Abstract
Serum ferritin (SF) levels are commonly elevated in patients with nonalcoholic fatty liver disease (NAFLD), due to systemic inflammation, increased iron stores or both. The aim of this study was to examine the relationship between elevated SF and NAFLD severity. Demographic, clinical, histologic, laboratory and anthropometric data were analyzed in 628 adult patients with NAFLD (age≥18 years) with biopsy-proven NAFLD and a serum ferritin measurement within six months of their liver biopsy. A threshold SF>1.5XULN (i.e. >300 ng/ml in women and >450 ng/ml in men) was significantly associated with male sex, elevated serum ALT, AST, iron, transferrin-iron saturation, iron stain grade and decreased platelets (p<0.01). Histologic features of NAFLD were more severe among patients with SF>1.5XULN including steatosis, fibrosis, hepatocellular ballooning and diagnosis of NASH (p<0.026). On multiple regression analysis, SF>1.5XULN was independently associated with advanced hepatic fibrosis (OR, 1.66, 95% CI, 1.05-2.62, p=0.028) and increased NAFLD Activity Score (NAS) (OR, 1.99, 95% CI, 1.06-3.75, p=0.033).
Conclusions
A SF >1.5XULN is associated with hepatic iron deposition, a diagnosis of NASH, and worsened histologic activity, and is an independent predictor of advanced hepatic fibrosis among patients with NAFLD. Furthermore, elevated SF is independently associated with higher NAS even among patients without hepatic iron deposition. We conclude that serum ferritin is useful to identify NAFLD patients at risk for NASH and advanced fibrosis.
Keywords: NAFLD, Steatohepatitis
Introduction
NAFLD is now recognized as the most common cause of liver disease and may be present in up to 20% of the U.S. population (1). Expression of ferritin, the primary tissue iron storage protein in the liver, where most extra body iron is stored, is induced in primary or secondary iron overload disorders, resulting in increased hepatic and circulating ferritin levels (2). However, ferritin is also an acute phase protein and can also be induced in the setting of systemic inflammation (3;4). Hyperferritinemia has been previously observed in obesity-related chronic inflammatory conditions such as diabetes and the metabolic syndrome (5–8). We and others have previously reported that serum ferritin may be increased in the general population in relationship to alcohol consumption, as well as in chronic liver disease due to hepatitis C and alcohol (9–16). In such conditions, serum ferritin elevation may or may not be accompanied by increased hepatic iron deposition (10–12;15;16). A number of in vitro and in vivo studies in hepatocytes and liver tissue suggest that inflammatory stimuli, particularly the pro-inflammatory cytokine TNFα, upregulates ferritin (17–23). This effect is additive upon addition of iron (24). Oxidative stress may also up-regulate ferritin depending on the specific oxidant stimuli (25–29) at the level of transcription and translation (24;25;27;30). Therefore, it is plausible that serum ferritin may reflect increased disease severity in NAFLD either due to increased ongoing hepatic or systemic inflammation or increased body iron stores, or a combination of these factors. Previous studies examining the relationship between serum ferritin level and histologic severity in NAFLD have found conflicting results, with some authors reporting that serum ferritin levels are associated with increased histologic severity and presence of NASH, while others have not (31–43). We have previously described that patients with NASH have significantly higher median serum ferritin levels compared to those with NAFLD (44). The goals of the current study were to: examine the relationship of serum ferritin to clinical, histologic, laboratory and anthropometric data in adult patients with NAFLD enrolled in the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN); to identify whether specific threshold levels of serum ferritin identify patients with more advanced disease; and to examine the relationship between serum ferritin level, hepatic iron stores and histologic features among these subjects.
MATERIALS AND METHODS
A total of 1,635 subjects were enrolled in the NASH CRN Database, PIVENS and TONIC studies between 2004 and 2008 - per inclusion criteria described elsewhere (44–46). Out of these, subjects that did not meet the following criteria were excluded: A) age less than 18 yrs of age at time of biopsy (368 subjects); B) lack of liver biopsy sample for central pathology review by the NASH CRN Pathology Committee (182 subjects); C) serum ferritin value not available within 6 months of biopsy (418 subjects); D) lack of NAFLD diagnosis due to less than 5% steatosis (39 subjects). The remaining 628 subjects constituted our study group. Patients with known hemochromatosis (defined as presence of hepatic iron index [hepatic iron (µmol/g)/age(years)] ≥1.9 or removal of more than 4 g of iron by phlebotomy), C282Y homozygosity for the HFE gene or unexplained substantial hepatic iron overload (≥3+ stainable iron on liver biopsy) were excluded from all NASH CRN studies. Demographic information such as age, sex, ethnicity, and race and medical history to identify co-morbidities and medications were obtained from patient interviews during screening. A physical exam including body weight and height measures was performed. Laboratory data including hepatic, hematologic, metabolic, lipid and serum iron assessments were analyzed for subjects with values collected within 6 months of the liver biopsy. Total dietary consumption of iron, vitamin C, tea and coffee were determined from the Block 98 food frequency questionnaire; (NutritionQuest, Berkeley, CA); alcohol consumption was determined from the AUDIT-C questionnaires completed during study visits closest to the time of biopsy (47). In addition, 552 of the 628 (88%) subjects included in this study had available hepatic iron staining which was routinely evaluated as part of the liver histologic assessment performed centrally by the NASH CRN Pathology Committee.
Histological assessment
Histologic features of NAFLD and iron accumulation were assessed by the Pathology Committee of the NASH CRN in a centralized consensus review format. Pathologists were blinded to all clinical, laboratory and demographic information. NAFLD features were scored as previously described (48). Semi-quantitative grading and pattern of hepatic iron staining using Perls’ iron stain was scored by the Pathology Committee as described (49).
Statistical analysis
The relationship between serum ferritin (SF) and variables of interest was examined with SF as a dichotomous variable, defined a priori to be practical in a clinical setting; ≤1.5XULN vs. >1.5XULN (ULN was defined as <200 ng/ml in women and <300 ng/ml in men, values adopted by the Hemochromatosis and Iron Overload Screening Study (HEIRS)(50). Baseline demographic, clinical, and laboratory characteristics were recorded as number and percentage for categorical data and means and standard deviation or medians and interquartile range for continuous data. Continuous variables including laboratory measures that were not normally distributed were analyzed using the Wilcoxon rank sum test. Categorical variables including histological features such as fibrosis stage, steatosis and lobular inflammation grade were analyzed using either Fisher’s exact or Chi-square tests where appropriate. Stepwise forward multivariate logistic regression was used to examine the relationship between threshold SF levels SF>ULN, SF>1.5XULN and SF>2.5XULN and presence of advanced fibrosis after controlling for the effect of the following variables selected a priori: age at biopsy, sex, presence of diabetes, BMI and ALT. Stepwise ordinal regression was used to examine the relationship between the threshold SF>1.5XULN levels and the NAFLD Activity Score (NAS) after controlling for the above covariates, separately among patients with each of the three distinct hepatic iron deposition patterns (49). Variables not independently associated with the dependant variable using a threshold p value of p≤0.20 were excluded from all models. All analyses were performed using STATA (version 9, College Station, TX, USA). Nominal, two-sided p-values were used and were considered to be statistically significant if p<0.05; no adjustments for multiple comparisons were made.
RESULTS
Patient characteristics
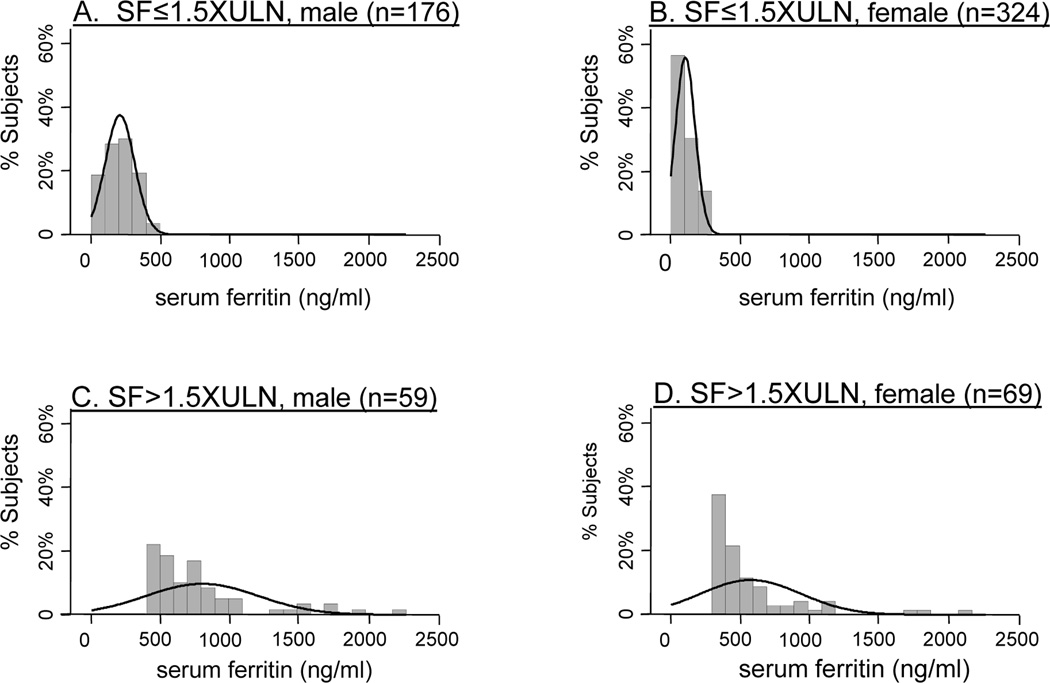
A total of 628 NASH CRN adult subjects (age≥18 years) with biopsy-proven NAFLD (defined as >5% steatosis) and a serum ferritin measurement within six months of their liver biopsy were evaluated in the present study. The distribution of SF values for the study cohort is presented graphically in Figure 1. Subjects were divided according to sex and threshold SF value of 1.5XULN (i.e., >300 ng/ml in women and >450 ng/ml in men). The majority of women tended to be in the lower range of this threshold (see Figure 1 B and D). In contrast, males were more evenly distributed throughout each threshold group (see Figure 1 A and C). More than half of all subjects (324 of 628) were women with SF≤1.5XULN. A comparison of clinical and demographic data for subjects with SF≤1.5XULN to those with SF>1.5XULN is shown in Table 1. Approximately 20% (128 of 628) of the study population had SF values >1.5XULN. There were 84 subjects (13%) which had SF>ULN but ≤1.5XULN (data not shown). The majority of patients with SF>1.5XULN were female (54%), however a greater proportion of males had SF>1.5XULN compared to females (25% vs. 18%, p=0.02). Increased SF was associated with lower BMI (p=0.004). There were no other significant differences in age at biopsy, race or ethnicity, or the presence of diabetes or the metabolic syndrome between normal and elevated SF groups. The proportion of subjects carrying HFE mutations was not significantly different between SF categories including SF>2.5XULN (Table 1 and data not shown).
Figure 1. Histogram of distribution of serum ferritin values according to sex, above and below the threshold SF>1.5XULN.
The relative proportion of subjects by serum ferritin values are shown for each of the four categories: A) SF≤1.5XULN, male; B) SF≤1.5XULN, female; C) SF>1.5XULN, male; D) SF>1.5XULN, female. Each bin corresponds to a range of SF values of 100ng/ml. The density estimation plot is shown by the smooth line.
Table 1.
Characteristics of patients above and below SF 1.5ULN
| Characteristic† | SF≤1.5ULN (N=500) |
SF>1.5ULN (N=128) |
Total (N=628) |
P value* |
|---|---|---|---|---|
| Sex [n (%)] | 0.02 | |||
| Male | 176 (75) | 59 (25) | 235 (37) | |
| Female | 324 (82) | 69 (18) | 393 (63) | |
| Age mean (years) | 47.7 ± 11.8 | 47.4 ± 11.8 | 47.7 ± 11.8 | 0.14 |
| Race [n (%)] | 0.14 | |||
| White | 404 (79) | 106 (21) | 510 (81) | |
| Black | 14 (93) | 1 (7) | 15 (2) | |
| Asian | 17 (65) | 9 (35) | 26 (4) | |
| American Indian/Alaskan Native | 17 (77) | 5 (23) | 22 (4) | |
| Other | 48 (87) | 7 (13) | 55 (9) | |
| Ethnicity [n (%)] | 0.30 | |||
| Non-Hispanic | 432 (79) | 115 (21) | 547 (87) | |
| Hispanic | 68 (84) | 13 (16) | 81 (13) | |
| BMI mean (kg/m2) | 34.4 ± 6.5 | 32.6 ± 5.5 | 34.0 ± 6.3 | 0.004 |
| BMI category [n (%)] | 0.01 | |||
| <25 (kg/m2) | 23 (79) | 6 (21) | 23 (1.3) | |
| 25<30 (kg/m2) | 110 (71) | 45 (29) | 148 (23) | |
| ≥30 (kg/m2) | 366 (83) | 77 (17) | 462 (73) | |
| Waist-hip ratio | 0.93 ± 0.08 | 0.94 ± 0.07 | 0.93 ± 0.07 | 0.15 |
| Diabetes diagnosis [n (%)] | 128 (78) | 36 (22) | 164 (26) | 0.56 |
| HFE genotype# | ||||
| WT/WT | 244 (81) | 57 (19) | 301 (61) | reference |
| C282Y/WT | 40 (77) | 12 (23) | 52 (11) | 0.49 |
| H63D/WT | 99 (76) | 31 (24) | 124 (25) | 0.25 |
| H63D/H63D | 6 (67) | 3 (33) | 9 (1.8) | 0.38 |
| C282Y/H63D | 3 (50) | 3 (50) | 6 (1.2) | 0.09 |
| Metabolic syndrome [n (%)] | 344 (80) | 88 (20) | 432 (69) | 0.99 |
| No of alcoholic drinks /week | 0.6 ± 1.3 | 0.8 ± 1.7 | 0.6 ± 1.4 | 0.67 |
| No of coffee/tea drinks /day | 1.7 ± 1.5 | 1.5 ± 1.4 | 1.7 ± 1.5 | 0.27 |
| Dietary iron consumed (mg/day) | 14 ± 8 | 14 ± 8 | 14 ± 8 | 0.64 |
| Dietary vitamin C (mg/day) | 107 ± 82 | 98 ± 72 | 105 ± 80 | 0.40 |
ULN=Serum ferritin upper limit of normal; defined as 200mg/dL in females and 300mg/dL in males
Values are number and percentage or mean ± SD
P values from Chi square or Fisher’s exact test for categorical variables; Wilcoxon rank sum test for continuous variables.
HFE genotype available in 492 subjects; P values compared to WT/WT
Relationship between laboratory data and SF level
Differences in laboratory tests between patients with SF≤1.5XULN vs. SF>1.5XULN are shown in Table 2. Patients with SF>1.5XULN have higher serum AST, ALT, GGT, direct bilirubin and lower platelet count (p<0.02). In contrast, metabolic abnormalities including fasting insulin, glucose, HOMA-IR and lipid levels were not different between groups (data not shown). As was expected, patients with hyperferritinemia had higher serum iron studies including iron, ferritin and percent transferrin-iron saturation (TS) and lower total iron binding capacity (TIBC), (p<0.0001).
Table 2.
Laboratory value differences among patients above and below SF 1.5ULN
| Variable* | SF≤1.5ULN (N=500) |
SF>1.5ULN (N=128) |
P value† |
|---|---|---|---|
| AST (U/L) | 43 (31–62) | 58 (35–85) | <0.0001 |
| ALT (U/L) | 58 (38–85) | 81 (53–132) | <0.0001 |
| Gamma glutamyl transferase (U/L) | 43 (28–77) | 59 (37–114) | 0.0001 |
| Direct bilirubin (mg/dL) | 0.10 (0.10–0.20) | 0.10 (0.10–0.20) | 0.02 |
| Platelets (K/cmm) | 246 (203–287) | 230 (195–273) | 0.015 |
| Hemoglobin (g/dL) | 14.3 (13.4–15.2) | 15.0 (13.8–15.7) | 0.0001 |
| Serum iron (μg/dL) | 84 (65–106) | 106 (80–128) | <0.0001 |
| Total iron binding capacity (μg/dL) | 371 (331–417) | 345 (301–388) | <0.0001 |
| Transferrin saturation (iron/TIBC) | 0.22 (0.17–0.29) | 0.29 (0.23–0.41) | <0.0001 |
| Serum ferritin (ng/mL) | 121 (71–216) | 547 (417–777) | <0.0001 |
Values are medians (IQR)
P values from Wilcoxon rank sum test.
Relationship between SF level and histologic severity of NAFLD
As shown in Table 3, increased histologic severity of NAFLD was associated with higher SF values. A higher proportion of patients with SF>1.5XULN showed increased severity of steatosis, lobular inflammation, hepatocellular ballooning and fibrosis compared to patients with SF≤1.5XULN (p≤0.026). Subjects with SF >1.5XULN were also more likely to have a definite diagnosis of NASH than those with SF ≤1.5XULN (70% vs. 59%, p=0.017). The proportion of subjects with advanced (stage 3 or 4) fibrosis at higher SF levels was 32% of SF>1.5XULN, 42% SF>2.5XULN and 34% SF>3.5XULN. To investigate whether various threshold levels of elevated SF were independently associated with advanced fibrosis, we used a multivariate stepwise logistic regression analysis including the following variables selected a priori, known to be associated with severity of NAFLD: age at biopsy, sex, BMI, presence of diabetes and ALT. Three separate models were created using SF as a dichotomous independent variable, as follows: A) SF >ULN, B) SF >1.5XULN, C) SF >2.5XULN. As shown in Table 4, there was a progressive trend towards an independent association between increasing SF level and advanced hepatic fibrosis. Both SF>1.5XULN and >2.5XULN were independent predictors of advanced fibrosis (OR 1.67, p=0.028 and OR 2.46, p=0.005, respectively) using logistic regression modeling controlling for BMI, age, sex, type 2 diabetes and serum ALT. In all three models, age, BMI and presence of diabetes were the only other variables independently associated with advanced hepatic fibrosis (p<0.002).
Table 3.
Histologic features among subjects above and below SF 1.5ULN
| Characteristic | SF≤1.5ULN (N=500) |
SF>1.5ULN (N=128) |
P value† |
|---|---|---|---|
| Steatosis grade [n (%)] | <0.001 | ||
| 5–33% | 215 (43) | 37 (29) | |
| 34–66% | 180 (36) | 34 (27) | |
| >66% | 105 (21) | 57 (45) | |
| Lobular inflammation [n (%)] | 0.026 | ||
| <2 under 20x | 266 (53) | 53 (41) | |
| 2–4 under 20x | 184 (37) | 54 (42) | |
| >4 under 20x | 50 (10) | 21 (16) | |
| Hepatocellular ballooning [n (%)] | 0.004 | ||
| None | 169 (34) | 30 (23) | |
| Mild | 129 (26) | 32 (25) | |
| More than mild | 202 (40) | 66 (52) | |
| Fibrosis stage* [n (%)] | <0.001 | ||
| None | 141 (28) | 19 (15) | |
| Mild to moderate, zone 3, perisinusoidal, or portal/periportal only | 151 (30) | 31 (24) | |
| Zone 3 and periportal, any combination | 85 (17) | 36 (28) | |
| Bridging | 78 (16) | 34 (27) | |
| Cirrhosis | 42 (8) | 7 (6) | |
| NASH diagnosis [n (%)] | 0.013 | ||
| No NASH | 111 (22) | 14 (11) | |
| Suspicious/borderline | 95 (19) | 24 (19) | |
| Definite | 294 (59) | 90 (70) |
Values are number and percentage
P values from Chi square or Fisher’s exact test
4 subjects were not scored for fibrosis
TABLE 4.
Independent predictors of advanced fibrosis on multivariate logistic regression modeling using different SF ULN levels.
| OR | 95% Conf. Int. | P value | |
|---|---|---|---|
| Model 1 (SF >ULN) | |||
| Diabetes present | 1.94 | 1.29 – 2.90 | 0.001 |
| Age, (yrs) | 1.05 | 1.03 – 1.07 | <0.001 |
| BMI mean (kg/m2) | 1.05 | 1.02 – 1.08 | 0.002 |
| Model 2 (SF >1.5ULN) | |||
| Diabetes present | 1.90 | 1.27 – 2.85 | 0.002 |
| SF >1.5ULN | 1.67 | 1.06 – 2.85 | 0.028 |
| Age, (yrs) | 1.05 | 1.03 – 1.07 | <0.001 |
| BMI mean (kg/m2) | 1.05 | 1.02 – 1.08 | 0.001 |
| Model 3 (SF >2.5ULN) | |||
| SF >2.5ULN | 2.46 | 1.30 – 4.64 | 0.005 |
| Diabetes present | 1.90 | 1.27 – 2.85 | 0.002 |
| Age, (yrs) | 1.05 | 1.03 – 1.07 | <0.001 |
| BMI mean (kg/m2) | 1.05 | 1.02 – 1.08 | 0.001 |
Each SF ULN cut-off was modeled individually including potential confounding variables age, sex, BMI, ALT, and presence of diabetes using stepwise forward multivariate logistic regression. Variables not independently associated with the advanced fibrosis on univariate analysis using a threshold P value of P≤0.20 were excluded from all models.
Relationship of serum ferritin and hepatic iron deposition pattern
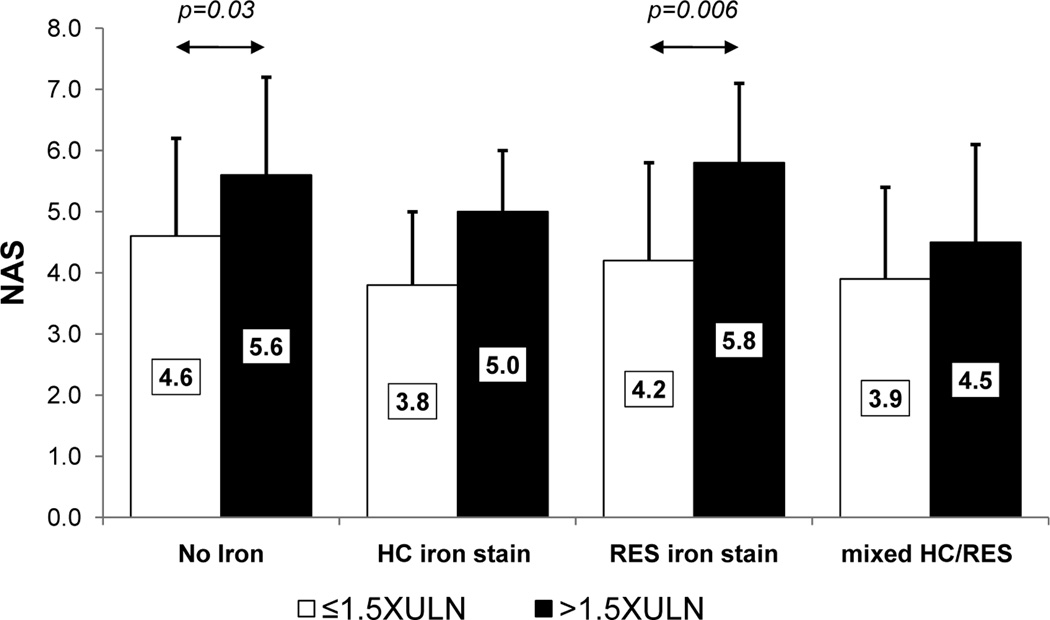
As described above, patients with elevated SF were also more likely to have increased serum iron and TS compared to those with SF≤ULN. Therefore, it is possible that increased hepatic iron loading may be responsible for the increased severity of liver disease among patients with elevated SF. We have previously shown that the presence and pattern of stainable hepatic iron was associated with advanced histologic features among patients with NASH; in addition, NAFLD patients with reticuloendothelial system (RES) iron deposition were more likely to have NASH and advanced fibrosis while those with hepatocellular (HC) iron were more likely to have milder disease (49). We therefore examined the relationship between SF level and stainable hepatic iron in the current cohort using ordinal regression. Serum ferritin >1.5XULN was significantly associated with greater iron content in both RES (OR, 2.22, 95% CI, 1.78-2.76, p<0.001) or HC cells (OR, 1.5, 95% CI, 1.03-1.91, p<0.001) compared to those with lower SF levels. To determine whether SF could identify patients with more advanced disease even in the absence of hepatic iron loading, we compared the NAS separately among patients with each of the three distinct hepatic iron deposition patterns using ordinal regression modeling after controlling for the covariates BMI, age, sex, type 2 diabetes and serum ALT. Shown in Figure 2 is a comparison of the mean differences of NAS for subjects above and below SF 1.5XULN according to each hepatic iron deposition pattern: HC only, RES only and mixed HC/RES. SF>1.5XULN was independently associated with increased NAS in both subjects without hepatic iron deposits (OR, 1.99, 95% CI, 1.06-3.75, p=0.033) and in subjects with RES iron stain only (OR, 4.59, 95% CI, 1.54-13.65, p=0.006).
Figure 2. Relationship between NAS, level of serum ferritin and presence and pattern of iron staining among subjects.
The mean NAS values are shown for subjects above and below SF 1.5XULN according to each hepatic iron deposition pattern including subjects without iron deposition; HC, RES and mixed HC/RES groups. Significant differences between groups, determined using multivariate ordinal regression modeling after controlling for the covariates BMI, age, sex, type 2 diabetes and serum ALT level, are indicated by arrows. Standard deviations are indicated by the error bars.
DISCUSSION
We have shown in the present study that serum ferritin has value as a non-invasive marker in identifying patients with NAFLD who are at increased risk of more advanced disease as indicated by a higher NAS, even among patients without hepatic iron deposition. Furthermore, we have found that SF>1.5XULN is independently associated with increased risk of advanced fibrosis in NAFLD patients. Our results indicate that use of a SF value >1.5XULN can add diagnostic value to the evaluation of patients with NAFLD. Unlike other case-control or epidemiologic survey studies making comparisons to non-disease control subjects, we did not find any differences in the presence of diabetes or the metabolic syndrome between normal and elevated SF groups, presumably due to the large proportion of NASH CRN subjects with these conditions (5–8). BMI and SF were inversely related in our study. We speculate that this may be related to production of hepcidin, the iron regulatory hormone, from adipose tissue (51). Hepcidin reduces iron absorption and recycling by binding to and internalizing the iron export protein ferroportin in enterocytes, macrophages and hepatocytes (52). Therefore, patients with higher BMI and thus more body fat, would have more circulating hepcidin due to secretion from an expanded fat mass. This in turn would result in lower body iron stores and an associated decreased SF level.
A number of previous studies have examined the frequent presence of hyperferritinemia with or without increased hepatic iron storage in patients with NAFLD (31–43). It was suggested that increased hepatic iron stores possibly via mutations in HFE, the hemochromatosis gene, may lead to NASH and advanced fibrosis in NAFLD via increased oxidative stress (53). In addition, recent murine studies suggest that hepatic iron loading or Hfe mutations in combination with a high fat diet result in upregulation of genes involved in cholesterol or lipid biosynthesis, providing another mechanism relating iron with NAFLD (54, 55). Another early study suggested that a large proportion of subjects referred for evaluation of hyperferritinemia had NASH, increased hepatic iron stores, features of metabolic syndrome despite normal TS and absence of HFE mutations (34). The emerging understanding of the intersection between abnormal iron metabolism and NAFLD has proved more complex, demonstrating that many patients with NAFLD had increased serum ferritin levels in the absence of increased hepatic iron stores or increased prevalence of HFE mutations (31;32;34;42;49;56). In the current study, hyperferritinemia was associated with a higher HC and RES iron stain grade, however, there was no association with HFE genotype and SF>1.5ULN was associated with a higher NAS even among subjects negative for iron staining. In comparison, Bugianesi and colleagues found serum ferritin to be a marker of increased histologic severity, but not of increased hepatic iron concentration (32). Most NAFLD patients in their study were lean males and information about features of metabolic syndrome was not reported; finally serum ferritin elevations appeared to be modest compared to the current series. The US population enrolled in the NASH CRN studies by contrast, is predominantly overweight or obese and has features of metabolic syndrome (44–46). These may explain the differences among our studies. It is noteworthy that the clinical and demographic features of a more recent cohort study from Italy examining risk factors for NASH and advanced fibrosis appear much more similar to the NASH CRN (35). The authors describe a similar relationship between multiples of SF level>ULN, NASH diagnosis and severity of hepatic fibrosis. Although the relationship between SF level and advanced histologic features was not examined separately among patients with and without stainable iron, the similarities between these two studies suggest our results may be applicable to other populations with NAFLD. In fact, recent studies in smaller cohorts from Japan and the UK have found similar results (38, 42).
Although several previous reports have attempted to create discriminant models to identify the presence or absence of severe disease, these are not widely used because of the need for specialized data (i.e. type IV collagen (40)) or need for use of a complex model that requires multiple variables (33, 57). Although serum ferritin has been identified as an independent marker for advanced fibrosis in regression models, previous studies have not examined whether threshold SF levels may provide value to clinicians. The current study provides evidence that serum ferritin may be a simple and useful adjunctive marker to clinicians in evaluation of patients with NAFLD. Another novel aspect of our study is the observation that elevated SF may identify patients with NAFLD who have more severe disease independent of the presence of iron overload. Furthermore, among patients with stainable hepatic iron, elevated serum ferritin was a marker for more severe disease.
It is unclear if hyperferritinemia in NAFLD is simply a consequence of disease severity or actively contributes to disease progression in NASH. Indeed, a number of signals which are thought to mediate NASH pathogenesis are also known to upregulate ferritin including pro-inflammatory cytokines TNFα (17–19, 23), IL-1β(18, 20, 23) and NFκB (21, 22) and oxidative stress (25–29). However, recent research suggests there are a number of potential mechanisms whereby ferritin could actively contribute to NASH pathogenesis. For example, ferritin has been shown to inhibit secretion of apolipoprotein B in vitro (58); an observation which may in part explain the association between ferritin level, degree of steatosis and increased NAS in this study through decreased VLDL secretion and increased lipotoxicity (59). Hepatocyte secreted ferritin has been shown to promote FAS-mediated apoptosis (60). Interestingly, a recent study has demonstrated that ferritin can act as a pro-inflammatory cytokine in activated hepatic stellate cells, in an iron-independent fashion, through induction of a signaling cascade involving PI3K, PKCζ, MEK1/2 and MAPK (61). This in turn resulted in phosphorylation of IKKα/β, activation of the p50/p65 NFκB heterodimer and the subsequent increased expression of NFκB-responsive genes known to be involved in hepatic fibrogenesis including IL-1β, iNOS , RANTES and ICAM1 (61). In summary, ferritin may be intimately involved in many important processes related to NASH pathogenesis including inflammation, apoptosis, oxidative stress, lipid transport and fibrogenesis.
Serum ferritin has also recently been shown to be a predictor of mortality in patients with end-stage liver disease (ESLD) both before (62, 63) and after liver transplant (63). Using SF cut-off values similar to the present study, these large multicenter studies have shown that hyperferritinemia with ESLD from all etiologies is associated with increased mortality after adjusting for a variety of potential confounding variables.
We recognize limitations to the current study. It can be argued that the threshold SF levels were arbitrarily selected. This is an inherent limitation with any study using “cut-off” levels. However, we set these threshold levels a priori and not from a post-hoc analysis. While we have adjusted the ULN in this study to be sex-specific, we recognize that in practice the “normal” reference range does vary somewhat between clinical labs and may also be influenced by age, facts that should be kept in mind when interpreting absolute SF values in NAFLD patients.
In summary, we have shown that elevated SF>1.5XULN is associated with hepatic iron deposition, a diagnosis of NASH, and worsened histologic activity, and is an independent predictor of advanced hepatic fibrosis among patients with NAFLD. We suggest that serum ferritin, an inexpensive and convenient clinical test, should be included in the laboratory evaluation of NAFLD patients.
Acknowledgments
Source of funding
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD). KVK is also supported by NIH grants K24DK002957 and R56DK087696.
Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, M01RR00188, UL1RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359).
This work was supported in part by the Intramural Research Program of the National Cancer Institute.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NAS
NAFLD activity score
- SF
serum ferritin
- TS
transferrin saturation
Reference List
- 1.McCullough AJ. The epidemiology and risk factors of NASH. In: Farrell GC, George J, de la Hall P, et al., editors. Fatty Liver Disease: NASH and Related Disorders Malden. MA: Blackwell Publishing; 2005. pp. 23–37. [Google Scholar]
- 2.Arosio P, Ingrassia R, Cavadini P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant. 2004;19:141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 4.Konijn AM. Iron metabolism in inflammation. Baillieres Clin Haematol. 1994;7:829–849. doi: 10.1016/s0950-3536(05)80127-1. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 6.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27:2422–2428. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- 7.Kaye TB, Guay AT. Increased serum ferritin levels in patients with diabetes mellitus. Mayo Clin Proc. 1994;69:498–499. doi: 10.1016/s0025-6196(12)61655-3. [DOI] [PubMed] [Google Scholar]
- 8.Tsimihodimos V, Gazi I, Kalaitzidis R, Elisaf M, Siamopoulos KC. Increased serum ferritin concentrations and liver enzyme activities in patients with metabolic syndrome. Metab Syndr Relat Disord. 2006;4:196–203. doi: 10.1089/met.2006.4.196. [DOI] [PubMed] [Google Scholar]
- 9.Bell H, Skinningsrud A, Raknerud N, Try K. Serum ferritin and transferrin saturation in patients with chronic alcoholic and non-alcoholic liver diseases. J Intern Med. 1994;236:315–322. doi: 10.1111/j.1365-2796.1994.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 10.Chapman RW, Morgan MY, Laulicht M, Hoffbrand AV, Sherlock S. Hepatic iron stores and markers of iron overload in alcoholics and patients with idiopathic hemochromatosis. Dig Dis Sci. 1982;27:909–916. doi: 10.1007/BF01316575. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza RF, Feakins R, Mears L, Sabin CA, Foster GR. Relationship between serum ferritin, hepatic iron staining, diabetes mellitus and fibrosis progression in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2005;21:519–524. doi: 10.1111/j.1365-2036.2005.02382.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara F, Ventura P, Vegetti A, Guido M, Abbati G, Corradini E, et al. Serum ferritin as a predictor of treatment outcome in patients with chronic hepatitis C. Am J Gastroenterol. 2009;104:605–616. doi: 10.1038/ajg.2008.126. [DOI] [PubMed] [Google Scholar]
- 13.Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293–1301. doi: 10.1053/j.gastro.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Olynyk JK, Knuiman MW, Divitini ML, Bartholomew HC, Cullen DJ, Powell LW. Effects of HFE gene mutations and alcohol on iron status, liver biochemistry and morbidity. J Gastroenterol Hepatol. 2005;20:1435–1441. doi: 10.1111/j.1440-1746.2005.03967.x. [DOI] [PubMed] [Google Scholar]
- 15.Tung BY, Emond MJ, Bronner MP, Raaka SD, Cotler SJ, Kowdley KV, Hepatitis C. iron status, and disease severity: relationship with HFE mutations. Gastroenterology. 2003;124:318–326. doi: 10.1053/gast.2003.50046. [DOI] [PubMed] [Google Scholar]
- 16.Valenti L, Pulixi EA, Arosio P, Cremonesi L, Biasiotto G, Dongiovanni P, et al. Relative contribution of iron genes, dysmetabolism and hepatitis C virus (HCV) in the pathogenesis of altered iron regulation in HCV chronic hepatitis. Haematologica. 2007;92:1037–1042. doi: 10.3324/haematol.11281. [DOI] [PubMed] [Google Scholar]
- 17.Miller LL, Miller SC, Torti SV, Tsuji Y, Torti FM. Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc Natl Acad Sci U S A. 1991;88:4946–4950. doi: 10.1073/pnas.88.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnov IM, Bailey K, Flowers CH, Garrigues NW, Wesselius LJ. Effects of TNF-alpha and IL-1beta on iron metabolism by A549 cells and influence on cytotoxicity. Am J Physiol. 1999;277:L257–L263. doi: 10.1152/ajplung.1999.277.2.L257. [DOI] [PubMed] [Google Scholar]
- 19.Torti SV, Kwak EL, Miller SC, Miller LL, Ringold GM, Myambo KB, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638–12644. [PubMed] [Google Scholar]
- 20.Wei Y, Miller SC, Tsuji Y, Torti SV, Torti FM. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289–296. doi: 10.1016/0006-291x(90)91466-6. [DOI] [PubMed] [Google Scholar]
- 21.Kwak EL, Larochelle DA, Beaumont C, Torti SV, Torti FM. Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem. 1995;270:15285–15293. doi: 10.1074/jbc.270.25.15285. [DOI] [PubMed] [Google Scholar]
- 22.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Tran TN, Eubanks SK, Schaffer KJ, Zhou CY, Linder MC. Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood. 1997;90:4979–4986. [PubMed] [Google Scholar]
- 24.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 25.Cairo G, Tacchini L, Pogliaghi G, Anzon E, Tomasi A, Bernelli-Zazzera A. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the "free" iron pool. J Biol Chem. 1995;270:700–703. doi: 10.1074/jbc.270.2.700. [DOI] [PubMed] [Google Scholar]
- 26.Jang MK, Choi MS, Park YB. Regulation of ferritin light chain gene expression by oxidized low-density lipoproteins in human monocytic THP-1 cells. Biochem Biophys Res Commun. 1999;265:577–583. doi: 10.1006/bbrc.1999.1725. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji Y, Ayaki H, Whitman SP, Morrow CS, Torti SV, Torti FM. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol Cell Biol. 2000;20:5818–5827. doi: 10.1128/mcb.20.16.5818-5827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson J, Pietsch EC, Torti SV, Torti FM. Ferritin regulation by oxidants and chemopreventive xenobiotics. Adv Enzyme Regul. 2003;43:135–151. doi: 10.1016/s0065-2571(02)00037-7. [DOI] [PubMed] [Google Scholar]
- 29.Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress. Biochem J. 2001;357:241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White K, Munro HN. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988;263:8938–8942. [PubMed] [Google Scholar]
- 31.Brudevold R, Hole T, Hammerstrom J. Hyperferritinemia is associated with insulin resistance and fatty liver in patients without iron overload. PLoS ONE. 2008;3:e3547. doi: 10.1371/journal.pone.0003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bugianesi E, Manzini P, D'Antico S, Vanni E, Longo F, Leone N, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–187. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 33.Cales P, Boursier J, Chaigneau J, Laine F, Sandrini J, Michalak S, et al. Diagnosis of different liver fibrosis characteristics by blood tests in non-alcoholic fatty liver disease. Liver Int. 2010;30:1346–1354. doi: 10.1111/j.1478-3231.2010.02314.x. [DOI] [PubMed] [Google Scholar]
- 34.Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, et al. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:2448–2455. doi: 10.1111/j.1572-0241.2001.04052.x. [DOI] [PubMed] [Google Scholar]
- 35.Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, et al. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244–1249. doi: 10.1016/j.jhep.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao TJ, Chen JC, Wang JD. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord. 2004;28:167–172. doi: 10.1038/sj.ijo.0802519. [DOI] [PubMed] [Google Scholar]
- 37.Licata A, Nebbia ME, Cabibbo G, Iacono GL, Barbaria F, Brucato V, et al. Hyperferritinemia is a risk factor for steatosis in chronic liver disease. World J Gastroenterol. 2009;15:2132–2138. doi: 10.3748/wjg.15.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011;31:730–739. doi: 10.1111/j.1478-3231.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa T, Muramoto Y, Hori M, Mihara S, Marubayashi T, Nakagawa K. A preliminary investigation of the association between haptoglobin polymorphism, serum ferritin concentration and fatty liver disease. Clin Chim Acta. 2008;398:34–38. doi: 10.1016/j.cca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 41.Valenti L, Swinkels DW, Burdick L, Dongiovanni P, Tjalsma H, Motta BM, et al. Serum ferritin levels are associated with vascular damage in patients with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Yoneda M, Nozaki Y, Endo H, Mawatari H, Iida H, Fujita K, et al. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig Dis Sci. 2010;55:808–814. doi: 10.1007/s10620-009-0771-y. [DOI] [PubMed] [Google Scholar]
- 43.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700–707. doi: 10.1016/j.jhep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- 48.Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 49.Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, et al. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 51.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 53.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 54.Graham RM, Chua AC, Carter KW, Delima RD, Johnstone D, Herbison CE, et al. Hepatic iron loading in mice increases cholesterol biosynthesis. Hepatology. 2010;52:462–471. doi: 10.1002/hep.23712. [DOI] [PubMed] [Google Scholar]
- 55.Tan TC, Crawford DH, Jaskowski LA, Murphy TM, Heritage ML, Subramaniam VN, et al. Altered lipid metabolism in Hfe-knockout mice promotes severe NAFLD and early fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011 Aug 4; doi: 10.1152/ajpgi.00150.2011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 58.Hevi S, Chuck SL, et al. Ferritins can regulate the secretion of apolipoprotein B. J Biol Chem. 2003;278:31924–31929. doi: 10.1074/jbc.M303081200. [DOI] [PubMed] [Google Scholar]
- 59.Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 60.Bresgen N, Ohlenschlager I, Fiedler B, Wacht N, Zach S, Dunkelmann B, et al. Ferritin--a mediator of apoptosis? J Cell Physiol. 2007;212:157–164. doi: 10.1002/jcp.21009. [DOI] [PubMed] [Google Scholar]
- 61.Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology. 2009;49:887–900. doi: 10.1002/hep.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker NM, Stuart KA, Ryan RJ, Desai S, Saab S, Nicol JA, et al. Serum ferritin concentration predicts mortality in patients awaiting liver transplantation. Hepatology. 2010;51:1683–1691. doi: 10.1002/hep.23537. [DOI] [PubMed] [Google Scholar]
- 63.Weismüller TJ, Kirchner GI, Scherer MN, Negm AA, Schnitzbauer AA, Lehner F, et al. Serum ferritin concentration and transferrin saturation before liver transplantation predict decreased long-term recipient survival. Hepatology. 2011 Sep 2; doi: 10.1002/hep.24635. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]