Abstract
The cucurbitacins are tetracyclic triterpenes found in plants of the family Cucurbitaceae. Cucurbitacins have been shown to have anticancer and anti-inflamatory activities. We investigated the anticancer activity of cucurbitacin B extracted from Thai medicinal plant Trichosanthes cucumerina Linn. Cell viability was assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Results indicated that cucurbitacin B from T. cucumerina Linn. has a cytotoxic effect on breast cancer cell lines SKBR-3 and MCF-7 with an IC50 of 4.60 and 88.75 μg/ml, respectively. Growth inhibition was attributed to G2/M phase arrest and apoptosis. Cyclin D1, c-Myc and β-catenin expression levels were reduced. Western blot analysis showed increased PARP cleavage and decreased Wnt-associated signaling molecules β-catenin, galectin-3, cyclin D1 and c-Myc, and corresponding changes in phosphorylated GSK-3β levels. Cucurbitacin B treatment inhibited translocation to the nucleus of β-catenin and galectin-3. The depletion of β-catenin and galectin-3 in the nucleus was confirmed by cellular protein fractionation. T-cell factor (TCF)/lymphoid enhancer factor (LEF)-dependent transcriptional activity was disrupted in cucurbitacin B treated cells as tested by a TCF reporter assay. The relative luciferase activity was reduced when we treated cells with cucurbitacin B compound for 24 hours. Our data suggest that cucurbitacin B may in part induce apoptosis and exert growth inhibitory effect via interruption the Wnt signaling.
Keywords: cucurbitacin B, Wnt signaling, Trichosanthes cucumerina Linn, apoptosis, breast cancer
Introduction
Breast cancer is the most common cancer in women in most parts of the world. Approximately 1,050,346 new cases of breast cancer were reported worldwide in the year 2000 [Parkin et al., 2001]. Mortality rates were generally highest in countries where the risk of developing breast cancer was greatest and declined in highly educated women as a consequence of the combined effects of earlier detection and improved treatment [Bray et al., 2002; Jemal et al., 2006].
Abnormalities in the Wnt signaling pathway are frequently observed in human cancers. In the absence of Wnt signals, the cytosolic pool of β-catenin is continuously degraded as a result of its phosphorylation by glycogen synthase kinase-3β (GSK-3β) and subsequent ubiquitination. Axin and APC are required to form a complex with β-catenin during the phosphorylation process. On the contrary, in the presence of Wnt signals, β-catenin levels increase and it accumulates in the cytosol and subsequently translocates to the nucleus to regulate expression of specific target genes such as c-myc and cyclin D1 [Brown, 2001; Polakis, 2000; Dihlmann and von Knebel Doeberitz, 2005]. Galectin-3, a beta-galactoside-binding protein, has been identified as an important component in canonical Wnt signaling since it can bind directly to the NH2 terminus of β-catenin and colocalizes with β-catenin in the nucleus [Shimura et al., 2004] Downregulation of galectin-3 results in GSK-3β dephosphorylation and reduces β-catenin and cyclin D1 levels [Song et al., 2009]. Growing evidences indicate that downstream components of Wnt signaling are activated in breast tumors. Activation of Wnt signal leads to mammary tumorigenesis in animal models [Brennan and Brown, 2004; Smalley and Dale, 2001]. Therefore, regulation of Wnt signaling components is believed to be a good molecular target for breast cancer therapy.
Surgical resection of the primary tumor and cytotoxic chemotherapy are the preferable procedures for breast cancer treatment. Currently, advanced estrogen receptor (ER) positive breast cancer is treated with hormonal therapies such as aromatase inhibitors [Gibson et al., 2007] and tamoxifen [Fisher et al., 2005]. Although these drugs can destroy dividing malignant cells, they usually cause side effects to the patients. Moreover, some differentiated tumor cells in a transient state may not be affected by the cytotoxic drugs and may account for tumor recurrence [Sinawat and Chiyabutra, 2004; Planas-Silva et al., 2007].
Herb extracts have been used as traditional medicines for cancer therapy. Recently studies showed that many herbs have been used against several cancer types such as breast, lung, colon, pancreatic and ovarian cancers [Lee and Houghton, 2005; McGovern et al, 2010; Tannin-Spitz et al., 2007; Usami et al, 2010]. Trichosanthes cucumerina Linn., a Thai medicinal plant, has been reported to have anti-inflammatory, antimicrobial and anticancer activities [Arawwawala, 2010; Wiwat and Silapa-archa, 1984; Kongtun et al., 2009]. In this report, we show that cucurbitacin B compound from T. cucumerina Linn. has anticancer property by inducing cell cycle arrest at G2/M as well as apoptosis. The underlying mechanisms of anticancer bioactivities of cucurbitacin B associated with Wnt signaling are also reported.
Material and Methods
Cell culture and extracts
Human breast cancer cell lines T47D, SKBR-3, MCF-7 and human breast epithelial cells HBL-100 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Meditech, Inc., Manassas, VA) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 50 U penicillin and streptomycin at 37°C in 5% CO2 humidified atmosphere. Trichosanthes cucumerina Linn. extracts including crude spray-dried extract, alcoholic fraction, bryonolic acid fraction and cucurbitacin B compound (approximately 95.96% of cucurbitacin B and 3.73% of dihydrocucurbitacin B) were obtained as previously described [Kongtun et al, 2009]. The T. cucumerina Linn. extracts was dissolved in 10% dimethylsulfoxide (DMSO) and diluted with DMEM to the desired concentrations prior to use, while DMSO alone was used as vehicle control.
Cell viability assay
Cell viability was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cells were seeded at 1×104 cells per well in 96-well plates (Becton, Dickinson and Company, Franklin Lakes, NJ) and allowed to attach overnight at 37°C. Each of DMEM containing T. cucumerina Linn. extracts was added to the cells and further incubated for 48 hours. The MTT assay was performed using a commercially available MTT assay kit (Millipore Corporation, Billerica, MA) according to the manufacturer’s instructions. The absorbance was measured at 570 nm using a microplate reader (Beckman Coulter, Mississauga, ON, Canada). Percent cell viability was calculated as (ODtreated/ODcontrol)×100. All experiments were performed in triplicate.
Apoptosis detection by Annexin V-FITC
FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, San Jose, CA) was used to determine apoptosis induction by T. cucumerina Linn. Cells were treated with crude spray-dried extract or cucurbitacin B for 24 hours and were then trypsinized and washed with phosphate buffer saline (PBS). The cells were resuspended in the staining solution containing Annexin V-FITC and propidium iodide (PI), mixed, and incubated for 10–15 min in the dark. Stained cells were analyzed by flow cytometry with Ex. = 488 nm and Em. = 530 nm for Annexin V-FITC detection (FL1 channel). Using a filter > 600 nm for PI detection (FL2 channel). Cells falling in the FITC (+)/PI(−) region are counted as early apoptotic cells.
Cell cycle analysis and DAPI staining
After treatment with cucurbitacin B, cells were harvested at indicated time intervals and fixed with 70% ethanol. Cells were then washed with PBS and stained with propidium iodide using the Cycle Test Plus DNA Reagent Kit (BD Biosciences, San Jose, CA) according to the manufacturer’s protocol. The DNA content was analyzed using flow cytometer (FACS Calibur, BD Biosciences) and CellQuest software (BD Biosciences). For apoptosis detection, cells were also grown on glass coverslips, fixed with 4% paraformaldehyde, washed with PBS, and stained with 300 nM 4,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). Images of the chromatin condensation and nuclear fragmentation were taken by a fluorescence microscope.
Real-time RT-PCR
Cells were seeded into 6-well plates and treated with cucurbitacin B at various concentrations for 24 hours. Total RNA was isolated from cell pellets using the TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Following reverse transcription, cDNA was subsequently amplified by real-time PCR using iQ SYBR Green Supermix (Bio-rad, Hercules, CA). The primers for PCR are shown in Table 1. The PCR was analyzed using the iCycler iQ Multi-color Real-time PCR detection system (Bio-rad). The β-actin expression was used for normalization. Relative expression of the target genes were calculated on the basis of the difference between treated and control.
Table 1.
The primer sequences for Real-time PCR
| Genes | Primers
|
Fragment Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| c-Myc | 5′ACCACCAGCAGCGACTCTGA3′ | 5′TCCAGCAGAAGGTGATCCAGACT3′ | 117 |
| β-catenin | 5′AAAGCGGCTGTTAGTCACTGG3′ | 5′GACTTGGGAGGTATCCACATCC3′ | 132 |
| Cyclin D1 | 5′AATGACCCCGCACGATT3′ | 5′GCACAGAGGGCAACGAAGG3′ | 108 |
| β-actin | 5′GATCATTGCTCCTCCTGAGC3′ | 5′ACTCCTGCTTGCTGATCCAC3′ | 101 |
Cellular protein fractionation and immunoblotting
Fractionated nuclear and cytosolic protein lysates were obtained as previously described [Hsu and Hung, 2007]. Briefly, after cells were treated with cucurbitacin B or vehicle control for 24 hours, They were harvested in ice-cold PBS, washed twice, and homogenized using a tightly fitting Dounce homogenizer. The cytoplasmic lysis buffer contains 10 mM KCl, 2 mM MgCl2, 0.5% Nonidet P-40 and 20 mM HEPES, pH7.0 and supplemented with Complete Protease Inhibitor Mix (Roche Molecular Biochemicals, Germany). The homogenate was centrifuged at 1,500 × g for 5 min to sediment the nuclei. The supernatant was then centrifuged at 14,000 × g for 20 min to get the non-nuclear supernatant fraction. The nuclear pellet was then washed three times with cytoplasmic lysis buffer and resuspended in NETN buffer (150 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40 and 20 mM Tris-Cl, pH8.0 and supplemented with Complete Protease Inhibitor Mix). The mixture was sonicated briefly to aid nuclear lysis. The nuclear lysates were collected after centrifugation at 14,000 × g for 20 min at 4°C.
To obtain whole-cell lysates, cells were harvested and washed with PBS, lysed in ice-cold lysis buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 1mM EDTA, 0.25% Na-Deoxycholate and Complete Protease Inhibitor Mix (Roche Molecular Biochemicals) for 20 min and centrifuged at 14,000 ×g at 4°C for 15 min. The protein lysates were then resolved by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (GE Healthcare Bio-Science Corp., Piscataway, NJ). After blocked with 5% nonfat dry milk in TBST, the membrane was incubated with the following primary antibodies: PARP, β-catenin, phosphorylated GSK-3β and GAPDH antibodies were purchased from Cell Signaling Technology (Danvers, MA); cyclin D1, c-myc, galectin-3 and GSK-3β antibodies were from Santa Cruze Biotechnology (Santa Cruz, CA). Blots were then washed and followed by incubation with suitable horseradish peroxidase-conjugated secondary antibodies, either the anti-rabbit IgG antibody (Cell Signaling Technology) or anti-mouse IgG antibody (GE Healthcare Bio-Science Corp.). The signal was detected using an enhanced chemiluminescence detection system (Pierce Biotechnology, Rockford, IL).
Immunofluorescent staining
Cells were grown on glass coverslips prior to the treatment with cucurbitacin B. The treated cells were fixed with 4% paraformaldehyde for 15 min, followed by permeabilization with ice-cold 100% methanol for 10 min. These cells were blocked with 5% normal goat serum in PBS/Triton for 60 min, then incubated for 60 min with primary antibody (diluted 1:200 in 1% NGS) and washed three times with PBS. The cells were then incubated with secondary antibody. The secondary antibody consists of 1:200 dilution of either Alexa Fluor 555 Goat anti Mouse or Alexa Fluor 488 Goat anti Rabbit from Invitrogen and 300 nM DAPI (Invitrogen). After mounting, the slides were examined for localization of certain proteins using fluorescence microscope.
TCF Reporter assay
TCF-reporter plasmids (TOPFLASH and the negative control counterpart FOPFLASH) and Renilla luciferase plasmid were kindly provided by Dr. Subhas Chakrabarty (Southern Illinois University School of Medicine, Springfield, IL). Cells were seeded in 6-well plates and the reporter plasmids contain TCF binding sites (TOPFLASH) or mutant, inactive TCF binding sites (FOPFLASH) was transiently transfected into cells as described previously [Chakrabarty et al., 2003]. Twenty four hours after cucurbitacin B treatment, TCF-mediated gene transcription was determined by the ratio of TOPFLASH:FOPFLASH luciferase activity, and each was normalized to the renilla luciferase activity (co-transfected as internal control). All experiments were performed in triplicate.
Statistical analysis
Data were expressed as mean ± standard error of the mean (S.E.M.). All experiments were performed at least three times with similar results. Statistically significant difference between the two groups was assessed using a two-tailed unpaired Student’s t-test. P value of less than 0.05 was considered to indicate a difference of statistically significant.
Results
Effect of T. cucumerina extracts on breast cancer cell growth
To evaluate the effect of T. cucumerina extracts on breast cancer cell proliferation, we initially treated ER+ cells (T47D and MCF-7) and ER- cells (SKBR-3 and HBL-100) with various concentrations of the following T. cucumerina extracts: crude spray-dried, alcoholic fraction, bryonolic acid fraction and cucurbitacin B compound. Cell viability was measured using an MTT assay after 48 hours of treatment. Cucurbitacin B showed the highest growth inhibition (Fig. 1D) while the bryonolic acid fraction showed the least effect on breast cancer cell growth (Fig. 1C). The viability of SKBR-3 cells was decreased the most among all cell types in all fractions (Fig. 1). Cucurbitacin B had a strong growth inhibitory effect on breast cancer cell lines, with an IC50 of 4.60 μg/ml, 55.46 μg/ml, 88.75 μg/ml and >100 μg/ml on SKBR-3, HBL-100, MCF-7 and T47D cells, respectively. These data suggest that cucurbitacin B is the most efficient active component among the four fractions from T. cucumerina for inhibiting proliferation. Moreover, our results indicate that the SKBR-3 cancer cell line is more sensitive to T. cucumerina extract than the ER- epithelium cell line HBL-100 and SKBR-3 is also more sensitive than the other two ER+ cells, T47D and MCF-7.
Figure 1.
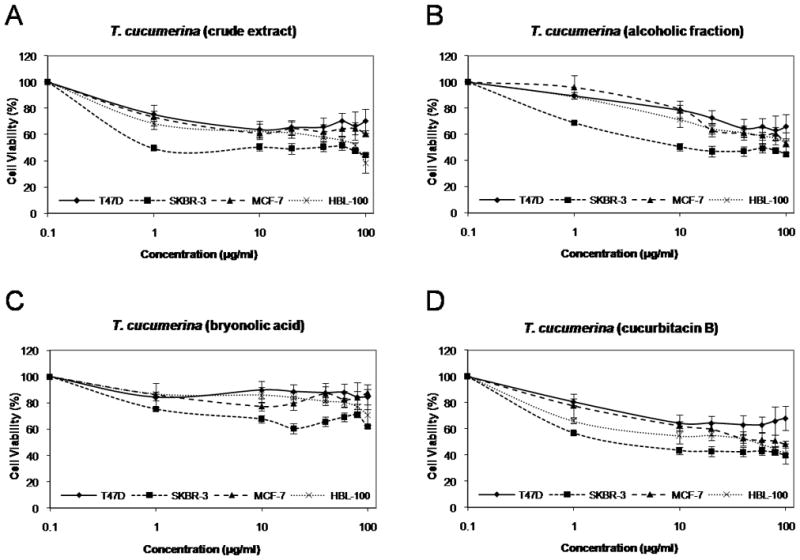
Effect of T. cucumerina extracts on the growth of breast cancer cells. Cell number was measured using the MTT assay after treatment with various T. cucumerina extracts; A: crude spray-dried extract, B: alcoholic fraction, C: bryonolic acid fraction or D: cucurbitacin B compound.
Cucurbitacin B promotes cell cycle arrest at G2/M and induces apoptosis
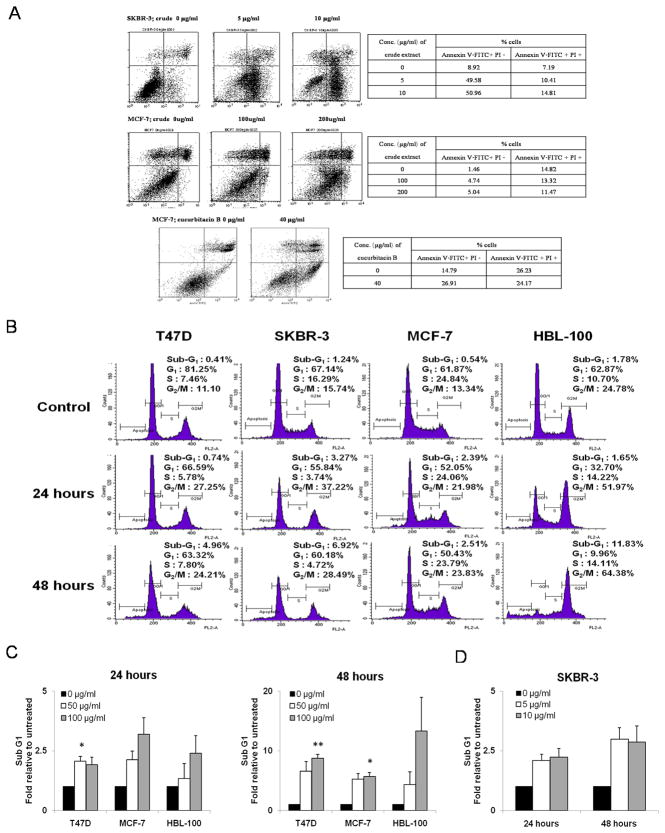
Treatment of SKBR-3 with T. cucumerina crude spray-dried extract and MCF-7 with either crude or cucurbitacin B was found to result in apoptosis after 24 hours. The apoptosis was verified using Annexin V-FITC and PI staining and determined by FACS. The percentage of cells in both early and late apoptosis was increased in a dose-dependent manner (Fig. 2A). In order to determine whether purified cucurbitacin B mediates cell cycle arrest and apoptosis, SKBR-3, HBL-100, MCF-7 and T47D cells were treated with cucurbitacin B compound for 24 and 48 hours. Treatment with cucurbitacin B caused cell cycle arrest at G2/M phase. The percentage of cells in the G2/M phase increased up to two-fold in the treated cells compared with the control cells (Fig. 2B). The sub G1 population, which is one of the hallmarks of apoptosis was also measured. Exposure of cells with cucurbitacin B increased the number of cells in the sub G1 population in a time- and dose-dependent manner (Fig. 2C and 2D). Apoptosis was induced up to 3- and 13-fold after 24 and 48 hours, respectively, of cucurbitacin B treatment.
Figure 2.
Cucurbitacin B promotes G2/M phase arrest and induces apoptosis. A: Percent positive cells after staining with Annexin V-FITC and PI after SKBR-3 and MCF-7 were treated with crude spray-dried T. cucumerina Linn. or cucurbitacin B for 24 hours. Percentage of Annexin V-FITC (+)/ PI (−) cells indicates the early apoptotic fraction and Annexin V-FITC (+)/ PI (+) indicates cells in late apoptosis. B: Cell cycle analysis was performed in T47D, SKBR-3, MCF-7 and HBL-100 cell lines treated with cucurbitacin B at half maximal concentration (IC50). Cell cycle/DNA content was analyzed by flow cytometry after 24 and 48 hours. The percentages of cells at each cell cycle phase are shown in each panel. C: Sub G1 population relative to untreated cells was measured when treated T47D, MCF-7 and HBL-100 with 50 and 100 µg/ml. D: SKBR-3 with 5 and 10 µg/ml of cucurbitacin B (10-times less concentration) for 24 and 48 hours. * P< 0.05, ** P< 0.01 compared with the untreated control.
Morphological changes, apoptotic bodies, nuclear fragmentation and PARP cleavage induced by cucurbitacin B
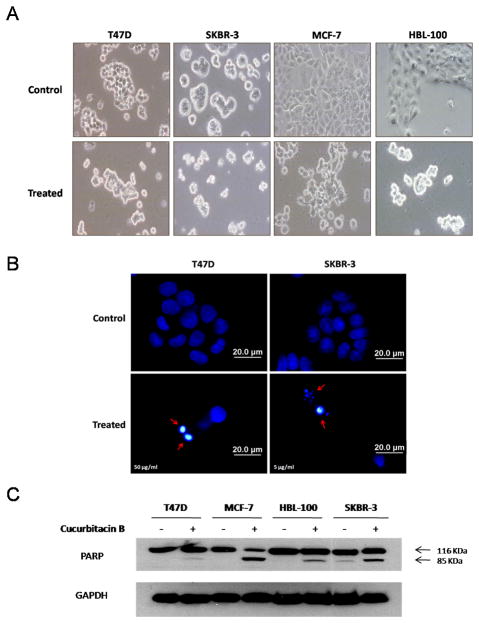
Cucurbitacin B induces morphological changes of breast cancer cells as observed by phase contrast microscopy. The cells became round up and shrank within 24 hours of the treatment (Fig. 3A). Apoptotic induction by cucurbitacin B was confirmed with DAPI nuclear staining. Regarding to more sensitivity of SKBR-3 to cucurbitacin B, SKBR-3 cells were exposed to 5 μg/ml while T47D cells and the other cell lines were exposed to 50 μg/ml cucurbitacin B for 24 hours prior to the staining. Increased fluorescent intensity of nuclear chromatin indicated nuclear condensation, fragmentation and apoptotic bodies (arrow head) in SKBR-3 and T47D are indicated in Fig. 3B. Another important apoptotic marker, PARP cleavage, was assessed by immunoblot with an antibody recognizing total PARP at 116 kDa and cleaved PARP at 85 kDa. The cleaved PARP was found in SKBR-3 treated with 10 μg/ml cucurbitacin B and in T47D, MCF-7 and HBL-100 treated with 100 μg/ml cucurbitacin B (Fig. 3C). These data, in combination with the results from flow cytometry indicate that cucurbitacin B promotes both cell cycle arrest and apoptosis.
Figure 3.
Morphological changes, apoptotic bodies, nuclear fragmentation and PARP cleavage induced by cucurbitacin B. Cells were treated with 50 µg/ml (T47D, MCF-7, HBL-100) and 5 µg/ml (SKBR-3) for 24 hours. A: Phase-contrast photomicrographs of untreated control (upper panel) and treated cells (lower panel). B: Cucurbitacin B treated cells were stained with DAPI to differentiate non-apoptotic from apoptotic cells (arrows). Staining was analyzed by fluorescence microscopy. C: Cell lysates were analyzed by Western blotting using anti-PARP which detects both intact PARP (116 kDa) and apoptotic marker PARP cleavage fragment (85 kDa). GAPDH was used as the loading control.
Expressions of c-Myc, β-catenin and cyclin D1 are abolished by cucurbitacin B
To determine whether cucurbitacin B affects expression of Wnt associated genes, mRNA levels of c-Myc, β-catenin and cyclin D1 were measured by real-time RT-PCR. Cucurbitacin B could inhibit expression of the three genes (Fig.4). Among the four cell lines, SKBR-3 exhibited the most reduction of mRNA transcripts upon cucurbitacin B treatment (Fig.4B). The concentration used in SKBR-3 was ten-times lower than the other cell lines.
Figure 4.
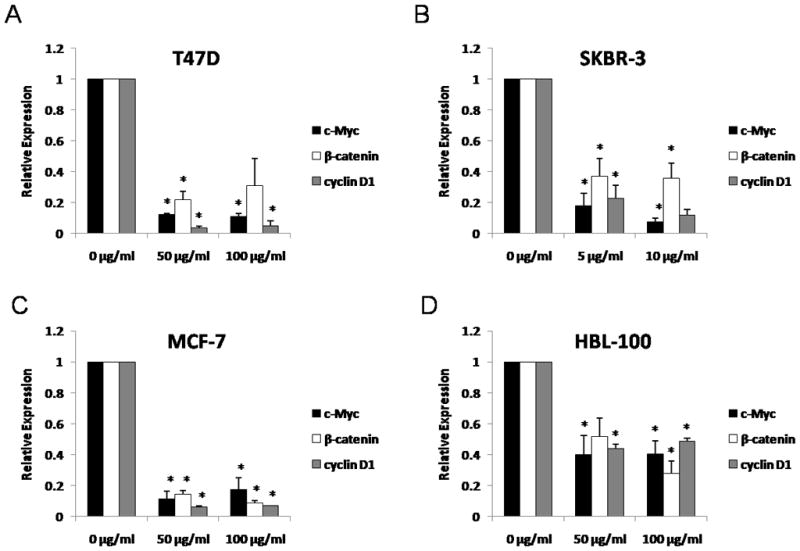
Expressions of c-Myc, β-catenin and cyclin D1 after cucurbitacin B treatment. A: T47D, B: SKBR-3, C: MCF-7 and D: HBL-100 cells were incubated with the specified concentrations of cucurbitacin B for 24 hours and the quantitative expression levels of c-Myc, β-catenin, and cyclin D1 were analyzed by real-time RT-PCR. Results shown are the average of three independent experiments. * P< 0.05 compared with the control group.
Cucurbitacin B inhibits canonical Wnt signaling by decreasing phosphorylated GSK-3β and reducing downstream effectors of Wnt
For further understanding the mechanisms by which cucurbitacin B inhibits cellular proliferation and induces apoptosis, we have studied on the Wnt signaling. This pathway regulates both cell growth and apoptosis [Pecina-Slaus, 2010]. Immunoblots from 24 hour-treated cell lysates were performed to determine the levels of Wnt signaling-associated proteins. β-catenin, the potential modulator of Wnt signaling, as well as the downstream targets of Wnt signaling, cyclin D1 and c-Myc were noticeably reduced compared to the untreated control (Fig. 5A). Galectin-3 protein expression was decreased in cells treated for 24 hours with cucurbitacin B and this correlated with the reduction of phosphorylated GSK- 3β (at Ser9) levels (Fig. 5B).
Figure 5.
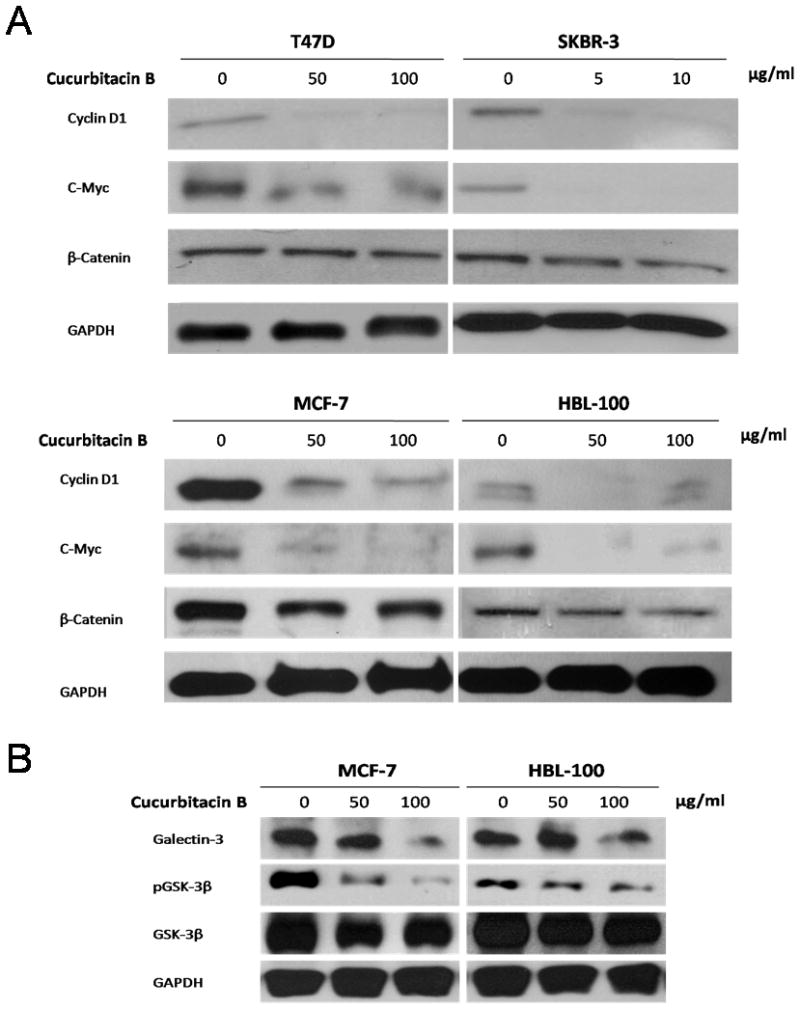
Differential expression of proteins associated with Wnt signaling. Western blot analysis was performed to compare expression levels of A: cyclin D1, c-Myc and β-catenin or B: galectin-3, GSK-3β and phosphorylated GSK-3β proteins among cucurbitacin B treated and untreated cells. Expression of GAPDH was used as protein loading control.
Inhibition of nuclear translocation of β-catenin and galectin-3 by cucurbitacin B
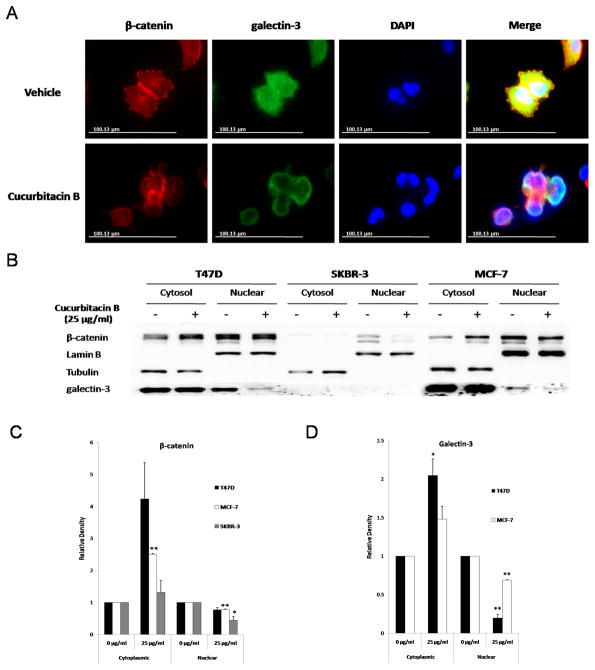
As cucurbitacin B was found to affect Wnt signaling proteins, we hypothesized that it may also inhibit translocation of β-catenin and galectin-3 to the nucleus and so disrupt a ternary complex of β-catenin, galectin-3 and TCF-4. We examined subcellular localization of β-catenin and galectin-3 in cucurbitacin B treated and untreated conditions. By immunofluorescent staining, both β-catenin and galectin-3 were distributed in the cytoplasm and in the nucleus in cells grown in control medium, whereas in the presence of 25 μg/ml cucurbitacin B, β-catenin and galectin-3 were localized within the cytoplasm and at the plasma membrane (Fig. 6A). This result was confirmed by Western blot analysis in which β-catenin and galectin-3 were increased in the cytoplasmic fraction while being decreased in the nuclear fraction in treated cells (Fig. 6B). Relative density of cytoplasmic and nuclear proteins was shown in Fig. 6C. A remarkable decreased nuclear β-catenin level was observed in SKBR-3 more than in T47D and MCF-7. To determine whether cucurbitacin B exerts its effect on the binding of β-catenin, galectin-3 and TCF-4, we performed immunoprecipitation using either anti-β-catenin or anti-galectin-3. We found that cucurbitacin B had no effect on a ternary complex of these three proteins (data not shown). Therefore, it is more likely that cucurbitacin B mediates its effect by inhibiting the translocation of β-catenin and galectin-3 to the nucleus rather than by disrupting β-catenin/galectin-3/TCF-4 complex formation itself.
Figure 6.
Nuclear translocation of β-catenin and galectin-3 was inhibited by cucurbitacin B. A: T47D cells were treated either with 25 µg/ml cucurbitacin B or control medium for 24 hours, fixed and immunostained with anti-β-catenin and anti-galectin-3 antibodies. Immunostaining was analyzed using fluorescence microscope. B: Three breast cancer cells T47D, SKBR-3 and MCF-7 were treated with 25 µg/ml cucurbitacin B for 24 hours. Cellular fractionation was carried out to determine the cellular localization of β-catenin and galectin-3. Lamin B was used as loading control for nuclear fraction and tubulin as control for cytoplasmic fraction. C: Relative density of cytoplasmic and nuclear proteins was analyzed. Results shown are the average of three independent experiments. * P< 0.05, ** P< 0.01 compared with the vehicle control.
TCF-dependent transcriptional activity, a downstream effector of Wnt signaling was decreased in cucurbitacin B treated cells
To confirm that cucurbitacin B down-regulates expression of Wnt target genes via inhibition of TCF-regulated promoters, we determined TCF reporter activity by transfection of TOPFLASH or FOPFLASH into T47D and SKBR-3 breast cancer cells. After treatment with 25 μg/ml cucurbitacin B, luciferase activities were measured and normalized with renilla luciferase. The relative TCF-reporter activities were significantly decreased from 100% (control) to 73.61 and 60.00% in T47D and SKBR-3, respectively (P<0.05) (Fig. 7). These results support our hypothesis that cucurbitacin B obstructs Wnt signaling by inhibition of translocation of β-catenin and galectin-3 to the nucleus and thus mediates down regulation of Wnt signaling targets.
Figure 7.
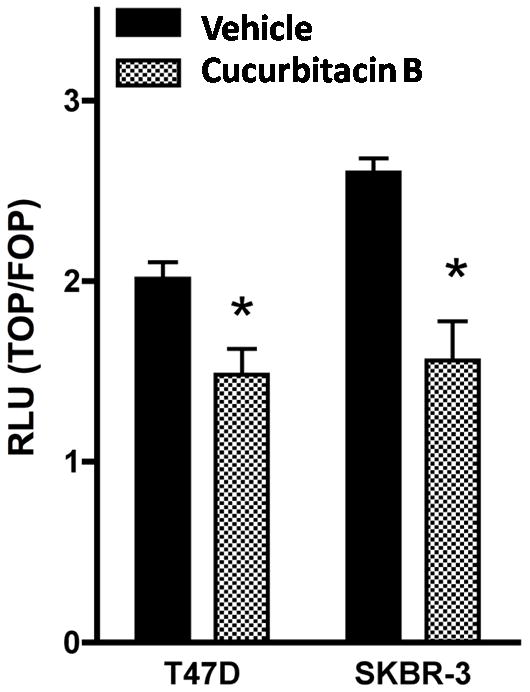
TCF reporter activity was decreased in cucurbitacin B treated cells. T47D and SKBR-3 were transiently transfected with either TOPFLASH or FOPFLASH together with renilla (as control expression). Cells were then treated with 25 µg/ml cucurbitacin B or vehicle control medium for 24 hours. Luciferase assay was measured and results were expressed as the ratio of TOPFLASH over FOPFLASH activity. The significance of the difference in the repression of TOPFLASH by vehicle and cucurbitacin B was analyzed using a paired Student’s t test. * P< 0.05 compared with the vehicle control.
Discussion
Cucurbitacins are natural biochemical compounds isolated from plants in the cucurbitaceae family. They are chemically classified as steroids, and formally derived from cucurbitane, a triterpene hydrocarbon. Twelve cucurbitacin sub-family members are divided according to their structural characteristics [Chen et al., 2005] Cucurbitacins have been reported to have anti-malarials, anti-inflammatory and anticancer activities [Ramalhete et al., 2010; Siqueira et al., 2007; Recio et al., 2004; Escandell et al., 2007; Jayaprakasam et al., 2003]. Indeed, cucurbitacins have been shown to have biological effects in multiple signal transduction pathways. For example, cucurbitacin E significantly inhibits cell proliferation, migration and tubulogenesis in vitro. An in vivo analysis revealed that cucurbitacin E blocks angiogenesis by inhibiting VEGFR2-mediated Jak-STAT3 and mitogen-activated protein kinases (MAPK) signaling pathways [Dong et al., 2010]. Another study showed that cucurbitacin B potentially inhibits STAT3 activation and the Raf/MEK/ERK pathway in leukemic cell line K562, which accounted for the obstruction of cellular proliferation and apoptosis induction [Chan et al., 2010].
Here we report the anticancer activity of cucurbitacin B compound, the most abundant active ingredients from Trichosanthes cucumerina Linn [Kongtun et al., 2009]. Previous studies have shown that both cucurbitacin B and dihydrocucurbitacin B exhibit antiproliferative effect, induce apoptosis against a variety of cancer cell types such as pancreatic, breast, glioblastoma and leukemia [Chan et al., 2010; Thoennissen et al., 2009; Wakimoto et al., 2008; Duangmano et al., 2010; Yin et al., 2008]. The cucurbitacin B compounds exert their biological effects mostly through interference of the signal transducers and activators of transcription (STAT) signal [Chan et al., 2010; Thoennissen et al., 2009] or disruption of cytoskeleton structure including F-actin filament [Wakimoto et al., 2008; Yin et al., 2008]. Glioblastoma cells obviously change their morphology within 15–30 min after exposure to cucurbitacin B. The cells become to round up and lose their pseudopodia. Disruption of actin and microtubules has been observed by immunoflourescence [Yin et al., 2008]. Similar morphological changes were also found in our results, where breast cancer cells exposed to cucurbitacin B became oval, shrank and formed clumps (Fig. 3A) due to morphological changes. Twenty-four hour-treated cells exhibited nuclear condensation, fragmentation and apoptotic bodies (Fig. 3B). Cucurbitacin B led to an accumulation of cells in G2/M phase (Fig. 2B) as well as an increase in PARP cleavage (Fig. 3C), indicating that this compound induces both growth inhibition and apoptosis. The same changes have also been demonstrated by the most potent microtubule stabilizing drugs such as paclitaxel [Ling et al., 2004; Blagosklonny and Fojo, 1999].
In human breast cancer, the evidence of β-catenin accumulation in cancer cells implies that Wnt signaling is active in over 50% of cases of this carcinoma. β-catenin acts by regulating transcription from a number of Wnt target genes including the oncogenes cyclin D1 and c-Myc [Brennan and Brown, 2004; Smalley and Dale, 2001]. Galectin-3 plays important role in this, as a β-catenin binding partner, assisting nuclear β-catenin accumulation, and activation TCF activity which positively regulates cyclin D1 and c-myc expression. [Shimura et al., 2004]. Interestingly, we found that cucurbitacin B inhibits expression of Wnt downstream effectors cyclin D1, c-Myc, β-catenin and galectin-3 (Fig. 5A and 5B). Exposure of breast cancer cells to cucurbitacin B led to a concurrent reduction in the expression of galectin-3 and phosphorylated GSK-3β (Fig. 5B). Therefore, our results suggest that cucurbitacin B down-regulates galectin-3, resulting in decrease phosphorylated GSK-3β and reduction of Wnt downstream effectors of β-catenin, cyclin D1 and c-Myc. Furthermore, treatment with cucurbitacin B caused inhibition of nuclear translocation of β-catenin and galectin-3 (Fig. 6) and reduction of TCF-reporter activity (Fig. 7). Although results suggest that cucurbitacin B obstructs Wnt/beta-catenin signaling and so inhibits TCF reporter activity, it is also possible that the reduction of TCF reporter activity might be partially caused by cucurbitacin B induced apoptosis. Noteworthy, the nuclear β-catenin levels were obviously reduced in ER-(SKBR-3) cells comparing with the ER+ (T47D and MCF-7) cells (Fig. 6B). This finding could be the good explanation why a stronger cucurbitacin B inhibition was seen in ER- cells than the ER+ cell lines in the cell viability tests (Fig. 1). Our results are consistent with previous studies showing that decreased galectin-3 expression in Gal-3 knockdown cells is associated with decreased phosphorylation of GSK-3β, decreased in total β-catenin and cyclin D1, inhibited TCF-reporter activity and induced apoptosis [Song et al., 2009; Shi et al., 2007]. However, since β-catenin and galectin-3 expression are relatively low in the estrogen receptor negative cell line SKBR-3, it is possible that the inhibiting effects of cucurbitacin B on nuclear translocation of β-catenin and galectin-3 is rather the estrogen receptor positive cell specific effects.
Taken together, we show for the first time the strong evidence that cucurbitacin B compound from T. cucumerina Linn. can induce apoptotic cell death and growth inhibition, at least in part, through the Wnt signaling pathway. Cucurbitacin B has more effects on estrogen receptor negative cells than estrogen receptor positive cells (Fig. 1). However, both estrogen receptor and c-Myc (downstream targets of Wnt signaling) are shown to increase hTERT mRNA levels and activate telomerase activity through estrogen responsive elements (ERE) and through the c-Myc binding site on the hTERT promoter region, respectively [Ducrest et al. 2002]. We believe that estrogen receptor in hormone dependent breast cancers may restore the growth inhibition effects of cucurbitacin B by activating telomerase activity. Therefore, the estrogen receptor negative cells are more sensitive to cucurbitacin B than the estrogen receptor positive cells.
From our results, cucurbitacin B showed a significant growth inhibitory effect against ER-cells (SKBR-3 and HBL-100) but less effect against ER+ cells (T47D and MCF-7), implying that ER- cells are more sensitive to the action of cucurbitacin B. Among ER- cells, human breast cancer cell line SKBR-3 is more vulnerable to cucurbitacin B than mammary epithelium cell line HBL-100. Duangmano et al. (2010) reported that telomerase activity was obviously reduced in ER- SKBR-3 cells, although the concentration of cucurbitacin B used for this SKBR-3 cancer cells was ten-times lower than those used for HBL-100 mammary epithelium cells. Accordingly, we believed that cucurbitacin B could be a good choice for treatment of estrogen receptor negative breast cancer since the ER negative cancer type is rather aggressive and resisted to some hormone therapy (estrogen receptor inhibitor based regimens).
Some other reports have shown that small molecule inhibitors of the Wnt signaling, such as CGP049090 and PKF115-584, significantly inhibit cell growth and induce apoptosis either in vitro or in vivo in leukemia and hepatocellular carcinoma [Minke et al., 2009; Gandhirajan et al., 2010; Wei et al., 2010]. The Wnt antagonists inhibit the formation of the β-catenin/TCF-4 complex and so its transcriptional activity, associated with downregulation of β-catenin/TCF-4 target genes c-Myc, cyclin D1 and survivin [Wei et al., 2010]. In contrast, we have found that cucurbitacin B inhibits the Wnt pathway by decreasing nuclear translocation of β-catenin and galectin-3 rather than disruption of β-catenin/galectin-3/TCF-4 complex. Therefore, the treatment with cucurbitacin B and antagonist of β-catenin/TCF-4 may lead to synergistic effects on suppressing canonical Wnt signaling and inducing apoptosis. In summary, our data indicate that cucurbitacin B targeting of the Wnt signaling pathway could be an innovative approach for breast cancer treatment.
Supplementary Material
HPLC chromatogram of the cucurbitacin B compound extracted from T. cucumerina Linn. The cucurbitacin B compound contains the following composition: cucurbitacin B 95.96%, dihydrocucurbitacin B 3.73% and other coexisting compound 0.31%.
Cucurbitacin B induces apoptosis. Percent Annexin V-FITC and PI positive cells after SKBR-3 were treated with cucurbitacin B for 24 hours. Percentage of Annexin V-FITC (+)/ PI (−) cells indicates the early apoptotic fraction and Annexin V-FITC (+)/ PI (+) indicates cells in late apoptosis.
Acknowledgments
Grant sponsor: Commission on Higher Education, Ministry of University Affairs, Thailand; Grant sponsor: Mahidol University Research Fund (National Budget); Grant number: 49439 to P.P.; Grant sponsor: National Cancer Institute/National Institutes of Health; Grant number: RO1CA128991 to O.B.; Grant sponsor: University of Texas M. D. Anderson Cancer Center.
S.D. is supported by a scholarship under the Commission on Higher Education, Ministry of University Affairs, Thailand. This work was supported by a Mahidol University Research Fund (National Budget), project #49439 grant to P.P., the National Cancer Institute/National Institutes of Health grant RO1CA128991 to O.B. and the University of Texas M. D. Anderson Cancer Center.
Abbreviations
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IC50
the half maximal inhibitory concentration
- PARP
poly ADP ribose polymerase
- GSK-3β
glycogen synthase kinase-3β
- TCF
T-cell factor
- LEF
lymphoid enhancer factor
- APC
adenomatous polyposis coli
- ER
estrogen receptor
- FITC
fluorescein isothiocyanate
- DAPI
4,6-diamidino-2-phenylindole
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- EDTA
ethylenediaminetetraacetic acid
- SDS
sodium dodecyl sulfate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IgG
immunoglobulin G
- S.E.M.
standard error of the mean
- FACS
fluorescence-activated cell sorting
- VEGFR
vascular endothelial growth factor receptor
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- MAPK
mitogen-activated protein kinases
- ERK
extracellular signal-regulated kinase
- Gal-3
galectin-3
References
- Arawwawala M, Thabrew I, Arambewela L, Handunnetti S. Anti-inflammatory activity of Trichosanthes cucumerina Linn. in rats. J Ethnopharmacol. 2010;131:538–543. doi: 10.1016/j.jep.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int J Cancer. 1999;83:151–156. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- Brown AM. Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res. 2001;3:351–355. doi: 10.1186/bcr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- Chan KT, Li K, Liu SL, Chu KH, Toh M, Xie WD. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 2010;289:46–52. doi: 10.1016/j.canlet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- Dihlmann S, von Knebel Doeberitz M. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li D, Wu Y, Song Y, Luo J, Pang X, Yi Z, Liu M. Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis. 2010;31:2097–2104. doi: 10.1093/carcin/bgq167. [DOI] [PubMed] [Google Scholar]
- Duangmano S, Dakeng S, Jiratchariyakul W, Suksamrarn A, Smith DR, Patmasiriwat P. Antiproliferative effects of cucurbitacin B in breast cancer cells: down-regulation of the c-Myc/hTERT/Telomerase pathway and obstruction of the cell cycle. Int J Mol Sci. 2010;11:5323–5338. doi: 10.3390/ijms11125323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducrest AL, Szutorisz H, Lingner J, Nabholz M. Regulation of the human telomerase reverse transcriptase gene. Oncogene. 2002;21:541–552. doi: 10.1038/sj.onc.1205081. [DOI] [PubMed] [Google Scholar]
- Escandell JM, Recio MC, Manez S, Giner RM, Cerda-Nicolas M, Rios JL. Cucurbitacin R reduces the inflammation and bone damage associated with adjuvant arthritis in lewis rats by suppression of tumor necrosis factor-alpha in T lymphocytes and macrophages. J Pharmacol Exp Ther. 2007;320:581–590. doi: 10.1124/jpet.106.107003. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Gandhirajan RK, Staib PA, Minke K, Gehrke I, Plickert G, Schlosser A, Schmitt EK, Hallek M, Kreuzer KA. Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia. 2010;12:326–335. doi: 10.1593/neo.91972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LJ, Dawson CK, Lawrence DH, Bliss JM. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2007;1:1–17. doi: 10.1002/14651858.CD003370.pub2. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem. 2007;282:10432–10440. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- Jayaprakasam B, Seeram NP, Nair MG. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett. 2003;189:11–16. doi: 10.1016/s0304-3835(02)00497-4. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Kongtun S, Jiratchariyakul W, Kummalue T, Tan-ariya P, Kunnachak S, Frahm AW. Cytotoxic properties of root extract and fruit juice of Trichosanthes cucumerina. Planta Med. 2009;75:839–842. doi: 10.1055/s-0029-1185455. [DOI] [PubMed] [Google Scholar]
- Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100:237–243. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Ling X, Bernacki RJ, Brattain MG, Li F. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J Biol Chem. 2004;279:15196–15203. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- McGovern PE, Christofidou-Solomidou M, Wang W, Dukes F, Davidson T, El-Deiry WS. Anticancer activity of botanical compounds in ancient fermented beverages (review) Int J Oncol. 2010;37:5–14. doi: 10.3892/ijo_00000647. [DOI] [PubMed] [Google Scholar]
- Minke KS, Staib P, Puetter A, Gehrke I, Gandhirajan RK, Schlosser A, Schmitt EK, Hallek M, Kreuzer KA. Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur J Haematol. 2009;82:165–175. doi: 10.1111/j.1600-0609.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Pecina-Slaus N. Wnt signal transduction pathway and apoptosis: a review. Cancer Cell Int. 2010;10:22. doi: 10.1186/1475-2867-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas-Silva MD, Bruggeman RD, Grenko RT, Smith JS. Overexpression of c-Myc and Bcl-2 during progression and distant metastasis of hormone-treated breast cancer. Exp Mol Pathol. 2007;82:85–90. doi: 10.1016/j.yexmp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Ramalhete C, Lopes D, Mulhovo S, Molnar J, Rosario VE, Ferreira MJ. New antimalarials with a triterpenic scaffold from Momordica balsamina. Bioorg Med Chem. 2010;18:5254–5260. doi: 10.1016/j.bmc.2010.05.054. [DOI] [PubMed] [Google Scholar]
- Recio MC, Prieto M, Bonucelli M, Orsi C, Manez S, Giner RM, Cerda-Nicolas M, Rios JL. Anti-inflammatory activity of two cucurbitacins isolated from Cayaponia tayuya roots. Planta Med. 2004;70:414–420. doi: 10.1055/s-2004-818968. [DOI] [PubMed] [Google Scholar]
- Shi Y, He B, Kuchenbecker KM, You L, Xu Z, Mikami I, Yagui-Beltran A, Clement G, Lin YC, Okamoto J, Bravo DT, Jablons DM. Inhibition of Wnt-2 and galectin-3 synergistically destabilizes beta-catenin and induces apoptosis in human colorectal cancer cells. Int J Cancer. 2007;121:1175–1181. doi: 10.1002/ijc.22848. [DOI] [PubMed] [Google Scholar]
- Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363–6367. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- Sinawat S, Chiyabutra T. Increased risk of endometrial abnormalities in breast cancer patients taking tamoxifen: the need for gynaecologic surveillance. Asian Pac J Cancer Prev. 2004;5:183–187. [PubMed] [Google Scholar]
- Siqueira JM, Jr, Peters RR, Gazola AC, Krepsky PB, Farias MR, Rae GA, de Brum-Fernandes AJ, Ribeiro-do-Valle RM. Anti-inflammatory effects of a triterpenoid isolated from Wilbrandia ebracteata Cogn. Life Sci. 2007;80:1382–1387. doi: 10.1016/j.lfs.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Dale TC. Wnt signaling and mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:37–52. doi: 10.1023/a:1009564431268. [DOI] [PubMed] [Google Scholar]
- Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–1349. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem Pharmacol. 2007;73:56–67. doi: 10.1016/j.bcp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Thoennissen NH, Iwanski GB, Doan NB, Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, Said JW, Koeffler HP. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009;69:5876–5884. doi: 10.1158/0008-5472.CAN-09-0536. [DOI] [PubMed] [Google Scholar]
- Usami Y, Nakagawa-Goto K, Lang JY, Kim Y, Lai CY, Goto M, Sakurai N, Taniguchi M, Akiyama T, Morris-Natschke SL, Bastow KF, Cragg G, Newman DJ, Fujitake M, Takeya K, Hung MC, Lee EY, Lee KH. Antitumor Agents 282. 2′-(R)-O-acetylglaucarubinone, a quassinoid from Odyendyea gabonensis as a potential anti-breast and anti-ovarian cancer agent. J Nat Prod. 2010;73:1553–1558. doi: 10.1021/np100406d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto N, Yin D, O’Kelly J, Haritunians T, Karlan B, Said J, Xing H, Koeffler HP. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008;99:1793–1797. doi: 10.1111/j.1349-7006.2008.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Chua MS, Grepper S, So S. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int J Cancer. 2010;126:2426–2436. doi: 10.1002/ijc.24810. [DOI] [PubMed] [Google Scholar]
- Wiwat C, Silapa-archa W. The antimicrobial activities of Cucurbitaceous plants. Mahidol Univ J Pharm Sci. 1984;11:12–18. [Google Scholar]
- Yin D, Wakimoto N, Xing H, Lu D, Huynh T, Wang X, Black KL, Koeffler HP. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int J Cancer. 2008;123:1364–1375. doi: 10.1002/ijc.23648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPLC chromatogram of the cucurbitacin B compound extracted from T. cucumerina Linn. The cucurbitacin B compound contains the following composition: cucurbitacin B 95.96%, dihydrocucurbitacin B 3.73% and other coexisting compound 0.31%.
Cucurbitacin B induces apoptosis. Percent Annexin V-FITC and PI positive cells after SKBR-3 were treated with cucurbitacin B for 24 hours. Percentage of Annexin V-FITC (+)/ PI (−) cells indicates the early apoptotic fraction and Annexin V-FITC (+)/ PI (+) indicates cells in late apoptosis.