Abstract
Autophagy can selectively remove damaged organelles, including mitochondria, and in turn protect against mitochondria damage induced-cell death. Acetaminophen (APAP) overdose can cause liver injury in animals and humans by inducing mitochondria damage and subsequent necrosis in hepatocytes. Although many detrimental mechanisms have been reported to be responsible for APAP-induced hepatotoxicity, it is not known whether APAP can modulate autophagy to regulate hepatotoxicity in hepatocytes. To test the hypothesis that autophagy may play a critical protective role against APAP-induced hepatotoxicity, primary cultured mouse hepatocytes and GFP-LC3 transgenic mice were treated with APAP. By using a series of morphological and biochemical autophagic flux assays, we found that APAP induced autophagy both in the in vivo mouse liver and in primary cultured hepatocytes. We also found that APAP treatment might suppress mTOR in hepatocytes and APAP-induced autophagy was suppressed by N-acetylcysteine, suggesting APAP mitochondrial protein binding and the subsequent production of reactive oxygen species may play an important role in APAP-induced autophagy. Pharmacological inhibition of autophagy by 3-methyladenine or chloroquine further exacerbated APAP-induced hepatotoxicity. In contrast, induction of autophagy by rapamycin inhibited APAP-induced hepatotoxicity.
Conclusion
APAP overdose induces autophagy which attenuates APAP-induced liver cell death by removing damaged mitochondria.
Keywords: acetaminophen, autophagy, reactive oxygen species, liver injury
INTRODUCTION
Acetaminophen (APAP) is a safe and effective analgesic at therapeutic doses. However, an overdose can cause liver injury and even liver failure in animals1 and in humans.2 The mechanism of APAP-induced liver injury includes the formation of a reactive metabolite (Nacetyl-p-benzoquinone imine, NAPQI), which depletes glutathione and binds to cellular proteins.1 The only clinically used antidote, N-acetylcysteine (NAC), acts as a prodrug for glutathione synthesis and is therefore most effective when administered very early when it prevents protein binding of NAPQI;3 however, later mechanisms of protection have also been identified.4
Although protein binding is a critical initiating event in the pathophysiology, it is insufficient to cause the massive cell injury typical of APAP overdose.5 Beginning with the identification of mitochondrial protein binding,6 inhibition of mitochondrial respiration,7 ATP depletion8 and the occurrence of a selective mitochondrial oxidant stress8 during APAP hepatotoxicity, the concept emerged that mitochondrial dysfunction and damage is a central propagation event responsible for cell necrosis. The oxidant stress leads to mitochondrial peroxynitrite formation, which causes mitochondrial DNA damage and nitration of mitochondrial proteins9 and triggers the membrane permeability transition (MPT) and subsequent cell necrosis.10,11 Given this central role of mitochondria in the pathophysiology and the fact that not all mitochondria are affected at the same time, it is feasible that removal of damaged mitochondria might be beneficial.
Macroautophagy (hereafter referred to as autophagy) is a bulk intracellular degradation system which is mainly responsible for the degradation of long-lived proteins and other cellular contents. Autophagy involves the formation of double-membrane autophagosomes, which enwrap cytoplasm and organelles and then fuse with lysosomes, thus degrading the enveloped contents. This process is tightly regulated and highly inducible. More than 30 Atg genes have been defined to participate in autophagy or autophagy-related processes.12,13 Under stress conditions, such as nutrient starvation, autophagy is induced largely due to the inhibition of mammalian target of rapamycin (mTOR) complex 1, a kinase complex which works as a nutrient sensor to initiate autophagy by activating ULK1/Atg1. Then two ubiquitin-like conjugation system including Atg7 (E1-like), Atg3 and Atg10 (E2-like) and the Atg5-Atg12-Atg16 complex promote the conjugation of light chain 3 (LC3), a mammalian homologue of yeast Atg8, with phosphatidylethanolamine (PE). The PE-conjugated form of LC3 (LC3-II) translocates to the autophagosomal membrane and promotes the formation a double membrane autophagosome. In addition, Beclin 1/Atg6 forms a complex with VPS34, VPS15 and Atg14. VPS34 is a class III PI-3-kinase that is required for autophagy induction. 3-Methyladenine (3-MA), a widely used autophagy inhibitor, inhibits the type III PI3K and autophagosome formation.14 Chloroquine (CQ), a clinically used anti-malarial drug, suppresses autophagy by increasing lysosomal pH.15,16
Autophagy is usually activated as a survival mechanism in response to an adverse environment, such as the deprivation of nutrients or growth factors.17 However, autophagy may also be involved in the pathogenesis of a number of human diseases.18,19 Most importantly, autophagy can eliminate damaged mitochondria and maintain mitochondrial homeostasis (Mitophagy).20 We have recently shown that removal of damaged mitochondria by mitophagy reduces alcohol-induced liver injury.21 Although mitochondrial damage is a key cellular event which contributes to APAP-induced hepatotoxicity, it is not known whether APAP can modulate autophagy in hepatocytes. Therefore, the objective of the current investigation was to assess the occurrence of autophagy in APAP-induced liver cell injury in vivo and in vitro and evaluate its potential pathophysiological relevance. We found that APAP induced autophagy both in the mouse liver and in primary cultured mouse hepatocytes. Moreover, pharmacological suppression of autophagy exacerbated APAP-induced liver injury whereas induction of autophagy protected against APAP-induced liver injury.
MATERIALS AND METHODS
Experimental Design
C57BL/6 wild type and GFP-LC3 transgenic mice as well as isolated primary mouse hepatocytes were used in this study. For in vivo studies, mice were either given saline (i.p.) or APAP (500 mg/kg, i.p.). Induction or suppression of autophagy was achieved by injection (i.p.) of rapamycin (2 mg/kg) or CQ (60 mg/kg). For in vitro studies, primary cultured hepatocytes were treated with various concentrations of APAP at different time points. Autophagic flux in APAP-treated cells was determined by quantifying the number of GFP-LC3 puncta, LC3-II levels, the number of autophagosomes (electron microscopy) as well as p62 degradation with or without the lysosomal inhibitor CQ. Necrosis was determined by propidium iodide (PI) staining and immunostaining for high mobility group box 1 (HMGB1). Liver injury was assessed by hematoxylin and eosin (HE) staining of liver sections and the serum alanine aminotransferase (ALT) activities.
For additional details on methods, please refer to the Supporting Material.
RESULTS
APAP induces autophagy in mouse liver
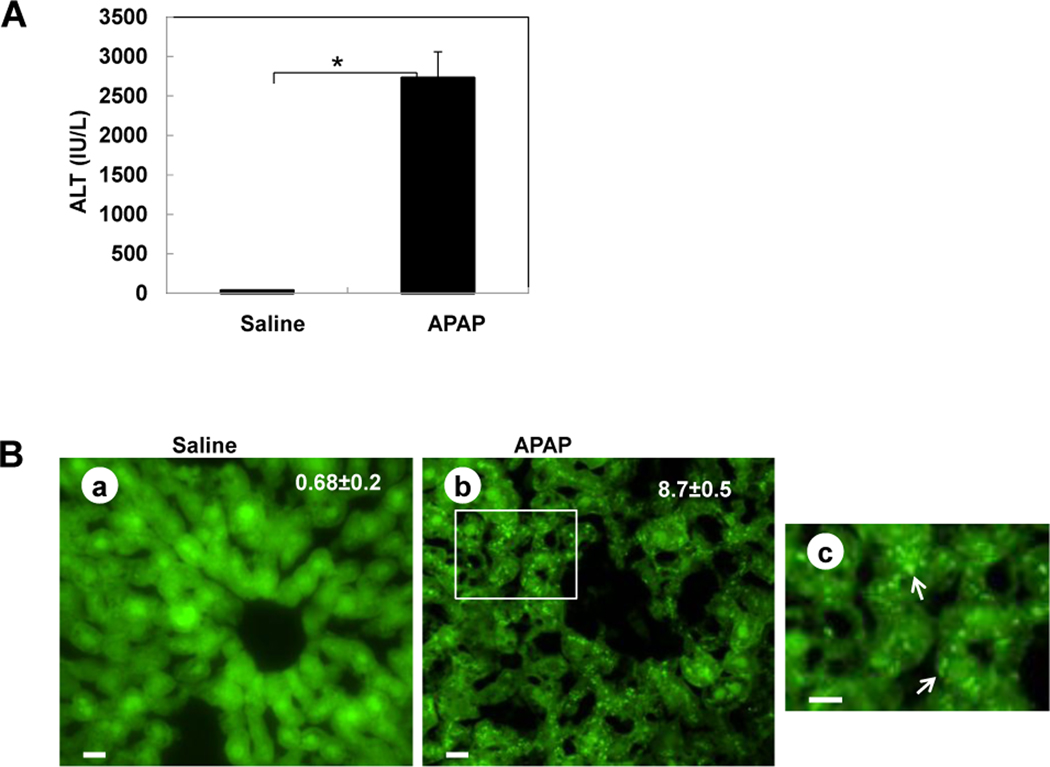
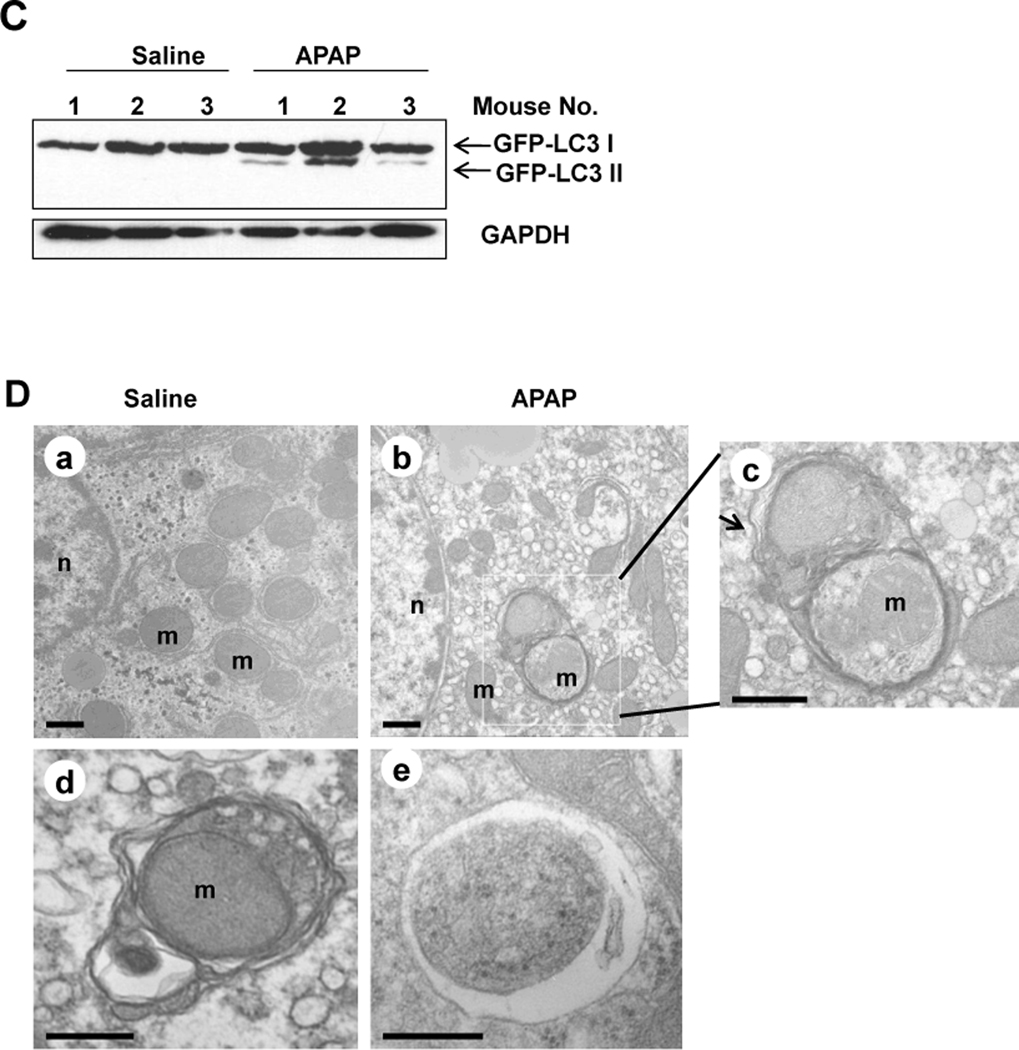
Activation of autophagy by APAP was first examined in GFP-LC3 transgenic mice. In agreement with previous reports that APAP induced liver injury,4,8 APAP treatment induced a significant elevation of serum ALT in GFP-LC3 transgenic mice (Figure 1A). Interestingly, APAP treatment also significantly increased GFP-LC3 puncta in the liver (Figure 1B), which represent autophagosomes. Immunoblot analysis confirmed the increase of the membrane-associated PE-conjugated form of GFP-LC3 (GFP-LC3-II) in APAP-treated mouse livers (Figure 1C). Importantly, EM analysis indicated an increased accumulation of autophagosomes following APAP treatment (Figure 1D). Interestingly, the double membrane autophagosomes often had enveloped mitochondria, suggesting that APAP-induced autophagy may help remove the damaged mitochondria caused by APAP (Figure 1D, panels c,d).
Figure 1. APAP overdose induces autophagy in the liver.
(A–D) GFP-LC3 mice (n=3) were treated either with saline or APAP (500 mg/kg) for 6 hrs, and blood was analyzed for ALT level (A) and the liver sections were analyzed by fluorescence microscopy (B). *: p<0.01. Panel a: saline; panel b: APAP; Panel c is enlarged photograph from the boxed area in panel b. Arrows denote GFP-LC3 puncta. The number of GFP-LC3 puncta (mean ± SEM) was quantified from each animal. More than 30 cells were counted in each individual experiment. Total lysates of the liver were analyzed by immunoblot assay using an anti-GFP antibody (C). (D) Wild-type mice were treated as in (A) and liver samples were processed for EM. Panel a: saline; panel b: APAP, panel c was from the boxed area in panel b; panels d–e: representative EM images of autophagosomes containing mitochondria from APAP treated mouse liver. Arrows denote autophagosomes. n: nucleus; m: mitochondria.
APAP induces autophagy in primary hepatocytes
We next examined the effects of APAP on primary cultured mouse hepatocytes. Consistent with the in vivo studies, APAP treatment also increased GFP-LC3 puncta in primary cultured mouse hepatocytes in a time- and dose-dependent manner (Figure 2A,B), indicating a significant accumulation of autophagosomes in APAP-treated hepatocytes. APAP treatment also increased the endogenous LC3-II levels. Importantly, p62, an autophagy substrate which is normally degraded during autophagy, was degraded dramatically by APAP treatment in a time- and dose-dependent manner (Figure 2C), indicating APAP induces autophagic flux. After 6 hrs treatment, all the various concentrations of APAP only mildly increased necrotic cell death (about 6 to 8%, Supplemental Figure 1). Similar to the in vivo studies, EM studies revealed that there was an increased amount of autophagosomes in APAP-treated cells compared to the non-treated control cells (Figure 2D–E). Enlarged photographs revealed that APAP-induced autophagosomes contained intracellular contents, including mitochondria (Figure 2D, panel c; Supplemental Figure 1B), suggesting APAP induces mitophagy. Indeed, results from western blot analysis revealed that APAP induced marked mitochondrial protein degradation including heat shock protein 60 (HSP 60, a mitochondrial matrix protein) and cytochrome c (Cyto c, a mitochondrial intermembrane space protein), and Tom20 (an outer mitochondrial membrane protein) (Figure 2F), further supporting that APAP induced mitophagy in hepatocytes.
Figure 2. APAP induces autophagy in primary mouse hepatocytes.
(A–B) Ad-GFP-LC3 infected hepatocytes were treated with APAP (5 mM) as indicated (for 3, 6 and 24 hrs) or with different concentrations of APAP (0, 2.5, 5, 10 mM) for 6 hrs and examined by fluorescence microscopy. Scale bar: 20 µm. GFP-LC3 puncta (mean ± SEM) were quantified for each experiment (n=3). At least 30 cells were counted in each individual experiment. *: p<0.01. Total lysates were subjected to immunoblot assay for LC3 and p62 (C). (D) Primary mouse hepatocytes were either treated with saline (panel a) or treated with APAP (5 mM, panels b &c) for 6 hrs and then processed for EM analysis. Panel c is an enlarged photograph from boxed area in panel b. Arrows: autophagosomes, n: nucleus, m: mitochondria; LD: lipid droplets. (E) The number of autophagosomes was quantified from more than 20 different cells (mean ± SD) *: p<0.01. (F) Hepatocytes were treated as in (A). Total cellular lysates were subjected to immunoblot assay for mitochondrial proteins: HSP60 (matrix protein); Cyto c (intermembrane space protein) and Tom20 (outer membrane protein).
Effects of APAP on autophagic flux and mTOR in primary hepatocytes
In addition to the determination of the degradation of p62, autophagic flux can also be assessed by using a combination of lysosomal inhibitors such as CQ.15 We found that in the presence of CQ, the average number of GFP-LC3 dots per cell induced by APAP was further increased than either APAP or CQ treatment alone (Figure 3A,B), although there is no statistical difference compared to the CQ treatment alone group. APAP-induced degradation of p62 was partially rescued by CQ in the APAP lower dose (2.5 mM) group but not in the APAP higher dose group (5 mM). However, combined CQ and APAP treatment did not further increase the level of endogenous LC3-II (Figure 3C). One possible explanation for these observations is likely the increased necrosis when hepatocytes were treated with both CQ and APAP (see below) which may suppress the further induction of autophagy.
Figure 3. APAP induces autophagic flux and suppresses mTOR in primary mouse hepatocytes.
(A) Ad-GFP-LC3 infected hepatocytes were treated with APAP (5 mM) in the absence or presence of CQ (20 µM) for 6 hrs and examined by fluorescence microscopy. Scale bar: 20 µm. (B) GFP-LC3 puncta (mean ± SEM) were quantified for each experiment (n=3). At least 30 cells were counted in each individual experiment *: p<0.01. (C)Total lysates were subjected to immunoblot assay for LC3 and p62. (D) Primary mouse hepatocytes were treated with different concentrations of APAP (0, 1.25, 2.5, 5 & 10 mM) for 6 hrs. Total cellular lysates were subjected to immunoblot assay.
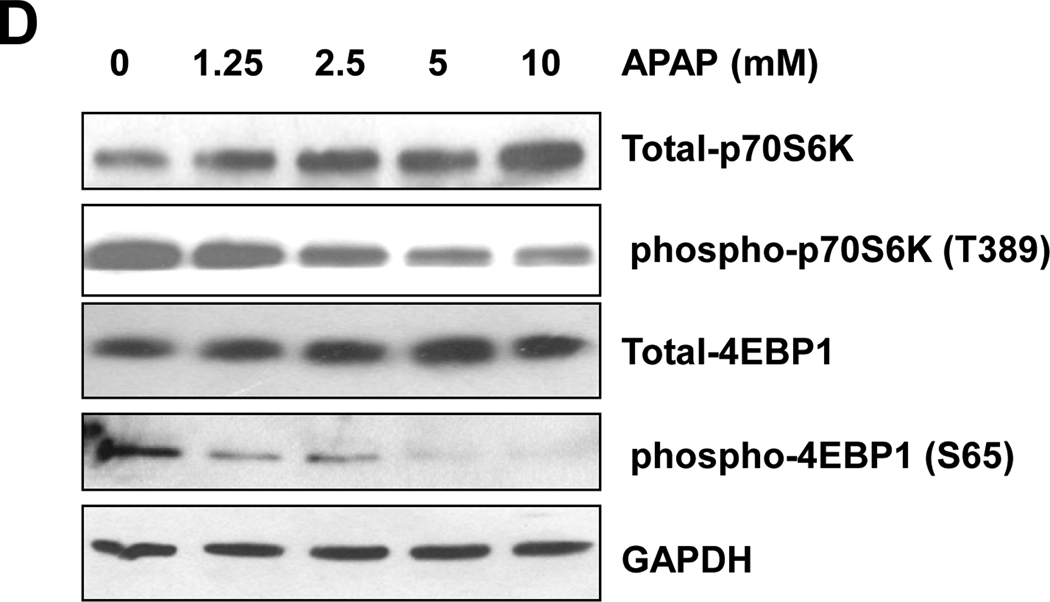
Because suppression of mTOR is one central molecular signaling pathway leading to autophagy induction, we next determined whether APAP treatment would affect mTOR activity in primary hepatocytes. We found that APAP treatment significantly decreased the levels of phosphorylated p70S6K and 4EBP-1, two well-known downstream phosphorylation targets of mTOR, in a dose-dependent manner (Figure 3D). Taken together, these data support that APAP induces autophagy and is involved in the suppression of mTOR in hepatocytes.
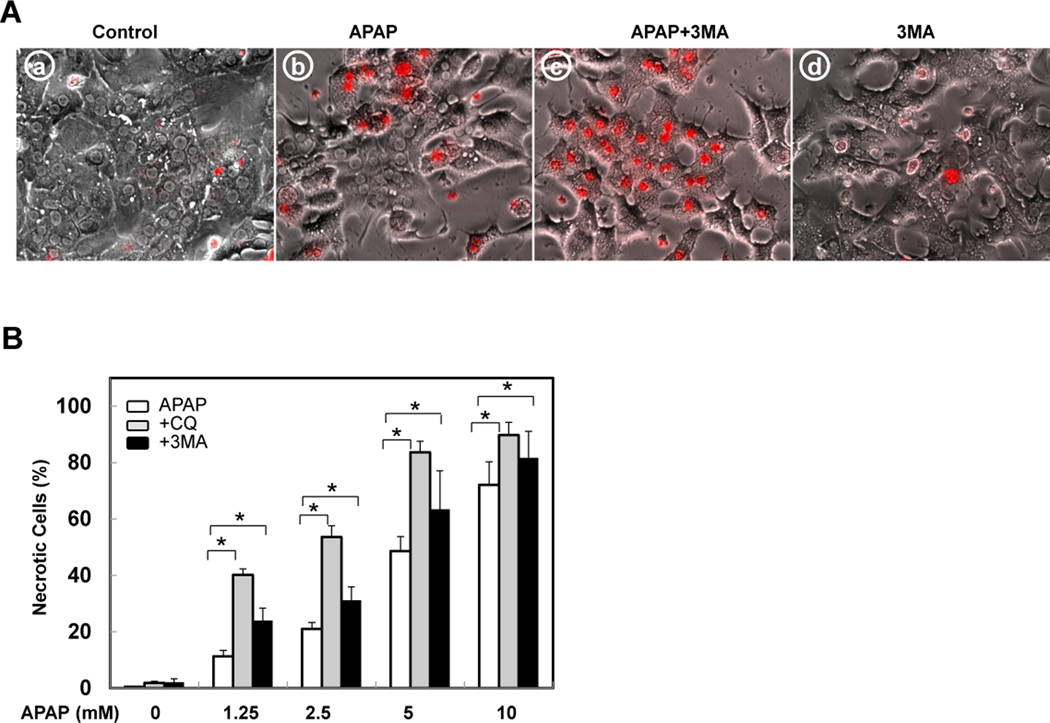
NAC and 3-MA suppress APAP-induced GFP-LC3 puncta
To determine whether APAP-induced protein binding and ROS would contribute to APAP-induced autophagy, we treated hepatocytes with APAP in the presence of NAC. 3-MA, the class-III PI3 kinase inhibitor, which has been shown to suppress autophagy in many models, suppressed APAP-induced GFP-LC3 puncta (Figure 4A,B). In addition, in the presence of NAC APAP-induced GFP-LC3 puncta were also completely blocked (Figure 4A,B). These results suggest that APAP-induced autophagy involves its metabolic activation and ROS production as well as the class-III PI3 kinase. To further determine whether suppression of autophagy would influence APAP-induced ROS production, we treated hepatocytes with APAP in the absence or presence of CQ for 6 hrs and stained the cells with DCFH-DA, a fluorescence dye which is widely used to assess ROS.24 We found that APAP treatment alone increased ROS production but suppression of autophagy by CQ further enhanced APAP-induced ROS production (Supplemental Figure 2).
Figure 4. NAC and 3MA suppress APAP-induced GFP-LC3 puncta.
(A) Ad-GFP-LC3 infected hepatocytes were treated with APAP (5 mM) in the absence or presence of NAC (10 mM) or 3-MA (10 mM) for 6 hrs and examined by fluorescence microscopy. Scale bar: 20 µm. (B) GFP-LC3 puncta (mean ± SEM) were quantified for each experiment (n=3). At least 30 cells were counted in each individual experiment *: p<0.01.
APAP induces typical necrosis in primary hepatocytes
To determine the nature of the cell death induced by APAP, we loaded the cells with PI, which stains the nuclei only after the cellular plasma membranes are permeabilized. At 24 hrs after treatment with APAP (10 mM), more than 80% of hepatocytes had PI-positive nuclei, suggesting permeabilization/disruption of the hepatocytes plasma membrane. We previously showed that TNF-α induces apoptosis in primary hepatocytes in the presence of actinomycin D (ActD).22 It can be seen that the apoptotic cells had shrunk and were often detached with multiple cellular blebbings in TNF-α/ActD treated cells, whereas APAP-treated hepatocytes were swollen and often still attached (Supplemental Figure 3A, panels b,c). Although most TNF-α/ActD treated cells eventually show PI-positive nuclei due to the secondary necrosis that occurs during the culture condition, all PI-positive nuclei were fragmented (Supplemental Figure 3A, panel b). In contrast, in APAP-treated cells, all PI-positive nuclei were intact without fragmentation (Supplemental Figure 3A, panels c,d). By quantifying the PI-positive cells with intact nuclei as necrotic cells, we found that APAP-induced necrosis increased in a dose-dependent manner (Supplemental Figure 3B). APAP-induced necrosis was further determined by EM studies. Almost all the cytosolic content and organelles were disrupted with numerous cytosolic vesicles in APAP treated necrotic cells (Supplemental Figure 3C). During necrotic, but not apoptotic cell death, HMGB1, a nuclear protein which binds to chromatin, is released from the nuclei.23 We determined whether APAP treatment would also induce HMGB1 release from the nuclei. In control hepatocytes, HMGB1 is exclusively located in the nuclei as demonstrated by its co-localization with nuclear staining by Hoechst 33342. However, HMGB1 displayed a marked cytosolic pattern in APAP-treated cells, indicating that APAP induces HMGB1 release (Figure 5A). The percentage of cells with the release of HMGB1 from the nuclei in APAP-treated cells increased in a dose-dependent manner (Figure 5B). These results indicate that APAP induced necrosis, but not apoptosis, in primary hepatocytes.
Figure 5. APAP induces necrotic cell death in primary mouse hepatocytes.
(A) Representative photographs of hepatocytes immunostained with an anti-HMGB1 antibody for cells either non-treated (panels a–d) or treated with APAP (10 mM, panels e–h) for 24 hrs. Cell nuclei were stained with Hoechst 33342. Panels d and h are enlarged photographs from the boxed areas from panels c and g respectively. (B) Cells with released HMGB1 from the nuclei were quantified for each experiment (n=3). More than 300 cells were counted in each individual experiment *: p<0.01.
Autophagy reduces APAP-induced hepatotoxicity in vitro
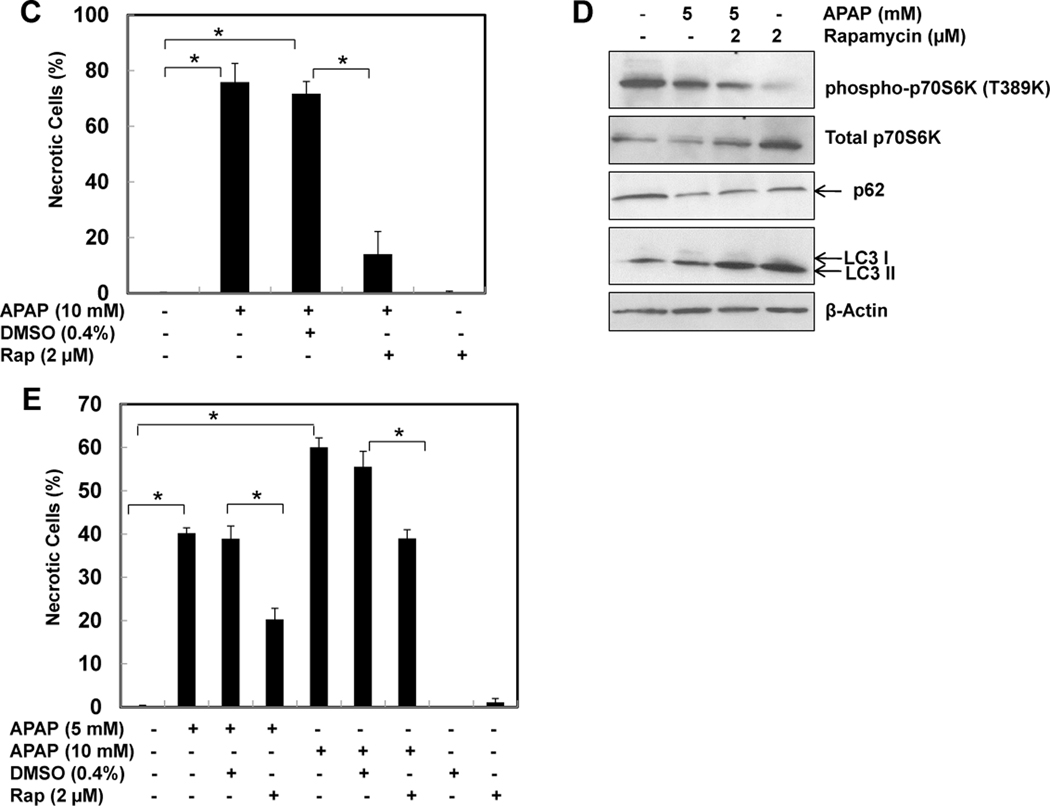
Because we observed that both 3-MA and CQ suppressed APAP-induced autophagy, we next determined the cell viability of cells treated with various concentrations of APAP in the presence of 3-MA or CQ. Whereas inhibition of basal autophagy alone by 3-MA or CQ did not affect cell death in hepatocytes (Figure 6A,B), both 3-MA and CQ significantly increased APAP-induced necrosis, indicating that APAP-induced autophagy is protective against cell death. Interestingly, suppression of autophagy clearly induced a shift in the dose-response of APAP-induced necrosis even in the low dose of APAP (1.25 mM). In contrast, co-treatment of APAP with rapamycin, a mTOR inhibitor and autophagy inducer, significantly inhibited APAP-induced necrosis (Supplemental Figure 3D & Figure 6C). The protective effects of rapamycin are not due to the solvent of DMSO because DMSO alone did not affect APAP-induced necrosis in primary cultured hepatocytes (Figure 6C). Rapamycin (2 µM) alone decreased the level of phosphorylated p70S6K, increased LC3-II and p62 degradation, suggesting that rapamycin induces autophagy in primary cultured hepatocytes (Figure 6D). APAP is metabolized via CYP2E1 in the first 2–3 hrs in hepatocytes after its exposure and generates the reactive metabolite NAPQI.5 To test whether induction of autophagy would still be protective after the initial phase of APAP metabolism, we treated hepatocytes with APAP for 3 hrs and then added rapamycin. Even the delayed treatment with rapamycin significantly attenuated APAP-induced necrosis (Figure 6E). Therefore, induction of autophagy protects against APAP-induced necrosis whereas inhibition of autophagy further exacerbates it.
Figure 6. Induction of autophagy protects against APAP-induced cell death.
(A) Representative overlayed images of phase-contrast with PI staining of primary cultured mouse hepatocytes treated for 24 hrs as indicated: panel a: non-treated; panel b: APAP (5 mM); panel c: APAP (5 mM) + 3-MA (10 mM); and panel d: 3-MA (10 mM). (B) Hepatocytes were either treated with saline or with various concentrations of APAP (1.25, 2.5, 5 &10 mM) in the absence or presence of CQ (20 µM) or 3MA (10 mM) for 24 hrs. PI positive cells with intact nuclei were quantified for each experiment (n=3). More than 300 cells were counted in each individual experiment *: p<0.01. (C) Primary mouse hepatocytes were co-treated for 24 hrs as indicated: panel a: DMSO; panel b: APAP (10 mM)+ DMSO (4 µL/mL); panel c: APAP (10 mM) + Rap (2 µM); and panel d: Rap (2 µM). PI positive cells with intact nuclei were quantified for each experiment (n=3). More than 300 cells were counted in each individual experiment *: p<0.01. (D) Primary hepatocytes were treated as indicated for 6 hrs and total cell lysates were subjected to immunoblot assay. (E) Primary mouse hepatocytes were treated with saline or APAP (5 & 10 mM) for 3 hrs, the cells were then further treated with or without DMSO or Rap (2 µM) for another 21 hrs. PI positive cells with intact nuclei were quantified for each experiment (n=3). *: p<0.01.
Induction of autophagy protects against APAP-induced liver injury in vivo
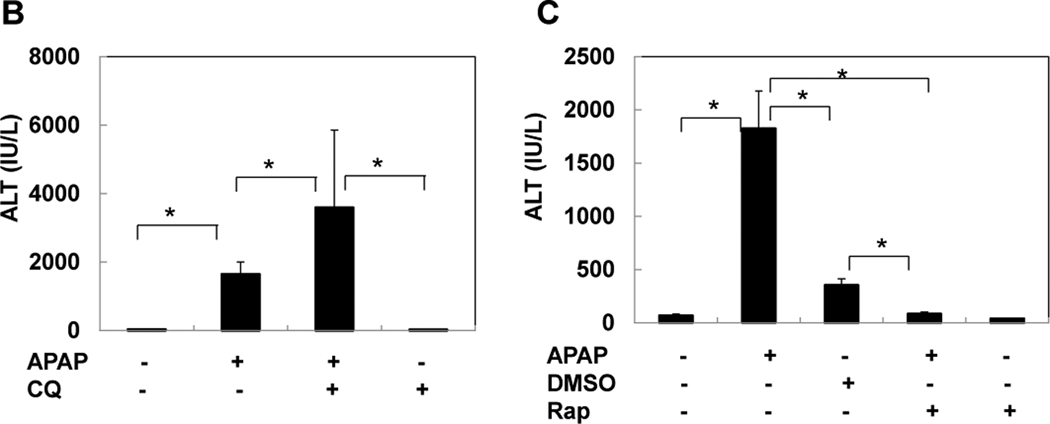
We next determined whether modulation of autophagy would affect APAP-induced liver injury in vivo. C57BL/6 mice were treated with APAP in the absence or presence of CQ and rapamycin for 6 hrs. Centrilobular necrosis was evident in livers of mice treated with APAP which was further exacerbated by CQ treatment but almost abolished in rapamycin-treated mice (Figure 7A). Furthermore, CQ treatment also further increased APAP-induced serum ALT levels (Figure 7B). In agreement with previous reports, DMSO alone (which was used to dissolve rapamycin) also partially reduced APAP-induced liver injury likely due to its inhibitory effects on CYP2E1.25 However, treatment with rapamycin completely eliminated APAP-induced liver injury (Figure 7A,C). Because hepatic GSH depletion 2 hrs after APAP was similar in DMSO- and rapamycin-treated animals (Supplemental Figure 4), the protection was not caused by inhibition of metabolism. Thus, these data indicate that induction of autophagy protects against APAP-induced liver injury in vivo.
Figure 7. Induction of autophagy suppresses APAP-induced liver injury in vivo.
(A) Wild type C57BL/6 mice were injected (i.p.) with CQ (60 mg/kg) or rapamycin (2 mg/kg) or DMSO (2% DMSO, 10 µL/g) immediately followed by APAP (500 mg/kg) injection for 6 hrs. Representative photographs of HE staining are presented. (B) Blood ALT levels were quantified (n=4–6 mice). *: p<0.01.
DISCUSSION
mTOR is an evolutionarily conserved nutrient-sensing serine/threonine protein kinase which plays a critical role in regulating protein synthesis and autophagy.26,27 mTOR regulates phosphorylation of at least two proteins important for translation: p70S6K and 4EBP1. mTOR signaling negatively regulates autophagy and mTOR suppression by rapamycin contributes to the induction of autophagy,27 which is in agreement with our present study in primary cultured mouse hepatocytes (Figure 6). We found that APAP decreased the phosphorylation levels of p70S6K and 4EBP1 in hepatocytes (Figures 3D,6D), suggesting that APAP may inhibit mTOR. How APAP suppresses mTOR in hepatocytes requires further studies.
In addition to the mTOR signaling pathway, we also found that co-treatment of NAC with APAP almost completely suppressed APAP-induced GFP-LC3 puncta (Figure 4). APAP is metabolized mainly by the CYP2E1 isoform of cytochrome P450 to NAPQI, which depletes intracellular glutathione and covalently binds to proteins, including many mitochondrial proteins. This triggers mitochondrial damage and production of ROS.5 NAC treatment promotes GSH synthesis in the cytosol and supports recovery of mitochondrial GSH levels and therefore improves the scavenging capacity for NAPQI and for mitochondrial ROS.3,4 The inhibitory effects of NAC on APAP-induced autophagy suggest that the metabolic activation of APAP is required for APAP-induced autophagy. Accumulating evidence now supports that ROS from damaged mitochondria can induce autophagy. For example, inhibition of mitochondrial respiration by mitochondrial electron transport chain inhibitors such as rotenone (complex I inhibitor) or thenoyl trifluoroacetone (TTFA, complex II inhibitor) induces autophagy.28,29. We also found that carbonyl cyanide m-chlorophenylhydrazone, a mitochondrial uncoupler, also induces autophagy in various mammalian cell lines. In the latter case, autophagy is also suppressed by NAC.29 Although the exact mechanisms are still unclear, it is proposed that ROS may induce autophagy by modulating the action of Atg4B on LC3. Atg4B is a cysteine protease which not only cleaves the full length LC3 to generate LC3-I but also delipidates LC3-II from the autophagosomal outer membrane to allow the recycling of LC3. ROS can modulate Atg4B activity by directly oxidizing a specific cysteine residue, Cys81 on Atg4B.30 It remains to be studied how exactly NAC suppresses APAP-induced autophagy.
It is well known that NAC can protect against APAP-induced liver injury3,4 and we also confirmed that NAC suppressed APAP-induced necrosis and liver injury (data not shown). Why would NAC suppress APAP-induced autophagy but also protect against APAP-induced liver injury? Early treatment of NAC results in rapid GSH formation, which enhances the capacity to scavenge NAPQI and thus prevents all upstream initiating events such as protein binding and mitochondrial ROS formation.3,4 Therefore, NAC prevents toxicity and eliminates also the stress that triggers the induction of autophagy.
In addition to its non-selective bulk degradation, accumulating evidence now suggests that macroautophagy can be selective. One of the well-studied forms of selective macroautophagy is mitophagy, a process where LC3 positive compartments enwrap mitochondria.20 In the present study, we have found that APAP-induced autophagosomes often contain mitochondria both in the mouse liver and in primary cultured hepatocytes (Figures 1D,2D & Supplemental Figure 1B). Moreover, many mitochondrial proteins were degraded in APAP-treated hepatocytes supporting the conclusion that APAP-induced autophagy removes damaged mitochondria (Figure 2F), which are the major source of intracellular ROS during APAP hepatotoxicity (Supplemental Figure 2).4,8 Timely removal of damaged mitochondria by mitophagy plays an important role in regulating cell death.31
Because we observed that rapamycin almost completely blocked APAP-induced liver injury in mouse livers, it is possible that rapamycin may have affected the metabolic activation of APAP. However, the >90% depletion of hepatic GSH levels in both APAP/rapamycin and APAP/DMSO-treated animals at 2 hours, i.e. before significant cell death (Supplemental Figure 4), suggests that the protection by rapamycin was not caused by inhibition of APAP metabolism but was due to induction of autophagy. Although we have demonstrated that rapamycin could be a potential therapeutic drug for treating APAP-induced liver injury, it should also be noted that rapamycin also has immunosuppression activity in addition to inducing autophagy. However, with the recent rapid progress on small molecule screening for autophagy inducers, future work should examine the protective effects against APAP-induced liver injury using more specific novel autophagy inducers.
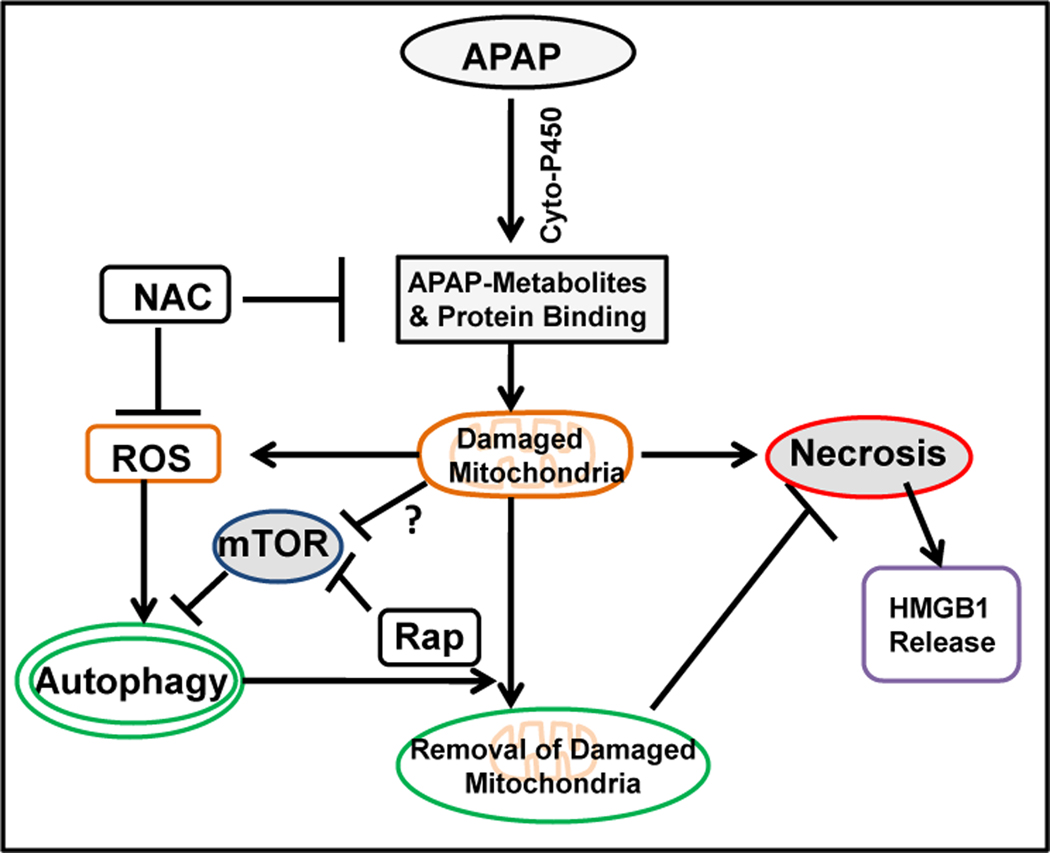
In conclusion, we have demonstrated that APAP overdose induces autophagy in both primary cultured mouse hepatocytes and in the mouse liver. Autophagy protects against APAP-induced hepatotoxicity. This protection could be mediated via the removal of damaged mitochondria, and thus a reduction to a major intracellular source of ROS production. The cellular and molecular events regulating APAP-induced necrosis and autophagy are summarized in Figure 8. These findings imply that induction of autophagy could be a novel therapeutic approach to mitigate APAP-induced hepatotoxicity and liver injury.
Figure 8. A proposed model for APAP-induced autophagy and necrosis.
In APAP-treated hepatocytes, APAP is first metabolized through the cytochrome P450 enzymes and generates reactive metabolites which bind to cellular and mitochondrial proteins to initiate mitochondrial damage. Damaged mitochondria can lead to necrotic cell death with nuclear HMGB1 release. On the other hand, damaged mitochondria can also lead to ROS generation or mTOR suppression through unknown mechanisms to trigger autophagy induction. Autophagy removes APAP-induced damaged mitochondria and in turn protects against APAP-induced necrosis.
Supplementary Material
Acknowledgments
Financial Support
This study was supported in part by the NIH funds R01 AA020518-01, R21 AA017421, P20 RR021940, and P20 RR016475 from the INBRE program of the National Center for Research Resources (W.X.D). H.J. was supported by the NIH funds R01 DK070195.
List of Abbreviations
- APAP
Acetaminophen
- ALT
Alanine aminotransferase
- CYP2E1
Cytochrome P450 2E1
- CQ
Chloroquine
- 3-MA
3-methyladenine
- mTOR
Mammalian target of rapamycin
- NAC
N-acetylcysteine
- PFA
Paraformaldehyde
- PE
Phosphatidylethanolamine
- ROS
Reactive oxygen species
- DMSO
Dimethylsulfoxide
- 4-EBP1
Translational initiation factor 4E binding protein-1
- EM
Electron microscopy
- HMGB1
High-mobility group box 1 protein
- p70S6K
70-kDa ribosomal protein S6 kinase-1
REFERENCES
- 1.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 2.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 3.Corcoran GB, Racz WJ, Smith CV, Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther. 1985;232:864–872. [PubMed] [Google Scholar]
- 4.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 6.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- 7.Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- 9.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 10.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding WX, Manley S, Ni HM. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med (Maywood) 2011;236:546–556. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy. 2011;7:188–204. doi: 10.4161/auto.7.2.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding WX, Ni HM, Gao W, Chen X, Kang JH, Stolz DB, Liu J, Yin XM. Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy. Mol Cancer Ther. 2009;8:2036–2045. doi: 10.1158/1535-7163.MCT-08-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell. 2003;4:422–424. doi: 10.1016/s1535-6108(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding WX, Ni HM, DiFrancesca D, Stolz DB, Yin XM. Bid-dependent generation of oxygen radicals promotes death receptor activation-induced apoptosis in murine hepatocytes. Hepatology. 2004;40:403–413. doi: 10.1002/hep.20310. [DOI] [PubMed] [Google Scholar]
- 23.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 24.Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 25.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Gibson SB. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008;4:246–248. doi: 10.4161/auto.5432. [DOI] [PubMed] [Google Scholar]
- 29.Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, 2nd, Yin XM. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem. 2010;285:27879–27890. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.