Abstract
Background
Intramuscular interstitial cells of Cajal (ICC-IM) have been shown to participate in nitrergic neuromuscular transmission (NMT) in various regions of the gastrointestinal (GI) tract but their role in the internal anal sphincter (IAS) is still uncertain. Contractile studies of the IAS in the W/Wv mouse (a model in which ICC-IM numbers are markedly reduced) have reported that nitrergic NMT persists and that ICC-IM are not required. However, neither the changes in electrical events underlying NMT nor the contributions of other non-nitrergic neural pathways have been examined in this model.
Methods
The role of ICC-IM in NMT was examined by recording the contractile and electrical events associated with electrical field stimulation (EFS) of motor neurons in the IAS of wildtype and W/Wv mice. Nitrergic, purinergic and cholinergic components were identified using inhibitors of these pathways.
Key Results
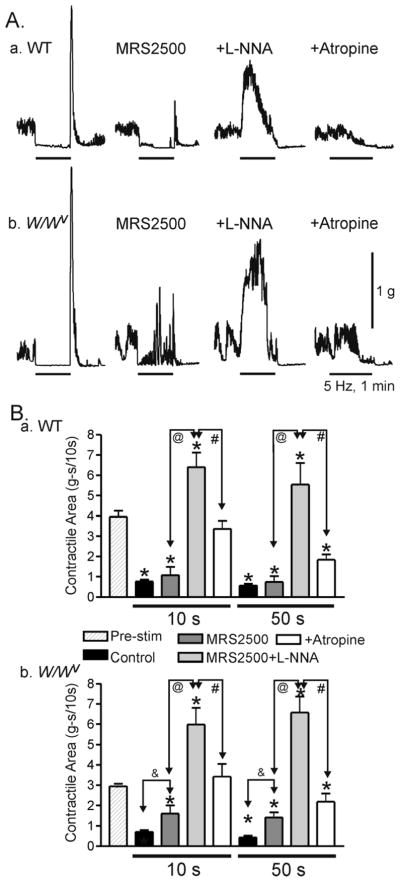
Under NANC conditions, purinergic, and nitrergic pathways both contribute to EFS induced inhibitory junction potentials (IJPs) and relaxation. Purinergic IJPs and relaxation were intact in the W/Wv mouse IAS whereas nitrergic IJPs were reduced by 50–60% while relaxation persisted. In the presence of L-NNA (NOS inhibitor) and MRS2500 (P2Y1 receptor antagonist), EFS gave rise to cholinergic depolarization and contractions that were abolished by atropine. Cholinergic depolarization was absent in the W/Wv mouse IAS while contraction persisted.
Conclusions and Inferences
ICC-IM significantly contribute to the electrical events underlying nitrergic and cholinergic NMT whereas contractile events persist in the absence of ICC-IM. The purinergic inhibitory neural pathway appears to be independent of ICC-IM.
Keywords: enteric, gastrointestinal, fibroblast-like cells, interstitial cells of Cajal, membrane potential, internal anal sphincter
Intramuscular interstitial cells of Cajal (ICC-IM) have been shown to be located in close proximity to nerves throughout most of the gastrointestinal (GI) tract 1 including the murine internal anal sphincter (IAS) 2. A variety of functional and morphological studies have provided evidence that ICC-IM participate in nitrergic and cholinergic neuromuscular transmission (NMT) in various regions of the GI tract 3–6. None-the-less, the role of these cells in NMT remains controversial 7–9.
ICC express the receptor tyrosine kinase Kit and in Kit mutant animal models, ICC numbers are greatly reduced in some portions of the GI tract 10, 11. These models have frequently been used to assess the role of ICC in mediating NMT. Immunohistochemical studies examining the presence of ICC in the Kit mutant W/Wv mouse IAS have yielded conflicting results with one study suggesting a complete absence of ICC 12 while another reported some “faintly stained” ICC at the submucosal edge 13. We recently re-examined this issue in the mouse IAS and found that ICC are absent from the myenteric edge (ICC-My) of both wildtype (WT) and W/Wv mice while stellate-shaped submucosal ICC (ICC-SM) are present in both. In contrast, ICC-IM are present in WT but not in W/Wv mice 2.
Studies of the W/Wv mouse IAS have also examined nerve evoked relaxations and the in vivo rectoanal inhibitory reflex (RAIR) 12, 13. One study reported that the RAIR was unchanged 12 whereas the other reported that the RAIR was reduced while nerve evoked relaxations were intact 13. The latter group suggested that the reduction in RAIR could be due to the contribution of ICC to the afferent limb of the response 13. In spite of these differences both studies concluded that ICC did not appear to be necessary for nitrergic inhibitory NMT in the mouse IAS 12, 13. However, the electrical events underlying NMT were not examined in these studies nor were possible changes in other non-nitrergic neural pathways. There is evidence that both purines such as ATP and peptides such as VIP contribute to inhibitory NMT in the mouse and rat IAS 14–16. It is therefore possible that changes could occur in inhibitory NMT in the W/Wv mouse IAS that were not detected using previous methodologies.
The present study examines the role of ICC-IM in enteric NMT in the mouse IAS in more depth by determining if there are differences in electrical and contractile events underlying the actions of various neurotransmitters in WT versus W/Wv mice. To do this we used the selective P2Y1 receptor antagonist, MRS2500 and the nitric oxide synthase (NOS) inhibitor L-NNA and measured membrane potential and contractile responses to activation of motor neurons under NANC conditions. Cholinergic NMT was also examined in the absence of atropine. Purines and NO were found to contribute to both the electrical and mechanical events underlying inhibitory NMT in the IAS whereas excitatory NMT was largely due to acetylcholine (ACh). Our results suggest that ICC-IM generate cholinergic depolarization and 50–60% of the nitrergic IJP whereas contractile events persist in the absence of ICC-IM. Purinergic electrical and contractile events were independent of ICC-IM. A preliminary report of this work has been published in abstract form 17.
Materials and methods
Tissue preparation
Mice used for these studies were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all experiments and procedures were approved by the Institutional Animal Use and Care Committee at the University of Nevada. C57BL/6 and W/Wv mice (30–90 days old; Jackson Laboratory, Bar Harbor, MN, USA) were killed with isoflurane (Baxter, Deerfield, IL, USA) followed by cervical dislocation. The rectoanal region was removed by dissecting away overlying tissue and was pinned in a dissecting dish containing cold Krebs bicarbonate solution (KRB) of the following composition (in mM): NaCl 118.5, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaHCO3, 23.8, KH2PO4, 1.2, dextrose, 11.0. This solution was maintained at a pH of 7.4 at 37°C by bubbling to equilibrium with 95%O2-5%CO2. The distal extremity of the IAS was identified and is referred to in this study as the anal verge. Overlying skeletal muscle, glands and mucosa were carefully dissected away before carrying out experiments.
Contractile Experiments
Muscle strips were attached with sutures to a stable mount and to a Gould strain gauge and immersed in tissue baths containing 15 ml of oxygenated KRB solution maintained at 37°C. A basal tension of 0.5 grams was applied to muscle strips. Tissues were equilibrated during the first 60 minutes after mounting. Applied tension declined to approximately 0.2–0.3 grams during this equilibration period. All experiments were performed in the presence of 10μM guanethidine. Data was stored and analyzed by computer using a data acquisition program (AcqKnowledge; Biopac systems, Inc., Goleta, CA). Neural responses were evaluated by the application of electrical field stimulation (EFS) using a Grass S48 stimulator (1–5Hz at 15V and 0.3ms pulse duration for 1 minute) as was determined by previous experiments. Platinum inoculation loops (3 mm diameter, separated from one another by 1 cm) were used as stimulating electrodes. Muscle strips were threaded through both loops. The muscle was attached at the bottom to a stable mount and at the top to a tension transducer. EFS gave rise to TTX-(1μM) sensitive neural responses.
Contraction analysis
Spontaneous contractions were evaluated using AcqKnowledge software. To determine phasic contractile amplitude a threshold value was selected (in grams) that bisected a group of phasic contractions to be measured. The program determines the peak and trough values for every phasic contraction that passes through the threshold on both rising and falling phases. Since fast phasic contractions (40–60 cpm) were usually superimposed upon a slower phasic rhythm (2–4 cpm), fast phasic contractile amplitude was averaged for 3 periods during the peak of the slow rhythm (“peak phasic”) and 3 periods during the trough of the slow rhythm (“trough phasic”). Mean values for each group were then reported (see SupFig. 1). The amplitude of the slow rhythm (“slow phasic”) was determined by subtracting the trough value during minimum contraction from the trough value during peak contraction. Tone was defined as the mean trough value during minimum contraction. Total spontaneous contraction was determined by summing together peak phasic contractile amplitude plus slow rhythm plus tone (see SupFig. 1 and Table I). Spontaneous contractile activity was calculated as a percentage of the contraction induced by 60mM KCl. Passive tension was determined at the end of the experiment with 10μM sodium nitroprusside (SNP) and 1μM nifedipine and was subtracted from spontaneous contractile amplitudes. Nerves were stimulated for 60s and the area of contraction during the initial 10s and the remaining 50s of electrical field stimulation (EFS) was quantitated using AcqKnowledge software. The initial 10s contractile response was determined for comparison to electrical responses. Contractile area during EFS was compared to control area (“Pre-Stimulus”) and expressed per 10s. Statistical analysis was performed using one-way ANOVA followed by a post-hoc Dunn’s or Tukey test. Data are expressed as mean±S.E. and values were considered significantly different when p<0.05.
Table I. Comparison of spontaneous contractile activity in the WT and W/Wv mouse IAS.
The various components of spontaneous contractile activity were determined in WT (n=7) and W/Wv mice (n=7) as outlined in the methods section and depicted in SupFig. 1.
| Mouse | 1) Peak Phasic | 2) Trough Phasic | 3) Slow Phasic | 4) Tone | 5) Total Phasic (1+3) | 6) Total Spontaneous (1+3+4) |
|---|---|---|---|---|---|---|
| WT (g) | 0.22±0.03 | 0.12±0.02 | 0.06±0.01 | 0.23±0.03 | 0.30±0.04 | 0.51±0.04 |
| WT (% Max) | 8.3±0.9 | 4.2±0.7 | 4.3±0.6 | 11.9±2.0 | 12.6±1.5 | 24.5±2.6 |
| W/Wv (g) | 0.2±0.03 | 0.1±0.02 | 0.1±0.01 | 0.27±0.04 | 0.28±0.04 | 0.58±0.05 |
| W/Wv (% Max) | 9.7±0.5 | 5.6±0.5 | 2.9±0.4 | 11.9±2.4 | 12.6±0.5 | 24.5±2.3 |
The mean ± S.E. for these contractions (g = grams) as well as the percentage of the maximum contractile force elicited with 60mM KCl (% Max) are shown in this table. Spontaneous contractile activity was not significantly different between WT and W/Wv mice.
Intracellular measurements
Muscle strips were pinned to the base ofan electrophysiological chamber with the submucosal surface facing upwards. Smooth muscle cell (SMC) impalements were made with glass microelectrodes (filled with 3M KCl; tip resistances ranging from 60–100 MΩ) as previously described 14. Nerves were stimulated (0.05–5Hz; 70V; 0.2 ms) via platinum electrodes (1 cm length) placed 1 mm away from the muscle at both sides along its entire length. The stimulus parameters for these experiments differ from the isolated tissue bath experiments since the depth of fluid was less. TTX-sensitive neural responses were elicited under these stimulation parameters. To maintain impalements and slow wave activity, experiments were carried out after super fusing tissue preparations with the myosin light chain kinase inhibitor wortmannin (25μM) for 20 minutes followed by a 60 minute washout period as previously described18. This treatment permanently blocked contraction while electrical activity recovered to that of untreated tissues, i.e., Em, slow waves and spikes were very similar to those reported for the mouse IAS in a previous study in the absence of wortmannin14.
Analysis of intracellular measurements
Resting membrane potential (Em) was determined as the average value of Em between slow waves. Slow wave amplitude, rise time and frequency were calculated with AcqKnowledge software. The amplitude of the composite inhibitory junction potentials (IJP) elicited with 5 Hz for 10s was measured as the most negative potential occurring during three different time periods; i.e., 0.3–1.3s, 5–6s, and 9–10s following the initiation of EFS. This was done so that the value of Em between slow waves could be selected.
Drugs
Atropine sulphate, N(ω)-nitro-L-arginine (L-NNA), guanethidine, sodium nitroprusside (SNP) tetrodotoxin (TTX) and nifedipine were all purchased from Sigma-Aldrich (Saint Louis, MO, USA). Wortmannin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). MRS2179 and MRS2500 were purchased from Tocris Bioscience (Ellisville, MO, USA). Atropine, L-NNA, guanethidine, SNP, MRS2179 and MRS2500 were dissolved in de-ionized water. Nifedipine and wortmannin were dissolved in ethanol and DMSO respectively.
Results
Basic electrical properties and inhibitory junction potentials in the wildtype mouse IAS
Electrical activity in the wildtype (WT) mouse IAS was measured with intracellular recording techniques. Oscillations in membrane potential (Em) were present that we have referred to as “slow waves” (Fig. 1A; SupFig. 2A). The average value of Em between slow waves was −49.1±0.5 mV (n=17). Slow waves in the IAS did not have an initial rapid depolarizing phase as described for other GI regions (e.g., 434 mV/sec upstroke in canine antrum, 19). Rather, they slowly depolarized (i.e., 10–80 mV/sec maximum rate of rise) reaching peak after 0.4–0.7 sec. The frequency of slow waves was relatively constant (38.2±1.5 cpm, n=17) whereas amplitude was quite variable; often exhibiting a “waxing and waning” pattern (see SupFig. 2A and 20, 21). Spikes (260–800 mV/sec) of variable amplitude were often superimposed upon slow waves (see Fig. 1A, SupFig. 2A,3A) Peak depolarization during the slow wave (including the spike) was determined for each tissue and averaged 26.0±1.4 mV (n=28).
Figure 1. Effect of various blockers on inhibitory junction potentials in the WT mouse IAS.
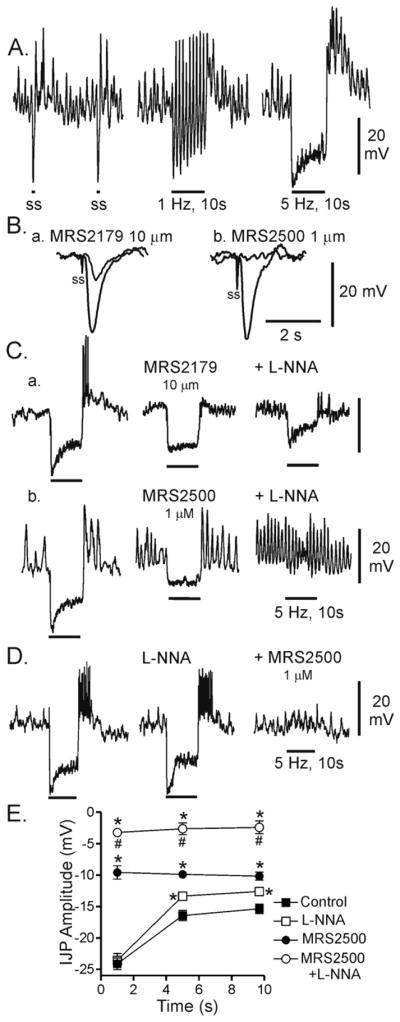
(A) Sample traces showing IJPs elicited with 0.05 Hz (left), 1 Hz (middle) and 5 Hz (right) EFS for 10s. Peak IJP amplitude always occurred with the first stimulus. Each stimulus in these recordings gives rise to rebound depolarization at 0.05 and 1 Hz whereas at 5 Hz rebound depolarization is limited to the end of the stimulus train. (B) Superimposed traces showing the effect of either MRS2179 (10 μM; Ba) or MRS2500 (1 μM; Bb) on IJPs elicited with a single shot (ss) electrical stimulus. L-NNA (100 μM was present throughout. While MRS2179 reduced IJPs (Ba), MRS2500 abolished them (Bb). (C) Sample traces showing composite IJPs elicited with 5 Hz EFS for 10s. Control composite IJPs (Ca,b left traces) were reduced by either MRS 2179 (Ca) or MRS2500 (Cb) (middle traces). However a residual purinergic component is apparent with combined addition of L-NNA (100 μM) and MRS2179 Ca), while the composite IJP is abolished with combined addition of L-NNA with MRS2500 (Cb) (right traces). (D) Sample traces showing the effect of first blocking nitrergic NMT on the composite IJP. Whereas there was a small reduction in the sustained component with L-NNA, the peak hyperpolarization was not reduced. (E) Summary graph of the effects of various blockers on the amplitude of the IJP measured during three different time periods (see methods for further details). Under control conditions (■, n=13), peak hyperpolarization occurs during the initial time period and this is followed by a smaller, more sustained period of hyperpolarization (second and third values). L-NNA (□, n=5) did not reduce peak IJP amplitude while the sustained component (second and third values) was significantly reduced (*, p<0.05). In contrast, MRS2500 (●, n=8) significantly (*) reduced IJP amplitude during all three time periods. Combined MRS2500 and L-NNA (○, n=10) significantly reduced IJP amplitude below the control level (*) and below the level observed with MRS2500 alone (#). All muscles were pre-exposed to 25 μM wortmannin for 20 min followed by at least 1 hour wash out before beginning experiments. Shown are mean values ±S.E.M.
A single shot electrical stimulus produced an inhibitory junction potential (IJP) averaging 22.0 ±1.1 mV (n=11). There was no further increase in IJP amplitude with repetitive stimulation (5Hz, 10s; atropine and guanethidine present throughout). At low frequencies of electrical field stimulation (EFS; 0.1–1Hz) Em following peak hyperpolarization returned at least to the “resting” value and in some cases this was followed by a spike (e.g., Fig. 1A). In contrast, at 5 Hz EFS individual hyperpolarizations fused together and rebound was confined to the end of the stimulus train although it was larger in amplitude and longer in duration than with a single shock (Fig. 1A). IJP amplitude during 5Hz EFS was measured during three different time periods (i.e., 0.3–1.3, 5–6 and 9–10s) to quantitate its time course. The initial peak hyperpolarization quickly declined in amplitude to a sustained level of hyperpolarization that persisted for the remainder of the stimulus train. At the end of stimulation, rebound depolarization occurred with superimposed slow waves and spikes (Fig. 1A,C,D).
Role of purinergic and nitrergic pathways in generation of inhibitory junction potentials in the wildtype mouse IAS
To investigate the contribution of P2Y1 receptors to generation of IJPs we first compared the effects of two different P2Y1 receptor antagonists, i.e., MRS2179 and MRS2500 (Fig. 1B,C) since MRS2500 has been reported to be a more potent blocker of P2Y1 receptors in rat colon than MRS2179 22. In the presence of L-NNA (100 μM), MRS2179 (10 μM) reduced IJPs elicited with a single stimulus from 23.0±1.2 mV to 8.0±1.6 mV (n=5; Fig. 1Ba) whereas MRS2500 (1 μM) abolished the single IJP (Fig. 1Bb). When MRS2179 (10 μM) and MRS2500 (1 μM) were tested on composite IJPs elicited with 5 Hz EFS for 10s IJPs and rebound were reduced by either P2Y1 blocker. However, there was still a residual IJP remaining following combined addition of MRS2179 with L-NNA (Fig. 1Ca) whereas combined MRS2500 and L-NNA abolished the composite IJP (Fig 1Cb). These data indicate that the IJP is composed of both purinergic and nitrergic components and supports the previous study of rat colon indicating that MRS2500 is a more potent P2Y1 antagonist than MRS2179 22.
To further examine the time course and amplitude of purinergic IJPs elicited with repetitive EFS (5Hz, 10s) we carried out additional experiments in which L-NNA was applied before MRS2500. Peak IJP amplitude was unchanged in the presence of L-NNA whereas there was a small but significant reduction in the sustained component (Fig. 1D,E). In contrast to P2Y1 blockers, rebound was not reduced by L-NNA. Interestingly, the remaining purinergic IJP also consisted of two phases, i.e., an initial peak hyperpolarization followed by rapid repolarization to a smaller sustained hyperpolarization. Since this study was primarily focused upon the role of ICC-IM in nitrergic NMT the remaining electrical measurements were carried out by first blocking the purinergic IJP with MRS2500 so that the nitrergic IJP could be examined in isolation.
Basal contractile properties and responses to stimulation of inhibitory nerves in the wildtype mouse IAS
Contractile activity in the WT mouse IAS was measured in strips of muscle in isolated tissue baths. Muscles developed spontaneous contractile activity over a 20–30 min period of time following immersion in the bath. The mean amplitude of peak spontaneous contraction (“total spontaneous” contraction) was 24.5±2.6% of the maximum contraction elicited with 60mM KCl (see Fig. 2A, Table I and SupFig. 1; n=7). Spontaneous contraction consisted of both phasic and tonic components as defined in the methods section (see also SupFig. 1). Phasic contractions were further divided into a fast rhythm (56.7±2.8 cpm) superimposed upon a slow rhythm (3.5±0.2 cpm) (see SupFig. 2C). The amplitude of fast phasic contractions superimposed upon the peak of the slow rhythm (“peak phasic”) was approximately twice as large as the fast phasic contractions occurring during the trough of the slow rhythm (“trough phasic”) (see Table I, n=7). Finally, the amplitude of total phasic activity (12.6±1.5% Max) was not different from the amplitude of tone (11.9±2.0% Max).
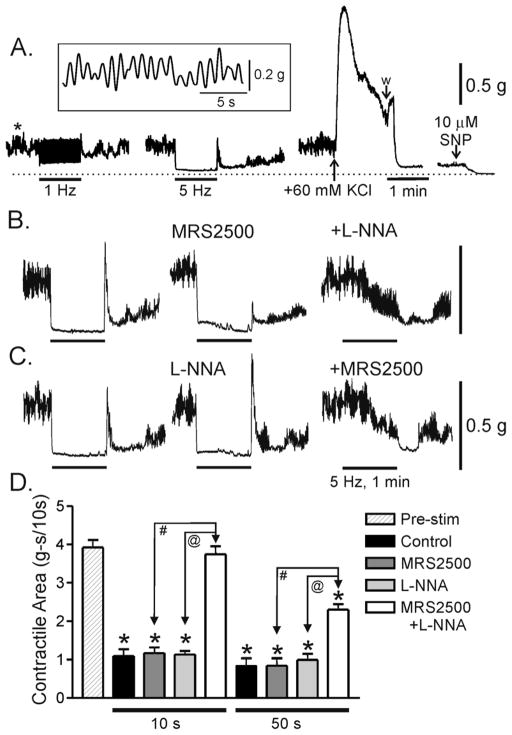
Figure 2. Effects of MRS2500 and L-NNA on nerve induced relaxations in the WT mouse IAS.
(A) Sample traces showing spontaneous phasic and tonic contractile activity along with several experimental procedures. Phasic contractions (*left panel) are shown at a more rapid sweep speed in the inset. 1 Hz (left trace) and 5 Hz (middle trace) EFS gave rise to frequency dependent relaxation. Peak contraction was determined at the end of the experiment with 60 mM KCl (w, wash; right panel) and zero tone with SNP (10 μM) plus nifedipine (1 μM, no further relaxation occurred in this muscle). (B) MRS2500 (1 μM) alone did not reduce relaxation at 5 Hz EFS but rebound contraction was reduced. MRS2500 also did not reduce relaxation with 1 Hz EFS (data not shown). (C) L-NNA (100 μM) alone also did not reduce relaxation but in this case rebound contraction increased. MRS2500 and L-NNA together abolished relaxation for approximately 20–30s of EFS at which point a slow onset relaxation began (B and C). (D) Summary graph of the effects of MRS2500 (n=8) and L-NNA (n=4) on contractile area during EFS. Contractile area during EFS (5 Hz, 60s) is compared to the area preceding EFS (“Pre-stim”, n=12). Contractile area during the first 10s of EFS and the subsequent 50s of EFS are shown separately (all areas have been normalized to area/10s). EFS gave rise to significant (*) relaxation under control conditions and in the presence of either MRS2500 or L-NNA. Neither MRS2500 nor L-NNA alone significantly reduced relaxation during either the initial 10s or the subsequent 50s of EFS, whereas combined MRS2500 and L-NNA (n=8) abolished the EFS-induced relaxation during the initial 10s of EFS and significantly reduced relaxation during the subsequent 50s of EFS (# and @). Shown are mean values ±S.E.M.
Low frequency EFS gave rise to brief relaxations followed by rebound contractions (e.g., 1Hz, Fig. 2A) whereas at 5 Hz there was continuous relaxation during EFS and rebound contraction at the termination of EFS (Fig. 2A). Rebound contraction with 5 Hz EFS was greater than with a single shock. The contractile patterns for lower (1Hz) and higher (5Hz) frequency EFS were therefore very similar to the patterns of junction potentials at lower and higher frequency EFS (Fig. 1A).
Role of purinergic and nitrergic pathways in nerve evoked relaxations in the wildtype mouse IAS
To examine the contribution of nitrergic and purinergic pathways to nerve evoked relaxation we compared the effects of MRS2500 (1μM) and L-NNA (100μM). Neither blocker significantly reduced the initial 10s or the subsequent 50s of relaxation (5Hz EFS 60s; guanethidine and atropine present throughout)(Fig 2B, C middle traces). However, rebound contraction was greatly reduced by MRS2500 (Fig. 2B) and increased by L-NNA (Fig. 2C). We have previously noted this effect in the BALB/c mouse IAS 14. In contrast, when both blockers were added relaxation was abolished during the initial 10s of EFS suggesting that both nitrergic and purinergic pathways contribute to this phase of the response. After an additional 10s of EFS a slowly developing relaxation commenced that reached maximum by the end of the 60s stimulus train (Fig. 2B,C right traces). Thus, contractile area from 10–60s (50s) was significantly less than control (Fig. 2D) suggesting that a third neurotransmitter is released with longer stimulus periods. This hypothesis was further supported by addition of the neurotoxin TTX (1 μM, n=3) which abolished the remaining ultraslow relaxation.
Comparison of basal electrical activity and inhibitory junction potentials in the wildtype and W/Wv mouse IAS
To examine the role of ICC-IM in the electrical events underlying inhibitory NMT in the IAS we first characterized the basal electrical activity of the W/Wv mouse IAS. Em was 4 mV more negative in W/Wv than in WT mice (−53.2 ±0.5 mV, n=14 W/Wv mice versus −49.1±0.5 mV, n=17 WT mice, p<0.05). In addition peak depolarization during the slow wave was 7 mV greater (33.3±0.9 mV, n=20 for W/Wv mice versus 26.0±1.4 mV, n=28 for WT mice, p<0.05) while slow wave frequency was not different (43.0±2.4 cpm, n=14 for W/Wv mice versus 38.2±1.5 cpm, n=17 for WT). Slow waves waxed and waned in W/Wv mice as they did in WT mice (SupFig. 2A,B) with rapidly rising spikes superimposed upon some slow waves (SupFig. 3A)
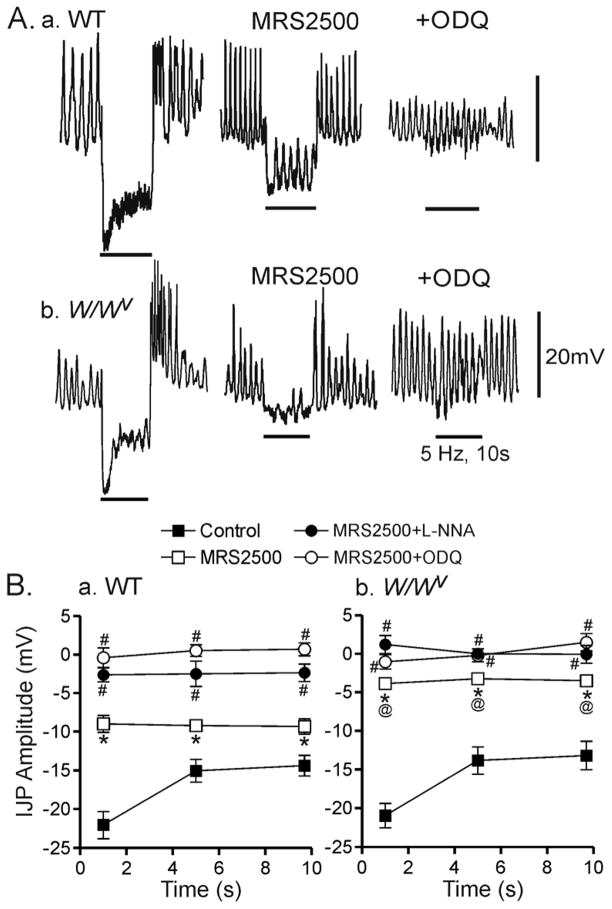
To examine the electrical events underlying NMT IJPs were elicited with 5 Hz EFS for 10s in the W/Wv mouse IAS (guanethidine and atropine present throughout) and compared to those of the WT mouse. Control IJPs in the W/Wv mouse were not significantly different from WT (Fig. 3A left traces and B) including the presence of rebound at the end of the stimulus train (Fig. 3Ab). In contrast, following MRS2500 addition (which did not depolarize Em in either WT or W/Wv mice), the remaining hyperpolarization was 50–60% smaller in W/Wv than in WT mice (Fig. 3A middle traces and B) suggesting that ICC-IM contribute to the generation of nitrergic IJPs. Nonetheless, slow waves were still greatly reduced or abolished with EFS in the W/Wv mouse IAS. Application of a single shock of EFS in the presence of MRS2500 gave rise to a significant nitrergic IJP in WT mice (4.5±0.6 mV, n=11), but not in W/Wv mice (n=12, see SupFig. 3) further confirming a role for ICC-IM in generating nitrergic IJPs. Combined addition of MRS2500 with either L-NNA or the guanylate cyclase inhibitor ODQ (10μM) eliminated all remaining electrical changes evoked with EFS in both animals (Fig. 3A right traces and B) confirming that the MRS2500-independent IJP is due to the nitrergic pathway and that guanylate cyclase is required. L-NNA depolarized 4 of 9 WT muscles (3.9±0.8 mV, n=4) while no W/Wv muscles depolarized (n=8).
Figure 3. Comparison of the effects of various blockers on inhibitory junction potentials in the WT and W/Wv mouse IAS.
(A) Sample traces showing the effects of MRS2500 and ODQ on composite IJPs elicited with 5 Hz EFS (10 s) in WT (Aa) and W/Wv (Ab) mice. Control IJPs (A, left traces) in WT and W/Wv mice are very similar and MRS2500 (1 μM) abolishes the initial fast component in both muscles (middle traces). However the IJP which persists in the presence of MRS2500 in the W/Wv mouse is smaller. Combined MRS2500 and ODQ (10 μM) abolishes the composite IJP in both muscles (right traces). (B) Summary graph of the effects of blockers on the amplitude of the IJP during 3 different time periods in WT (Ba) and W/Wv (Bb) mice. The IJP was not significantly different in WT (n=9) and W/Wv (n=10) mice under control conditions (■) while MRS2500 (□) significantly (*) reduced the IJP in both WT (n=9) and WWv (n=9) animals. The IJP which persists in the presence of MRS2500 was consistently smaller (@) in W/Wv than in WT mice (B) while the variation between cells within either group was very similar. The IJP recorded in the presence of MRS2500 plus L-NNA (●, n=5) or MRS2500 plus ODQ (○, n=5) was significantly less (#) than with MRS2500 alone in both animals. All muscles were pre-exposed to 25 μM wortmannin for 20 min followed by at least 1 hour wash out before beginning experiments. Shown are mean values ±S.E.M..
Comparison of basal contractile activity and nerve evoked relaxations in the wildtype and W/Wv mouse IAS
As described for WT mice, spontaneous contractions developed in W/Wv mice over a 20–30 minute time period following immersion in the tissue bath and consisted of both phasic and tonic components (SupFig. 2D). The fast phasic (53.9±1.7 cpm, n=7 W/Wv vs 56.7±2.8 cpm, n=14 in WT IAS) and slow phasic (3.3±0.2 cpm, n=7 W/Wv, 3.5±0.2 cpm, n=7 WT) contractile frequencies were not different between W/Wv and WT mice nor were mean amplitudes of total spontaneous contractile activity (i.e., 24.5±2.3%, n=7 for W/Wv versus 24.5±2.6%, n=7 for WT mice, see Table I). In addition there were no significant differences in the various components contributing to overall spontaneous contractile activity (see Table I).
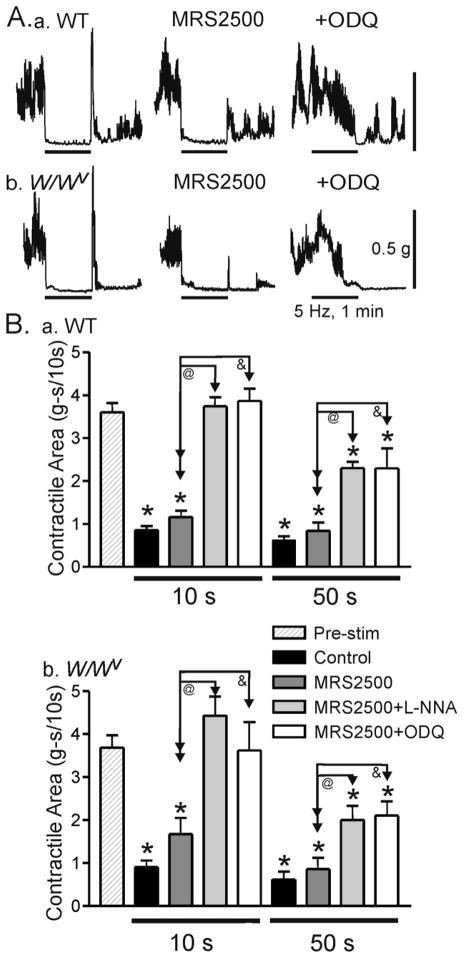
To investigate whether nerve evoked relaxations differ in the absence of ICC-IM we repeated contractile measurements in the W/Wv mouse IAS. Relaxations elicited with 5 Hz EFS (60s; guanethidine and atropine present throughout) were compared to those of WT mice. Under control conditions nerve evoked relaxations were not significantly different between animals either during the first (10s) or second (10–60s) time period of EFS (Fig. 4A, left traces and B). In addition, MRS2500 did not significantly reduce relaxation in either WT or W/Wv mice (Fig. 4A, middle traces and B) while rebound contractions were reduced in both. As noted for WT mice (Fig. 2B–D), combined addition of MRS2500 with either L-NNA or ODQ completely abolished relaxation in the W/Wv mouse IAS during the first 20–30s of EFS while some relaxation developed with longer periods again indicating the presence of a third inhibitory neurotransmitter (Fig. 4A,B). Neither MRS2500 nor MRS2500 in combination with either L-NNA or ODQ increased spontaneous contractile activity in WT and W/Wv mice. Although not seen during the period of contraction shown in Fig. 4, contractile activity always returned to the control amplitude 3–5 minutes after termination of EFS in both WT and W/Wv mice.
Figure 4. Comparison of the effects of various blockers on nerve induced relaxations in the WT and W/Wv mouse IAS.
(A) Sample traces showing the effects of MRS2500 and ODQ on relaxations elicited with 5 Hz EFS (60s) in WT (Aa) and W/Wv (Ab) mice. MRS2500 did not reduce EFS-induced relaxation in either animal model (A, middle traces) whereas combined MRS2500 and ODQ led to complete blockade of relaxation during the first 30s of EFS (A, right traces). (B) Summary graph of the effects of blockers on nerve-induced relaxation during the initial 10s of EFS and the subsequent 50s of EFS in WT (Ba) and W/Wv (Bb) mice. Contractile area during EFS (5 Hz, 60s) is compared to the contractile area preceding EFS (“Pre-stim”, n=11 WT; n=10 W/Wv). All areas are normalized to area/10s. EFS gave rise to a significant relaxation (control, black bars, n=11 WT; n=10 W/Wv). MRS2500 alone (dark grey bars, n=8 WT; n=10 W/Wv) did not significantly reduce relaxation during the first 10s or the subsequent 50s of EFS in either animal. Combined MRS2500 plus L-NNA (light grey bars, n= 7 WT; n=6 W/Wv) or ODQ (white bars, n=4 WT; n=4 W/Wv) abolished the EFS-induced relaxation during the initial 10s of EFS and significantly reduced relaxation during the subsequent 50s of EFS in both animals (@ and &). Shown are mean values ±S.E.M.
Differences in nerve evoked depolarizations in the wildtype and W/Wv mouse IAS
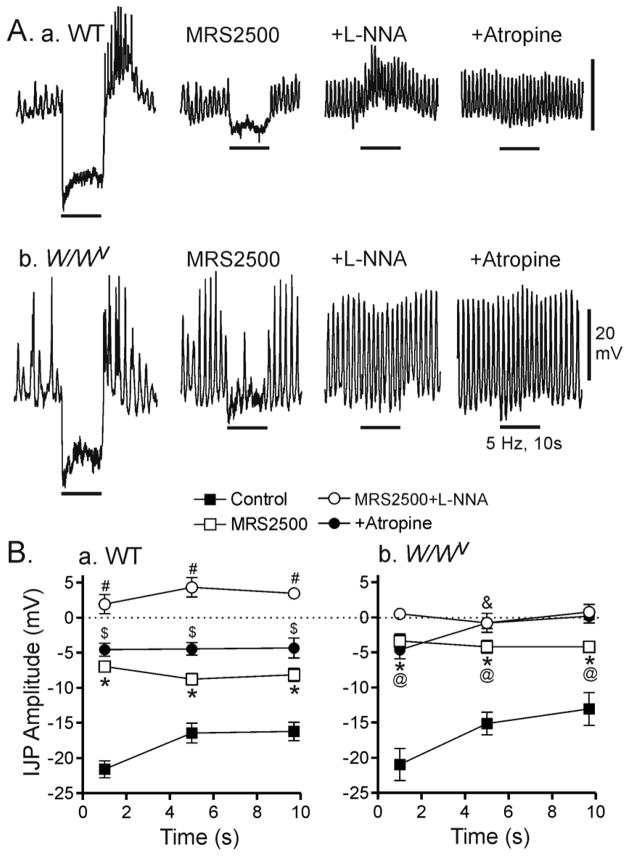
In previous studies we have shown that excitatory motor innervation is sympathetic in the monkey but not the mouse IAS 23. For this study we therefore examined whether cholinergic nerves serve as excitatory motor neurons in the mouse IAS and whether this pathway is modified in the W/Wv mouse IAS. Atropine was omitted from the initial bathing conditions and nerves were repetitively stimulated (5Hz EFS, 10s; guanethidine present throughout). The composite IJP was not significantly different between WT and W/Wv mice (Fig. 5A, 1st traces and B) nor was rebound depolarization. In contrast, following addition of MRS2500, the remaining hyperpolarization elicited with EFS was significantly smaller in W/Wv than in WT mice (Fig. 5A, 2nd traces and B) again suggesting that ICC-IM are important for generation of the nitrergic IJP. As observed in the WT mouse, MRS2500 reduced or abolished rebound depolarization (Fig. 5Ab) The most significant difference noted between animals was the appearance of a slowly developing depolarization in WT mice with combined MRS2500 and L-NNA (Fig. 5Aa, 3rd trace and Ba). This depolarization was blocked by atropine confirming its cholinergic nature (Fig. 5Aa, 4th trace and B). In contrast, slow depolarization was not observed in the W/Wv mouse IAS (Fig. 5Ab, 3rd trace and Bb) suggesting that ICC-IM are required for generation of the cholinergic depolarization.
Figure 5. Comparison of nerve-evoked junction potentials in the WT and W/Wv mouse IAS in the absence of atropine.
(A) Sample traces showing the effects of MRS2500 and L-NNA on junction potentials elicited with 5 Hz EFS (10 s) in WT (Aa) and W/Wv (Ab) mice in the absence of atropine. The inhibition of IJPs by MRS2500 (1 μM) in the absence of atropine (2nd traces) was similar to that seen in the presence of atropine (see Fig. 3A, middle traces). However, with combined addition of L-NNA and MRS2500 a slow depolarization is revealed in WT but not W/Wv mice (3rd traces). This depolarization is abolished by atropine (1 μM; 4th traces). (B) Summary graph of the effects of MRS2500 and L-NNA in the absence of atropine on junction potentials during 3 different time periods in WT (Ba) and W/Wv (Bb) mice. IJPs elicited under control conditions (■, n= 6 WT; n=5 W/Wv) were significantly (*) reduced by MRS2500 (□, n= 6 WT; n= 5 W/Wv) in both animals. The IJP remaining in the presence of MRS2500 in W/Wv mice was significantly smaller (@) than in WT mice. In both animals IJPs were abolished (#) by combined addition of MRS2500 and L-NNA (○, n= 5 WT; n=5 W/Wv) revealing a 5 mV depolarization in WT mice that was absent from W/Wv mice (&). Atropine abolished ($) the slow depolarization in WT mice (●, n= 5 WT; n=5 W/Wv). All muscles were pre-exposed to 25 μM wortmannin for 20 min followed by at least 1 hour wash out before beginning experiments. Shown are mean values ± S.E.M.
Comparison of nerve evoked contractions in the wildtype and W/Wv mouse IAS
The role of ICC-IM in cholinergic NMT was also examined by comparing contractile responses to EFS (5Hz, 60s) in WT and W/Wv mice in the absence of atropine (guanethidine present throughout). Relaxations elicited with EFS were not significantly different between animals (Fig. 6A, 1st traces and B) nor were they different from relaxations elicited in the presence of atropine. However, rebound contraction was at least twice as large in the absence of atropine (compare Fig. 4A and 6A). Responses in WT and W/Wv mice differed in that MRS2500 significantly reduced nerve evoked relaxation during both the initial 10s and the subsequent 50s of EFS in W/Wv mice (Fig. 6Ab, 2nd trace and Bb) but not in WT mice (Fig. 6Aa, 2nd trace and Ba) suggesting that the strength of nitrergic NMT was reduced in the W/Wv mouse. Following combined addition of MRS2500 and L-NNA EFS gave rise to a large contractile response in both WT and W/Wv mice that approximately doubled spontaneous contractile activity (Fig. 6A, 3rd traces and B). This contraction was abolished with atropine confirming its cholinergic origin (Fig. 6A, 4th traces and B). There was no significant difference in the nerve evoked contraction between animals (Fig. 6B) suggesting that ICC-IM independent pathways play an important role in the generation of cholinergic motor responses in this muscle.
Figure 6. Comparison of nerve-induced contractile responses in the WT and W/Wv mouse IAS in the absence of atropine.
(A) Sample traces showing the effects of MRS2500 and L-NNA on contractile responses elicited with 5 Hz EFS (10 s) in WT (Aa) and W/Wv (Ab) mice in the absence of atropine. Under control conditions EFS gave rise to relaxation (1st traces). MRS2500 did not significantly reduce nerve evoked relaxation in WT mice but it did in W/Wv mice (2nd traces). Following combined L-NNA and MRS2500 addition a large contractile response was seen in both animals (3rd traces). These contractions were abolished by atropine (1 μM; 4th traces). (B) Summary graph of the effects of blockers on nerve-induced changes in contractile activity during the initial 10s and the subsequent 50s of EFS in WT (Ba) and W/Wv (Bb) mice. Contractile area during EFS (5 Hz, 60s) is compared to the contractile area preceding EFS (“Pre-stim”, n=10 WT; n=6 W/Wv). All areas are normalized to area/10s. EFS gave rise to significant (*) relaxation in both WT and W/Wv mice (black bars, n=4 WT; n=6 W/Wv)). In the presence of MRS2500 (dark grey bars, n=4 WT; n=6 W/Wv) relaxation in WT mice was unchanged whereas it was significantly (&) reduced during both time periods of EFS in W/Wv mice. Combined addition of MRS2500 plus L-NNA (light grey bars, n=10 WT; n=6 W/Wv) resulted in significant (*) contraction in both animals during both time periods of EFS. Contractions were abolished by atropine (white bars, n=10 WT; n= 6 W/Wv) and a significant (*) relaxation was revealed during the latter 50s time period in both animals. Shown are mean values ±S.E.M.
Discussion
The role of ICC-IM in NMT was investigated in the mouse IAS by comparing the contractile and electrical responses to stimulation of motor neurons in WT and W/Wv mice. Nitrergic and cholinergic junction potentials were reduced or abolished in the W/Wv mouse IAS whereas nitrergic and cholinergic contractile effects were unchanged. In contrast, purinergic hyperpolarization and relaxation were unchanged in the W/Wv mouse IAS. These data suggest that ICC-IM participate in the generation of nitrergic and cholinergic junction potentials but not in purinergic NMT. Other ICC-IM independent pathways also play an important role in nitrergic relaxation and cholinergic contraction.
Basic contractile and electrical properties of wildtype and W/Wv mice
Spontaneous contractile activity in the mouse IAS consisted of fast and slow phasic events superimposed upon tone. Interestingly the pattern and amplitude of this activity was not significantly different between WT and W/Wv mice. However, in the W/Wv mouse Em was 4 mV more negative while slow waves (SW) were 7 mV larger in amplitude. It is possible that the greater polarization of Em gives rise to the larger SW. A similar relationship between Em and SW amplitude has previously been reported in other GI muscles (e.g., 24). The lack of difference in contractile activity in WT and W/Wv mice (see Table I) may reflect a balance between the more polarized Em and the larger SW in the W/Wv mouse IAS.
The persistence of SW in the W/Wv mouse is significant since SW are usually attributed to ICC (25). Although ICC-IM are absent from the W/Wv mouse IAS, ICC-SM persist2. Thus, it is possible that ICC-SM give rise to SW in the mouse IAS as previously reported for the mouse colon26. The greater polarization of Em in the W/Wv mouse IAS suggests that ICC-IM play a role in setting “resting” Em. ICC-IM in mouse GI muscles have been reported to express the Ca2+ activated Cl− channel ANO1. These channels give rise to depolarizing currents27, 28 and we have confirmed with immunohistochemical techniques that ANO1 is expressed in ICC of the mouse IAS (Cobine and Keef, unpublished observation). Thus, Em may be more negative in the W/Wv mouse IAS because of loss of inward ANO1 currents generated by ICC-IM.
The frequency of SW was somewhat less than the frequency of phasic contractions in both WT and W/Wv mice. The most likely explanation for this difference is that electrical and contractile recordings sample different populations of cells, i.e., the microelectrode records Em from a single cell whereas the contractile recording represents the summed activity of the entire cross section of the tissue. Spikes (which are especially important for the generation of phasic contractions) tend to be local events that do not conduct over large distances29. In addition, the waxing and waning pattern observed in this muscle (see SupFig. 2) is commensurate with SW being generated by multiple sites30. It is also possible that subtle differences in the recording conditions used for contractile and microelectrode measurements contributed to the apparent differences between SW and phasic contractile frequency in these muscles.
Neuromuscular transmission in the wild type mouse IAS
The mouse IAS exhibits ongoing SW, rhythmic (“phasic”) contractions and tone that are all reduced or abolished by inhibitory motor neurons. Nitrergic and purinergic NMT each contribute to nerve evoked relaxation and hyperpolarization but the time course of junction potentials elicited by these two pathways differ. Repetitive EFS (5 Hz) gives rise to a biphasic response consisting of an initial peak hyperpolarization that is almost entirely purinergic in nature followed by rapid repolarization to a more sustained level of hyperpolarization. NOS blockade with L-NNA significantly reduced the sustained but not the peak hyperpolarization, although the remaining IJP was still biphasic. The L-NNA-resistant IJP was entirely abolished by the P2Y1 blocker MRS2500 (see Fig. 1D) suggesting that purinergic NMT is comprised of two phases, i.e. a large rapidly desensitizing phase and a smaller more sustained phase. This observation is similar to the rat IAS15 whereas in non-rodents the purinergic IJP is more transient31.
By using the more potent P2Y1 receptor antagonist MRS2500 we were able to isolate and characterize the nitrergic IJP. This was not possible in our previous study which used MRS2179 since purinergic NMT contaminated the nitrergic response 14. MRS2179 has since been shown to be a significantly less potent and efficacious inhibitor of P2Y1 receptors in rat colon 22 and we have confirmed this difference in the mouse IAS (see Fig. 1B,C). MRS2500 (1 μM) has also been shown to be an effective antagonist of the purinergic IJP in the rat IAS15. The nitrergic IJP reached maximum within 1s of EFS at 5 Hz. It was smaller in amplitude than the purinergic IJP and remained relatively constant during 10s of EFS. Thus the nitrergic pathway contributes to the sustained phase of hyperpolarization whereas peak hyperpolarization is determined by the purinergic pathway. A similar conclusion has been reported for IJPs in the rat IAS15.
A single shock of EFS also generated transient relaxations in the IAS while the relaxation with repetitive EFS (5Hz) was sustained. Interestingly the response to 5 Hz EFS was not reduced by selective blockade of either nitrergic or purinergic NMT. However when both pathways were simultaneously blocked relaxation was completely abolished for ~20s EFS (5Hz) indicating that this component of the response is mediated by both pathways. The fact that each pathway independently produced the same amount of relaxation suggests that they are of similar “strength” to one another. However they clearly do not add together in a straightforward manner.
The redundant nature of relaxations elicited by the nitrergic and purinergic pathways may be due to their shared ability to hyperpolarize Em. Rhythmic contractions and tone in the mouse IAS are blocked by the L-type Ca2+ channel (Cav) blocker nifedipine 14, 23 and by the Katp channel opener pinacidil (Keef and Cobine, unpublished observation) suggesting that Cav and Em play a critical role in regulating contractile activity in the IAS. Cav is a voltage dependent conductance with a relatively discrete threshold potential for channel activation. At potentials negative to this value almost all Cav channels are closed whereas at potentials positive to this value a steep relationship between Em and Cav activity exists. In GI muscles this voltage-dependence of CaV in turn gives rise to a “mechanical threshold” for electromechanical coupling with values between −55 and −40mV reported for various GI muscles 32, 33. In the mouse IAS Em averages −49mV and both purinergic and nitrergic pathways hyperpolarize Em by at least 9mV (see Fig. 1E) bringing Em to at least −58mV. This potential is negative to the range of values listed above for mechanical threshold. Once Cav are closed, further hyperpolarization will not produce a further reduction in calcium entry. Thus, nitrergic and purinergic pathways may be “non-additive” in nature because each hyperpolarizes Em below the mechanical threshold.
Differences in interstitial cell populations in the W/Wv mouse IAS
In a previous study of the mouse IAS we compared the distribution of ICC and “fibroblast-like cells” (FLC) in the IAS of WT (C57BL/6) and W/Wv mice along with their relationship to nitrergic nerves 2. The term FLC has been proposed to denote common features shared between these cells in the GI tract and true fibroblasts 34, 35. Recent studies have shown that FLC express high levels of the receptor tyrosine kinase PDGFRα+ 2, 36, 37. Because of this, we and others have referred to intramuscular FLC as PDGFRα+-IM 2, 36, 38. Several studies have suggested that PDGFRα+-IM may participate in inhibitory NMT in the GI tract 2, 36, 39, 40. In the mouse IAS we found that ICC-IM and PDGFRα+-IM were each distributed throughout the thickness of the muscle layer and formed close associations with nitrergic nerves and with each other 2. Thus, ICC-IM, PDGFRα+-IM and smooth muscle cells (SMC) all represent viable candidates for participation in NMT in the mouse IAS. The present study compares the electrical and contractile events associated with NMT in WT and W/Wv mice to further explore the role of ICC-IM in NMT.
Changes in nitrergic neuromuscular transmission in the W/Wv mouse IAS
Previous studies have concluded that ICC are not involved in nitrergic NMT since nerve evoked relaxations were not reduced in the W/Wv mouse IAS 12, 13. Our data confirms this observation. However, given the non-additive nature of nitrergic and purinergic NMT in the mouse IAS it is possible that a significant reduction in nitrergic NMT could occur without a change in the overall relaxation response. To further address this question we selectively isolated the nitrergic IJP and relaxation in the W/Wv mouse IAS using MRS2500. Under these conditions the IJP evoked with 5 Hz EFS was reduced by 50–60% in the W/Wv mouse (see Figs. 3,5) while the response to a single shock of EFS was absent (see SupFig 3B). In addition, L-NNA depolarized Em in 44% of WT mice but did not depolarize any muscles in W/Wv mice; again implicating a role for ICC-IM in mediating NO effects. Finally, in the absence of atropine we found that MRS2500 significantly reduced relaxation in W/Wv but not WT mice suggesting that the nitrergic pathway is less effective at opposing the actions of cholinergic nerves when ICC-IM are absent (see Fig. 6Ab,Bb).
Taken together, these data suggest that ICC-IM significantly contribute to the generation of nitrergic IJPs and possibly to relaxation. This conclusion is in agreement with some studies of other GI regions 3, 4. However, studies of the W/Wv mouse lower esophageal sphincter (LES) have suggested that nitrergic NMT is compromised in this animal because of changes in calcium handling by the smooth muscle sarcoplasmic reticulum rather than to a loss of ICC-IM 41. The changes reported for NMT in the W/Wv LES differ significantly from those we observed for the W/Wv IAS including: 1) cholinergic depolarization persisted in the W/Wv LES but not in the W/Wv IAS and 2) nitrergic IJP amplitude varied widely between animals in the W/Wv LES whereas nitrergic IJPs were consistently reduced in the W/Wv IAS (see Fig. 3B). These substantial differences make it difficult to extrapolate between studies.
Besides hyperpolarizing Em, the nitrergic pathway was also associated with blockade of SW. Blockade was sometimes complete (e.g., Figs. 1A,Cb; 5Aa) and sometimes partial (e.g., Fig. 3Aa). Interestingly, this effect persisted in the W/Wv mouse IAS (e.g., Fig. 3Ab). SW and contraction in the mouse IAS are also blocked by Cav antagonists 14, 23 indicating that SW are an important source of calcium for contraction (see review25 for related discussion). The continued blockade of SW during nitrergic NMT in the W/Wv mouse IAS therefore provides a plausible explanation for why most nitrergic relaxation persists.
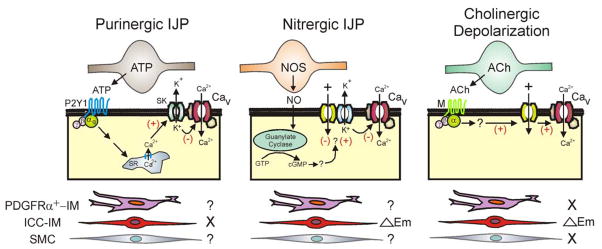
In summary, these data suggest that ICC-IM play a significant role in the generation of nitrergic IJPs in the mouse IAS (see Fig. 7). However, approximately 40–50% of the nitrergic IJP elicited with 5 Hz EFS still remains in the W/Wv mouse and SW are still blocked. Taken together our results suggest that other cell types also contribute to generation of the electrical events underlying nitrergic NMT in the IAS, particularly when trains of stimuli are applied. Both SMC and PDGFRα+-IM represent viable candidates for this role (Fig. 7). Finally, pharmacomechanical coupling mechanisms such as a decrease in myofilament sensitivity may also contribute to relaxation.
Figure 7. Diagram depicting proposed pathways giving rise to changes in membrane potential with neuromuscular transmission in the mouse IAS.
Shown are inhibitory motor neurons releasing either purines (e.g., ATP) or nitric oxide (NO) and excitatory motor neurons releasing acetylcholine (ACh). The postjunctional cells giving rise to changes in Em are depicted in the center along with proposed second messenger pathways. The three possible cell types considered for these responses are shown at the bottom of the diagram. Left panels: Purinergic IJPs persisted in the W/Wv mouse IAS suggesting that they were independent (X) of ICC-IM leaving either PDGFRα+-IM or SMC as possible candidates (?). ATP or a related purine binds to P2Y1 receptors leading to the release of Ca2+ from the sarcoplasmic reticulum (SR) which activates SK channels (see 14, 36) hyperpolarizing Em and closing L-type Ca2+ channels (Cav). Middle panels: Nitrergic IJPs were greatly diminished in the W/Wv mouse suggesting that ICC-IM play an important role in the generation of these events (△Em). Since nitrergic NMT still blocks slow waves in the W/Wv mouse IAS, other ICC-IM independent pathways are likely involved (?). NO activates guanylate cyclase leading to generation of cGMP. This in turn hyperpolarizes Em via an unknown mechanism that may involve either inhibition of inward current or activation of potassium (K+) channels. Hyperpolarization in turn closes Cav. Right panels: Cholinergic depolarization was only observed in WT mice suggesting that ICC-IM play a critical role in generating this event (△Em) whereas PDGFRα+-IM and SMC do not (X). ACh binds to muscarinic receptors (M) leading to depolarization via an unknown mechanism that perhaps involves stimulation of inward currents. Depolarization in turn opens Cav.
Purinergic neuromuscular transmission is not different in the W/Wv mouse IAS
The peak hyperpolarization elicited with 5 Hz EFS was not different in WT and W/Wv mice and this component was abolished by MRS2500 (see Fig. 3B) suggesting that ICC-IM are not required for generation of the purinergic IJP (Fig. 7). This conclusion is in agreement with several studies of other GI muscles 39, 41, 42. In addition, “resting” Em in both WT and W/Wv mice was unaffected by addition of the P2Y1 antagonist MRS2500 suggesting that “resting” Em is not modulated by basal purine release. A similar conclusion was reached for the rat IAS15. An alternative cellular candidate for purinergic NMT in the IAS is PDGFRα+-IM. This cell has a number of features supporting a role in purinergic NMT including: 1) association with intramuscular nerves 2, 39, 43, 2) the presence of gap junctions with SMC 35, 39, 44, 45, 3) the expression of two proteins critical for purinergic NMT, i.e., small conductance calcium activated potassium channels, SK3 (Kcnn3), and P2Y1 (P2ry1) receptors 36, 45–48 and 4) the ability to generate large Ca2+-activated, apamin-sensitive K+ currents in response to purines while SMC in the same preparation do not 36. Thus, we propose PDGFRα+-IM as possible mediators of purinergic NMT in the mouse IAS. SMC have been reported to express P2Y1 receptors 15 making these cells candidates as well (Fig. 7).
Changes in cholinergic neuromuscular transmission in the W/Wv mouse IAS
The neural pathways involved in excitatory NMT in the IAS are species dependent. In dog, cat, sheep, monkey and humans extrinsic sympathetic nerves play the predominant role as excitatory motor neurons whereas in smaller mammals such as rabbit and mouse this is not the case (e.g., 23, 49). We have now characterized excitatory NMT in the mouse IAS. EFS (5Hz) gave rise to contraction and a small (i.e., ~5mV), slowly developing depolarization in the presence of guanethidine, MRS2500 and L-NNA. Since both depolarization and contraction were abolished by atropine they are due to cholinergic excitatory motor neurons. In contrast, depolarization was absent from the W/Wv mouse IAS. Thus, ICC-IM appear to be important for generation of cholinergic depolarization in this muscle (Fig. 7) as previously described in several other studies 4, 5. However, although depolarization was absent in the W/Wv mouse IAS, cholinergic contraction at 5 Hz EFS was unchanged. This result does not preclude a role for depolarization in the generation of cholinergic contraction but it does suggests that other Em-independent pathways such as calcium release from the sarcoplasmic reticulum and an increase in myofilament sensitivity to calcium (for reviews see 25, 50) must also play important roles in cholinergic contraction.
Purinergic and nitrergic independent relaxation is not different in the W/Wv mouse IAS
Contractile and electrical responses were abolished following blockade of purinergic, nitrergic and cholinergic pathways during 10s of EFS@5Hz. However, relaxation started to develop in these muscles after 20–30s of EFS and by 60s tone had usually declined to near baseline. Interestingly, this slowly developing relaxation also reversed slowly following termination of EFS taking several minutes to return to the pre-stimulus level. This relaxation was abolished by TTX indicating its neural origin. The nature of this slower component was not further investigated in this study but we have obtained preliminary evidence that it is due to VIP since it is abolished by VIP6–28 (10 μM) 51. It is of interest that, like purinergic NMT, the slow relaxation was unchanged in the W/Wv mouse IAS suggesting that this pathway is also independent of ICC-IM. Although non-cholinergic excitatory NMT was not observed in the mouse IAS it is possible that it was masked by purinergic and nitrergic independent inhibitory NMT.
In summary, we have re-examined the hypothesis that ICC-IM are not required for nitrergic NMT in the mouse IAS 12, 13. Our results provide evidence that ICC-IM do indeed contribute to generation of nitrergic IJPs but that other ICC-IM independent pathways are also important. In addition we could find no role for ICC-IM in either purinergic NMT or in the slowly developing relaxation that accompanies longer duration EFS when adrenergic, cholinergic, purinergic and nitrergic pathways are blocked. Given these significant redundancies in neural inhibitory pathways it is not surprising that some investigators have reported an intact RAIR in the W/Wv mouse12. Our results also suggest that ICC-IM are required for generation of cholinergic depolarization but that other ICC-IM independent pathways also play an important role in cholinergic contraction. Since PDGFRα+-IM persist in the W/Wv mouse IAS, both PDGFRα+-IM and SMC are viable cellular candidates for aspects of NMT that persist in the absence of ICC-IM.
Supplementary Material
Acknowledgments
This work was supported by grant DK078736 to KDK. The authors would like to thank Dr. Kenton Sanders for critical evaluation of the manuscript.
Biography
AMD performed most intracellular microelectrode recordings and some data analysis; CAC performed and analyzed all contractile experiments and analyzed most microelectrode recordings. She also contributed to the writing of this paper; KDK was the principal investigator of this study including intellectual development, data analysis and writing the paper. She also participated in collecting some of the contractile and intracellular data.
References
- 1.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006 November 1;576(Pt 3):675–82. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011 April;344(1):17–30. doi: 10.1007/s00441-011-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93(21):12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115(2):314–29. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 5.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000 February 15;20(4):1393–403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002 September 15;543(Pt 3):871–87. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol. 2010 January;298(1):G10–G13. doi: 10.1152/ajpgi.00426.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009 November;137(5):1548–56. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol. 2008 February;294(2):G372–G390. doi: 10.1152/ajpgi.00344.2007. [DOI] [PubMed] [Google Scholar]
- 10.Iino S, Horiguchi S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal in the gastrointestinal musculature of W mutant mice. Arch Histol Cytol. 2007 October;70(3):163–73. doi: 10.1679/aohc.70.163. [DOI] [PubMed] [Google Scholar]
- 11.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007 January 1;578(Pt 1):33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terauchi A, Kobayashi D, Mashimo H. Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol. 2005 August;289(2):G291–G299. doi: 10.1152/ajpgi.00005.2005. [DOI] [PubMed] [Google Scholar]
- 13.de Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut. 2005 August;54(8):1107–13. doi: 10.1136/gut.2004.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonnell B, Hamilton R, Fong M, Ward SM, Keef KD. Functional evidence for purinergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2008 April;294(4):G1041–G1051. doi: 10.1152/ajpgi.00356.2007. [DOI] [PubMed] [Google Scholar]
- 15.Opazo A, Lecea B, Gil V, Jimenez M, Clave P, Gallego D. Specific and complementary roles for nitric oxide and ATP in the inhibitory motor pathways to rat internal anal sphincter. Neurogastroenterol Motil. 2011 January;23(1):e11–e25. doi: 10.1111/j.1365-2982.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 16.Rattan S, Regan RF, Patel CA, De Godoy MA. Nitric oxide not carbon monoxide mediates nonadrenergic noncholinergic relaxation in the murine internal anal sphincter. Gastroenterology. 2005 December;129(6):1954–66. doi: 10.1053/j.gastro.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Cobine CA, Duffy AM, Yan W, Ward SM, Sanders KM, Keef KD. Comparison of the morphological and functional properties of the internal anal sphincter in wild type mice (C57BL/6) and mice containing the reduced function Kit allele (Wv) Neurogastroenterol Motil. 2010 Aug 1;22(Suppl 1):86. [Google Scholar]
- 18.Burke EP, Gerthoffer WT, Sanders KM, Publicover NG. Wortmannin inhibits contraction without altering electrical activity in canine gastric smooth muscle. Am J Physiol. 1996;270(5 Pt 1):C1405–12. doi: 10.1152/ajpcell.1996.270.5.C1405. [DOI] [PubMed] [Google Scholar]
- 19.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol. 2007 November;293(5):C1645–C1659. doi: 10.1152/ajpcell.00165.2007. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N, Prosser CL, DeVos W. Waxing and waning of slow waves in intestinal musculature. Am J Physiol. 1986 January;250(1 Pt 1):G28–G34. doi: 10.1152/ajpgi.1986.250.1.G28. [DOI] [PubMed] [Google Scholar]
- 21.Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987;252:C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- 22.Grasa L, Gil V, Gallego D, Martin MT, Jimenez M. P2Y(1) receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009 November;158(6):1641–52. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobine CA, Fong M, Hamilton R, Keef KD. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol Motil. 2007 November;19(11):937–45. doi: 10.1111/j.1365-2982.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 24.Nose K, Suzuki H, Kannan H. Voltage dependency of the frequency of slow waves in antrum smooth muscle of the guinea-pig stomach. Jpn J Physiol. 2000 December;50(6):625–33. doi: 10.2170/jjphysiol.50.625. [DOI] [PubMed] [Google Scholar]
- 25.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008 May;20(Suppl 1):39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoneda S, Fukui H, Takaki M. Pacemaker activity from submucosal interstitial cells of Cajal drives high-frequency and low-amplitude circular muscle contractions in the mouse proximal colon. Neurogastroenterol Motil. 2004 October;16(5):621–7. doi: 10.1111/j.1365-2982.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- 27.Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009 October 15;587(Pt 20):4887–904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009 June;296(6):G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer NJ, Hennig GW, Smith TK. Electrical rhythmicity and spread of action potentials in longitudinal muscle of guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2002 May;282(5):G904–G917. doi: 10.1152/ajpgi.00345.2001. [DOI] [PubMed] [Google Scholar]
- 30.Bortoff A. Electrical transmission of slow waves from longitudinal to circular intestinal muscle. Am J Physiol. 1965 December;209(6):1254–60. doi: 10.1152/ajplegacy.1965.209.6.1254. [DOI] [PubMed] [Google Scholar]
- 31.Gallego D, Gil V, Aleu J, Auli M, Clave P, Jimenez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008 September;295(3):G522–G533. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- 32.Morgan KG, Szurszewski JH. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980 April;301:229–42. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer AJ, Sanders KM. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol (Lond) 1985;369:283–94. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou DS, Komuro T. Interstitial cells associated with the deep muscular plexus of the guinea-pig small intestine, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 1992 May;268(2):205–16. doi: 10.1007/BF00318788. [DOI] [PubMed] [Google Scholar]
- 35.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000 May 12;80(3):142–7. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 36.Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011 February 1;589(Pt 3):697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009 June;131(6):691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 38.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010 December 1;588(Pt 23):4621–39. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farre R, Wang XY, Vidal E, Domenech A, Pumarola M, Clave P, Huizinga JD, Jimenez M. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil. 2007 June;19(6):484–96. doi: 10.1111/j.1365-2982.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 40.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience. 2008 March 18;152(2):437–48. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol. 2010 January;298(1):G14–G24. doi: 10.1152/ajpgi.00266.2009. [DOI] [PubMed] [Google Scholar]
- 42.Sergeant GP, Large RJ, Beckett EA, McGeough CM, Ward SM, Horowitz B. Microarray comparison of normal and W/Wv mice in the gastric fundus indicates a supersensitive phenotype. Physiol Genomics. 2002 October 2;11(1):1–9. doi: 10.1152/physiolgenomics.00052.2002. [DOI] [PubMed] [Google Scholar]
- 43.Komuro T. Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microsc Res Tech. 1999 November 15;47(4):267–85. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 44.Komuro T. Three-dimensional observation of the fibroblast-like cells associated with the rat myenteric plexus, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 1989 February;255(2):343–51. doi: 10.1007/BF00224117. [DOI] [PubMed] [Google Scholar]
- 45.Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003 May;92(1):35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- 46.Vanderwinden JM, Rumessen JJ, de Kerchove d’Exaerde A, Jr, Gillard K, Panthier JJ, De Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002 December;310(3):349–58. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- 47.Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009 July;72(2):107–15. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
- 48.Klemm MF, Lang RJ. Distribution of Ca2+-activated K+ channel (SK2 and SK3) immunoreactivity in intestinal smooth muscles of the guinea-pig. Clin Exp Pharmacol Physiol. 2002 January;29(1–2):18–25. doi: 10.1046/j.1440-1681.2002.03601.x. [DOI] [PubMed] [Google Scholar]
- 49.Brading AF, Ramalingam T. Mechanisms controlling normal defecation and the potential effects of spinal cord injury. Prog Brain Res. 2006;152:345–58. doi: 10.1016/S0079-6123(05)52023-5. [DOI] [PubMed] [Google Scholar]
- 50.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003 October;83(4):1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 51.Keef KD, Kaminski RE, McDowall RA, Duffy AM, Cobine CA. Peptidergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Neurogastroenterol Motil. 2011 September;23(s1):49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.