Abstract
C-reactive protein (CRP) is a component of non-specific immune defense and is a reliable marker of low-grade inflammation involved in obesity, type 2 diabetes and cardiovascular disease. Genome-wide association studies (GWAS) in middle-aged and elderly populations, predominantly of European descent, demonstrated associations of CRP levels with SNPs at several loci. To examine whether the variants identified are replicated in Filipino young adults, we applied Tobit regression models to study the association of plasma CRP with 12 SNPs at seven loci in a cohort of 1,691 Filipino young adults (aged 21.5 ± 0.3 years) from the Cebu Longitudinal Health and Nutrition Survey (CLHNS). SNPs in or near CRP (P = 3.2 × 10-11), HNF1A, IL6R, APOE-APOC1 and LEPR showed significant associations (P < 0.05) and together explained 4.8% of the total variation in CRP. Modest interactions were observed between LEPR rs1892534 and waist circumference (uncorrected Pinteraction = 0.020) and between APOE rs769449 and pathogen exposure (uncorrected Pinteraction = 0.0073) in models predicting CRP. Our results demonstrated that variants in several loci are significantly associated with plasma CRP in Filipino young adults, suggesting shared genetic influences on circulating CRP across populations and age groups.
Keywords: C-reactive protein (CRP), Filipinos, genetic association, interaction, SNP, young adults
INTRODUCTION
C-reactive protein (CRP) is a component of the acute-phase response and a marker of chronic inflammation, which plays important roles in the co-morbidities that accompany obesity, dyslipidemia, type 2 diabetes and cardiovascular diseases1-4. In a large US-based study, an estimated 35-40% of the variability in circulating CRP was explained by genetic variation5. Studies using a candidate gene strategy have described associations linking circulating CRP with several SNPs and haplotypes in the gene regions of CRP, LEPR and APOE6-8. A prior genome-wide association (GWA) study based on individuals of European ancestry from the women's genome health study (WGHS) has provided evidence of associations between CRP and SNPs at seven genetic loci, including CRP, HNF1A, LEPR, IL6R, APOE, GCKR and 12q23.2, together accounting for 10.1% of the total variation in circulating CRP in this population9. Similar findings of association were reported by subsequent GWA studies conducted in individuals of middle-aged and elderly European descent10-11 and in Japanese12. A recent meta-analysis confirmed the reported 7 loci and identified 11 additional loci associated with CRP levels13.
Quantitative traits such as CRP concentrations are known to be influenced by both genetic and environmental factors, and interactions between them are likely to be important predictors as well14. Evidence exists that an interaction between adiposity and CRP gene variants influences CRP levels. An interaction analysis conducted in Taiwanese noted that the association between CRP haplotypes and CRP level predominantly occurred in obese subjects15, and a CRP variant by adiposity interaction analysis in Europeans showed that the correlated increase in CRP levels with adiposity was accentuated by the presence of the CRP rs1205 G allele16.
Genetic background may vary across populations, and the genetic contribution of identified variants on circulating CRP remains unclear in populations of non-European descent. The purpose of this study is to assess whether the CRP-associated loci identified in Europeans are associated with plasma CRP in a cohort of Filipino young adults living in the Metro Cebu area of the Philippines. In light of the roles of infectious agents17 and adipose tissue18 in up-regulating inflammatory pathways, prior work in this population demonstrated that CRP levels were influenced by a dual burden of environmental pathogenicity and central adiposity19-21. Thus, we also aim to determine whether measures of individual adiposity and household-level pathogenicity modify any effects of these polymorphisms on CRP levels.
MATERIALS AND METHODS
Samples and data come from the Cebu Longitudinal Health and Nutrition Survey (CLHNS), an on-going community-based cohort study of births between May 1, 1983 and April 30, 1984. The CLHNS is based in Metro Cebu, Philippines, which encompasses three central cities within Cebu province as well as contiguous peri-urban and rural municipalities. The original study design and recruitment protocols have been previously described in detail22. Written informed consent was obtained from all participants, and study protocols were approved by the University of North Carolina Institutional Review Board for the Protection of Human Subjects. In the 2005 survey, the available sample included 1,779 Filipino young adults. A pathogen exposure score was constructed based on the mean value of five interviewer-assigned variables, each scored on a three point scale (0=low exposure, 1=moderate, 2=high): cleanliness of the food preparation area, means of garbage disposal, presence of excrement near the house, level of garbage and excrement present in the neighborhood surrounding the household19. In addition, to control for any effects of active infection on CRP levels, participants were asked whether they were experiencing any symptoms of infection at the time of blood collection. Reported symptoms included runny nose, cough, fever, diarrhea, sore throat and the more general categories of flu, cold and sinusitis20.
Overnight fasting blood samples were obtained at the 2005 survey and collected into EDTA-coated tubes. Plasma CRP concentration was determined using a high sensitivity immunoturbidimetric method (Synchron LX20 with lower detection limit of 0.1mg/L; Beckman Coulter, Fullerton, CA) with between-assay CVs < 7.6 across the assay range19. Following AHA/CDC guidelines, participants with CRP level > 10 mg/L were interpreted as having an acute infection23 and were therefore excluded from analyses. The final sample set for analysis included 1,691 unrelated CLHNS young adults with complete data on plasma CRP, all genotypes and covariates.
Selection of SNPs for analyses was based on published data regarding their association with CRP concentrations9-10. At the seven loci (CRP, LEPR, IL6R, GCKR, 12q23.2, HNF1A and APOE-APOC1) for which we have > 80% power to detect the reported variance explained by SNPs, we selected SNPs with the strongest evidence of association at a given locus or with potential functional significance based on annotation24-25. The nine SNPs included rs1205 (CRP), rs3091244 (CRP), rs1892534 (LEPR), rs2228145 (IL6R), rs1260326 (GCKR), rs10778213 (12q23.2), rs1169288 (HNF1A), rs7310409 (HNF1A) and rs769449 (APOEAPOC1). Three additional SNPs in APOE or APOC1 were selected to better characterize this locus. SNPs rs429358 and rs7412 were selected to construct the reported APOE haplotypes ε2 (rs429358-rs7412: T-T), ε3 (rs429358-rs7412: T-C), and ε4 (rs429358-rs7412: C-C)8. SNP rs4420638 in APOC1 was selected based on reproducible evidence of association with CRP level in previous reports11-13.
Genotyping was performed using TaqMan fluorogenic 5' nuclease assays (Applied Biosystems) using an ABI PRISM® 7900 system. The triallelic SNP rs3091244 was genotyped based on two separate assays (C/T and C/A)26, and the final genotypes were assigned as shown in Supplementary Table 1. Genotyping success rates for all assays were > 96.5%, and all SNPs were in agreement with Hardy-Weinberg equilibrium (P > 0.1). SNP rs4420638 in APOC1 was imputed using MACH27 from MetaboChip (Illumina, San Diego, CA, USA) SNP genotypes in CLHNS young adults [Damien C. Croteau-Chonka, et.al., manuscript submitted] based on CEU, CHB and JPT haplotypes created from the 1000 Genomes Project pilot (June 2011 data release). Based on the high imputation quality (r2 = 0.96) of rs4420638, we used the posterior expected genotypes in statistical analyses.
A total of 574 (33.9%) individuals among the 1,691 CLHNS young adults had CRP concentrations below the detectable level (0.1 mg/L), and log-CRP concentrations produced a left-censored distribution. Given the markedly skewed distribution of CRP concentrations and the presence of many values < 0.1, we used Tobit regression that applies linear regression to a mixture of censored discrete data and normally distributed continuous distributions28 to test association between natural log-transformed CRP (left-censored at value of -3, corresponding to natural log of 0.05) and genotype in the CLHNS young adults. In addition, we applied linear regression, in which CRP values were natural log-transformed after adding the constant 0.10. In initial analysis for main effects, an additive genetic model was assumed. Sex, pathogenic score and infection status were included as covariates. In secondary analyses, both models were further adjusted for waist circumference to examine whether the associations of SNPs and plasma CRP were mediated by central adiposity. Interactions between SNPs and waist circumference and between SNPs and pathogen exposure score were evaluated in Tobit regression models, accounting for the same covariates used in the main effect analyses. Waist circumference and pathogenic score were treated as dichotomous variables, using the medians (waist circumference < 69 or ≥ 69 cm and pathogen exposure score <0.5 or ≥ 0.5) as the threshold to stratify the study samples.
We further assessed the combined effect of multiple SNPs on plasma CRP using a cumulative genotype score as an independent variable. The cumulative genotype score was calculated based on the number of alleles associated with elevated CRP level, weighted by the fitted linear regression model coefficients. A total of five SNPs (CRP-rs1205, LEPR-rs1892534, IL6R-rs2228145, HNF1A-rs7310409 and APOE-rs429358) representing each of the five CRP-associated loci in the CLHNS were used for the combined effect estimation. Individuals with genotypes available for all the 5 selected SNPs (n = 1,592) were categorized into quintiles based on the cumulative genotype score.
All analyses for association with single SNPs were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA). The ‘haplo.score’ and ‘haplo.glm’ functions implemented in the ‘haplo.stats’ R package were applied for haplotype analysis.
RESULTS
Twelve SNPs at seven loci were investigated in 1,691 CLHNS young adults (Table 1). Based on the results from Tobit regression models, we observed evidence of significant association with plasma CRP for SNPs at five loci, including CRP (P for rs1205 = 3.2 × 10-11 and P for rs3091244 = 5.6 × 10-10), HNF1A (P for rs7310409 = 4.1 × 10-5 and P for rs1169288 = 1.0 × 10-4), IL6R (P for 2228145 = 6.2 × 10-4), APOE-APOC1 (P for rs769449 = 0.0068, P for rs429358 = 0.0060, and P for rs4420638 = 0.044), and LEPR (P for rs1892534 = 0.018), all showing the same direction of effect as previously reported9, 13 (Table 2). The associations for variants at CRP, HNF1A and IL6R remained significant after Bonferroni correction for multiple testing (P < 0.0042, 0.05/12 tests). No evidence for association was observed for variants at GCKR (P = 0.46 for rs1260326) or the gene desert region of 12q23.2 (P = 0.73 for rs10778213). A third SNP in the APOE gene, rs7412, showed no association with CRP level in CLHNS young adults (P = 0.51). Consistent results for association were observed using linear regression models (data not shown). We further examined whether these associations with CRP level were affected by adjustment for waist circumference. None of the associations substantially changed, suggesting that the genetic associations with plasma CRP were independent of central adiposity (Supplementary Table 2).
Table 1.
Characteristics of the CLHNS young adult cohort
| Females (n=799) | Males (n=892) | Total (n=1,691) | |
|---|---|---|---|
| Age (years) | 21.5 ± 0.3 | 21.5 ± 0.3 | 21.5 ± 0.3 |
| CRP (mg/L) | 0.3 (0, 1.0) | 0.2 (0, 0.8) | 0.2 (0, 0.9) |
| Waist circumference (cm) | 68.0 ± 7.6 | 72.2 ± 7.5 | 70.3 ± 7.8 |
| BMI (kg/m2) | 20.4 ± 3.2 | 21.1 ± 3.1 | 20.8 ± 3.2 |
| Symptoms of infection (%) | 13.9 | 12.8 | 13.3 |
| Pathogen exposure score | 0.54 ± 0.39 | 0.55 ± 0.40 | 0.54 ± 0.40 |
Data are mean ± SD, median (25th percentile, 75th percentile) or %.
Table 2.
Candidate SNP association with CRP levels in 1,691 CLHNS young adults
| SNP | Locus | Annotation | Reported Effect Allele | Effect Allele | Non-effect Allele | β (SE) | P |
|---|---|---|---|---|---|---|---|
| rs1205 | CRP | 3’UTR | C | C | T | 0.518 (0.077) | 3.2E-11 |
| rs3091244 | CRP | Promoter | A or T | A or T | C | 0.575 (0.092) | 5.6E-10 |
| rs1892534 | LEPR | Downstream | C | C | T | 0.258 (0.109) | 0.018 |
| rs2228145 | IL6R | Asp358Ala | A | A | C | 0.313 (0.091) | 6.2E-04 |
| rs7310409 | HNF1A | Intron | G | G | A | 0.347 (0.084) | 4.1E-05 |
| rs1169288 | HNF1A | Ile27Leu | A | A | C | 0.324 (0.083) | 1.0E-04 |
| rs1260326 | GCKR | Pro446Leu | T | T | C | 0.060 (0.080) | 0.46 |
| rs10778213 | 12q23.2 | Intergenic | T | T | C | 0.034 (0.097) | 0.73 |
| rs769449 | APOE | Intron | G | G | A | 0.376 (0.139) | 0.0068 |
| rs429358 | APOE | Cys130Arg | T | T | C | 0.377 (0.137) | 0.0060 |
| rs7412 | APOE | Arg176Cys | N.A. | T | C | 0.083 (0.125) | 0.51 |
| rs4420638 | APOC1 | 3'UTR | A | A | G | 0.260 (0.129) | 0.044 |
Adjusted for sex, infectious status, and pathogen score. Individuals with CRP > 10mg/L were excluded from analyses. Reported effect allele: the allele that is associated with elevated CRP levels in previously reported GWAS; Effect Allele: the allele associated with elevated CRP level in CLHNS young adults.
An association between CRP level and APOE haplotype consisting of the SNPs rs429358 and rs7412 was previously reported in middle-aged and elderly Caucasians8. Our results provided confirmatory evidence of the haplotype association in this cohort of Filipino young adults (Table 3). Haplotype analysis based on score statistic revealed an overall association between haplotypes and CRP levels (global P value = 0.018). Specifically, the APOE ε4 haplotype with a frequency of 0.099 was significantly associated with lower CRP level (P = 0.0047). Complementary analysis that assessed the effect on CRP level of each additional copy of the specific haplotype compared to the homozygote reference haplotype found that the APOE ε4 haplotype was significantly associated with decreased plasma CRP (β = -0.210, P = 5.4 × 10-3) compared to the most common APOE ε3 haplotype. We next performed conditional analysis at APOE-APOC1 to examine whether the associated SNPs represent a single signal. Reciprocal conditional analysis on APOC1-rs4420638 and APOE-rs429358 (LD r2 = 0.51) showed that the effect of association with rs4420638 was substantially attenuated (Pconditional = 0.76) whereas the association with rs429358 remained significant (Pconditional = 0.0093). Therefore, the SNPs at the APOE-APOC1 cluster appear to represent a single signal best represented by APOE-rs429358.
Table 3.
CRP association with APOE haplotypes
| Haplotype | rs429358-rs7412 | Freq | Haplo.score |
Haplo.glm |
||
|---|---|---|---|---|---|---|
| score | P | β (SE) | P | |||
| APOE ε2 | T-T | 0.018 | 0.58 | 0.56 | 0.018 (0.070) | 0.79 |
| APOE ε3 | T-C | 0.794 | 1.59 | 0.11 | reference | reference |
| APOE ε4 | C-C | 0.099 | -2.83 | 0.0047 | -0.210 (0.075) | 5.4 × 10-3 |
Using the “haplo.score” function implemented in the “haplo.stats” R package, the global P values for association between haplotypes and CRP level were 0.018. The “haplo.glm” function implemented in the “haplo.stats” R package was used to calculate the coefficient β and P value for each haplotype compared with the reference haplotype (APOE ε3). The same covariates used for genotype analysis were applied in haplotype analysis.
We next investigated whether the genetic associations with plasma CRP are modified by waist circumference or pathogen exposure (Supplementary Table 3), both of which are predictors of baseline CRP level. Modest evidence was detected for the interaction of waist circumference with rs1892534 at LEPR (uncorrected Pinteraction = 0.020). In a secondary analysis stratifying by waist circumference above or below the median value, the estimated increase in log-CRP level for each additional C allele of rs1892534 was 0.232 (P = 0.0062) in individuals with smaller waist circumference and 0.015 (P = 0.86) in those with larger waist circumference. We also observed evidence for interaction between APOE variant rs769449 and pathogen exposure on plasma CRP (uncorrected Pinteraction = 0.0073). When stratified analysis was conducted, we found that the significant and strong association between CRP level and the APOE variant was predominantly observed in individuals with lower exposure to a pathogenic environment (β = 0.419, P = 6.8 × 10-5) compared to those with higher exposure (β = 0.017, P = 0.88). Given that the observed interaction was not significant after Bonferroni correction (corrected Pinteraction > 0.0045, 0.05/ 11 tests), further studies in larger populations are warranted.
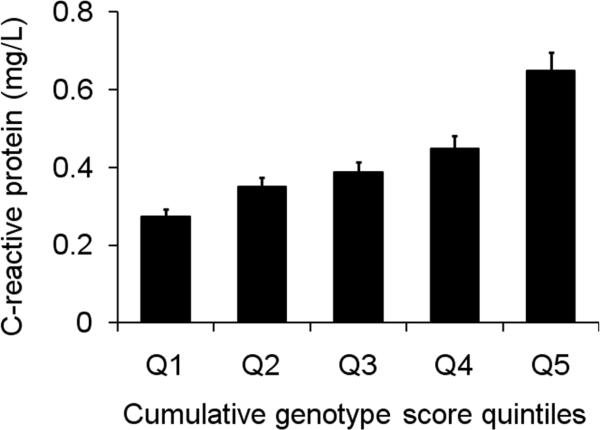
We observed no evidence of multiplicative SNP-SNP interaction among variants CRP-rs1205, LEPR-rs1892534, IL6R-rs2228145, HNF1A-rs7310409 and APOE-rs429358 (all uncorrected Pinteraction > 0.12). In assessing the combined effects of multiple SNPs on plasma CRP, we observed a dose response. Individuals carrying a greater number of alleles associated with elevated level of CRP had increased circulating CRP level (P for trend = 1.0 × 10-17, Figure 1). The geometric mean of plasma CRP in the group of individuals in the highest quintile (Q5) was 0.648 mg/L (SE = 0.048), a 2.4-fold increase compared to that in the lowest quintile group (geometric mean = 0.273, SE = 0.019).
Figure 1.
CRP level increases with genotype score in 1,592 CLHNS Filipino young adults. The cumulative genotype score was calculated based on the number of alleles associated with elevated CRP level, weighted by the fitted linear regression model coefficients. Individuals with genotypes available from five selected SNPs (CRP-rs1205, LEPR-rs1892534, IL6R-rs2228145, HNF1A-rs7310409 and APOE-rs429358) were categorized into quintiles based on the cumulative genotype score.
DISCUSSION
In this study of Filipino young adults, we replicated associations for plasma CRP with several variants previously identified in middle-aged or elder Europeans9. SNPs at five loci of CRP, HNF1A, LEPR, IL6R, APOE-APOC1 were significantly associated with CRP level and together explained 4.8% of the variance in CRP in this population. In addition, modest interactions were observed between LEPR rs1892534 and waist circumference and between APOE rs769449 and pathogen exposure in models predicting plasma CRP, suggesting that the impact of these variants may be contingent upon environmental exposures.
In past studies, variants at the CRP locus, which encodes the CRP protein, were reproducibly reported to show significant association with circulating CRP not only in populations of European ancestry, but also in Asians such as Chinese and Japanese15, 29. Specifically, the triallelic SNP rs3091244 was suggested to be functionally relevant30. In our Filipino young adult sample, the SNPs rs1205 and rs3091244 at the CRP gene region showed the strongest associations with plasma CRP, consistent with the previous findings from candidate gene and GWAS9-10, 15. We also observed a strong association between plasma CRP and HNF1A variants rs7310409 and rs1169288. Because the protein HNF1A is involved in modulating CRP gene expression by directly binding to its promoter31, the gene region of HNF1A had convincing prior evidence of association with CRP as a candidate gene and was further confirmed by GWAS in European-based populations9-10. We also replicated associations with CRP related variants at IL6R32, APOE8, APOC113, and LEPR7, providing the first confirmation of genetic association for these loci in a young Asian population.
We did not find evidence for an association between plasma CRP and the SNP in GCKR (rs1260326) or the intergenic SNP 12q23.1-rs10778213, despite our >95% power to detect the previously reported variance explained by these SNPs9. Similar to our results, the association with these SNPs failed to reach significant levels in a prior GWAS conducted in the Pharmacogenomics and Risk of Cardiovascular Disease (PARC) study10, thus suggesting that the association for variants at these loci might be population-specific. We did not test additional CRP associated loci reported by a recent meta-analysis of GWA studies13 because our study was poorly powered (< 35%) to detect the reported variance explained by those SNPs. In addition, given the relatively younger age of our study sample compared to those previously reported9, the potential interactive influence between gene and age may also partially explain the discrepancy. Furthermore, because the environmental factors and behavior such as infection, smoking, diet pattern and physical activity may contribute to baseline CRP variation33, we cannot exclude the possibility that the long-term exposure to certain environmental factors may accentuate the genetic susceptibility on CRP level in middle-aged or elderly populations.
In conclusion, our results demonstrate that SNPs at CRP, HNF1A, IL6R, APOE-APOC1 and LEPR are associated with plasma CRP in a sample of Filipino young adults. This study expands our understanding of the genetic associations with CRP level to a younger population of mostly non-European ancestry and suggests a shared genetic influence between ethnic groups.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the USC-Office of Population Studies Foundation research and data collection teams and the study participants who generously provided their time for this study. This work was supported by National Institutes of Health grants DK078150, TW05596, HL085144, RR20649, ES10126, and DK56350.
Footnotes
Supplementary information is available at The Journal of Human Genetics’ website.
REFERENCES
- 1.Chen TH, Gona P, Sutherland PA, Benjamin EJ, Wilson PW, Larson MG, et al. Long-term C-reactive protein variability and prediction of metabolic risk. Am J Med. 2009;122:53–61. doi: 10.1016/j.amjmed.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currie CJ, Poole CD, Conway P. Evaluation of the association between the first observation and the longitudinal change in C-reactive protein, and all-cause mortality. Heart. 2008;94:457–462. doi: 10.1136/hrt.2007.118794. [DOI] [PubMed] [Google Scholar]
- 3.Hu G, Jousilahti P, Tuomilehto J, Antikainen R, Sundvall J, Salomaa V. Association of serum C-reactive protein level with sex-specific type 2 diabetes risk: a prospective finnish study. J Clin Endocrinol Metab. 2009;94:2099–2105. doi: 10.1210/jc.2008-2260. [DOI] [PubMed] [Google Scholar]
- 4.Jiang S, Bao Y, Hou X, Fang Q, Wang C, Pan J, et al. Serum C-reactive protein and risk of cardiovascular events in middle-aged and older chinese population. Am J Cardiol. 2009;103:1727–1731. doi: 10.1016/j.amjcard.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, et al. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 6.Kathiresan S, Larson MG, Vasan RS, Guo CY, Gona P, Keaney JF, Jr., et al. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006;113:1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YY, Gottardo L, Mlynarski W, Frazier W, Nolan D, Duffy J, et al. Genetic variability at the leptin receptor (LEPR) locus is a determinant of plasma fibrinogen and C-reactive protein levels. Atherosclerosis. 2007;191:121–127. doi: 10.1016/j.atherosclerosis.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 8.Judson R, Brain C, Dain B, Windemuth A, Ruano G, Reed C. New and confirmatory evidence of an association between APOE genotype and baseline C-reactive protein in dyslipidemic individuals. Atherosclerosis. 2004;177:345–351. doi: 10.1016/j.atherosclerosis.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, et al. Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am J Hum Genet. 2008;82:1185–1192. doi: 10.1016/j.ajhg.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiner AP, Barber MJ, Guan Y, Ridker PM, Lange LA, Chasman DI, et al. Polymorphisms of the HNF1A gene encoding hepatocyte nuclear factor-1 alpha are associated with C-reactive protein. Am J Hum Genet. 2008;82:1193–1201. doi: 10.1016/j.ajhg.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada Y, Takahashi A, Ohmiya H, Kumasaka N, Kamatani Y, Hosono N, et al. Genome-wide association study for C-reactive protein levels identified pleiotropic associations in the IL6 locus. Hum Mol Genet. 2011;20:1224–1231. doi: 10.1093/hmg/ddq551. [DOI] [PubMed] [Google Scholar]
- 13.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore JH, Williams SM. Epistasis and its implications for personal genetics. Am J Hum Genet. 2009;85:309–320. doi: 10.1016/j.ajhg.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng MS, Hsu LA, Wu S, Chang HH, Chou HH, Ko YL. Association between C-reactive protein gene haplotypes and C-reactive protein levels in Taiwanese: interaction with obesity. Atherosclerosis. 2009;204:e64–69. doi: 10.1016/j.atherosclerosis.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Eiriksdottir G, Smith AV, Aspelund T, Hafsteinsdottir SH, Olafsdottir E, Launer LJ, et al. The interaction of adiposity with the CRP gene affects CRP levels: age, gene/environment susceptibilty-Reykjavik study. Int J Obes (Lond) 2009;33:267–272. doi: 10.1038/ijo.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr., Sacco RL, et al. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 19.McDade TW, Rutherford JN, Adair L, Kuzawa C. Adiposity and pathogen exposure predict C-reactive protein in Filipino women. J Nutr. 2008;138:2442–2447. doi: 10.3945/jn.108.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009;89:1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutherford JN, McDade TW, Lee NR, Adair LS, Kuzawa C. Change in waist circumference over 11 years and current waist circumference independently predict elevated CRP in Filipino women. Am J Hum Biol. 2010;22:310–315. doi: 10.1002/ajhb.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, et al. Cohort Profile: The Cebu Longitudinal Health and Nutrition Survey. Int J Epidemiol. 2010 doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 24.Ge D, Zhang K, Need AC, Martin O, Fellay J, Urban TJ, et al. WGAViewer: software for genomic annotation of whole genome association studies. Genome Res. 2008;18:640–643. doi: 10.1101/gr.071571.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36:D820–824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita A, Nakayama T, Doba N, Hinohara S, Mizutani T, Soma M. Genotyping of triallelic SNPs using TaqMan PCR. Mol Cell Probes. 2007;21:171–176. doi: 10.1016/j.mcp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: Using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genetic Epidemiology. 2010 doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly MP, Wolfe ML, Localio AR, Rader DJ. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Ognjanovic S, Yamamoto J, Saltzman B, Franke A, Ognjanovic M, Yokochi L, et al. Serum CRP and IL-6, genetic variants and risk of colorectal adenoma in a multiethnic population. Cancer Causes Control. 2010;21:1131–1138. doi: 10.1007/s10552-010-9540-7. [DOI] [PubMed] [Google Scholar]
- 30.Szalai AJ, Wu J, Lange EM, McCrory MA, Langefeld CD, Williams A, et al. Single-nucleotide polymorphisms in the C-reactive protein (CRP) gene promoter that affect transcription factor binding, alter transcriptional activity, and associate with differences in baseline serum CRP level. J Mol Med. 2005;83:440–447. doi: 10.1007/s00109-005-0658-0. [DOI] [PubMed] [Google Scholar]
- 31.Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, Song J, et al. Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven C-reactive protein gene expression. J Immunol. 2008;180:3492–3501. doi: 10.4049/jimmunol.180.5.3492. [DOI] [PubMed] [Google Scholar]
- 32.Qi L, Rifai N, Hu FB. Interleukin-6 receptor gene, plasma C-reactive protein, and diabetes risk in women. Diabetes. 2009;58:275–278. doi: 10.2337/db08-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hage FG, Szalai AJ. C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol. 2007;50:1115–1122. doi: 10.1016/j.jacc.2007.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.