–3487. doi: 10.1128/MCB.22.10.3474-3487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.She R, Shih-Chieh Chu J, Uyar B, Wang J, Wang K, et al. genBlastG: extending BLAST to be a high performance gene finder. Bioinformatics. 2011;27:2141–2143. doi: 10.1093/bioinformatics/btr342. [DOI] [PubMed] [Google Scholar]
- 51.Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mulder N, Apweiler R. InterPro and InterProScan: tools for protein sequence classification and comparison. Methods Mol Biol. 2007;396:59–70. doi: 10.1007/978-1-59745-515-2_5. [DOI] [PubMed] [Google Scholar]
- 53.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter. 2002;2:Unit 2 3. doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- 55.Remm M, Storm CE, Sonnhammer EL. Automatic clustering of orthologs and in-paralogs from pairwise species comparisons. J Mol Biol. 2001;314:1041–1052. doi: 10.1006/jmbi.2000.5197. [DOI] [PubMed] [Google Scholar]
- 56.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertz-Fowler C, Peacock CS, Wood V, Aslett M, Kerhornou A, et al. GeneDB: a resource for prokaryotic and eukaryotic organisms. Nucleic Acids Res. 2004;32:D339–343. doi: 10.1093/nar/gkh007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartfai R, Hoeijmakers WA, Salcedo-Amaya AM, Smits AH, Janssen-Megens E, et al. H2A.Z demarcates intergenic regions of the plasmodium falciparum epigenome that are dynamically marked by H3K9ac and H3K4me3. PLoS Pathog. 2010;6:e1001223. doi: 10.1371/journal.ppat.1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Improved P. vivax and P. knowlesi gene models in GFF3 format.
(TXT)
Detected imperfect synteny blocks between P. falciparum and P. vivax . OrthoCluster ‘.cluster’ output file showing both syntenic and non-syntenic genes of identified imperfect synteny blocks.
(TXT)
Detected imperfect synteny blocks between P. vivax and P. knowlesi . OrthoCluster ‘.cluster’ output file showing both syntenic and non-syntenic genes of identified imperfect synteny blocks.
(TXT)
Additional revised P. vivax and P. knowlesi gene models in GFF3 format. This file contains GeneWise gene models produced during syntenic examination of putative parasite-specific genes using genes from the syntenic genomic region of P. falciparum genes as query. Some predictions are based on low sequence similarity (down to ∼15% global protein sequence PID) and should therefore be considered preliminary.
(TXT)
Phylogeny (A) and selected genome features (B) of the six Plasmodium genomes compared in this study. The phylogenetic tree was computed with proml/PHYLIP v3.68 based on concatenated protein sequences from 50 randomly chosen well conserved nuclear 1-to-1 orthologs (see Text S1). Out-group species Babesia bovis not shown. Branch lengths drawn to scale. Scale bar represents 0.025 amino acid substitutions per site, and numbers at branch points represent bootstrap values from 1,000 iterations. Abbreviations: n/a … not available or not applicable; n/d … not determined; CDS: coding sequence; EST: expressed sequence tag. ¥ Includes mitochondrial and apicoplast genome. †Excluding 447 contigs likely representing DNA contamination from host species Saimiri boliviensis boliviensis. ‡Inferred from presence of consensus telomere tandem repeat sequence GGGTT(T/C)A at chromosome ends. § Only longest isoforms.
(DOC)
Examples of improved gene models in P. vivax and P. knowlesi . Panel A shows a newly identified 60S ribosomal protein (L39) in P. knowlesi (PKH_113715), which has 86% global protein sequence identity (PID) and 98% coverage with its P. falciparum ortholog PFF0573c. Panel B shows two P. vivax split genes (PVX_090855 and PVX_090860) merged into a single gene (PVX_090860). The revised gene model is supported by EST evidence and, after improvement, recognized as CPW-WPC domain containing protein by InterProScan. Panel C shows a merged gene in P. knowlesi (PKH_132400) annotated as dynein-associated protein that was split into two genes (PKH_132400a and PKH_132400b), one of which is subsequently recognized as membrane occupation and recognition nexus (MORN)-motif containing protein. Panel D shows the replacement of a truncated hypothetical protein in P. vivax (PVX_088280) with a longer gene model, facilitating its recognition as putative acetyltransferase. Improved gene models shown in yellow. Existing PlasmoDB 7.1 gene models shown in blue (forward strand) or red (reverse strand). The complete set of improved gene models is provided in Dataset S1 (GFF format).
(DOC)
Different types of non-syntenic regions. Subtelomeric regions (STR) range from the first gene on a chromosome arm to the first syntenic gene (two STRs on each chromosome). Synteny breakpoint regions (SBR) are defined as genomic regions between imperfect synteny blocks, which may or may not contain genes. Synteny gap regions (SGR) are defined as genomic regions that interrupt synteny within imperfect synteny blocks due to the presence of one or more parasite-specific genes or non-syntenic orthologs (seven SGRs shown). Shown are two real imperfect synteny blocks identified between P. falciparum (chromosome 4) and P. vivax. The syntenic architecture depicted here (large STRs, few SBRs, many SGRs) is typical for all investigated Plasmodium chromosomes.
(TIF)
Influence of OrthoCluster -ip and -op parameter on the number of identified imperfect synteny blocks between P. falciparum and P. vivax . The graph shows the number of identified imperfect synteny blocks between P. falciparum and P. vivax as a function of allowed in-map mismatches (-ip parameter) and out-map mismatches (-op parameter). After allowing for 10% in-map mismatches and 40% out-map mismatches the number of identified synteny blocks reaches a plateau and does not decrease further. Similar results were observed between P. vivax and P. knowlesi (data not shown).
(XLS)
Gene organization of thiamine biosynthesis genes in Clostridium ljungdahlii suggesting an operonic gene structure. Protein sequences of the three thiamine biosynthesis genes of P. falciparum (PFL1920c, PFE1030c, and PFF0680c) were used as NCBI BLASTP queries to search for homologous proteins (nr database). For all three genes top hits outside Plasmodium were found in bacteria, with top hits in Clostridium ljundahlii in two of three cases. In Clostridium ljundahlii the three enzymes are located next to each other and on the same strand, suggesting that they could form an operon in this species. Screenshot adapted from the NCBI Entrez database (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene&cmd=retrieve&list_uids=9446921).
(DOC)
List of improved gene models in P. vivax . The list includes new and replaced gene models together with characteristics of query and target genes (length, number of exons), EST support, genomic location, method of prediction, sequence conservation levels, query coverage, and newly predicted domains after improvement.
(XLS)
List of improved gene models in P. knowlesi . The list includes new and replaced gene models together with characteristics of query and target genes (length, number of exons), genomic location, method of prediction, sequence conservation levels, query coverage, and newly predicted domains after improvement.
(XLS)
Gene orthology and synteny between P. falciparum and P. vivax as well as between P. vivax and P. knowlesi . Ortholog groups and synteny blocks were identified with Inparanoid4 and OrthoCluster, respectively. Only chromosomal contigs considered for synteny analysis. Orthology prediction included genes from all contigs, chromosomal and non-chromosomal. ‡ Number of protein-coding genes after gene model improvement and excluding annotated pseudogenes and shorter isoforms. § Average percent identity of global protein sequence alignments of all one-to-one orthologs computed with ClustalW. Abbreviations: bp … base pairs; chr … chromosome totals, excluding non-chromosomal contigs.
(DOC)
Complete list of identified parasite-specific genes between P. falciparum and P. vivax . The list includes both subtelomeric and chromosome-internal parasite-specific genes. Chromosome-internal parasite-specific genes are listed together with non-syntenic regions they fall into (SGR or SBR).
(XLS)
Complete list of identified parasite-specific genes between P. vivax and P. knowlesi . The list includes both subtelomeric and chromosome-internal parasite-specific genes. Chromosome-internal parasite-specific genes are listed together with non-syntenic regions they fall into (SGR or SBR). Note that numbers of questionable parasite-specific genes in this table are lower than in Figure 7 because questionable parasite-specific identified in the previous comparison with P. falciparum were excluded for this table.
(XLS)
Number of parasite-specific genes found in non-syntenic regions before (top) and after (bottom) excluding questionable differences. Questionable cases of parasite-specific genes and non-syntenic orthologs are detected and removed through a combination of both automatic and manual examination of SGRs and SBRs (see Materials and Methods). ‡ Includes 374 genes (P. vivax) and 75 genes (P. knowlesi) currently located on non-chromosomal contigs.
(DOC)
Enriched GO terms in P. falciparum -specific genes. P. falciparum-specific genes identified in comparison with P. vivax were subjected to GO term enrichment analysis using Ontologizer. GO terms statistically significantly enriched after correcting for multiple testing (P-value<0.05, Benjamini-Hochberg correction) are highlighted in red.
(XLS)
List of species subset-specific genes as identified by BLAST and genBlastG. Genes in this table underlie the Venn diagram of Figure 1. They are listed together with functional annotations and sequence conservation levels as determined with BLASTP and genBlastG.
(XLS)
Genes conserved in primate parasites but absent in rodent parasites. Same genes as in Table 1 but including graphical RNA-seq expression profiles.
(PDF)
Genes shared between P. falciparum and P. vivax but absent in P. knowlesi . Same genes as in Table 2 but including graphical RNA-seq expression profiles.
(PDF)
P. falciparum genes absent in P. vivax with possible role in human virulence. Same genes as in Table 3 but including graphical RNA-seq expression profiles.
(PDF)
Supplementary information. Additional information about Plasmodium genome features and species phylogeny, homology-based gene model improvement, synteny block analysis, syntenic examination of chromosome-internal parasite-specific genes, compositional bias of Plasmodium genomes, and enriched GO terms of P. falciparum-specific genes.
(DOC)