Abstract
The cross talk of CD40/CD40 ligand (CD40L) plays a key role in CD4+ T cell priming, B-cell terminal maturation, and immunoglobulin (Ig) class-switch recombination. Genetic defects in the CD40L lead to a disorder characterized by elevated concentrations of serum IgM and immunodeficiency. Patients with Primary Biliary Cirrhosis (PBC) characteristically show circulating anti-mitochondrial antibodies (AMAs), liver infiltrating autoreactive T lymphocytes against mitochondrial antigens, and high levels of IgM. We hypothesized that CD40L may play a key role in the pathogenesis of the elevated serum IgM and analyzed genetic and epigenetic modifications of the gene coding for CD40L in CD4+ and CD8+ T cells isolated from circulating mononuclear cells from PBC patients and healthy controls. We herein demonstrate significantly lower levels of DNA methylation of the CD40L promoter in CD4+ T cells from PBC patients as compared with controls, and this decreased methylation was inversely correlated with levels of serum IgM in PBC patients. In conclusion, the findings of an absence of genetic modifications of the CD40L gene in concert with decreased DNA methylation of the CD40L promoter in PBC patients suggests that environmental factors rather than genetics must play a major role in the pathogenesis of elevated serum IgM in PBC.
Keywords: Epigenetics, autoimmunity, methylation, CD40-CD40L
Although mechanisms underlying the loss of self-tolerance in autoimmunity remain largely unknown, recent data have shown that the CD40 ligand (CD40L) plays an important role in the pathogenesis of a number of autoimmune diseases (1-2). Naive T cells require contact with appropriately activated antigen presenting cells (APC) in order to be primed, and the CD40-CD40L system constitutes one of the fundamental accessory systems in T cell priming (3). CD40 is expressed on all APCs and is up-regulated upon cell activation secondary to infection or inflammation (4). CD40 binds to its natural ligand CD40L, which is expressed primarily on activated CD4+ T cells. Moreover, CD40 is constitutively expressed by B cells and its interaction with CD40L is critical for immunoglobulin (Ig) class-switch recombination (5); mutations of the X linked CD40L gene leads to a disorder characterized by elevated levels of IgM in the blood, immunodeficiency, and a high incidence of opportunistic infections (6). Finally, CD40-CD40L interactions have also been shown to be essential for peripheral B cell tolerance (7).
Primary biliary cirrhosis (PBC) is an autoimmune disease of the liver, characterized by the presence of high titers of circulating anti-mitochondrial antibodies (AMAs) and liver infiltrating autoreactive T lymphocytes, leading to the progressive destruction of small intrahepatic bile ducts (8). Other characteristics of PBC include high levels of serum IgM and a strong gender bias with a female:male ratio of 9:1 (8). Similarly to most autoimmune diseases, PBC is reasoned to result from the combined effects of genetics and the environment (9-10). Epigenetic modifications, particularly DNA methylation, are known regulatory mechanism of gene expression and appear as ideal candidates to explain the environmental influence on individual susceptibility to complex diseases such as PBC (11). However, although abnormal DNA demethylation has been shown in CD4+ T cells in women with lupus (12), the actual involvement of epigenetic mechanisms exemplified by abnormal DNA methylation in PBC has not been studied (13). It is reasoned that genetic and epigenetic aberrancies of the X chromosome would be most likely involved in female predominant diseases, such as PBC (14).
We thus hypothesized that abnormal DNA methylation modifications of the X chromosome genes, such as CD40L may be involved in the pathogenesis of PBC because of the definite role of activated T cells in disease initiation and progression. A specific role of the CD40L gene is suggested by the high IgM titers commonly found in the sera from patients with PBC. We herein demonstrate, that PBC is associated with significantly lower levels of DNA methylation of the CD40L promoter in CD4+ T cells and that these lower levels of DNA methylation inversely correlate with serum IgM levels in PBC patients. These results identify a major role for CD40L in the pathogenesis of PBC and potentially in the induction of abnormal humoral immune responses contributing to the disease process.
MATERIALS AND METHODS
Subjects
Fresh heparinized peripheral blood samples were obtained from Italian female patients diagnosed with PBC (n=20), and unaffected controls (n=20) (8). In addition, female patients with psoriasis vulgaris (n=9) and type 1 diabetes (n=9) were recruited as disease controls from the outpatient clinics in the Second Xiangya Hospital, Central South University.
All patients with PBC (Table 1) were women and had readily detectable AMA; the diagnosis was made based on internationally accepted criteria (8). The mean age was 64 years old (range 44-87 years) and 70% of them were taking ursodiol. The PBC patients included in this study were histologically characterized as belonging to stage I (n=7), stage II (n=10) or stage III (n=3). Serum liver function and levels of immunoglobulins were assessed utilizing routine laboratory methods. The diagnosis of psoriasis was based on characteristic clinical features and histological confirmation (15); type 1 diabetes was diagnosed based on the ADA diagnostic criteria (16). Subjects were excluded from the study if they had malignancies or were using immunosuppressive drugs. Patients and controls were matched for sex (all female subjects). After approval from appropriate institutional review boards in Italy, China and USA, all subjects provided written informed consent prior to enrollment in the study.
Table 1.
Clinical, biochemical and serological characteristics of PBC patients.
|
PBC patients (n=20) |
Healthy controls (n=20) |
|
|---|---|---|
| Mean age (years) (range) | 64 (44-87) | 60 (42-79) |
| Females (n, %) | 20 (100%) | 20 (100%) |
| Duration of disease (years) (range) |
13 (5-24) | n.a. |
| AMA positivity (n, %) | 20 (100%) | n.a. |
| Liver cirrhosis (n, %) | 3 (15%) | n.a. |
| Total bilirubin (mg/dl) (n.v. < 1.0) |
0.76 ± 0.21 | 0.23 ± 0.05 |
| Alkaline phosphatase (IU/l) (n.v. < 279) |
415 ± 291 | 173 ± 27 |
| Alanine aminotransferase (U/l) (n.v. < 50) |
34 ± 21 | 34 ± 9 |
| Albumin (g/dl) (n.v. > 3,5) | 4.29 ± 0.40 | 4.70 ± 0.03 |
| IgG (mg/dl) (n.v. < 1700) | 1410 ± 377 | - |
| IgM (mg/dl) (n.v. < 280) | 403 ± 192 | - |
| IgA (mg/dl) (n.v. <400) | 325 ±139 | - |
Mean values ± standard deviation unless otherwise stated.
Abbreviation: n.a. not applicable
CD4+ and CD8+ T cell purification
Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation on a Ficoll-Hypaque gradient for 30 min at 500 g. First, CD8+ cells were isolated from PBMCs by positive selection under endotoxin-free conditions using anti-CD8 conjugated microbeads (Miltenyi Biotec). The CD4+ T cells utilized in the studies were isolated by negative selection using a cocktail of antibodies against CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCR γ/δ, and CD235a (Miltenyi Biotec). Aliquots of the CD8 + and CD4+ T cells were subjected to viability assays and flow cytometric analysis. The purity of these lymphocytic populations was >95% and the viability of cells was always >95%.
RNA isolation and CD40L messenger RNA quantification
cDNA was synthesized from CD4+ T cells using the SuperScript III CellsDirect cDNA Synthesis System (Invitrogen). 100 ng of cDNA in a total volume of 20 μL were amplified for 40 cycles on an Applied Biosystems 7900 HT Sequence Detection System, using TaqMan Gene Expression Assay specific for CD40L (Applied Biosystems); products were detected with FAM. All reactions were run in duplicate. The relative mRNA expression level of CD40L was quantitated using mRNA level of the internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and compared to a calibrator (2−(ΔΔCt)).
Genomic DNA extraction and bisulfite sequencing
Genomic DNA from CD4+ and CD8+ T cell populations was isolated using Qiagen Blood Mini kits (Qiagen). Bisulfite conversion of genomic DNA was performed using the EpiTect Bisulfite Kit (Qiagen). The CD40L promoter fragment was amplified by nested PCR and cloned into the pGEM-T easy vector (Promega). Seven independent clones from each subject were sequenced for each of the amplified fragments. Primers are described as follows: Round I, forward: (−448 to −407) GAAGAATTCAGTTGATGGGATATTAGTTATAAAATTAATTT, reverse: (−194 to −153) AAATCTAGACCCAATCATCTAAATAATAAAAAAAACAA. Round II: forward: (−402 to −366) TTTGAATTCATGTGTTTTTTTTTTTATATATTAGGTTTT, reverse: (+150 to +116) AATTCTAGAAAATTTTCATACTAATAAACTATCCAATAA.
CD40L sequencing
All 5 exons of CD40L (accession number NG_007280) were amplified by PCR from genomic DNA isolated from CD4+ T cells from PBC patients using primers spanning the intron/exon boundary.
Amplification of CD40L promoter was performed using primers described previously (17). PCR products were purified by Centrifugal Filter Units (Millipore) and sequencing was performed using BigDye Terminator cycle sequencing kit (Applied Biosystems) and analyzed with the Applied Biosystems Prism 3130 genetic Analyzer.
Statistical analysis
Statistical differences between groups were determined using a two tailed Mann-Whitney non-parametric test with 95% confidence interval (CI). All results were expressed as mean ± standard error (SEM). Statistical comparisons were made using GraphPad Prism 5.0 Software (GraphPad Software, Inc, La Jolla, CA, USA).
RESULTS
CD40L promoter is down-methylated in CD4+ T cells from PBC patients
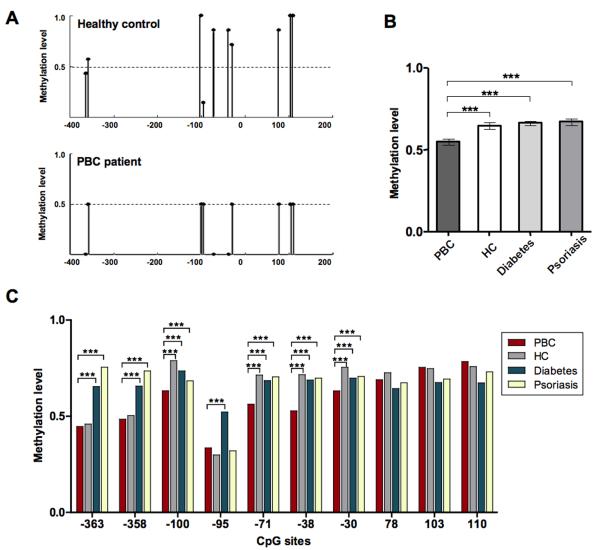
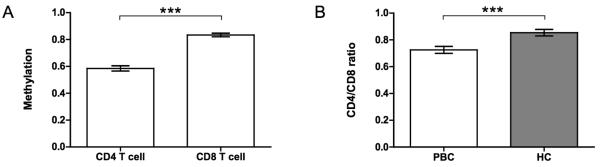
DNA methylation was examined for the CD40L promoter that lacks a CpG island (http://genome.ucsc.edu, CpG island track). Bisulfite sequencing primers were designed to amplify a 473 bp region 5′ of the transcription start site containing ten CpG sites (Figure 1). CD40L bisulfite sequencing data were obtained on a minimum of 7 clones prepared from each of both CD4+ and CD8+ T cells isolated from the PBMC of 20 PBC patients and 20 unrelated controls. The CD40L promoter sequences amplified from CD4+ T cells of PBC patients showed significantly lower methylation as compared to healthy controls. The methylation patterns of individually sequenced clones are shown for two representative subjects in Figure 2A. Overall promoter methylation was determined as the percentage of methylated CpG sites out of all possible CpG sites which indicated a significant reduction in CD4+ T cells from patients compared to healthy controls (0.54 versus 0.64, p< 0.001), to subjects with type I diabetes (0.54 in PBC versus 0.66, p< 0.001), and to psoriasis patients (0.54 in PBC versus 0.67, p< 0.001) (Figure 2B). Similarly, site-specific methylation was calculated for each of the ten CpG sites in the CD40L promoter region (Figure 2C), and ranged from 0.33 to 0.61 in PBC patients versus 0.29 to 0.78 in healthy subjects, 0.29 to 0.78 in type I diabetes, and 0.32 to 0.75 in psoriasis patients (Figure 2C). No detectable difference in the level of CD40L promoter methylation in isolated CD8+ T cells was observed between PBC patients and controls (data not shown). However, in general, the levels of CD40L promoter methylation was significantly downmethylated in CD4+ T cells compared to CD8+ T cells from both PBC patients (Figure 3A) and controls (data not shown), as confirmed by a significantly lower CD4+/CD8+ methylation ratio in PBC patients (0.72 versus 0.85, p=0.0002) (Figure 3B).
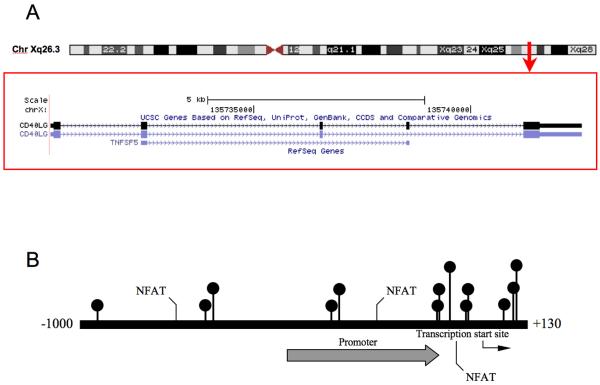
Figure 1. CD40L promoter.
A. Chromosomal sequence location of the region of the CD40L promoter analyzed by bisulfite sequencing. B. CD40L promoter numbered relative to the transcription start site (bent arrow), is shown. The promoter is identified by the broad arrow, three Nuclear Factor of Activated T-cells (NFAT) sites and all CG pairs are also included. This region contains 10 CpG sites (circles) within a 473 bp region overlapping the transcription start site as shown (22).
Figure 2.
CD4+ T cell CD40L promoter methylation patterns in PBC patients and controls. A. Representative bisulfite sequencing data from peripheral CD4+ T cells from a healthy donor and a PBC patient; the region indicated on the x-axis was amplified, cloned, and 7 clones per donor were sequenced. The average fraction methylated of each CG pair is shown on the y-axis. B. Overall methylation was determined by the total number methylated sites out of the total CpG sites in all clones and graphed as mean ± SEM for PBC (n=20) versus healthy subjects (n=20), type I diabetes (n=9), and psoriasis (n=9). C. Average methylation of 10 CG sites of the CD40L promoter in CD4+ T cells from PBC patients (n=20), healthy controls (n=20), subjects affected from type I diabetes (n=9), and psoriasis (n=9). Statistical difference was analyzed by a two tailed Mann-Whitney test (95% CI), *** p< 0.001.
Figure 3.
Comparison of CD40L promoter methylation between CD4+ T and CD8+ T cells. A. Overall methylation from both CD4+ T and CD8+ T cells from PBC patients was determined by the total number of methylated sites out of the total CpG sites in all clones and graphed as mean ± SEM. CD4+ T cells were shown to be down methylated compared to CD8+ T cells. B. CD4+/CD8+ methylation ratio was significantly lower in PBC patients compared to controls. Statistical difference was analyzed by a two tailed Mann-Whitney test (95% CI). *** p< 0.001.
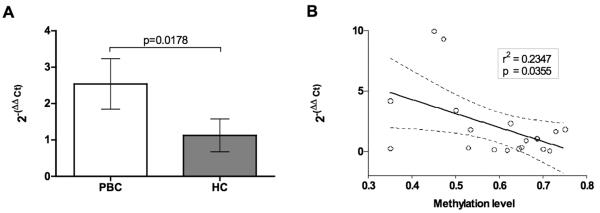
The levels of CD40L mRNA were also evaluated in CD4+ T cells from both patients and healthy controls by RT-PCR. As seen in Fig. 4, the levels of CD40L mRNA expression was increased in CD4+T cells from PBC patients compared to controls (2−(ΔΔCt)=2.53 versus 1.12, p=0.0178) and inversely correlated with levels of CD40L promoter methylation (r2=0.2347, p=0.0355) (Figure 4).
Figure 4.
CD40L expression in CD4+ T cells is higher in PBC patients (A) and inversely correlate with methylation levels (B). Real-time threshold cycle values for CD40L cDNA were normalized with GADPH and compared to a calibrator. Calibrated CD40L expression levels were correlated to levels of CD40L promoter overall methylation. 95% confidence limits of the best-fit line are shown.
PBC patients with high IgM levels show lower levels of CD40L promoter methylation
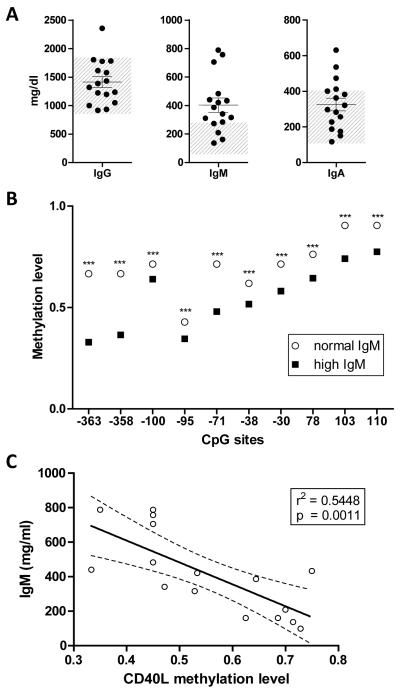
Based on the fact that patients with mutations of the X linked CD40L gene exhibit high titers of serum IgM (18), we evaluated the potential correlation between levels of CD40L methylation and serum IgM levels. The sera from sixteen of the twenty PBC patients (80%) included in the study had high relative levels of IgM (Figure 5A) and showed significantly lower levels of CD40L promoter methylation within CD4+ T cells compared to their normal IgM counterparts (Figure 5B). Interestingly, IgM levels inversely correlated with levels of CD40L promoter methylation (r2=0.5448, p=0.0011) (Figure 5C).
Figure 5. High IgM levels in PBC patients inversely correlate with CD40L promoter methylation.
A. Serum IgG, IgM and IgA levels (mg/dl) of PBC patients included in the study, the grey area shows normal Ig range. B. Single CpG site methylation levels are significantly lower in PBC patients with high levels of IgM (> 280 mg/dl) compared to those with normal values. *** p< 0.001. C. CD40L promoter overall methylation inversely correlates with IgM levels. 95% confidence limits of the best-fit line are shown.
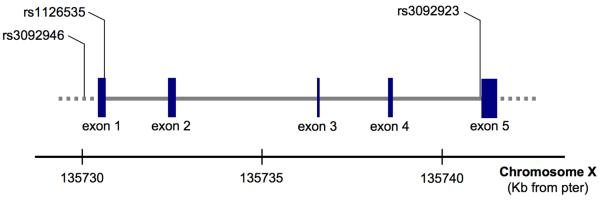
In order to determine the potential contribution of the presence of mutations of the CD40L gene that could influence IgM levels (17-18), we sequenced CD40L in genomic DNA samples isolated from each of the PBC patients and analyzed them for the presence of mutations previously documented for the CD4L gene (Figure 6). Results from such analysis failed to identify the presence of genetic variants for the CD40L in CD4+ T cells from PBC patients. While three SNPs were found within the sequences for the CD40L gene among the PBC patients, the frequency of the genotypes was not different from the general Caucasian population (Table 2).
Figure 6. Genomic organization of CD40L.
distribution of exons and location of the SNPs that were found to demonstrate some variability in PBC patients.
Table 2.
CD40L sequencing in PBC patients compared to general Caucasian population, as obtained from HapMap-CEU (http://hapmap.ncbi.nlm.nih.gov/index.html.en)
| Genotype frequency | PBC patients | General population |
p | ||
|---|---|---|---|---|---|
| rs3092946 | T/T | 0.8 | T/T | 0.78 | n.s. |
| A/T | 0.2 | A/T | 0.13 | n.s. | |
| A/A | 0.0 | A/A | 0.08 | n.s. | |
|
| |||||
| rs1126535 | T/T | 0.8 | T/T | 0.76 | n.s. |
| C/C | 0.0 | C/C | 0.00 | n.s. | |
| C/T | 0.2 | C/T | 0.24 | n.s. | |
|
| |||||
| rs3092923 | T/T | 0.6 | T/T | 0.85 | n.s. |
| C/T | 0.2 | C/T | 0.08 | n.s. | |
| C/C | 0.2 | C/C | 0.06 | n.s. | |
DISCUSSION
The present study demonstrates for the first time that patients with PBC demonstrate relatively lower levels of DNA methylation of the CD40L promoter in CD4+ T cells which accordingly results in higher levels of CD40L expression in CD4+ T cells. These findings provide a reasonable explanation for the elevated levels of serum IgM that are characteristic of PBC patients. CD40 has a key role in generating effective immune responses, and consequently abnormalities associated with its expression or function also play an important role in the pathogenesis of autoimmune diseases (1, 19).
The importance of CD40-CD40L interactions is highlighted by the phenotype of transgenic mice over-expressing CD40L that demonstrate systemic autoimmunity including dermatitis, nephritis, the presence of auto-antibodies in the serum and polyclonal autoreactive T cells (1, 20). CD40 potentially contributes to T-cell dependent autoimmune diseases in several ways. Thus, abnormal expression in thymus promotes autoreactive T cell clones to escape deletion (21). Abnormal expression in secondary lymphoid organs mediates enhanced T cell priming by B cells or other APC. Finally within the target tissue, enhanced CD40 signaling leads to the production of high levels of pro-inflammatory cytokines and chemokines which contribute to tissue destruction and inflammatory cell influx (3). CD40L has been reported to be overexpressed on lupus T cells, contributing to overproduction of pathogenic autoantibodies. The CD40L regulatory sequences demethylate in CD4+ T cells from women with lupus; lupus CD4+ T cells and demethylated CD4+ T cells express high level of CD40L and overstimulate B cells to produce IgG (22-23). Interestingly, T cell activation has been excluded as a mechanism for overexpression (24). Herein we also detected the levels of DNA methylation of CD40L promotor in CD4+ T cells from psoriasis vulgaris patients and type 1 diabetes patients respectively as disease controls. We chose these diseases as both have chronic CD4+ T cell activation. Importantly, our data confirm that both disease controls are comparable to healthy controls.
Our data highlight an important role of the CD40-CD40L axis in PBC. Previous work has noted that the hilar lymph nodes and the liver of PBC patients compared with matching PBMC samples have a 100- to 150-fold increase in the number of HLA DRB4*0101 restricted PDC-E2163-176 lipoyl domain peptide specific auto reactive CD4+ T cells (25). These findings, associated with a relatively higher prevalence of X chromosome monosomy in PBC patients (26), could be the basis for selective dysregulation of CD40–CD40L interaction mediated by abnormal levels of DNA methylation.
A major unanswered question in PBC is that although all nucleated cells have mitochondria, the damage is limited to small biliary epithelial cells (BEC) (27-28). In this regard, there have been a number of studies that have focused on identifying the unique properties of BECs as compared wih epithelial cells from other tissues. One such finding has been the unique process of apoptosis in BEC’s following exposure of PDC-E2 to the effector processes of the immune system. The data presented herein adds significance to the concept of a role for unique pathways involved in the apoptosis of BEC’s in PBC. Thus, BECs express CD40 and are exquisitely sensitive to CD40Lmediated apoptosis (29); indeed, after stimulation with CD40L there is a sustained up-regulation of FasL and induction of apoptosis is accompanied by activation of the AP-1 (c-Fos/c-Jun) and pSTAT-3 signaling pathways (30-31). It is important to note that inadequate glutathiolation has been reasoned to lead to exposure of PDC-E2 by biliary cells making the BEC’s a potential source of neoantigens responsible for the activation of autoreactive T lymphocytes (32-33). We extended this work and demonstrated that in contrast to other epithelial cells, PDC-E2 remains immunologically intact within the apoptotic bleb when BECs undergo apoptosis (34). We also demonstrated that there was a marked increase in inflammatory cytokine production in the presence of the unique triad of normal BEC blebs, PBC monocytes derived macrophages and AMA (35). We interpret these data to suggest that the presence of intact immunologically active PDC-E2 within the blebs of BECs gives rise to a local proinflammatory milieu. Importantly, it has also been suggested that macrophages can directly kill BECs via CD40-CD40L interaction (36). This insight into innate immunity provides one explanation for our understanding of biliary epithelial cell destruction and the key role of CD40-CD40L axis in this process. In a larger context, it has implication in our understanding of the tissue specificity of many autoimmune diseases.
Finally, high levels of CD40L expression in PBC patients appear to be related to elevated levels of serum IgM, a common and distinct feature of PBC. Little is known about the mechanism of hyper-IgM in PBC. CD40L has a crucial role in immunoglobulin class switching in B cells and mutations in the gene encoding CD40L are known to induce X-linked hyper-IgM syndrome (5). An early study by Higuchi and colleagues investigated the presence of mutations in the CD40L gene in PBC patients by single-strand conformational polymorphism (SSCP). However, the results of these studies led to a failure to identify any differences between patients and controls (37). Studies reported herein show that the levels of IgM levels are inversely co-related with the levels of CD40L methylation, suggesting that the CD40-CD40L interaction is directly involved in the production of high amounts of IgM. Mutations in the CD40 gene have also been reported in select patients with hyper-IgM syndrome. However, there is no defect in the CD40 gene suggesting that the hyper-IgM observed in PBC has a different origin. In conclusion, these findings suggest an important role of CD40L modulation in PBC and emphasize the importance of mechanisms that disrupt epigenetic regulation of CD40L.
Acknowledgments
FINANCIAL SUPPORT: This work was supported by National Institutes of Health grant DK39588, the National Natural Science Foundation of China (No 30730083), the National Basic Research Program of China (973 Plan) (2009CB825605) and the Aid program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province [2008] 244. Dr Bernuzzi was in part supported by Clonit srl, Milan, Italy.
ABBREVIATIONS
- PBC
primary biliary cirrhosis
- AMA
antimitochondrial antibodies
- APC
antigen presenting cells
- IgM
immunoglobulin M
- BEC
biliary epithelial cells
REFERENCES
- 1.Mehling A, Loser K, Varga G, Metze D, Luger TA, Schwarz T, Grabbe S, et al. Overexpression of CD40 ligand in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J Exp Med. 2001;194:615–628. doi: 10.1084/jem.194.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98:826–837. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 5.Pessach IM, Notarangelo LD. X-linked primary immunodeficiencies as a bridge to better understanding X-chromosome related autoimmunity. J Autoimmun. 2009;33:17–24. doi: 10.1016/j.jaut.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Lanzi G, Ferrari S, Vihinen M, Caraffi S, Kutukculer N, Schiaffonati L, Plebani A, et al. Different molecular behavior of CD40 mutants causing hyper-IgM syndrome. Blood. 2010 doi: 10.1182/blood-2010-03-274241. [DOI] [PubMed] [Google Scholar]
- 7.Herve M, Isnardi I, Ng YS, Bussel JB, Ochs HD, Cunningham-Rundles C, Meffre E. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Invernizzi P, Lu Y, Kosoy R, Bianchi I, Podda M, Xu C, et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42:658–660. doi: 10.1038/ng.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewagama A, Richardson B. The genetics and epigenetics of autoimmune diseases. J Autoimmun. 2009;33:3–11. doi: 10.1016/j.jaut.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao M, Sun Y, Gao F, Wu X, Tang J, Yin H, Luo Y, et al. Epigenetics and SLE: RFX1 downregulation causes CD11a and CD70 overexpression by altering epigenetic modifications in lupus CD4+ T cells. J Autoimmun. 2010;35:58–69. doi: 10.1016/j.jaut.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell MM, Lleo A, Zammataro L, Mayo MJ, Invernizzi P, Bach N, Shimoda S, et al. Epigenetic investigation of variably X chromosome inactivated genes in monozygotic female twins discordant for primary biliary cirrhosis. Epigenetics. 2011;6 doi: 10.4161/epi.6.1.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M. Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun. 2009;33:12–16. doi: 10.1016/j.jaut.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 16.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 17.Van Hoeyveld E, Zhang PX, De Boeck K, Fuleihan R, Bossuyt X. Hyper-immunoglobulin M syndrome caused by a mutation in the promotor for CD40L. Immunology. 2007;120:497–501. doi: 10.1111/j.1365-2567.2006.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notarangelo LD, Hayward AR. X-linked immunodeficiency with hyper-IgM (XHIM) Clin Exp Immunol. 2000;120:399–405. doi: 10.1046/j.1365-2249.2000.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato K, Santana-Sahagun E, Rassenti LZ, Weisman MH, Tamura N, Kobayashi S, Hashimoto H, et al. The soluble CD40 ligand sCD154 in systemic lupus erythematosus. J Clin Invest. 1999;104:947–955. doi: 10.1172/JCI7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 21.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 22.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol. 2007;179:6352–6358. doi: 10.4049/jimmunol.179.9.6352. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Yuan J, Pan Y, Fei Y, Qiu X, Hu N, Luo Y, et al. T cell CD40LG gene expression and the production of IgG by autologous B cells in systemic lupus erythematosus. Clin Immunol. 2009;132:362–370. doi: 10.1016/j.clim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cron RQ. CD154 transcriptional regulation in primary human CD4 T cells. Immunol Res. 2003;27:185–202. doi: 10.1385/IR:27:2-3:185. [DOI] [PubMed] [Google Scholar]
- 25.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, et al. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–535. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 27.Watt FE, James OF, Jones DE. Patterns of autoimmunity in primary biliary cirrhosis patients and their families: a population-based cohort study. QJM. 2004;97:397–406. doi: 10.1093/qjmed/hch078. [DOI] [PubMed] [Google Scholar]
- 28.Gershwin ME, Van de Water J. Cholangiocytes and primary biliary cirrhosis: prediction and predication. J Clin Invest. 2001;108:187–188. doi: 10.1172/JCI13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afford SC, Ahmed-Choudhury J, Randhawa S, Russell C, Youster J, Crosby HA, Eliopoulos A, et al. CD40 activation-induced, Fas-dependent apoptosis and NF-kappaB/AP-1 signaling in human intrahepatic biliary epithelial cells. FASEB J. 2001;15:2345–2354. doi: 10.1096/fj.01-0088com. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed-Choudhury J, Williams KT, Young LS, Adams DH, Afford SC. CD40 mediated human cholangiocyte apoptosis requires JAK2 dependent activation of STAT3 in addition to activation of JNK1/2 and ERK1/2. Cell Signal. 2006;18:456–468. doi: 10.1016/j.cellsig.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Humphreys EH, Williams KT, Adams DH, Afford SC. Primary and malignant cholangiocytes undergo CD40 mediated Fas dependent apoptosis, but are insensitive to direct activation with exogenous Fas ligand. PLoS One. 2010;5:e14037. doi: 10.1371/journal.pone.0014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, Huebert RC, et al. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27:232–241. doi: 10.1016/j.jaut.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alabraba EB, Lai V, Boon L, Wigmore SJ, Adams DH, Afford SC. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2008;47:552–562. doi: 10.1002/hep.22011. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi M, Horiuchi T, Kojima T, Nishizaka H, Ishibashi H, Hayashi K, Niho Y, et al. Analysis of CD40 ligand gene mutations in patients with primary biliary cirrhosis. Scand J Clin Lab Invest. 1998;58:429–432. doi: 10.1080/00365519850186418. [DOI] [PubMed] [Google Scholar]