Abstract
The ESCRT (Endosomal Sorting Complex Required for Transport) complexes and associated proteins mediate membrane scission reactions, such as multi-vesicular body formation, the terminal stages of cytokinesis and retroviral particle release. These proteins are believed to be sequentially recruited to the site of membrane scission, and then complexes are disassembled by the ATPase Vps4A. However these events have never been observed in living cells and their dynamics are unknown. By quantifying the recruitment of several ESCRT and associated proteins during the assembly of two retroviruses, we show that Alix progressively accumulated at viral assembly sites, coincident with the accumulation of the major viral structural protein, Gag, and was not recycled after assembly. In contrast, ESCRT-III and Vps4A were only transiently recruited when the accumulation of Gag was complete. These data suggest that the rapid and transient recruitment of proteins that act late in the ESCRT pathway and carry out membrane fission is triggered by prior and progressive accumulation of proteins that bridge viral proteins and the late-acting ESCRT proteins.
Introduction
The ESCRT (Endosomal Sorting Complex Required for Transport) complexes and associated proteins function in membrane fission events, such as multivesicular body (MVB) formation and the terminal stages of cytokinesis 1, 2. The ESCRT machinery is also required for the budding of numerous enveloped viruses to cut the membranous neck that connects the virion to the plasma membrane 3, 4. There are more than 20 ESCRT members in mammalian cells and they are all connected into a coherent network by protein-protein interactions 5. The network consists of 3 complexes, ESCRT-I, ESCRT-II and ESCRT-III, and other associated proteins such as the ATP-ase Vps4. ESCRT proteins are soluble and are thought to be recruited at the site of membrane fission in an ordered manner 6–8, with ESCRT-I proteins (such as Tsg101) and associated proteins (Alix) acting early in the pathway, and ESCRT-III proteins (Chmp proteins) acting late, and then disassembled by the ATPase Vps4A9. Indeed, recent in vitro data indicates that ESCRT-III proteins are responsible for the scission of the membrane neck and that Vps4 acts after the scission step to recycle the complex 10, 11. However, the in vivo kinetics of assembly and disassembly of ESCRT proteins at site of membrane fission is unknown. There are no direct observations available to support the idea that there is a sequential recruitment of the ESCRT machinery to viral assembly sites, or during MVB formation, or cytokinesis. The recycling of ESCRT proteins by Vps4 has not been directly observed in live cells either.
Numerous enveloped viruses, including all retroviruses 3, 4 hijack the ESCRT machinery for budding. Retroviruses recruit the machinery through specific sequences, called late domains, that are contained within their major structural protein, Gag 3, 4. There are 3 known types of late domain which all recruit different ESCRT proteins via direct interactions: PTAP sequences recruit Tsg101 12, 13, YPDL or LxxLF sequences recruit Alix14, 15 and PPXY interacts with ubiquitin ligases, such WWP-1 16. HIV-1 Gag contains two late domains, PTAP and LxxLF, with PTAP being the functionally more important motif 3, 4 and recruiting the ESCRT complex via a direct interaction with Tsg101 12, 13. The second L-domain, LxxLF, interacts with Alix 14, 15. The retrovirus Equine Infectious Anemia Virus (EIAV) Gag possess a unique YPxL late domain, which recruits the ESCRT complexes via a direct interaction with Alix 14, 15. In mammalian cells, Alix in turn recruits ESCRT-III proteins 14, 17, 18.
The assembly of HIV-1 and EIAV virions or virus-like-particles (VLPs) is driven by Gag proteins and takes place at the plasma membrane 19-21. Using total-internal-reflection fluorescent microscopy (TIR-FM), which selectively excites fluorophores near the coverslip (within ~<70 nm) 22 and C-terminally tagged fluorescent versions of Gag in transfected Hela cells23, there is sufficient signal-to-noise ratio to allow dynamic quantification of the assembly of individual VLPs, from initiation of assembly to budding and release 23,24,25. We have previously determined, using FRAP analysis, that completion of particle assembly occurs when the recruitment of Gag molecules stopped, which corresponds to the point when the intensity of Gag signal reaches a plateau 23. The time to complete assembly is the elapsed time from the first image at which a fluorescent puncta is first detectable to the point when its intensity reaches a plateau.
Here, we quantified the recruitment of ESCRT and associated proteins during the assembly of mCherry tagged HIV-1 and EIAV VLPs using cell lines stably expressing GFP fused ESCRT proteins. These studies reveal that there two clearly distinct behaviors among members of the ESCRT pathway and that the Vps4 removes some, but not all, ESCRT pathway components from sites for viral budding.
Results
Characterization of cell lines stably expressing GFP-tagged ESCRT proteins
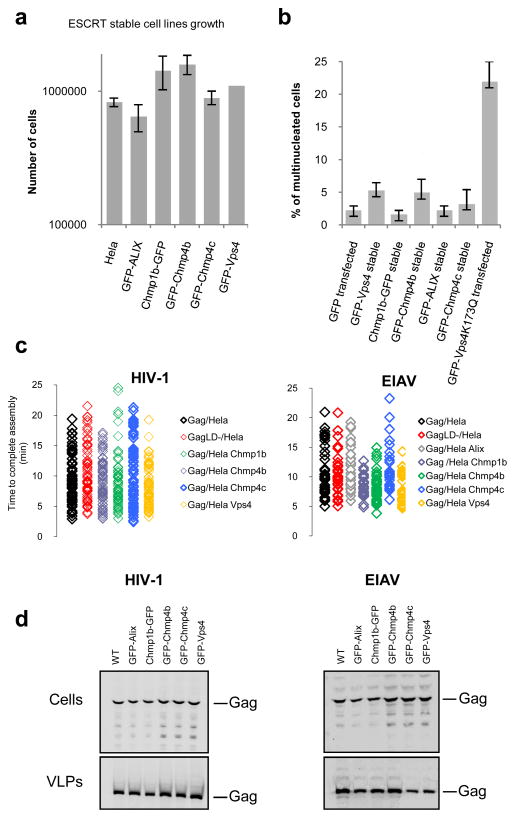
Our goal was to use fluorescence to determine the dynamics of ESCRT protein recruitment during assembly of HIV-1 and EIAV VLPs. However, overexpression of ESCRT proteins fused to bulky tags such as fluorescent proteins can be deleterious to cell physiology and virion release 14, 17, 26–28. Thus, we established criteria for selecting fusion proteins and stable cell lines for our analyses. First, the GFP tagged ESCRT proteins had to interact with the endogenous components of the ESCRT pathway as assessed by localization of the GFP-ESCRT fusion to the midbody during cytokinesis (Fig. 1 and Supplementary Figs. 1–4) and recruitment to class E compartments, induced by expression of catalytically inactive Vps4A mutant (Vps4A-DN). Second, there had to be no gross effects on cell physiology or division (Fig. 2a–b) on the kinetics of HIV-1 VLP assembly or release (Fig. 2c–d) that sometimes accompany ESCRT protein overexpression 29, 30. Third, the GFP fusion proteins had to remain intact, as assayed by western blot assays (Supplementary Fig. 5a). Fourth, all the cells of a clone had to express relatively homogeneous level of the fluorescent proteins (Supplementary Fig. 5b). Finally, in situations where antibodies to the ESCRT protein were available, the level of expression of the GFP-tagged ESCRT protein had to be close to the level of the native protein, as assayed by western blot (Supplementary Fig. 5c).
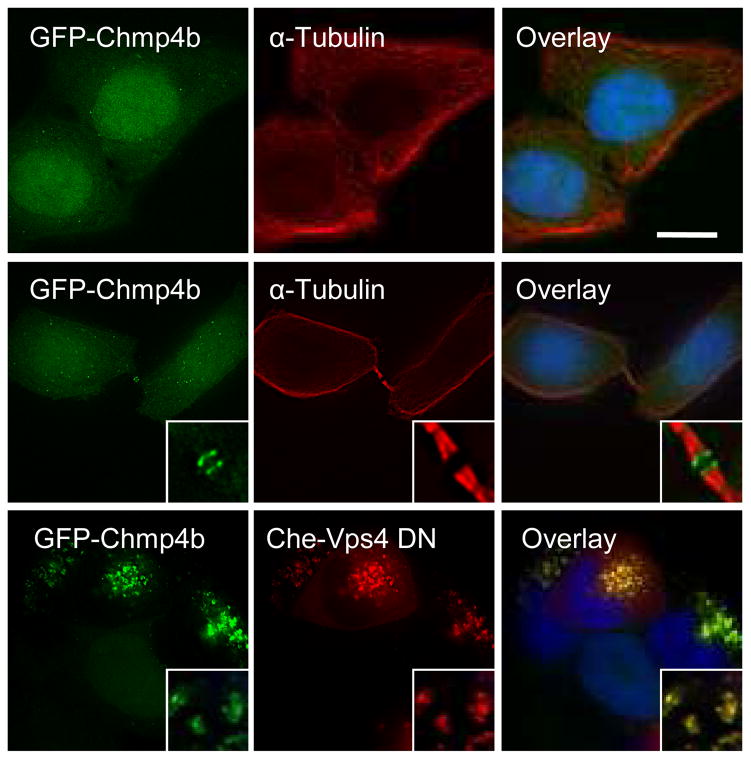
Figure 1. Characterization of the GFP-Chmp4b-expressing cell clone.
Hela cells stably expressing GFP-Chmp4b (green) were fixed and stained with anti-α-tubulin antibodies (red) and with DAPI (blue). Images show the distribution of GFP-Chmp4b in interphase cells (top panels) and in telophase cells (middle panels). Alternatively, cells were transfected with mCherry-Vps4-DN (bottom panels), fixed 18 hours post-transfection and stained with DAPI (blue). Samples were observed with an epifluorescence microscope. Deconvolved optical sections acquired at the center of the vertical dimension of the cell are shown. Expanded views are shown in insets. The scale bars represent 10μm.
Figure 2. Effect of stably expressed GFP fused ESRCT proteins on cell proliferation, cytokinesis and virion assembly and release.
a. The stable expression of GFP-tagged ESCRT proteins does not affect cell proliferation. 105 cells were plated into each well of a 24-well plate, harvested and counted 48h later. Error bars indicate s.d from 3 independent experiments.
b. Stable expression of GFP-tagged ESCRT proteins does not disrupt cytokinesis. Hela cells stably expressing GFP-tagged ESCRT proteins or transfected with GFP or GFP-Vps4-K173Q were fixed, stained with both anti-α-tubulin antibodies and DAPI, and scored for multinucleated cells. 300 cells from 3 independent experiments were analyzed for the presence of more than one nucleus per cell for each factor. Error bars indicate s.d.
c. Kinetics of HIV-1 and EIAV assembly were not affected in the cell lines stably expressing GFP-tagged ESCRT proteins. The plots show the time to complete assembly for individual HIV-1 and EIAV VLPs, including wild-type and late domain mutants (LD-) Gag proteins, in unmodified Hela cells, or cell lines expressing the indicated GFP-ESCRT proteins lines. Each symbol represents an individual VLP. The time to complete assembly was defined for each VLP as the interval between the points of inflection on plots of fluorescence intensity versus time.
d. The stable cell lines stably expressing GFP-tagged ESCRT proteins support the release of HIV-1 and EIAV Gag VLPs. Western blot analysis (LICOR) of Hela cells and Hela cells stably expressing GFP-tagged ESCRT transfected with HIV-1 (left panel) or EIAV (right panel) Gag. Samples were probed with an anti-p24 monoclonal antibody for HIV-1 or anti-EIAV horse serum. Fig. S6 is showing the corresponding unprocessed western blots.
Based on these criteria, a collection of clonal cell lines were developed that stably expressed Alix, Chmp1b, Chmp4b, Chmp4c, Chmp6, Tsg101 and Vps4A, each fused to GFP. Each cell clone exhibited either a diffuse cytoplasmic fluorescence (Tsg101, Alix, Chmp4c and Chmp6), or diffuse cytoplasmic and nuclear fluorescence (Chmp1b, 4b, and Vps4A) (Fig.1, supplementary Figs. 1–4 and data not shown) when imaged by epi-illumination. Some bright cytoplasmic GFP puncta were also detected in each cell line, possibly representing endosomes or centrosomes31 (Fig1 and supplementary Figs. 1–4). Using TIR-FM, these GFP puncta occasionally appeared transiently in the vicinity of the plasma membrane (data not shown). As expected 27, when co-expressed with mCherry-Vps4A-DN, each of these GFP-fused ESCRT fusion proteins accumulated on a characteristic class E compartment (Fig.1 and supplementary Figs. 1–4). Moreover, as previously reported 32, 33, these GFP tagged proteins localized to the midbody at late stages of cell division (Fig. 1 and supplementary Figs. 1–4).
Evidence that association of some ESCRT proteins with HIV-1 and EIAV Gag is transient due to the action of Vps4
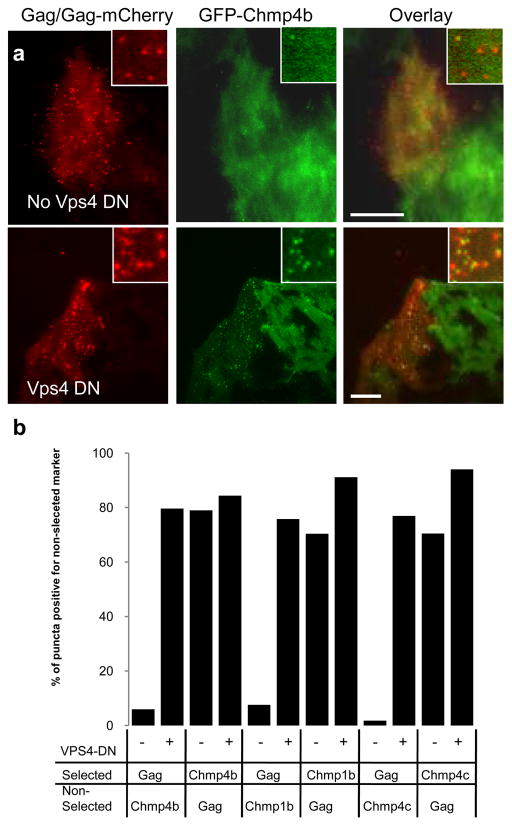
When cells stably expressing GFP-tagged ESCRT proteins were transiently transfected with a mixture of plasmids expressing HIV-1 Gag and Gag-mCherry and imaged at a single time point, fluorescent puncta of the some of the GFP-ESCRT proteins (specifically GFP-Chmp4b, GFP Chmp4c and Chmp1b-GFP) were observed at the plasma membrane, as visualized by TIR-FM. Most of the fluorescent puncta of GFP-ESCRT fluorescence co-localized with puncta of Gag-mCherry (Fig 2a top row, Fig 1b). However, the converse was not the case. At many puncta of Gag-mCherry the GFP-ESCRT fusion proteins were not detectable (Fig 2a top row, Fig 2b). In contrast, when a catalytically inactive Vps4A-DN was also expressed, GFP-Chmp4b puncta were detected at most Gag-mCherry puncta (Fig 2a bottom row, Fig. 2b). Similarly, Chmp1b-GFP and GFP-Chmp4c puncta colocalized with HIV-1 Gag-mCherry puncta, but most of the Gag-mCherry puncta colocalized with the Chmp1b-GFP and GFP-Chmp4c puncta, only when Vps4A-DN was also expressed (Fig. 2b). There was no detectable GFP signal co-localized with HIV-1 Gag-mCherry puncta in the cell lines expressing GFP-Tsg101, GFP-Alix or Chmp6-GFP (data not shown).
When EIAV Gag/Gag-mCherry were used in place of HIV-1 Gag, a similar pattern of colocalization was observed with GFP-tagged Chmp1b, Chmp4b, Chmp4c and Vps4A. Namely, most GFP-Chmp protein puncta localized with Gag puncta, but only a subset of Gag puncta localized with Chmp protein puncta (data not shown). In contrast, GFP-Alix puncta were observed coincident with nearly all EIAV Gag puncta, even in the absence of Vps4A-DN (Supplementary Fig 4c).
These results suggested that the association of many of the ESCRT proteins, particularly the Chmp proteins, with Gag might be transient, but stabilized when Vps4A activity was inhibited. Thus, we examined the dynamics of the localization of the ESCRT proteins, during assembly of VLPs.
Dynamics of HIV-1 and EIAV assembly
We have previously published criteria for assaying HIV-1 particle assembly by following the quantitative recruitment of Gag, and demonstrated that Gag recruitment into nascent VLPs becomes irreversible once it reaches a plateau (by FRAP) and that Gag is closely packed in VLPs (by FRET)23.
Similar to our previous findings23, 24, recruitment of HIV-1 Gag/Gag-mCherry into individual VLPs was completed in a mean of 9.6 minutes after the initial detection of a punctum (n=65, range 4–19.4 min, Fig. 2c). EIAV Gag assembly was similarly completed in a mean of 11.5 minutes (n=36, range 5 to 21.4 min) (Fig. 2c). In both cases the rate of VLP assembly was indistinguishable in unmodified cells versus those expressing the GFP-ESCRT proteins (Fig. 2c).
Interestingly, the assembly kinetics of VLPs composed of L-domain deficient HIV-1 Gag (10.6 min, range 4.2 to 21.5 min, n= 63) and EIAV Gag (10.4 minutes, range 5 to 21.2 min, n=34) (Fig 2c), was indistinguishable from the 9.6 minutes and 11.5 minutes assembly kinetics of the corresponding WT HIV-1 and EIAV VLPs. This finding is similar to a previous report 25 and suggests that the ESCRT proteins do not affect the rate of assembly.
Dynamics of ESCRT protein recruitment during retroviral assembly
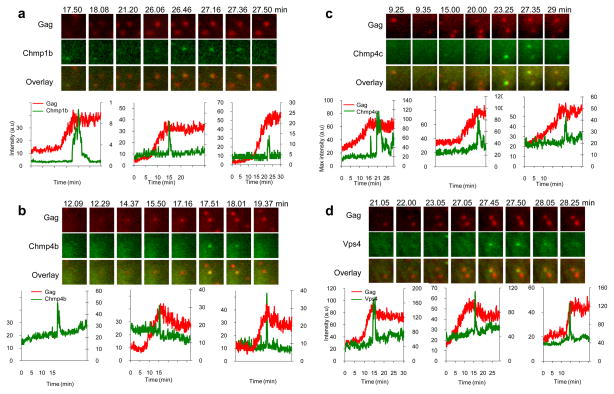
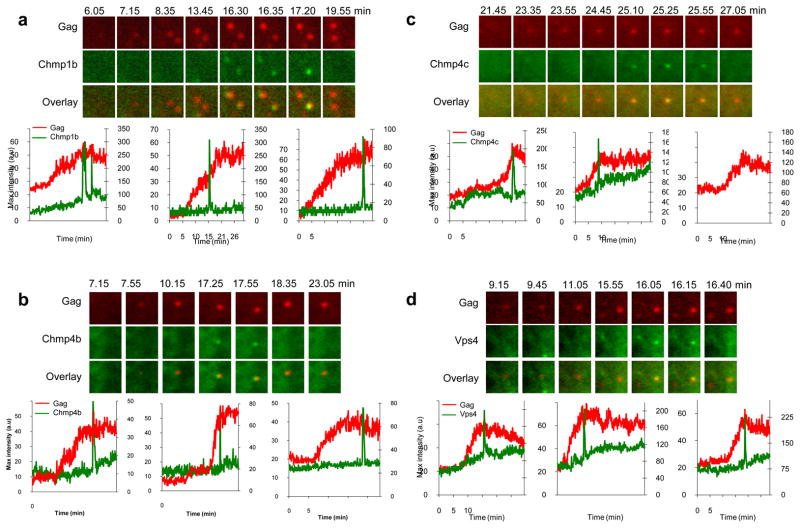
When HIV-1 VLP assembly was imaged in cells stably expressing Chmp1b-GFP, GFP-Chmp4b, GFP-Chmp4c, or GFP-Vps4A, there was transient increase of GFP fluorescence at nascent HIV-1 VLPs. This transient appearance of GFP-tagged ESCRT proteins was, typically, coincident with the termination of the recruitment of Gag to the corresponding VLP (Fig. 4). The dynamics of the recruitment of Chmp1b-GFP, GFP-Chmp4b, GFP-Chmp4c, or GFP-Vps4A to nascent EIAV VLPs was strikingly similar to that observed with HIV-1 (Fig. 5). At each VLP there was transient recruitment of each GFP-ESCRT fusion protein at the site of VLP assembly, close to the termination of EIAV Gag recruitment. At most VLPs, a single pulse of GFP-ESCRT protein recruitment was observed, but occasionally a second, or even a third pulse was detected (Fig. 7d, examples in Fig. 4c and fig. 5a, left panels).
Figure 4. Imaging Chmp1b, Chmp4b, Chmp4c and Vps4A recruitment during HIV-1 Gag assembly.
Hela cells stably expressing Chmp1b-GFP (a), GFP-Chmp4b (b), GFP-Chmp4c (c) or GFP-Vps4A (d) were transfected with HIV-1 Gag/Gag-mCherry and observed under TIR-FM beginning at 6 hours post-transfection. Each set of images illustrates the recruitment of GFP-labeled ESCRT proteins (green) during the genesis of an individual VLP (red). The time after the commencement of observation is given in minutes:seconds. Fields are 2.5x2.5μm. Plots of fluorescence intensity in arbitrary units (a.u) over time for the GFP-ESCRT protein (green, right axis) and Gag-mCherry signals (red, left axis) associated with the assembly of 3 individual VLPs are shown.
Figure 5. Imaging Chmp1b, Chmp4b, Chmp4c and Vps4A recruitment during EIAV Gag assembly.
Hela cells stably expressing Chmp1b-GFP (a), GFP-Chmp4b (b), GFP-Chmp4c (c) or GFP-Vps4A (d) were transfected with EIAV Gag/Gag-mCherry and observed under TIR-FM beginning at 6 hours post-transfection. Each set of images illustrates the recruitment of GFP-labeled ESCRT proteins during the genesis of an individual VLP. The time after the commencement of observation is given in minutes:seconds. Fields are 2.5x2.5μm. Plots of fluorescence intensity in arbitrary units (a.u) over time for the GFP-ESCRT protein (green, right axis) and Gag-mCherry signals (red, left axis) associated with the assembly of 3 individual VLPs are shown, the left chart in panels a and d correspond to the microscopic images shown above.
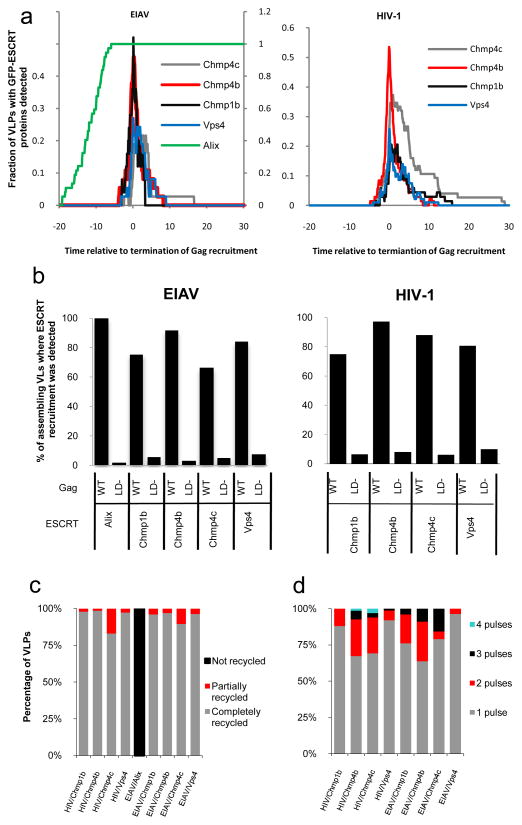
Figure 7. Dynamics and pattern of ESCRT proteins recruitment during retroviral assembly.
a. The fraction of EIAV (left panel) and HIV-1 (right panel) VLPs at which GFP-ESCRT-protein was detectable is plotted as a function of time. For this analysis, T=0 was set as the point at which Gag recruitment to each VLP reached a plateau.
b. Proportion (%) of VLP assembly events for which the recruitment of GFP-tagged ESCRT proteins was detected. Hela cells stably expressing GFP-tagged ESCRT proteins were transfected with wild-type or L-domain mutant (LD−) HIV-1 (left panel) or EIAV (right panel) Gag/Gag-mCherry. Cells were observed live under TIR-FM beginning at 6 hours post-transfection, for a period of 25 to 50 minutes.
c. Quantification of the number of pulses of ESCRT protein recruitment (percentage of VLPs for which each behavior is observed) during HIV-1 and EIAV VLP assembly
d. Removal of the GFP-ESCRT proteins from sites of HIV-1 and EIAV assembly. ESCRT-III and Vps4 proteins are generally completely recycled (and signified by the GFP signal at VLP assembly sites returning to baseline levels following the pulse), but in some cases, the proteins appear to be only partially recycled (e.g. Fig. S8, left panel). Alix is not recycled (i.e the GFP signal remains at a plateau after reaching its maximum, see Fig 3c). The percentage of VLPs showing each behavior is plotted.
The aforementioned Chmp and Vps4A proteins are not thought to bind directly to Gag. Rather, HIV-1 and EIAV Gag proteins engage the ESCRT machinery via late domains that directly bind Tsg101 (PTAP in HIV-1)12, 13, 34 or Alix (LxxLF in HIV-1, YPxL in EIAV)14, 15, 17, 18. These L-domain binding proteins are presumed to act as bridging factors to the ESCRT-III proteins that are responsible for membrane scission. Indeed, interactions between Alix and Chmp4 proteins are well-described14, 17, 18 and are essential for the ability of Alix to promote HIV-1 release and cytokinesis15, 35, 36.
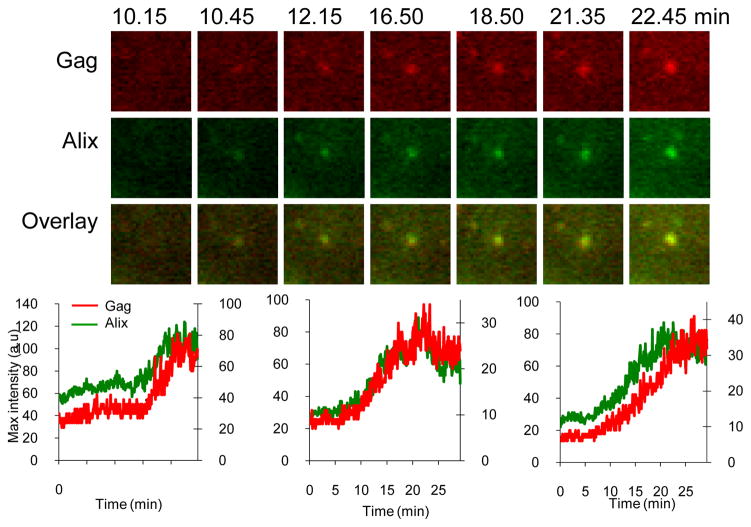
Upon expression of HIV-1 Gag-mCherry in cell lines expressing GFP-Tsg101 or GFP-Alix we were not able to detect either GFP fusion protein at sites of HIV1-Gag-mCherry VLP assembly (data not shown). Presumably, the numbers of molecules of GFP-Tsg101 or GFP-Alix that were recruited fell below the detection threshold. The EIAV L-domain (YPxL) has a higher affinity for Alix than does the HIV-1 LxxLF motif 37 and we were readily able to detect GFP-Alix recruitment to EIAV VLPs. The dynamics of Alix recruitment were completely different to those of Chmp and Vps4A protein recruitment. Specifically, Alix progressively accumulated at the sites of VLP assembly with the same dynamics as Gag, and remained there throughout the period of observation (Fig. 6).
Figure 6. Imaging Alix recruitment during EIAV Gag assembly.
Hela cells stably expressing GFP-Alix were transfected with EIAV Gag/Gag-mCherry and observed under TIR-FM beginning at 6 hours post-transfection. Each set of images illustrates the recruitment of GFP-labeled ESCRT proteins during the genesis of an individual VLP. The time after the commencement of observation is given in minutes:seconds. Fields are 2.5x2.5μm. Plots of fluorescence intensity in arbitrary units (a.u) over time for the GFP-ESCRT protein (green, right axis) and Gag-mCherry signals (red, left axis) associated with the assembly of 3 individual VLPs are shown, the left chart correspond to the microscopic images shown above.
We next quantified the time at which each ESCRT protein appeared and disappeared relative to the termination of HIV-1 and EIAV Gag recruitment (Fig. 7a, n=477). All of the ESCRT-III proteins (Chmp1b, Chmp4b, Chmp4c), as well as Vps4A, were recruited transiently at approximately the same time as recruitment of HIV-1 or EIAV Gag ended, while Alix was recruited at the same time as Gag and remained detectable at all VLPs thereafter (Fig. 7a). Quantification of the time course of residency at the membrane for each of the ESCRT III proteins, and Vps4A, showed they are recruited for only ~1–3 minutes, although Chmp4c seemed to persist for 1–2 min longer (Fig. 7a). The different dynamics of Alix recruitment during EIAV-Gag assembly is consistent with its role as a bridge between Gag and Chmp4, and its apparent failure to dissociate from VLP assembly sites (Fig. 7c) is consistent with biochemical data14, 18, showing that it is incorporated into viral particles.
Notably, ESCRT protein recruitment was observed at the great majority of assembling VLPs. Specifically, Alix recruitment was detectable in 100% of EIAV assembly events (n=36) and recruitment of ESCRT-III/Vps4A was detectable in 84% (n=441) of HIV-1 and EIAV assembly events (Fig. 7b). In contrast, the recruitment of each of these ESCRT and associated proteins was greatly reduced (to between 3 to 10% of assembly events, n=323) when Gag mutants that lacked L-domains (Fig. 7b). were used.
Discussion
The curvature of cellular membranes is away from the cytosol during formation of multivesicular bodies, formation of the cleavage furrow during cell division and during the budding of viruses off the plasma membrane. Common to each of these events is the recruitment of the ESCRT complexes and associated proteins 1, 2. There is considerable biochemical and genetic evidence for the involvement of these proteins in these processes. However, studying the dynamics of the ESCRT molecules has been limited by the adverse effects of expression of fluorescently tagged forms of these proteins. Here, we demonstrate that cell lines stably expressing low-levels of these proteins fused to fluorescent proteins show none of the potential adverse effects thereby allowing studying these molecules with live cell microscopy.
At individual assembling retrovirus particles, the dynamics of recruitment of the ESCRT and associated proteins could be categorized into two groups. Alix, which acts early in the pathway and binds directly to Gag, was recruited along with the viral proteins and remained with the viral particles (Fig. 6). The recruitment of Vps4A and the ESCRT-III proteins (Chmps) showed a strikingly different dynamics. First, the Vps4A and ESCRT-III were only recruited concomitant with the termination of the recruitment of Gag (Fig. 7a). Second, the Vps4A and ESCRT-III were recruited transiently, with a typical residence time of few minutes (Fig. 7a and c).
The ESCRT-III and Vps4 proteins were most often recruited in a single pulse. However, in rare cases, 2 or 3 pulses of recruitment were detected (Fig. 7c). The timing of the arrival and recycling of Vps4 and the Chmp proteins were not distinguishable under our conditions. Rather, they appeared to be tightly coupled temporally in their appearance at and disappearance from the membrane. Displacement of the ESCRTIII proteins is due to the ATPase activity of Vps4: in the presence of WT Vps4 the ESCRT-III proteins were observed only transiently at the membrane (Fig. 3). In contrast, in the presence of a dominant negative Vps4, the ESCRT-III proteins were observed statically with many assembled Gag puncta (Fig. 3). Thus it is possible that if the Vps4 is recruited or activated too quickly, the first pulse of recruitment of the ESCRT-III may be prematurely terminated, requiring another round of recruitment.
Figure 3. Catalytically inactive Vps4A increases localization of stably expressed GFP tagged ESCRT-III proteins at sites of HIV-1 assembly.
a. Hela cells stably expressing GFP-Chmp4b (green) were transfected with HIV-1 Gag/Gag-mCherry (red), in the absence (top panel) or presence (bottom panel) of Vps4A- DN. Cells were fixed 24 hours later and observed with a TIR-FM microscope. Expanded views are shown in insets. The scale bar represents 10μm.
b. Quantification of the co-localization between VLPs and puncta of ESCRT proteins. Hela cells stably expressing GFP-fused ESCRT-III proteins were transfected with Gag/Gag-mCherry, in the absence(−) or presence (+) of Vps4A-DN, as indicated. Cells were observed under TIR-FM at 18 hours post-transfection and the co-localization between puncta of Gag-mCherry and puncta of GFP was quantified by randomly selecting puncta of one marker (selected) and then enumerating what percentage of these puncta were coincident with puncta of the other, non selected marker.
Interestingly, there was no diminution in the level of GFP-Alix associated with VLPs following the completion of assembly (Fig. 7a and c). This is consistent with previous findings that Alix is incorporated into HIV-1 particles with reasonable efficiency 14, 18. Conversely the ESCRT-III and Vps4A proteins were most often completely removed from site of assembly through the action of the ATPase (Fig. 7c). Thus, Vps4 appears to selectively remove ESCRT proteins, particularly those that are thought to mediate the membrane fission reaction, from the fission site. Occasionally, residual molecules of ESCRT-III and Vps4 were observed to remain co-localized with VLPs after assembly was apparently completed. However, this almost always constituted a small minority of the ESCRT-III and Vps4 molecules that were present at the peak of recruitment. From our results it cannot be determined if these molecules were left associated with the cytosolic membrane or within the nascent virion.
The ESCRT-III and Vps4A proteins were detectable at 84% of HIV-1 and EIAV assembly events (Fig. 7b). Moreover, the intensity of the signal emitted by each ESCRT proteins varied greatly from one assembly event to another, even within in a single cell. Possibly, this could be explained by heterogeneity in the ratio of GFP-tagged proteins/endogenous proteins in individual budding events. Alternatively, it there may be an overlap of function between the different ESCRT-III proteins and they may be recruited to varying degrees at different budding sites. Thus, in those assembly events where they were not detected, it may be that the fraction of the GFP-tagged ESCRT protein examined in that particular assembly event was below our detectable limit. A further source of heterogeneity may derive from variability in the number of ESCRT molecules recruited. Potentially the number recruited at assembly sites is determined by the number of Gag molecules per virons, which is known to vary considerably 38.
Importantly, when mutant Gag proteins that lacked L-domains were used, the recruitment of each of these ESCRT and associated proteins was greatly reduced (Fig 7b), but not completely abolished. The residual apparent recruitment of ESCRT proteins by Gag mutants could be explained by the presence of another, less efficient, late domain. Such a late domain, contained within the nucleocapsid domain of HIV-1 Gag and that can binds Alix, was recently described39, 40. Another possibility is that some of these events might not represent proper recruitment but rather the ‘random’ appearance of ESCRT puncta - which are observed in the absence of Gag at the plasma membrane- at the same location as assembling VLPs.
Two independent observations suggest that the ESCRT-III proteins do not play a role in initiation, execution or termination of recruitment of Gag. First, the ESCRT-III and Vps4A proteins were only recruited coincident with the completion of Gag accumulation (Fig. 7a). Thus, they are not likely to be involved in initiating or facilitating recruitment. If they were responsible for terminating Gag recruitment, the recruitment of Gag lacking a late domain should extend longer than the corresponding WT VLPs. However, the assembly kinetics of VLPs composed of Gag mutant (Fig. 2c) was indistinguishable from the assembly kinetics of WT VLPs. Similar findings were reported by others 25.
The expression of Vps4A-DN blocks release of virions from cells indicating the requirement of Vps4 for particle release 13. This could be explained by a requirement for Vps4 to remove ESCRT proteins to enable the fission reaction, or recycle components of the ESCRT complexes from nascent virions for subsequent rounds of assembly. Alternatively, Vps4A-DN expression could result in the sequestration of ESCRT proteins on class E compartments. Thus, in the presence of VpsA-DN, ESCRT proteins may simply not be available to mediate particle release. This hypothesis predicts that over time there should be an increase in VLPs that do not have ESCRT complexes associated with them. Our observations demonstrate that the presence of Vps4A-DN increases the number of ESCRT complexes that are observed to be associated with VLPs. This suggests that the Vps4 plays a more active function in scission than simply recycling the ESCRT components.
Thus, our data support a model in which ESCRT-III functions may be limited to events after the recruitment of Gag. Our data are also consistent with a model in which the progressive accumulation of an ‘early’ ESCRT protein or complex (e.g. Alix or ESCRT-I) to a threshold level, potentially together with other factors (e.g. curvature of the nascent virion or formation of a membraneous neck) triggers the rapid deposition of ESCRT-III and Vps4A proteins that carry out membrane scission and ESCRT protein recycling. This model might be generalized to similar reactions at the MVB limiting membrane and at the midbody and provides a simple potential mechanism by which the fission machinery is temporally and spatially regulated.
Supplementary Material
Acknowledgments
We thank Wes Sundquist for reagents, Juan Martin-Serrano for helpful discussions, Vincent Sahi for FACS analysis and Alexa Mattheyses for TIRFM settings. This work was supported by NIH grant K99AI87368 (to NJ), NIH grant R01AI50111 (to PDB) and NSF grant BES-0620813 and NIH GM87977 and R01 AI089844 (to SMS). PDB is a Howard Hughes Medical Institute investigator.
Footnotes
The authors declare no competing financial interests.
Author contribution
NJ, SMS and PDB conceived and designed the experiments. NJ performed the experiments with help from MZ (Figure 2d and 5). NJ, SMS and PDB analyzed the data and wrote the paper.
Supplementary Information accompanies the paper
References
- 1.McDonald B, Martin-Serrano J. No strings attached: the ESCRT machinery in viral budding and cytokinesis. J Cell Sci. 2009;122:2167–2177. doi: 10.1242/jcs.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wollert T, et al. The ESCRT machinery at a glance. J Cell Sci. 2009;122:2163–2166. doi: 10.1242/jcs.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 4.Morita E, Sundquist WI. Retrovirus budding. Annu Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- 5.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 6.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 7.Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 8.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 9.Saksena S, Wahlman J, Teis D, Johnson AE, Emr SD. Functional reconstitution of ESCRT-III assembly and disassembly. Cell. 2009;136:97–109. doi: 10.1016/j.cell.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 13.Garrus JE, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 14.Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 15.Fisher RD, et al. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell. 2007;128:841–852. doi: 10.1016/j.cell.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Serrano J, Eastman SW, Chung W, Bieniasz PD. HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J Cell Biol. 2005;168:89–101. doi: 10.1083/jcb.200408155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Serrano J, Yarovoy A, Perez-Caballero D, Bieniasz PD. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc Natl Acad Sci U S A. 2003;100:12414–12419. doi: 10.1073/pnas.2133846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Schwedler UK, et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 19.Jouvenet N, et al. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 2006;4:e435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finzi A, Orthwein A, Mercier J, Cohen EA. Productive human immunodeficiency virus type 1 assembly takes place at the plasma membrane. J Virol. 2007;81:7476–7490. doi: 10.1128/JVI.00308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsch S, et al. HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007;3:e36. doi: 10.1371/journal.ppat.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon SM. Partial internal reflections on total internal reflection fluorescent microscopy. Trends Cell Biol. 2009;19:661–668. doi: 10.1016/j.tcb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouvenet N, Bieniasz PD, Simon SM. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature. 2008;454:236–240. doi: 10.1038/nature06998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jouvenet N, Simon SM, Bieniasz PD. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc Natl Acad Sci U S A. 2009;106:19114–19119. doi: 10.1073/pnas.0907364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanchenko S, et al. Dynamics of HIV-1 assembly and release. PLoS Pathog. 2009;5:e1000652. doi: 10.1371/journal.ppat.1000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Kimpler LA, Naismith TV, Lauer JM, Hanson PI. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. J Biol Chem. 2005;280:12799–12809. doi: 10.1074/jbc.M413968200. [DOI] [PubMed] [Google Scholar]
- 27.Howard TL, Stauffer DR, Degnin CR, Hollenberg SM. CHMP1 functions as a member of a newly defined family of vesicle trafficking proteins. J Cell Sci. 2001;114:2395–2404. doi: 10.1242/jcs.114.13.2395. [DOI] [PubMed] [Google Scholar]
- 28.Stuchell MD, et al. The human endosomal sorting complex required for transport (ESCRT-I) and its role in HIV-1 budding. J Biol Chem. 2004;279:36059–36071. doi: 10.1074/jbc.M405226200. [DOI] [PubMed] [Google Scholar]
- 29.Zamborlini A, et al. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc Natl Acad Sci U S A. 2006;103:19140–19145. doi: 10.1073/pnas.0603788103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goila-Gaur R, Demirov DG, Orenstein JM, Ono A, Freed EO. Defects in human immunodeficiency virus budding and endosomal sorting induced by TSG101 overexpression. J Virol. 2003;77:6507–6519. doi: 10.1128/JVI.77.11.6507-6519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita E, et al. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1005938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita E, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. Embo J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 34.VerPlank L, et al. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag) Proc Natl Acad Sci U S A. 2001;98:7724–7729. doi: 10.1073/pnas.131059198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usami Y, Popov S, Gottlinger HG. Potent rescue of human immunodeficiency virus type 1 late domain mutants by ALIX/AIP1 depends on its CHMP4 binding site. J Virol. 2007;81:6614–6622. doi: 10.1128/JVI.00314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlton JG, Agromayor M, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc Natl Acad Sci U S A. 2008;105:10541–10546. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhai Q, et al. Structural and functional studies of ALIX interactions with YPX(n)L late domains of HIV-1 and EIAV. Nat Struct Mol Biol. 2008;15:43–49. doi: 10.1038/nsmb1319. [DOI] [PubMed] [Google Scholar]
- 38.Briggs JA, et al. The stoichiometry of Gag protein in HIV-1. Nat Struct Mol Biol. 2004;11 :672–675. doi: 10.1038/nsmb785. [DOI] [PubMed] [Google Scholar]
- 39.Popov S, Popova E, Inoue M, Gottlinger HG. Divergent Bro1 domains share the capacity to bind human immunodeficiency virus type 1 nucleocapsid and to enhance virus-like particle production. J Virol. 2009;83:7185–7193. doi: 10.1128/JVI.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dussupt V, et al. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPXnL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 2009;5:e1000339. doi: 10.1371/journal.ppat.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.