To the Editor
Nonalcoholic fatty liver disease (NAFLD) is a burgeoning medical problem that affects 20–34% of the population in Western countries (1). Although the prevalence of NAFLD is somewhat lower in Asia, the frequency is increasing and the disorder is now being seen in younger individuals (2,3).
NAFLD is a multifactorial disorder. Major risk factors include obesity, insulin resistance and a variation (rs738409) in patatin-like phospholipase domain-containing protein 3 (PNPLA3) that substitutes methionine for isoleucine at residue 148 (I148M) (1). Only one prior study has examined the relationship between this variant and NAFLD in China. In that study the variant was associated with fibrosis, but not steatosis (4). Here we examined the relationship between PNPLA3-I148M and liver TG content in 203 unrelated adults with NAFLD who were recruited from an outpatient liver clinic at the First Hospital of China Medical University. Hepatic steatosis was diagnosed by liver ultrasonography using established criteria (5); all other known causes of hepatic steatosis were excluded (see legend to Table 1). A total of 202 ethnically-matched controls with normal liver enzyme levels and no steatosis by ultrasonography were recruited from primary care outpatient clinics at the same institution.
Table 1.
Demographic and clinical characteristics of subjects.
| Characteristic | NAFLD (n-203) | Control (n=202) | P-value |
|---|---|---|---|
| Men/women | 103/100 | 81/121 | 0.04 |
| Age (years) | 46.6 ± 13.4 | 41.9 ± 13.1 | 1.80E-04 |
| BMI (kg/m2) (median) | 26.6 ± 4.9 | 23.2 ± 4.3 | 2.10E-17 |
| ALT (IU/L) | 44.3 ± 39.8 | 17.0 ± 8.0 | 1.00E-18 |
| AST (IU/L) | 31.4 ± 28.0 | 19.8 ± 4.3 | 1.80E-08 |
| GGT(IU/L) | 61.2±16.1 | 24.8±16.2 | 2.00E-04 |
| Cholesterol (mmol/L) | 5.2±1.0 | 4.5±1.1 | 1.20E-09 |
| Triglyceride (mmol/L) | 1.7 ± 1.2 | 1.0 ± 0.6 | 9.00E-18 |
| LDL-C (mmol/L) | 3.3 ± 1.1 | 2.6 ± 1.1 | 4.30E-12 |
| HDL-C (mmol/L) | 1.3 ± 0.6 | 1.9 ± 0.9 | 8.50E-14 |
| Glucose (mmol/L ) | 6.0 ±1.3 | 5.6 ± 1.3 | 9.80E-04 |
| PNPLA3-I148M allele | 0.45 | 0.31 | 1.50E-04 |
| APOC3 C-482T | 0.43 | 0.45 | 0.57 |
| APOC3 T-455C | 0.47 | 0.46 | 0.94 |
| APOC3 combined variant | 0.65 | 0.66 | 0.92 |
Values are described by mean ± standard deviation for continuous variables; The median values ± interquartile range are provided fro the BMI and triglyceride. NAFLD was diagnosed by ultrasonography (10). Secondary causes of steatosis were excluded (ethanol >30/20 g/day for men/women, total parenteral nutrition, hepatitis B and hepatitis C virus, autoimmune liver disease, hemochromatosis, alpha1-antitrypsin deficiency, Wilson’s disease or use of drugs that promote steatosis. Nominal P-values were calculated by a two-sample t-test for continuous variables, a proportion test for categorical variables, and Fisher’s exact test for genotypes (http://www.R-project.org/.) PNPLA3 and APOC3 genotypes were assayed as described (7, 11).
BMI, body mass index,; ALT, alanine aminotransferase, AST, aspartate aminotranferase; GGT, Gamma-glutamyl transpeptidase; LDL-C, low density lipoprotein-cholesterol; HDL-C, high density lipoprotein-cholesterol; PNPLA3, patatin-like phospholipase domain-containing protein 3; APOC3, apolipoprotein C3
After obtaining IRB-approved written informed consent, fasting blood samples were collected. A higher proportion of cases were men (60% versus 49%; nominal P=0.04) and their mean age was greater than that of controls (46.6 versus 41.9 years old; P=1.8×10−4). Levels of circulating liver enzymes, LDL-C, triglyceride and glucose were significantly higher, and HDL-C levels were significantly lower, in cases than in controls (Table 1).
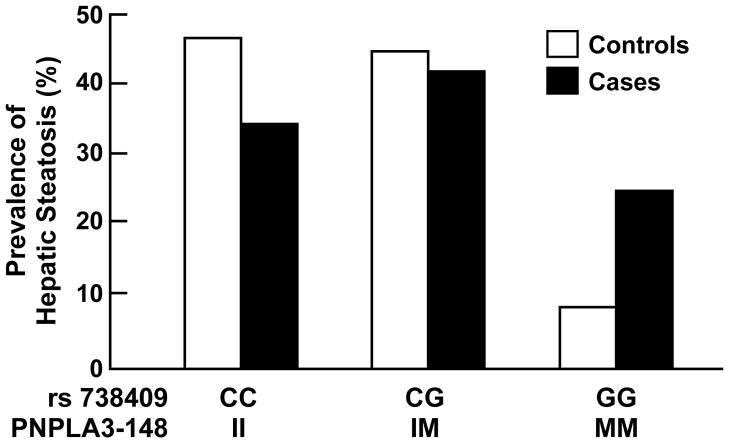
The frequency of the I148M variant was significantly higher in cases (0.45) than in controls (0.31) (P=1.5×10−4) and the association remained robust after adjusting for age, sex, and BMI (P=3.7×10−3) (Fig. 1). The odds ratio for hepatic steatosis was 1.73 for each copy of the G allele (95% CI: [1.49, 1.99]). The I148M variant was also associated with higher serum ALT levels (nominal P=1.1×10−7, adjusted P=2.0×10−6), but not with BMI, fasting glucose or with lipid/lipoprotein levels (data not shown).
Figure 1.
Genotype frequencies of rs738409 in cases with hepatic steatosis (n=203) and controls (n=202).
This is the first report to document an association between PNPLA3-I148M and hepatic steatosis in a population from mainland China. The relatively high frequency of the risk allele in China makes it imperative that efforts are made to control weight gain with increasing urbanization and adoption of Western eating habits.
Acknowledgments
Financial support: NIH (RL1HL092550 and RO1DK090066)
References
- 1.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 26(Suppl 1):163–172. doi: 10.1111/j.1440-1746.2010.06548.x. [DOI] [PubMed] [Google Scholar]
- 3.Fan JG, Peng YD. Metabolic syndrome and non-alcoholic fatty liver disease: Asian definitions and Asian studies. Hepatobiliary Pancreat Dis Int. 2007;6:572–578. [PubMed] [Google Scholar]
- 4.Li X, Zhao Q, Wu K, Fan D. I148M variant of PNPLA3 confer increased risk for nonalcoholic fatty liver disease not only in European population, but also in Chinese population. Hepatology. 2011 doi: 10.1002/hep.24567. [DOI] [PubMed] [Google Scholar]
- 5.Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, Mao YM. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]