Abstract
Swarming motility is the movement of bacteria over a solid surface powered by rotating flagella. The expression of flagellar biosynthesis genes is governed by species-specific master regulator transcription factors. Mutations that reduce or enhance master regulator activity have a commensurate effect on swarming motility. Here we review what is known about the proteins that modulate swarming motility and appear to act upstream of the master flagellar regulators in diverse swarming bacteria. We hypothesize that environmental control of the master regulators is important to the swarming phenotype perhaps at the level of controlling flagellar number.
Introduction
Many bacterial species move by rotating flagella. Flagella are complex, membrane-anchored, proteinaceous structures, in which one or more helical filaments are rotated to generate force like a propeller. Flagella allow bacteria to swim as individuals in three-dimensions through an aqueous environment. Additionally, flagella in a subset of bacteria coordinate movement of groups of cells across the surface of solid media in a process called swarming (Henrichsen, 1972). The factors that allow some flagella to power swimming motility and others to power swarming motility are poorly understood.
Swarming has a number of characteristics that distinguish it from swimming (Harshey, R.M., 1994; Copeland and Weibel, 2009; Kearns, 2010). First, swarming requires either the secretion of wetting agents by the bacterium or the use of solid surfaces with inherently reduced surface tension (Neu, 1996; Gygi et al., 1995; Chen et al., 2007, Copeland and Weibel, 2009). Second, isolated cells on surfaces are largely non-motile and rapid swarming is facilitated by the collective movement of dynamic cell assemblages (Morrison and Scott, 1966; Henrichsen, 1972). Finally, liquid grown cells remain immobile at the point of surface inoculation for an experimentally reproducible period of time, called the “swarm lag”, before swarm expansion begins (Hoeniger, 1964; Morrison and Scott, 1966; Belas et al., 1986; Harshey and Matsuyama, 1994; Rauprich et al., 1996; Eberl et al., 1999; Kearns and Losick, 2003; Wang et al., 2004). The swarm lag may represent a time for cells become physiologically proficient for swarming motility (Allison and Hughes, 1991; Eberl et al., 1999; McCarter, 1999). The precise nature and extent of the physiological differences during swarming are unknown but the change to the swarming proficient-state is thought to be induced environmental signals.
Swarming motility ultimately depends on flagella function. The expression of flagellar biosynthesis genes are organized in a hierarchy, the top of which is governed by a master regulatory transcription factor (“master regulator”). Master regulators serve as an integration point for environmental signaling, activate flagellar gene expression, and govern the production of flagellar basal bodies. Flagellar assembly is complex and there are species-specific mechanisms of transcriptional and post-transcriptional regulation. In this review we compare what is currently understood of the diverse regulatory mechanisms that control flagellar gene expression during swarming motility in various bacterial species.
Master regulators control flagellar gene expression
The flagellum is assembled from the interior to the exterior of the cell in a highly organized fashion. The basal body is the first structural intermediate built within the lipid bilayer. Decorating the basal body are the motor proteins—ion channels that harvest the energy for flagellar rotation. A rod passes through the basal body rings and transits the peptidoglycan (and the outer membrane in the case of Gram-negative bacteria) to the cell exterior. Attached to the rod is a flexible hook, which changes the angle of rotation and serves as the foundation for the filament. The filament is the last structure assembled and forms the semi-rigid helical propeller (Macnab, 2003; Chevance and Hughes, 2008). The genes that encode the flagellar components are expressed in roughly the same order in which the components are assembled, and master regulatory proteins sit atop the entire heirarchy to govern flagellar gene expression. Whereas flagellar biosynthesis genes are highly conserved between bacterial species, the master regulators and the regulatory mechanisms that control them are divergent.
Master regulators are typically transcriptional activators that bind to DNA upstream of promoters and recruit RNA polymerase holoenzyme to initiate transcription of basal body genes. Since each flagellum originates from and is anchored by a basal body, control of flagella number is intimately linked with basal body gene expression. Some swarming bacteria appear to increase the number of flagella per cell during the swarm lag (Figure 1) (Hoeniger, 1965; Belas and Colwell, 1982; Kearns and Losick, 2003). Artificial overexpression of the master regulator is sufficient to increase flagella number per cell and reduce the swarming lag period in multiple species (Kutsukake and Iino, 1994; Kearns and Losick, 2005, Aldridge et al., 2010; Erhardt et al., 2010). Likewise mutations in proteins that decrease master regulator activity reduce or abolish swarming motility. Here we focus on the control of master regulator proteins from three bacteria, P. mirabilis, B. subtilis, and V. parahaemolyticus in the context of swarming motility.
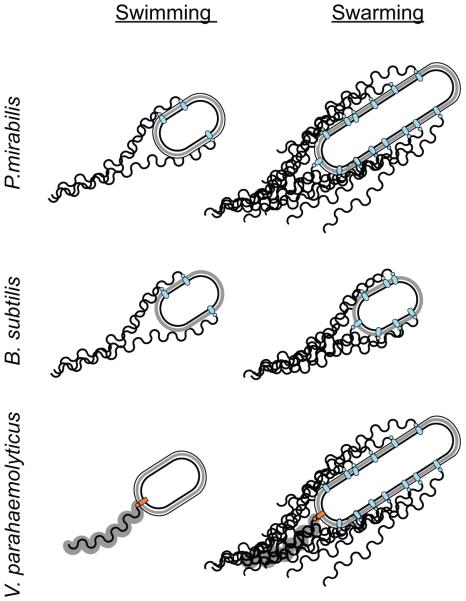
Figure 1. The flagellar number of Proteus mirabilis, Bacillus subtilis, and Vibrio parahaemolyticus appears to increase during swarming.
Both P. mirabilis and V. parahaemolyticus appear to increase cell length during swarming but B. subtilis does not. V. parahaemolyticus encodes two different flagella systems, a single polar sheathed flagellum (orange basal body) is synthesized in liquid cultures and multiple unsheathed lateral flagella (blue basal bodies) are induced by surface contact and swarming conditions.
FlhDC: Proteus mirabilis
The best-studied flagellar master regulator is FlhDC, of E. coli, S. enterica, P. mirabilis, and Serratia. FlhD and FlhC assemble into the heterohexamer FlhD4C2, and bind upstream of flagellar operons, interact with RNA polymerase holoenzyme, and activate transcription of basal body genes (Liu and Matsumura, 1994; Wang et al., 2006). FlhC dimerizes and binds to DNA independently of FlhD, but the addition of FlhD to the complex increases both the stability and the specificity of the FlhC-DNA interaction (Claret and Hughes, 2000a; Claret and Hughes, 2002). The FlhD4C2 complex recognizes large inverted repeats and causes reflex bending of the DNA (Liu and Matsumura, 1994; Claret and Hughes, 2002, Wang et al., 2006). Deletion and complementation experiments have shown that the flhDC operon is necessary for basal body gene expression, as cells lacking flhDC are aflagellate and nonmotile (Silverman and Simon, 1977; Kutsukake and Iino, 1994; Eberl et al., 1996; Liu et al., 2000). Artificial overexpression of flhDC increases flagellar number in a variety of bacteria (Furness et al., 1997; Eberl et al., 1996; Aldridge et al., 2010; Erhardt et al., 2010).
The genes encoding FlhD and FlhC, flhD-flhC, are transcribed as a dicistronic operon by RNA polymerase and the vegetative sigma factor σ70 (Furness et al., 1997). The flhDC promoter is extensively regulated and expression is activated by Catabolite Repressor Protein (CRP) and the nucleoprotein H-NS, and is further repressed by LrhA, HdfR and multiple two component systems (EnvZ/OmpR, RcsC/RcsB, QseB/QseC) (Shin and Park, 1995; Soutourina et al., 1999; Ko and Park, 2000; Lehnen et al., 2002; Sperandio et al., 2002; Pearson et al., 2010; Girgis et al., 2007). In wild type P. mirabilis cells, flhDC transcription is upregulated at the transition from swimming to swarming, consistent with an observed increase in flagella number (Furness et al., 1997; Pearson et al., 2010). Furthermore, overexpression of flhDC decreased the swarming lag period in P. mirabilis suggesting that flagellar induction is an important event that takes place prior to swarming initiation (Furness et al., 1997). Finally, mutations that effect the regulation of flhDC and swarming have been best studied in P. mirabilis and we will focus on these regulators below (Figure 2A).
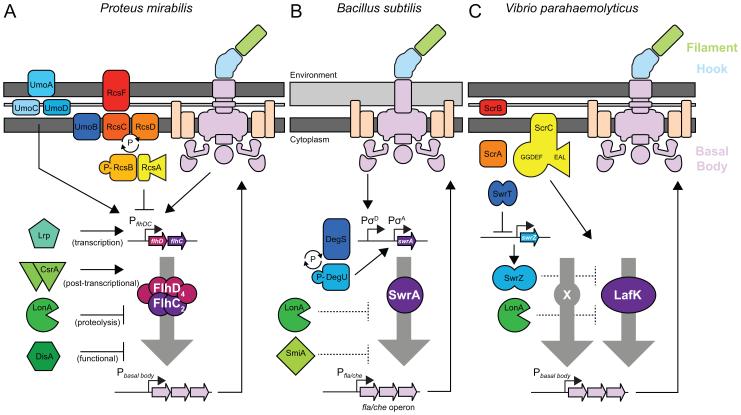
Figure 2. Regulation of master flagellar regulators during swarming motility.
Each panel indicates what is known about the master regulators (A) FlhDC in P. mirabilis, (B) SwrA in B. subtilis, and (C) LafK in V. parahaemolyticus. In all cartoons, block arrows represent genes and thin bent arrows that precede genes represent promoters. All other arrows indicate activation and T-bars indicate inhibition. Colored shapes represent the various proteins mentioned in the text. Dark grey bars represent membranes and light gray bars represent peptidoglycan. For each model organism, a flagellum is included where the basal body is colored purple, the hook is colored blue, the filament is colored green and the motor proteins are colored orange. Note that the basal body operons and their respective promoters are generalized and abbreviated. The one operon that encodes basal body proteins in B. subtilis is referred to as the fla/che operon.
In P. mirabilis, a transposon mutant in an open reading frame with homology to the E. coli leucine-responsive regulatory protein Lrp was isolated that reduced swimming and abolished swarming. Lrp is a highly conserved global regulator that controls the transcription of multiple operons, including those for amino acid synthesis and degradation, nutrient transport, and pili production (Calvo and Matthews 1994). Expression of Lrp was increased in differentiating swarm cells and mutation of the P. mirabilis gene encoding Lrp resulted in a decrease in flhDC mRNA. Furthermore, swarming could be restored to lrp mutants by artificially overexpressing the flhDC operon from an inducible promoter indicating that Lrp acted upstream of flhDC expression (Hay et al., 1997). Swarming motility requires a high energy medium and Lrp may be involved in directly or indirectly integrating nutritional signals into the flagella cascade.
Flagella are complex structures and mechanisms of regulatory feedback couple assembly intermediates to the expression of flagellar genes (Chevance and Hughes, 2008). In P. mirabilis, flhDC expression is dependent on the flagellar secretion apparatus component FlhA, which participates in the secretion of the hook and filament proteins (Bange et al., 2010). Cells mutated for flhA show a severe defect in flhDC mRNA transcript accumulation when incubated on a solid surface. Further, the flhA gene is upregulated during swarmer cell differentiation and thus may create a positive feedback loop on flhDC expression (Gygi et al., 1995a; Furness et al., 1997). Similarly, mutation of flgN, encoding FlgN the secretion chaperone for hook-associated proteins (Fraser et al., 1999; Gygi, et al., 1997), reduces flhDC expression and the number of flagellar filaments such that swimming is permitted but swarming is abolished (Gygi et al., 1997). That mutations in flhA and flgN have an effect of flhDC gene expression suggests that there is positive feedback from flagellar completion to the assembly of new basal bodies. Controlled positive feedback between flagellar assembly and gene expression could increasing flagella number and perhaps promote swarming motility.
To further understand the regulation of flhDC in P. mirabilis, a suppressor analysis was conducted to discover genes, which when overexpressed on multicopy plasmids, restored swarming to a flgN mutant and four previously uncharacterized proteins, UmoA, UmoB, UmoC, and UmoD were identified (Dufour et al., 1998). Each of the Umo proteins (upregulator of the master operon) activated flhDC expression and were themselves upregulated during swarming (Dufour et al., 1998; Pearson et al., 2010). Loss of umoA, umoC, or umoD reduced flhDC mRNA by increasing transcript turnover, whereas loss of umoB completely abolished flhDC transcript accumulation (Dufour et al., 1998). All four of the Umo proteins appear to be associated with the cell envelope: UmoA appears to be an outer membrane protein, UmoC and UmoD are periplasmic proteins, and UmoB is found in the cytoplasmic membrane (Dufour et al., 1998). The umoB homologue, yrfF (mucN), is related to the Rcs signal transduction pathway of S. enterica serovar Typhimurium and S. marcescens (Costa et al., 2003; Castelli and García Véscovi, 2011).
Like the Umo proteins, the Rcs pathway has also been shown to be an important regulator of flhDC. RcsB is a response regulator that, when phosphorylated and in a complex with RcsA, controls gene transcription by binding to DNA. RcsB is phosphorylated by phosphotransfer from the relay protein RcsD which is phosphorylated by the sensor kinase RcsC (Majdalani and Gottesman, 2006). RcsC is activated by the outer membrane protein RcsF (Majdalani et al., 2005; Castenié-Cornet et al., 2006). In E. coli, the Rcs pathway inhibits flhDC transcription by binding of RcsBA to DNA downstream of the flhDC promoter region (Francez-Charlot et al., 2003). The Rcs system has been linked to swarming in P. mirabilis and the RcsD (formerly RsbA) phosphorelay protein was originally discovered as a mutation that disrupted RcsD and reduced the swarming lag period (Belas et al., 1998).
The Rcs system is activated in response to outer membrane perturbations. Mutations that disrupt the outer membrane components lipopolysaccharide (LPS) O-antigen, the enterobacterial common antigen (ECA), or the osmoregulated periplasmic glycans (OPG) decrease flhDC expression and abolish swarming motility in E. coli, P. mirabilis, or S. marcescens (Belas et al., 1995; Girgis et al., 2007; Inoue et al., 2007; Castelli et al., 2008; Morgenstein et al., 2010). In each case, mutations in the Rcs system restored both flhDC transcription and swarming motility to the outer membrane mutants (Girgis et al., 2007; Morgenstein et al., 2010; Castelli and García Véscovi, 2011). Consistent with signal transduction from the outer membrane, the addition of membrane active surfactants partially restored swarming to O-antigen mutants of S. enterica (Toguchi et al., 2000). We speculate that the addition of surfactant may have disrupted Rcs signaling and restored flhDC expression. Thus, Rcs senses stress on the outer membrane, perhaps related to surface contact, and appears to be an important input for control of the flagellar master regulator and swarming motility (Majdalani and Gottesman, 2006).
In addition to transcriptional regulation, there are also post-transcriptional regulators of flhDC in the control of swarming motility. For example, flhDC is regulated post-transcriptionally by the RNA binding protein CsrA in E. coli. CsrA binds to the untranslated leader of flhDC mRNA and stabilizes the transcript, resulting in increased translation (Wei et al., 2001). The CsrA homologue, RsmA of P. mirabilis, has also been shown to be involved in swarming in as much as overexpression of RsmA inhibited swarming but not swimming motility (Liaw et al., 2003). It is not yet clear if RsmA represses flhDC transcript accumulation in P. mirabilis or whether RsmA negatively regulates other targets in the flagellar hierarchy.
FlhDC is also subject to regulation at the level of proteolysis and activity. Although flhDC is upregulated when swarming is initiated in P. mirabilis, the FlhD and FlhC proteins only accumulate transiently (Furness et al., 1997; Claret and Hughes, 2000b). FlhD and FlhC half-lives decrease in swarming cells due to an increased rate of proteolysis (Claret and Hughes, 2000b). When P. mirabilis FlhD and FlhC were heterologously expressed in E. coli, both proteins were stabilized in a mutants defective for the Lon protease but not in other protease mutants, suggesting that FlhDC turnover is primarily mediated by Lon (Claret and Hughes, 2000b). Proteolytic degredation of master regulator proteins may be a shared mechanism of controlling swarming as the Lon protease has also been demonstrated to inhibit swarming in V. parahaemolyticus and B. subtilis (Stewart et al., 1997; Chen et al., 2009). Finally, FlhD4C2 activity may be antagonized directly or indirectly by the P. mirabilis amino acid decarboxylase DisA (Stevenson and Rather, 2006). Overexpression of DisA abolishes flagellar gene expression perhaps due to synthesis of the putative product, decarboxylated phenylalanine. How DisA or decarboxylated phenylalanine inhibits FlhD4C2 activity is unknown.
FlhDC is an important regulator of motility and it is regulated at many levels. Although flhDC transcription is dramatically increased in swarming P. mirabilis cells, flhDC does not appear to be induced in swarming cells of S. liquefaciens (Tolker-Nielsen et al., 2000). FlhDC activity may be regulated post-transcriptionally in S. liquefaciens as artificial induction of FlhDC shortened swarming lag period (Givskov et al., 1995). Likewise, swarming motility required a higher level of artificially-induced FlhDC than swimming motility in Y. enterocolitica (Young et al., 1999). Thus regulation at the level of FlhDC is a good starting point for extending our understanding of swarming motility. Swarming-specific mutants have been identified in P. mirabilis, E. coli, and S. typhimurium further investigation into if and how these proteins feed in to the master regulators could offer greater insight into the induction of swarming behavior (Sturgill and Rather, 2004; Stevenson and Rather, 2006; Girgis et al., 2007; Stafford and Hughes, 2007).
SwrA and DegU: B. subtilis
The regulation of flagellar biosynthesis in Bacillus subtilis is still poorly understood but the best candidate for a master regulator of flagellar gene expression is the protein SwrA. Unlike FlhDC, SwrA of B. subtilis is not obligately required for flagellum production, as swrA mutants swim but do not swarm (Kearns et al., 2004). SwrA has no homology to any known proteins, has no identifiable DNA-binding motifs, and increases transcription basal body gene expression (Kearns and Losick, 2005). Furthermore, spontaneous bypass suppressor mutations of non-swarming swrA mutants increase expression of the fla/che operon that encodes basal body genes by mutation of a promoter sequence closer to consensus, or by deletion of a stem-loop terminator from the upstream gene allowing transcriptional read-through (Kearns and Losick, 2005). Finally, overexpression of swrA abolishes the swarming lag period (Kearns et al., 2004; Kearns and Losick, 2005). Thus, SwrA appears to regulate the expression of basal body gene expression and can be considered a master regulator of flagellar biosynthesis.
Domesticated laboratory strains of B. subtilis do not swarm due, in part, to a loss-of-function mutation in swrA (Patrick and Kearns, 2009). In fact, SwrA was originally identified as the increased frequency of motility locus (ifm) in which gain-of-function mutations in this locus resulted in an increase in the proportion of motile cells in the population and the number of flagella per cell (Grant and Simon, 1969; Kearns and Losick, 2005; Calvio et al., 2005). Domesticated strains (168 and its derivatives) contain a frameshift mutation early in the swrA open reading frame located within a homopolymeric tract of A-T base pairs and the ifm allele was a deletion of one A-T base pair that restored the open reading frame and SwrA functionality (Calvio et al., 2005). Insertions and deletions occur within the swrA homopolymeric tract at a high frequency (10−4 to 10−5) on par with high frequency phase variation mechanisms that permit/abolish expression of virulence factors in many pathogens (Henderson et al., 1999; Kearns et al., 2004). Thus, one possible mechanism for the regulation of swarming motility is genetic as phase variation toggles the swrA reading frame between the functional and nonfunctional alleles (Kearns et al., 2004).
SwrA is transcribed from two promoters, one is dependent upon the housekeeping sigma factor, σA, the other upon the motility alternative sigma factor σD that expresses own expression indirectly via its σD-dependent promoter (Calvio et al., 2008) as the gene encoding σD (sigD) is activated by SwrA (Márquez-Magaña and Chamberlin, 1994). The relative contribution of the two promoters upstream of swrA has been investigated in liquid grown cells and fusions of each promoter to lacZ expression indicated that swrA was transcribed from the σD promoter alone and no expression was detected from the σA promoter. Under swarming conditions however, deletion of either promoter resulted in swarming proficient cells suggesting that both promoters are functional (Calvio et al., 2008). Finally, the σA dependent promoter was expressed in the presence of an activated form of the response regulator DegU, suggesting that DegU might become activated during swarming conditions (Calvio et al., 2008).
DegU has been called a global regulator because it has been implicated in the transcriptional regulation of various multicellular processes such as sporulation, competence, biofilm formation, and motility (reviewed in Murray et al., 2009). DegU seems to differentially coordinate the various phenotypes because its binding affinity for promoters is altered when phosphorylated by its cognate cytoplasmic kinase DegS (Dahl, et al., 1992; Verhamme et al., 2007; Kobayashi, 2007). Unphosphorylated DegU binds to an inverted repeat-like sequence upstream of the fla/che promoter and activates transcription (Amati et al., 2004; Kobayashi, 2007; Verhamme et al., 2007; Calvio et al., 2008; Tsukahara and Ogura, 2008). When phosphorylated, DegU-P inhibits fla/che operon expression by binding to a second inverted repeat-like sequence downstream of the fla/che promoter and also activates expression of the FlgM anti-sigma factor that inhibits σD and flagellin expression (Amati et al., 2005; Tsukahara and Ogura, 2008; Hsueh et al., 2011). Thus DegU behaves as both an activator and a repressor of motility depending on its phosphorylation state. The stimulus that triggers phosphorylation of DegU is unknown.
SwrA activation of basal body gene expression may require DegU. SwrA does not resemble a DNA binding protein and the positive effect of SwrA on basal body genes is lost in a degU mutant (Calvio et al., 2008; Tsukahara and Ogura, 2008). Like mutation of swrA, mutation of degU abolishes swarming motility but not swimming (Verhamme et al., 2007; Kobayashi, 2007). In addition, the non-swarming phenotypes of swrA and degU mutants can each be bypassed by the same mutations in the fla/che promoter region (Amati et al., 2004; Kearns and Losick, 2005). Finally, overexpression of swrA cannot compensate for a loss of degU in restoring swarming motility, suggesting either that the two proteins function at the same regulatory level or that DegU activates other components of the flagellar machinery downstream of the fla/che promoter (Calvio et al, 2008). Thus, the functions of SwrA and DegU seem to be related in the regulation of swarming motility and other phenotypes (Osera et al., 2009).
Much remains unknown about flagellar regulation in B. subtilis. Future studies must determine the mechanism by which SwrA activates flagellar gene expression including if and how SwrA is related to DegU. The function of DegU, its regulation, and the regulon it controls remains complicated and mysterious. Two inhibitors the Lon protease and a protein of unknown function SmiA both inhibit swarming at an unknown step but abolish the swarm lag when mutated (Chen et al., 2009). Finally, other motility regulators likely exist in B. subtilis that remain as-yet-undiscovered.
LafK: Vibrio parahaemolyticus
Vibrio parahaemolyticus encodes two types of flagella. A single, polar, sheathed flagellum consumes the sodium motive force and provides the power for swimming in liquid media (Kim and McCarter, 2000; McCarter, 2001). Upon contact with a surface, multiple, peritrichous lateral flagella are synthesized, that consume the proton motive force to provide the power for swarming motility (Allen and Bauman, 1971; Shinoda and Okamoto, 1977; Atsumi et al.,1992). The lateral flagella system (laf) contains over 30 genes arranged into at least seven operons many of which are transcribed by RNA polymerase and σ54, and are activated by the transcriptional regulator LafK (McCarter and Wright, 1993; Stewart and McCarter, 2003). Luciferase reporter fusions and transcriptome analysis indicated that the lateral flagellar basal body genes were expressed upon contact with a solid surface, and activation of many but not all of the genes was dependent on LafK (Belas et al., 1986; Stewart and McCarter, 2003; Gode-Potratz et al., 2011). Thus LafK may be a master regulator but it is not alone at the apex of the lateral flagella hierarchy (Gode-Potratz et al., 2011). The identity of another regulator controlling surface-induced gene expression and whether that regulator acts upstream of, or in parallel to, LafK is unknown.
One set of master regulator candidates are encoded within the scrABC operon that encodes ScrA, an aminotransferase homolog, ScrB a homolog of a periplasmic solute binding protein, and ScrC a di-guanidylate cyclase/phosphodiesterase GGDEF-EAL domain protein which activates lateral flagella gene expression. Mutation in the scr genes reduces swarming motility and expression of the lateral flagella genes (Boles and McCarter, 2002; Ferreria et al., 2008). Overexpression of the scrABC operon increases swarming and lateral flagella expression (Boles and McCarter, 2002). The scr operon is induced upon surface growth, and is transcribed independently of LafK (Gode-Potratz et al., 2011). Finally, the ScrABC module represses biofilm genes while activating lateral flagella and thus it may have a more general role in controlling the lifestyle switch between motility and biofilm formation (Boles and McCarter, 2002; Ferreria et al., 2008)
Two other swarming regulators, SwrT and SwrZ, control lateral flagella expression (Jaques and McCarter, 2006). SwrT encodes a TetR-like transcriptional regulator, and mutation of SwrT abolished swarming motility. Bypass suppressors that restored swarming to the swrT mutant disrupted swrZ, encoding SwrZ a homolog of a GntR-like transcriptional regulator. Thus, SwrT represses swrZ expression and high level SwrZ is sufficient to inhibit the expression of lateral flagella basal body genes. (Jaques and McCarter, 2006). It is not yet known if SwrZ regulation of flagellar gene expression is direct or indirect, nor is it known whether SwrZ inhibits lafK expression or some other level of the flagella hierarchy. The signal that regulates SwrT is also unknown.
Vibrio parahaemolyticus is the best understood model of surface-induced initiation of swarming—the single sheathed polar flagellum acts as a surface sensor (Belas et al., 1986; McCarter et al., 1988). When the cells encounter increased viscosity in the environment, rotation of the polar flagellum slows. Very elegant experiments by Kawagishi et al. (1996) showed that decreased sodium ion flow through the motor proteins by increased media viscosity or the sodium channel inhibitor phenamil acts as a signal for lateral-flagella induction. How ion current is recognized by the cell to activate expression of lateral flagella genes still remains a mystery. Further, it remains an open question as to whether LafK is a master regulator of flagellar synthesis or whether there exists a higher order regulator above LafK in the hierarchy.
Conclusions
It is perhaps not surprising that the expression of flagellar genes is under the control of master regulatory proteins: motility is but one of many conditionally advantageous, energy costly, physiological states that cells can adopt and is regulated accordingly. Rather, it is the exquisitely fine-tuned regulation of the master regulators themselves that is remarkable as their output results in variation at the level of basal body gene expression and therefore the number of flagella per cell. Thus, we infer from the modulation in master regulator activity that the number of flagella a cell synthesizes is of consequence beyond the simple issue of the presence or absence of motility. Bacterial species are known to differ in the number, location, and organization of the flagella but the biological relevance of this variation is poorly understood. Swarming motility is one phenotype in which flagellar number seems to matter.
That bacteria control flagellar number during swarming motility is controversial. Some swarming publications make qualitative observations stating that the number of flagella appears by microscopy to be greater on swarming cells than swimming cells (Hoeniger, 1965; Alberti and Harshey, 1990; Harshey and Matsuyama, 1994; Kearns and Losick, 2003). Others publications observe no significant change during swarming in flagellar gene expression by transcriptome analysis (Wang et al., 2004; Overhage et al., 2008; Tremblay and Deziel, 2010) and attribute the apparent increase in flagellar number to an optical illusion (Tolker-Neilsen et al., 2000; Harshey, 2010). In fact, few if any swarming publications have counted flagella in swimming and swarming cells due to the fact that flagellar number is surprisingly difficult to count. Biologically, flagella filaments are long, have a natural tendency to form bundles, and may become tangled or sheared. Experimentally, common light microscopy techniques to stain flagella use mordants that cause flagella to adhere to one another, and high-resolution electron microscopy is technically-challenging, requires small sample sizes, and is no less susceptible to counting problems (Mayfield and Innes, 1977). Rather, we infer that controlling flagellar number is important from the study of mutations that reduce or enhance swarming motility with a corresponding effect on gene flagellar gene expression.
Flagellar number is defined at the level of basal body assembly and basal body gene expression is controlled by master regulatory proteins (Aldridge et al., 2010; Erhardt and Hughes, 2010). Here we discuss three organisms which appear to control flagellar number during swarming and the upstream regulators that govern master regulator activity. The swarming stimulus that controls the master regulators remains poorly understood but is thought to involve contact with a solid surface. If true, a putative mechanosensitive receptor must pass a signal through a transduction system that transits the cell envelope to regulate cytoplasmic gene expression. The surface-accessible outer membrane of Gram-negative bacteria and the flagellum are candidates for the sensor but if and how contact is recognized and transduced remains a mystery. The proteins that are required for swarming and act upstream of the master flagellar regulators provide the good candidates for a surface-sensing signal transduction cascade.
The regulation of swarming motility has been best explored in P. mirabilis in which both highly-conserved and novel proteins have been shown to be required for swarming through regulation of FlhDC at multiple levels. Even in E. coli which does not appear to regulate flagellar number during swarming (Harshey, 2010), initiation of flagellar assembly is one of the most extensively regulated processes in the cell and is modulated by at least a dozen various factors (Erhardt and Hughes, 2010). The lessons learned from FlhDC are informative for other swarming model organisms such as B. subtilis and V. parahaemolyticus, and it will be important to determine how proteins such as SwrA and LafK are regulated. Thus the study of master flagellar regulatory proteins will hopefully answer questions about the nature of the swarming signal, how the information is transduced, and perhaps even the relevance of flagella number and organization. Finally, we may discover the elusive mechanism(s) of surface sensing in bacteria which may be as diverse as the master flagellar regulators themselves.
ACKNOWLEDGEMENTS
Work in the Kearns laboratory is supported by the US National Institutes of Health grant GM093030.
References
- Aldridge C, Poonchareon K, Saini S, Ewen T, Soloyva A, Rao CV, Imada K, Minamino T, Aldridge PD. The interaction dynamics of a negative feedback loop regulates flagellar number in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2010;78:1416–1430. doi: 10.1111/j.1365-2958.2010.07415.x. [DOI] [PubMed] [Google Scholar]
- Allen RD, Baumann P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol. 1971;107:295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison C, Hughes C. Bacterial swarming: an example of prokaryotic differentiation and multicellular behavior. Sci Progress. 1991;75:403–422. [PubMed] [Google Scholar]
- Amati G, Bisicchia P, Galizzi A. DegU-P represses expression of the motility fla/che operon in Bacillus subtilis. J Bacteriol. 2004;186:6003–6014. doi: 10.1128/JB.186.18.6003-6014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi T, McCarter L, Imae Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature. 1992;355:182–184. doi: 10.1038/355182a0. [DOI] [PubMed] [Google Scholar]
- Bange G, Kümmer N, Engel C, Bozkurt G, Wild K, Sinning I. FlhA provides the adapter for coordinated delivery of late flagella building blocks to the Type III secretion system. PNAS. 2010;107:11295–11300. doi: 10.1073/pnas.1001383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DH, Frantz BB, Matsumura P. Flagellar transcriptional activators FlbB and FlaI: Gene sequences and 5′ consensus sequences of operons under FlbB and FlaI control. J Bacteriol. 1988 doi: 10.1128/jb.170.4.1575-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas MR, Colwell RR. Scanning electron microscope observation of the swarming phenomenon of Vibrio parahaemolyticus. J Bacteriol. 1982;150:956–959. doi: 10.1128/jb.150.2.956-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R, Goldman M, Ashliman K. Genetic analysis of Proteus mirabilis mutants defective in swarmer cell elongation. J Bacteriol. 1995;177:823–828. doi: 10.1128/jb.177.3.823-828.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R, Schneider R, Melch M. Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior. J Bacteriol. 1998;180:6126–6139. doi: 10.1128/jb.180.23.6126-6139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R, Simon M, Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, McCarter LL. Vibrio parahaemolyticus scrABC, a novel operon affecting swarming and capsular polysaccharide regulation. J Bacteriol. 2002;184:5946–5954. doi: 10.1128/JB.184.21.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvio C, Celandroni F, Ghelardi E, Amati G, Salvetti S, Ceciliani F, Galizzi A, Senesi S. Swarming differentiation and swimming motility in Bacillus subtilis are controlled by swrA, a newly identified dicistronic operon. J Bacteriol. 2005;187:5356–5366. doi: 10.1128/JB.187.15.5356-5366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvio C, Osera C, Amati G, Galizzi A. Autoregulation of swrAA and motility in Bacillus subtilis. J Bacteriol. 2008;190:5720–5728. doi: 10.1128/JB.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo JM, Matthews RG. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli ME, Fedrigo GV, Clementín AL, Ielmini MV, Feldman MF, García Véscovi E. Entrobacterial common antigen integrity is a checkpoint for flagellar biogenesis in Serratia marcescens. J Bacteriol. 2008;190:213–220. doi: 10.1128/JB.01348-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli ME, García Véscovi E. The Rcs signal transduction pathway is triggered by enterobacterial common antigen structure alterations in Serratia marcescens. J Bacteriol. 2011;198:63–74. doi: 10.1128/JB.00839-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenié-Cornet MP, Cam K, Jacq A. RcsF is an outer membrane lipoprotein involved in the RcsCDB phosphorelay pathway in Escherichia coli. J Bacteriol. 2006;188:4264–4270. doi: 10.1128/JB.00004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BG, Turner L, Berg HC. The wetting agent required for swarming in Salmonella enterica Serovar Typhimurium is not a surfactant. J Bacterial. 2007;189:8750–8753. doi: 10.1128/JB.01109-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Guttenplan SB, Blair KM, Kearns DB. Role of the σD-dependent autolysins in Bacillus subtilis population heterogeneity. J Bacteriol. 2009;191:5775–5784. doi: 10.1128/JB.00521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevance FFV, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nature. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret L, Hughes C. Functions of the subunits in the FlhD2C2 transcriptional master regulator of bacterial flagellum biogenesis and swarming. J Mol Biol. 2000a;303:467–478. doi: 10.1006/jmbi.2000.4149. [DOI] [PubMed] [Google Scholar]
- Claret L, Hughes C. Rapid turnover of FlhD and FlhC, the flagellar regulon transcriptional activator proteins, during Proteus swarming. J Bacteriol. 2000b;182:833–836. doi: 10.1128/jb.182.3.833-836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret L, Hughes C. Interaction of the atypical prokaryotic transcription activator FlhD2C2 with early promoters of the flagellar gene hierarchy. J Mol Biol. 2002;321:185–199. doi: 10.1016/s0022-2836(02)00600-9. [DOI] [PubMed] [Google Scholar]
- Copeland MF, Weibel DB. Bacterial swarming: a model system for studying dynamic self-assembly. Soft Matter. 2009;5:1174–1187. doi: 10.1039/B812146J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa CS, Pettinari MJ, Méndez BS, Antón DN. Null mutation in the essential gene yrfF (mucN) are not lethal in rcsB, yojN, and rcsC strains of Salmonelle enterica serovar Typhimurium. FEMS Microbiol Lett. 2003;222:25–32. doi: 10.1016/S0378-1097(03)00221-0. [DOI] [PubMed] [Google Scholar]
- Dahl MK, Masdek T, Kunst F, Rapoport G. The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degredative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J Biol Chem. 1992;267:14509–14514. [PubMed] [Google Scholar]
- Dufour A, Furness RB, Hughes C. Novel genes that upregulate the Proteus mirabilis flhDC master operon controlling flagellar biogenesis and swarming. Mol Microbiol. 1998;29:741–751. doi: 10.1046/j.1365-2958.1998.00967.x. [DOI] [PubMed] [Google Scholar]
- Eberl L, Christiansen G, Molin S, Givskov M. Differentiation of Serratia liquifaciens into swarm cells is controlled by the expression of the flhD master operon. J Bacteriol. 1996;178:554–559. doi: 10.1128/jb.178.2.554-559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl L, Molin S, Givskov M. Surface motility of Serratia liquifaciens MG1. J Bacteriol. 1999;181:1703–1712. doi: 10.1128/jb.181.6.1703-1712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt M, Hughes KT. C-ring requirement in flagellar type III secretion is bypassed by FlhDC upregulation. Mol Microbiol. 2010;75:376–393. doi: 10.1111/j.1365-2958.2009.06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RBR, Antunes LCM, Greenberg EP, McCarter LL. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J Bacteriol. 2008;190:851–860. doi: 10.1128/JB.01462-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francez-Charlot A, Laugel B, Von Gemert A, Dubarry N, Wiorowski F, Castanie-Cornet M, Gutierrez C, Cam K. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol Microbiol. 2003;49:823–832. doi: 10.1046/j.1365-2958.2003.03601.x. [DOI] [PubMed] [Google Scholar]
- Fraser GM, Bennett JC, Hughes KT. Substrate specific binding of hook-associated proteins by FlgN and FliT, putative chaperones of flagellum assembly. Mol Microbiol. 1999;32:569–580. doi: 10.1046/j.1365-2958.1999.01372.x. [DOI] [PubMed] [Google Scholar]
- Furness RB, Fraser GM, Hay NA, Hughes C. Negative feedback from Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J Bacteriol. 1997;179:5585–5588. doi: 10.1128/jb.179.17.5585-5588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis HS, Liu Y, Riu WS, Tavozoie S. A comprehensive genetic characterization of bacterial motility. PLOS Genet. 2007;3e:e154. doi: 10.1371/journal.pgen.0030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givskov M, Eberl L, Christiansen G, Benedik MJ, Molin S. Induction of phospholipase- and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhD. Mol. Microbiol. 1995;15:445–454. doi: 10.1111/j.1365-2958.1995.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL. Surface sensing in Vibrio parahaemolyticus triggers a program of gene expression that promotes colonization and virulence. Mol Microbiol. 2011;79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GF, Simon MI. Synthesis of bacterial flagella. J Bacteriol. 1969;99:116–124. doi: 10.1128/jb.99.1.116-124.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi D, Bailey MJ, Allison C, Hughes C. Requirement of FlhA in flagella assembly and swarm-cell differentiation by Proteus mirabilis. Mol Microbiol. 1995a;15:761–769. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Gygi D, Fraser G, Dufour A, Hughes C. A motile but nonswarming mutant of Proteus mirabilis lacks FlgN, a facilitator of flagella filament assembly. Mol Microbiol. 1997;25:597–604. doi: 10.1046/j.1365-2958.1997.5021862.x. [DOI] [PubMed] [Google Scholar]
- Harshey RM. Bees are the only ones: swarming in Gram negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Harshey RM. Maloy SM, et al., editors. Swarming Adventures. The Lure of Bacterial Genetics: A Tribute to John Roth. 2010:163–172. [Google Scholar]
- Harshey RM, Matsuyama T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. PNAS. 1994;91:8631–8635. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay NA, Tipper DJ, Gygi D, Hughes C. A nonswarming mutant of Proteus mirabilis lacks the Lrp global transcriptional regulator. J Bacteriol. 1997;179:4741–4746. doi: 10.1128/jb.179.15.4741-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Owen P, Nataro JP. Molecular switches - the ON and OFF of bacterial phase variation. Mol Microbiol. 1999;33:919–932. doi: 10.1046/j.1365-2958.1999.01555.x. [DOI] [PubMed] [Google Scholar]
- Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeniger JFM. Cellular changes accompanying the swarming of Proteus mirabilis. I. Observations of living cultures. Can J Microbiol. 1964;10:1–9. doi: 10.1139/m64-001. [DOI] [PubMed] [Google Scholar]
- Hoeniger JFM. Development of flagella by Proteus mirabilis. J Gen Microbiol. 1965;40:29–42. [Google Scholar]
- Hsueh Y-H, Cozy LM, Sham L-T, Calvo RA, Gutu AD, Winkler ME, Kearns DB. DegU-phosphate activates expression of the anti-sigma factor FlgM in Bacillus subtilis. Mol Microbiol. 2011;81:1092–1108. doi: 10.1111/j.1365-2958.2011.07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Shingaki R, Hirose S, Waki K, Mori H, Fukui K. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J Bacteriol. 2007;189:950–957. doi: 10.1128/JB.01294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques S, McCarter LL. Three new regulators of swarming in Vibrio parahaemolyticus. J Bacteriol. 2006;188:2625–2635. doi: 10.1128/JB.188.7.2625-2635.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol Microbiol. 1996;20:693–699. doi: 10.1111/j.1365-2958.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DB, Losick R. Swarming motility in undomesticated Bacillus subtilis. Mol Microbiol. 2003;49:581–590. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- Kearns DB, Chu F, Rudner R, Losick R. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol Microbiol. 2004;52:357–369. doi: 10.1111/j.1365-2958.2004.03996.x. [DOI] [PubMed] [Google Scholar]
- Kearns DB, Losick R. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 2005;19:3083–3094. doi: 10.1101/gad.1373905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, McCarter LL. Analysis of the polar flagellar gene system of Vibrio parahaemolyticus. J Bacteriol. 2000;182:3643–3703. doi: 10.1128/jb.182.13.3693-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Park C. H-NS-dependent regulation of flagellar synthesis is mediated by a LysR family protein. J Bacteriol. 2000;182:4670–4672. doi: 10.1128/jb.182.16.4670-4672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol. Microbiol. 2007;66:395–409. doi: 10.1111/j.1365-2958.2007.05923.x. [DOI] [PubMed] [Google Scholar]
- Kutsukake K, Iino T. Role of the FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagella formation in Salmonella typhimurium. J Bacteriol. 1994;176:3598–3605. doi: 10.1128/jb.176.12.3598-3605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnen D, Blumer C, Polen T, Wackwitz B, Wendisch VF, Unden G. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol Microbiol. 2002;45:521–532. doi: 10.1046/j.1365-2958.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- Liaw S, Lai H, Ho S, Luh K, Wang W. Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. 2003;52:19–28. doi: 10.1099/jmm.0.05024-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Matsumura P. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J Bacteriol. 1994;176:7345–7351. doi: 10.1128/jb.176.23.7345-7351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lai M, Ang S, Shu J, Soo P, Horng Y, Yi W, Lai H, Luh K, Ho S, Swift S. Role of flhDC in the expression of the nuclease gene nucA, cell division and flagellar synthesis in Serratia marcescens. J Biomed Sci. 2000;7:475–483. doi: 10.1007/BF02253363. [DOI] [PubMed] [Google Scholar]
- Macnab RM. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- Macnab RM. How bacteria assemble flagella. Annu Rev Microbio. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol. 2006;58:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- Majdalani N, Heck M, Stout V, Gottesman S. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J Bacteriol. 2005;187:6770–6778. doi: 10.1128/JB.187.19.6770-6778.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez-Magaña LM, Chamberlin MJ. Characterization of the sigD transcriptional unit of Bacillus subtilis. J Bacteriol. 1994;176:2427–2434. doi: 10.1128/jb.176.8.2427-2434.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield CI, Inniss WE. A rapid, simple method for staining bacterial flagella. Can J Microbiol. 1977;23:1311–1313. doi: 10.1139/m77-198. [DOI] [PubMed] [Google Scholar]
- McCarter LL. The multiple identities of Vibrio parahaemolyticus. J Molec Microbiol Biotechnol. 1999;1:51–57. [PubMed] [Google Scholar]
- McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- McCarter LL, Wright ME. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J Bacteriol. 1993;175:3361–3371. doi: 10.1128/jb.175.11.3361-3371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter LL. Polar flagellar motility of the Vibrionaceae. Microbiol Mol Biol Rev. 2001;65:445–462. doi: 10.1128/MMBR.65.3.445-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstein RM, Clemmer KM, Rather PN. Loss of the WaaL O-antigen ligase prevents surface activation of the flagellar gene cascade in Proteus mirabilis. J Bacteriol. 2010;192:3213–3221. doi: 10.1128/JB.00196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RB, Scott A. Swarming of Proteus—a solution to an old problem. Nature. 1966;211:255–257. doi: 10.1038/211255a0. [DOI] [PubMed] [Google Scholar]
- Murray EJ, Kiley TB, Stanley-Wall NR. A pivitol role for the response regulator DegU in controlling multicellular behavior. Microbiol. 2009;155:1–8. doi: 10.1099/mic.0.023903-0. [DOI] [PubMed] [Google Scholar]
- Neu TR. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev. 1996;60:151–166. doi: 10.1128/mr.60.1.151-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osera C, Amati G, Calvio C, Galizzi A. SwrAA activates poly-gamma-glutamate synthesis in addition to swarming in Bacillus subtilis. Microbiol. 2009;155:2282–2287. doi: 10.1099/mic.0.026435-0. [DOI] [PubMed] [Google Scholar]
- Overhage J, Bains M, Brazas MD, Hancock RE. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J Bacteriol. 2008;190:2671–2679. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JE, Kearns DB. Laboratory strains of Bacillus subtilis do not exhibit swarming motility. J Bacteriol. 2009;191:7129–7133. doi: 10.1128/JB.00905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MM, Rasko DA, Smith SN, Mobley HL. Transcriptome of swarming Proteus mirabilis. Infect Immun. 2010;78:2834–2845. doi: 10.1128/IAI.01222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauprich O, Matsushita M, Weijer CJ, Siegert F, Esipov S, Shapiro JA. Period phenomena in Proteus mirabilis swarm colony development. J Bacteriol. 1996;178:6525–6538. doi: 10.1128/jb.178.22.6525-6538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Park C. Modulation of flagellar expression in Escherchia coli by acetyl phosphate and the osmoregulator OmpR. J Bacteriol. 1995;177:4696–4702. doi: 10.1128/jb.177.16.4696-4702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S, Okamato K. Formation and function of Vibrio parahaemolyticus lateral flagella. J Bacteriol. 1977;129:1266–1271. doi: 10.1128/jb.129.3.1266-1271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M, Simon MI. Bacterial flagella. Ann Rev Microbiol. 1977;31:397–419. doi: 10.1146/annurev.mi.31.100177.002145. [DOI] [PubMed] [Google Scholar]
- Soutourina O, Kolb A, Krin E, Laurent-Winter C, Rimsky S, Danchin A, Bertin P. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite actvator protein complex in transcription of the flhDC master operon. J Bacteriol. 1999;181:7500–7508. doi: 10.1128/jb.181.24.7500-7508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V, Torres AG, Kaper JB. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two component regulatory system involved in the regulation of flagella motility by quorum sensing in E. coli. Mol Microbiol. 2002;43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- Stafford GP, Hughes C. Salmonella typhimurium flhE, a conserved flagellar regulon gene required for swarming. Microbiology. 2007;153:541–547. doi: 10.1099/mic.0.2006/002576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson LG, Rather PN. A novel gene involved in regulating the flagellar gene cascade in Proteus mirabilis. J Bacteriol. 2006;188:7830–7839. doi: 10.1128/JB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BJ, Enoserlage JL, McCarter LL. The lonS gene regulates swarmer cell differentiation of Vibrio parahaemolyticus. J Bacteriol. 1997;179:107–114. doi: 10.1128/jb.179.1.107-114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BJ, McCarter LL. Lateral flagellar gene system of Vibrio parahaemolyticus. J Bacteriol. 2003;185:4508–4518. doi: 10.1128/JB.185.15.4508-4518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill G, Rather PN. Evidence that putrescine acts as an extracellular signal required for swarming in Proteus mirabilis. Mol Microbiol. 2004;51:437–446. doi: 10.1046/j.1365-2958.2003.03835.x. [DOI] [PubMed] [Google Scholar]
- Toguchi A, Siano M, Burkhart M, Harshey RM. Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysacharide. J Bacteriol. 2000;182:6308–6321. doi: 10.1128/jb.182.22.6308-6321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolker-Nielsen T, Christiansen AB, Holmstrøm K, Eberl L, Rasmussen TB, Sternberg C, Heydorn A, Molin S, Givskov M. Assessment of flhDC mRNA levels in Serratia liquefaciens swarm cells. J. Bacteriol. 2000;182:2680–2686. doi: 10.1128/jb.182.10.2680-2686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay J, Déziel E. Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genomics. 2010;11:587. doi: 10.1186/1471-2164-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara K, Ogura M. Promoter selectivity of the Bacillus subtilis response regulator DegU, a positive regulator of the fla/che operon and sacB. BMC Microbiol. 2008;8:8. doi: 10.1186/1471-2180-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhamme DT, Kiley TB, Stanley-Wall NR. DegU co-ordinates multicellular behavior exhibited by Bacillus subtilis. Mol. Microbiol. 2007;65:554–568. doi: 10.1111/j.1365-2958.2007.05810.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Fleming RT, Westbrook EM, Matsumura P, McKay DB. Structure of the Escherichia coli FlhDC complex, a prokaryotic heteromeric regulator of transcription. J Mol Biol. 2006;355:798–808. doi: 10.1016/j.jmb.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Wang Q, Frye JG, McClelland M, Harshey RM. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol Microbiol. 2004;52:169–187. doi: 10.1111/j.1365-2958.2003.03977.x. [DOI] [PubMed] [Google Scholar]
- Wei BL, Brun-Zinkemagel A, Simecka JW, Prüßa BM, Babitzke P, Romeo T. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol Microbiol. 2001;40:245–256. doi: 10.1046/j.1365-2958.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- Young GM, Smith MJ, Minnich SA, Miller VL. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 1999;1999;181:2823–2833. doi: 10.1128/jb.181.9.2823-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]